Abstract
The zebrafish is a valuable model for teaching developmental, molecular, and cell biology; aquatic sciences; comparative anatomy; physiology; and genetics. Here we demonstrate that zebrafish provide an excellent model system to teach engineering principles. A seven-member undergraduate team in a biomedical engineering class designed, built, and tested a zebrafish microfluidic bioreactor applying microfluidics, an emerging engineering technology, to study zebrafish development. During the semester, students learned engineering and biology experimental design, chip microfabrication, mathematical modeling, zebrafish husbandry, principles of developmental biology, fluid dynamics, microscopy, and basic molecular biology theory and techniques. The team worked to maximize each person's contribution and presented weekly written and oral reports. Two postdoctoral fellows, a graduate student, and three faculty instructors coordinated and directed the team in an optimal blending of engineering, molecular, and developmental biology skill sets. The students presented two posters, including one at the Zebrafish meetings in Madison, Wisconsin (June 2008).
Introduction
Our objective was to provide a team of students in a biomedical engineering (BME) design course with an opportunity to apply bioengineering/microfluidic experimental approaches to some important developmental biological problems, using the zebrafish as a model system. Our laboratory and colleagues have been studying development and regeneration of the nervous system1–3 and have recently found that there is important cross-talk between the immune system and the nervous system.2,4,5 Immune system inflammatory cytokines, which are vital for T-cell maturation and function,6 also act as neurotrophins in the developing nervous system (Shen et al., unpublished observations; Gerlach-Bank et al., unpublished observations; Ramamurthy et al., unpublished observations; Ebisu et al., unpublished observations). Secreted cytokines act on cells at a distance, and their effects on early nervous system development are well documented.7–14 However, their effects on whole embryos immersed in varying concentrations of the cytokines or with circumscribed regions of the embryo's exterior exposed to the same concentrations of cytokines have not been examined.
We wanted to present some technological,15–17 theoretical, and mathematical/statistical approaches that would be of use to students in their future research endeavors in both engineering and biology. We also tried to impart experimental design skills in both disciplines and at the interdisciplinary interface and to emphasize the importance of a thorough literature search before beginning any project.
The project we offered in a multi-team BME senior undergraduate design class was based on the general observation that morphogenetic growth factors, including the bone morphogenetic protein 4 antagonist noggin,18–23 the cytokine macrophage migration inhibitory protein (MIF),2,10–13 and retinoic acid (all trans Retinoic acid, at-RA24,25), have profound effects at the cellular level during neural axis formation and neural and sensory cell development. All of these molecules are known to help shape the embryo and its neural axis from the earliest embryonic times. However, until we developed the bioreactor described in this report, there was no means of exposing a limited area of the surface of the embryo to these growth factors. For example, an excess of the BMP antagonist noggin introduced into a pregastrula frog embryo through implantation of a second noggin-secreting region called the organizer26–30 in the classic embryological experiments of Mangold and Spemann can produce a two-headed embryo, with two neural axes. This occurs if the cells of the “organizer” region are introduced into the blastula at a site different from the evolving dorsal lip of the blastopore,27,28 the place where gastrulation begins in the frog. Noggin is a secreted protein and is transcribed initially in the dorsal lip of the blastopore and subsequently in head mesoderm and the notochord.18–22,29,30 In amphibian embryos, overexpression of noggin results in the formation of a secondary axis that is primarily made of head tissue (see review26). Noggin binds to BMP4 (as well as BMP2) with high affinity and competes with their receptors.19 Noggin's neural inducing activity is based on its ability to prevent BMP4 signaling in the organizer and the tissue that will become the neural plate. It is unknown what effect a highly focal application of a growth factor such as noggin applied externally might have on a developing embryo. We wanted to restrict secreted growth factor (noggin, aT-RA, and MIF) exposure to very circumscribed regions of the embryo exterior and determine what effect(s), if any, they had on development. Eventually, receptors and downstream targets of these growth factors will be assessed as we have done previously.31,32
The zebrafish is an ideal model system in which to test such growth factor exposure,24 because embryos are plentiful at early stages. We also have a great deal of experience analyzing development of nervous and sensory systems in this model.3,33–35 In a microfluidic bioreactor, each developing zebrafish embryo can be separated in an individual, tiny, well barely larger than the embryo itself.
In the ideal bioreactor, several conditions should be met, including restriction of limited regions of the embryo's exterior to the fluidic streams, a means of identifying which cells have been exposed to the stream, and a way to track the exposed cells through early development. It is well known that other soluble growth factors have profound effects at a distance on the developing nervous system, including MIF10 and RA.24 However, until now there has not been a means of restricting the exposure and monitoring the limited number of exposed cells exposed through time in development.
To solve this problem, we administered bodipy ceramide (BDC), a fluorescent cell marker, along with the growth factor. The cells that were exposed to both BDC and the growth factor incorporate BDC into their cell membranes and are thus marked for a considerable time period postlabeling since the dye becomes incorporated into the cell membranes of daughter cells as well and can be detected for up to 72 h postlabeling.36
The science of microfluidics, a popular branch of bioengineering technology, could theoretically provide a means of effecting the exposure of limited and circumscribed regions of a developing embryo to growth factors, with economy of scale both for the number of embryos used and for the amount of expensive reagents that need to be used in the experiments. Microfluidic devices15 are tools used to manipulate fluids in small capillaries or microchannels with dimensions of tens of micrometers. The development of these tools and their use to approach problems in analytical chemistry, organic synthesis, genomics, proteomics, and cell biology has emerged as a distinct new field called microfluidics. Due to microfluidic devices' unique characteristics, including small size and laminar flow, microfluidics offers a number of useful capabilities compared to macroscale fluidics: the ability to use very small quantities of samples and reagents (particularly important in this context), to perform analysis within short times, and to carry out separations and detections with high resolution and sensitivity. It also offers fundamentally new capabilities in the control of concentrations of molecules in space and time. Moreover, microfluidic systems can be low cost (as in this instance) and have small footprints as analytic devices. Here, we apply microfluidics to the manipulation of the zebrafish embryo, to accomplish goals that could not be approached using any other system, with an economy of scale and reagents.
To date, no one has tested the effects of external focal application of any of these cytokines, growth factors, or growth factor antagonists (including Noggin, which is an antagonist for BMP423 on a developing embryo of any type, including zebrafish embryos). Bath application of 10−7 M aT-RA to the 16 h postfertilization zebrafish24 has marked effects on embryonic nervous system development and morphogenesis. External exposure of the whole zebrafish embryo to at-RA markedly affects brain, eye, and nervous system development. These observations were thought by the authors24 to be consistent with an integral role for specific genes downstream of RA in the retinoid signaling network during hindbrain and eye development. A reduction in size of the nascent inner ear (the otic vesicles) is also apparent from the photomicrographs shown in the figures of this paper, although these were not discussed by the authors. The sizes of the vesicles post-aT-RA treatment were one third to one half the size of the vesicles in untreated controls, although it is not known whether this was a direct or indirect effect of the treatment. It is also not known which, if any, cells normally present in the developing inner ear are missing (cells contributing to the semicircular canals or to the vestibular or auditory maculae). We also did not assess the otolith number.
Scientific objectives of the project
The student team's scientific objectives were therefore (1) to design a microfluidic bioreactor that would provide continuous external focal application of MIF, retinoic acid, BMPs, and BMP antagonists, including the BMP4 antagonist noggin (singly and in combination), to selected circumscribed external areas of the developing zebrafish embryo for periods from 3 to 24 h in the device itself, (2) to document the exposed regions by BDC marking of either the anterior or posterior dorsal neural axis and to follow the marked cells (either by the marker dye or rough positional identification through photomicrography) and the embryo itself through development on a daily basis for at least a week after removal of the embryo from the device, and (3) to observe the results of circumscribed exposure compared to immersion of the fish in the same growth factors on embryonic development in general and nervous system development in particular.
The effects of such externally localized growth factor delivery on embryos are still unknown, but previously, no device was available with the required size/scale and ability to support a living vertebrate embryo as well as deliver spatially localized growth factors. The objective was to support the living dechorionated zebrafish embryo and expose either the anterior or posterior neural axis to a microfluidic stream containing growth factors and/or cytokines.
Pedagological objectives of the project
We (Barald and Takayama) offered this project in Dr. Rachael Schmedlen's semester-long BME 450 design course, in which faculty from different engineering discipline mentor teams of students attempting to accomplish goals set out by the faculty mentors. The course instructors, led by Dr Schmedlen, requested projects for the course from the faculty of the BME Department and affiliated faculty, some of whom have links to local companies. All faculty and local entrepreneurs who offered projects were asked to present them to the whole class (about 60 students). Although the course directors prescreened the projects for feasibility, the students were the ultimate deciders. Each would-be faculty supervisor presented his or her project in a 15-min PowerPoint presentation to the class and then answered questions from the audience. The students were asked to rank their first three choices of projects and commence work on them in the following week.
Barald, who conceived the project, and Takayama, who provided the microfluidic expertise, were the overall directors of this project and met with students on a weekly basis to discuss progress and give advice. The design team was composed of seven undergraduate students (Li, Al-Shoaibi, Ali, Flak, Perrin, Winslow, and Shah), who worked directly and on a daily basis with two postdoctoral fellows (Shen from the Barald lab and Bersano-Begey from the Takayama lab). A graduate student instructor for the course (Chen), who also worked on the project after the term finished, provided day-to-day guidance for the students and hands-on participation. A BME graduate student from the Barald lab (Ramamurthy), who has microfluidic experience in our collaborative studies,37 gave advice and grew the transfected CHO cell lines that produced noggin,18 determined the noggin concentration in the conditioned medium, and harvested the protein for use in the experiments by our previous methods.23
The students were instructed to think of themselves as a company; present a timeline for accomplishing the project; delegate major tasks among themselves; research the background to the project (literature review and patent searches); prepare a budget and prepare periodic written and oral reports and a final paper, poster, and of course the device itself with specifications; and test/analyses results. They also provided failure risk analyses periodically as the device evolved. The final recapitulation of this analysis is provided in Supplemental Fig. S1 (available online at www.liebertonline.com).
All oral presentations were videotaped and critiqued by the whole class, class instructors, and participating postdoctoral fellows and team instructors. The periodic written reports received comments from the course instructor, the graduate student instructor (GSI), the faculty, and postdoctoral fellows involved in each interdisciplinary project. Frequent brainstorming sessions were held spontaneously in the labs or in meetings at either the Engineering College or Medical School, particularly when problems arose with the design. Several information sessions were also held, in which the faculty and/or postdoctoral fellows gave chalk talks that incorporated principles of developmental, engineering, cell, and organismal biology and microfluidics.
Some students on the team were adept at theoretical calculations and applications, mathematical modeling through MATLAB, and the other design programs (COMSOL), which they used. Others had more of a flair for fish handling. Few of these students had been exposed to biology and molecular and cellular bench science. Although each person on the team worked on every aspect of the project and contributed to the written and oral reports (as well as the many appendices in the final report that outlined the tests performed on the device and the modeling approaches), more biologically oriented students who could tell the zebrafish sexes apart (not easy even for those of us who have worked in the field a long time), set up matings, inserted embryos into and removed them from the device, and the like, took on these tasks and improve them during the course of the term. Other students, with skills in device design, blueprint production, clean room processes, and IT did more of the microchip design fabrication and testing, using embryo surrogates (e.g., the embryo-sized ball bearing) and fluidic modeling software. Those with mathematical and modeling skills also concentrated on calculating the important parameters that were used to model (theoretically) and to analyze (on a day-to-day basis) the use of the device. This specialization allowed the students to cross-inform each other during brainstorming sessions and during the preparation of the written and oral reports as well as to receive advice from their fellow team members. They taught each other as they prepared for the submission of written and presentation of oral reports.
Assessment
Students were graded on (1) the amount and use (prioritization) of time they devoted to the project, (2) their comprehension of all (engineering and biology) aspects of the project, (3) their written reports, (4) their individual presentations in the oral sessions, which were videotaped and critiqued by the whole class and instructors, and (5) their final report and its oral delivery as a PowerPoint presentation. In the assessment of the team, faculty instructors were asked to rate the team and individuals on the following criteria (using a scale of 1 to 10, with a grade of 10 the highest).
Detailed comments on each of a set of criteria devised by the course director were also requested from the participating faculty and postdocs. These included
1. Professionalism: Did the team show up on time to meetings? Did they come prepared to meetings? Did they demonstrate enthusiasm and sincere effort? Did they handle communications in a professional manner? Were they considerate of your time and resources? Did they demonstrate the ability to work independently (and not rely on you)? Team strengths? Team weaknesses?
2. Deliverables: Did they apply science, engineering, and biological concepts where appropriate? Did they understand the principles and background concepts involved? On the engineering side? On the biology side? Did they formulate creative solutions? Did they present logical, well-thought-out rationale for their selected design? Did the reports and presentations meet your expectations? Was the information presented in a clear and effective way? Were key points and information easy to find in the final report? Did the user manual students produced provide an accurate account of the device's operation?
3. Final Prototype: Did the prototype meet your design requirements? Were issues of safety, quality, and performance adequately addressed? Are you satisfied with their solutions? Will you be able to use the prototype? How does the prototype rank (please circle the appropriate response)? Working, better than expected; Working, satisfactory; Mostly working; Mostly non-working; Incomplete; Not built? Did the prototype meet your expectations?
4. Using the following scale, what grade would you give the prototype: (Excellent, 95–100%; Very good, 90–94%; Above average, 85–89%; Average, 80–84%; Below average, 75–79%; Unprepared/Big gaps, 74% and below).
5. Did you feel that the students had adequate course preparation and technical skills to successfully tackle the project? If not, what type of skills or preparation would you have liked them to know before the course?
The students were also requested to provide recommendations to any students following them in the project. They were directed to comment on the limitations of their design, propose improvements, and provide guidance for the next team. They were also directed to identify problems with the current design, suggest alternatives, and recommend improvements based on validation of their test results. They were also asked to recommend the next steps for the project (e.g., if they had another 2 months to work on it). The directive was, “Be specific about all methods and modifications such that someone taking over the project could quickly execute them.”
The biological problem
In the design course, we took a microfluidic approach to the problem of achieving focal exposure of a small region of the zebrafish embryonic surface. The objective was to determine if such focal exposure had effects on general development or neural axis development of the zebrafish over time. In the project, we combined our expertise and capitalized on our previous collaborative efforts in this field.27
Previous studies of embryos in microfluidic devices
Mammalian embryos have been cultured in microfluidic devices for purposes of enhancing their development for in vitro fertilization applications.3,15 Drosophila embryos and larvae have also been studied in microfluidic devices,39,40 and we have used microfluidic devices in previous collaborative efforts to study the differentiation of embryonic stem cells/embryoid bodies into a variety of cell types.37
For this project, the problem, as presented to the students, was to build a microfluidic device that could stably hold zebrafish embryos in place, mark the region of the embryo exposed, allow the embryos to develop normally in the device over 3–24 h, and deliver growth factors and nutrients in two separate streams to circumscribed area(s) of the embryos' exterior. It was essential that the growth factor or cytokine be delivered only to a specific site on the embryo's exterior and that the cells exposed be marked and followed during the subsequent course of development. It is not possible to orient any or all of the embryos—either experimental or control embryos—identically in the device; therefore, an additional constraint was that to generate statistically reliable data, a large number of embryos will eventually need to be analyzed in a high-throughput manner.
During the course of the term, the design team of BME students modeled and tested several designs, constructed and validated sequential prototypes, tested embryonic zebrafish in the microfluidic bioreactors with and without growth factors in the fluid streams, formulated recommendations for improvements, and proposed further experiments. After the close of the term, two students (Li and Al-Shoaibi) and the GSI (Chen) worked on the project in either the spring and summer (Li and Chen) or fall term (Al-Shoaibi).
During the course itself, students had frequent opportunities to present their work to other design teams working on different projects and to the course faculty, including other design team faculty facilitators and to present research posters on their work, one of which was presented5 at the 8th International conference on Zebrafish Development and Genetics meeting in Madison, Wisconsin (June 2008) (Fig. 1).
FIG. 1.
Shen et al.'s poster presented by the design team at the Zebrafish International Meeting in Madison, Wisconsin (June 2008).
Pedagological Approaches
The target audience
Undergraduate BME students at the University of Michigan are a unique group of engineering students. In addition to the traditional engineering curriculum, which includes engineering courses in various disciplines, mathematics, computer science, and physics, these students also take chemistry, physiology, cell biology, biomaterials, and design courses that attempt to synergize among these disciplines both theoretically and practically. “BME is the newest engineering discipline, integrating the basic principles of biology with the tools of engineering.” (http://www.bme.umich.edu/programs/undergrad/). Although many of these students will attend medical school, work in the biotech industry or bioengineering industries, and design institutes, a substantive number of them will attend graduate school in engineering, physics, or biology-based disciplines.
The BME design course
Incorporation of the zebrafish project Dr. Schmedlen's BME 450 design course recruits faculty members from the BME Department to oversee projects during the winter term (January to late April). The projects should ideally pose questions that involve engineering approaches to their solutions. Each year the projects differ, as do the faculty sub-project leaders.
The project described in this report was an extension of work done in the Barald lab, which is a developmental neurobiology lab interested in embryonic body and neural axis development as well as inner ear sensory cell and neural cell development41–43 in a number of model systems, including the zebrafish,3,5,33–35 and the Takayama lab, which has applied its expertise in microfluidics to many different projects on scales from single cells in culture15 to culture of stem cells and embryoid bodies in collaboration with the Barald lab.37
In the course of the ensuing week, students elected their first, second, and third choices of projects on which they wished to work. Five to seven students were then assigned to each project team depending on interest and availability. The students then met with faculty sponsors to clarify the goals and requirements of the project and to establish criteria for progress toward the achievement of these goals and for grading. Students and faculty met on at least a weekly basis for the rest of the term, and students met almost daily with their immediate supervisors, as well as with faculty in the labs in more informal meetings. In the case of the zebrafish microfluidic project, the supervisors were two postdoctoral fellows, Dr. Shen from the Barald lab and Dr. Bersano-Begey from the Takayama lab. The students also received a good deal of advice and both theoretical and hands-on help from the course's graduate student instructor, Mr. Chen.
Delegating specific roles in the project and assuring exposure of all students to multiple aspects of the project
After meeting to delegate initial assignments on the project, the seven student team members took on specific tasks in the project. The team members themselves met to decide how to divide the work and how to make sure that each of them got ample exposure to the various aspects of the project, including the microfluidic design principles, engineering design, mathematical modeling, the theoretical and practical aspects of the biological system, and zebrafish care and developmental biology. They self-identified to some degree (Mr. Li did most of the mathematical modeling, for example) and Mr. Al-Shoaibi, Mr. Ali, and Mr. Winslow, as well as Mr. Li, were the key zebrafish lab hands-on students. Ms. Flak and Ms. Perrin were the overall organizers. Some specialized in the design, micro-fabrication, and modeling of the device, particularly determining the fluid mechanical properties of the device and doing theoretical and mathematical modeling experiments. Some students specialized in learning the zebrafish biology and developmental biology necessary to evaluate the efficacy of the device. They learned how to distinguish male and female fish, set up matings, recovered the eggs from the matings, and learned how to do the molecular, cellular, and behavioral tests by which viability/survivability and developmental influences of the device were measured. Although there were areas in which each student member of the team specialized, each person took part in all of the engineering and biological assessment tasks during the course of the term.
Frequent written and oral prospective and retrospective reports were the hallmark of the course
The students were also required to report their progress many times during the course of the semester to their fellow students on the other project teams using PowerPoint presentations. They presented their design model iterations, budgets, and timelines and received feedback from the course director, the GSI, the involved faculty on their and other design projects, fellow students, and the postdoctoral fellows from their own and other teams. There is a practical entrepreneurial aspect to the course (involving a budget) and a client (the faculty who devised the project) and company (the student team) aspects that are also meant to prepare the students to work in a team in an industrial design firm or think tank setting. The students' presentations were videotaped and also available as podcasts to allow review and constructive suggestions to be given by course faculty who did not attend the presentation sessions as well as the course directors and GSI and other course participants besides the team.
Written reports
Several written reports, along with the oral presentations, were given during the course of the term, including a major presentation that accompanied the final report, which was the collective work of the entire team.
Troubleshooting and brainstorming sessions
Periodic brainstorming sessions among the design team and immediate supervisors/faculty were carried out during the course of the term to discuss roadblocks and to prepare the written and oral reports and a final group poster as well as a final oral presentation and written report. The students also carefully prepared budgets for materials, chemicals, biological reagents, fees for use of clean rooms and the plasma oxidizer used to make the microfluidic devices, and identified sources of the materials they needed. The team kept within the budget (US$1500 for the whole project), although the time of the faculty and overseeing postdoctoral fellows was donated.
They solved some problems ingeniously, for example, substituting small ball bearings and large hydrated grains of pearl tapioca for embryos in some of the early design and modeling experiments, because during the course of the term, the ability to get enough embryos—particularly, dechorionated embryos—for statistically significant results was rate-limiting, and the stand-ins were excellent substitutes in the modeling experiments.
Hands on lab work in microfluidics, fabrication of the microfluidic design models (chips), and the biology of the project
Students worked with Drs. Bersano-Begey and Takayama to design the microfluidic bioreactor, to determine how to fabricate it (with and without the use of plasma oxidation,16,17), and to assess different materials (PDMS vs. agarose) and prepare detailed design blueprints for the various iterations of the devices during the course of the term. They based much of the practical design work on theoretical modeling experiments done using a variety of computer design programs. Cost and feasibility as well as rate-limiting access to facilities such as the plasma oxidation facility had to be taken into account by the students.
The modeling and fabrication had to be accomplished in the context of the biology of the problem and the questions that were being addressed. This added a level of uncertainty, which is familiar to biologists dealing with living systems, but which was novel and at times disconcerting to the engineering students, who are more accustomed to theoretical aspects of biology and biological concepts on the molecular and cellular levels than the organismal level. The students met this challenge admirably, and spent many long hours in the lab learning zebrafish husbandry skills, developmental biology skills, and underlying principles and experimental design and interpretation. They were enthusiastic, bench-competent, and curious about the biological tenets of the system that affected their designs' successes and failures.
Three of the participants (Li, Chen, and Al-Shoaibi) and Dr. Shen continued work on the project after the term finished. The team produced a poster including their extended multi-funnel design and presented it in a poster session at the end of the course and at the Zebrafish meetings in Madison, Wisconsin (June 2008) (Fig. 1).
Biological Materials and Methods (Brief Description)
Zebrafish maintenance, embryo collection, and embryo husbandry
Zebrafish were obtained from a local pet store (University Aquarium, Ann Arbor, MI) or from the Zebrafish Stock Center at the University of Oregon (Eugene, OR) and kept on a 14:10 h. light/dark cycle at 28.5°C. The embryos were collected 30–45 min after removing dividers that had separated the two males and four females in the mating tanks overnight. All mating tanks were kept in a water bath at 28.5°C. Each tank is composed of an inner and outer tank. The inner tank has a perforated insert through which eggs can fall. Embryos were raised in filtered methylene blue–containing fish water, which prevents fungal growth on fish eggs (2 mL of 0.1% methylene blue to 1 L of fish water) at 28.5°C.44 Between 12 and 20 h postfertilization, 0.03% 1-phenyl-2-thiourea (chemical formula, C6H5NaHCSNH2/PTU) was added in a ratio of 1:10 to prevent pigmentation from developing. All students participated in embryo husbandry and in setting up fish matings, and learned to stage the embryos.45
The basic microfluidic device
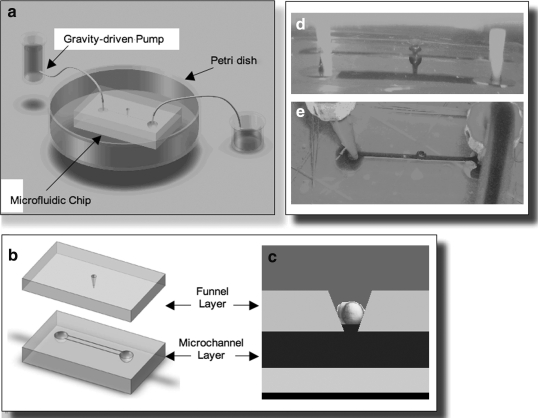
The zebrafish microfluidic bioreactor/device was composed of five basic parts: a gravity-driven pump, which was capable of delivering the growth factors in fish water; 30 cm of connecting tubing; the microfluidic chip (described below); a Petri dish providing a general temperature-controlled (28.5°C) environment containing fish water; and a collection reservoir for the collection of medium to be recycled (Figs. 1 and 2).
FIG. 2.
(a) The funnel device consists of a gravity-driven pump, microfluidic chip submerged in a Petri dish filled with fish water, and collecting reservoir connected by tubing with an interior diameter of 0.4 mm. (b) The microfluidic chip contains, from the top, a funnel layer and a microchannel layer. The combined components are placed in a Petri dish containing fish water. (c) The embryo is immobilized in the funnel and separates fluid in the microchannel below from the fluid above. (d) Side view of bead in funnel: red dye in the microchannel exposes the bottom portion of the immobilized bead but does not mix with the fluid above. (e) The overhead view also indicates that there is no mixing between fluid in the lower channel and fluid in the upper part of the funnel.
Microfluidic chip
The microfluidic chip consisted of two polydimethyl-siloxane (PDMS) (Dow Corning) layers: the top layer of the final device contained funnel-shaped holes that immobilized the embryos, and the lower layer contained a microchannel that delivered growth factors or cytokines along with BDC. Gravity, hydrostatic pressure, and the sticky surface of PDMS kept the embryo from rotating or being expelled from the funnel. However, although the portion of the embryonic surface that was exposed to this fluid channel was constant, the exact region of the surface for each embryo was random, and once the embryo was in position, it was not possible to rotate it. However, the embryo itself effectively separated the stream of fish water containing growth factors from the fish water alone that surrounded the microfluidic chip in the Petri dish. (Fig. 2). It is critical to understand that large numbers of embryos in a multi-well device are needed to achieve statistically significant numbers of embryos in each experimental group. To date, we have not yet achieved this milestone.
Simulations/calculations/modeling
Flow modeling was done using the COMSOL® program version 3.5 (http://www.comsol.com/). Specifically, the MEMS Module in this program was used to model physical phenomena, including flow and the effect of temperature on flow in the microfluidic device.
Statistical analysis of viability studies
To quantify the impact of our device on zebrafish embryo survival and development, we statistically analyzed embryo survival data over 5 h to 6 days postfertilization. We calculated mean survival rates and standard deviations across viability, aT-RA, and MIF studies. Next, we used a 22 factorial fit across all experiments, with treatment and location of the embryo designated as the two factors. A 2k factorial design was chosen for the analysis of the data, as it allows for the study of both individual and joint effects of the factors on a specific response, which in this case is embryo survival. Additionally, a 2k factorial design allows for each factor to be tested at two levels. For this analysis, the treatment factor levels were defined as GF treatment versus treatment with standard fish water and the location of the factor defined as embryo loaded in the device (with a limited portion of the exterior exposed) versus embryo grown (immersed) in a Petri dish. All embryos analyzed were dechorionated, as our device is not intended for the study of embryos within the chorion. Using Minitab™, we analyzed the survival data and generated an analysis of variance (ANOVA) for survival, using a p-value of 0.10. Because there is naturally a large variation in survival rates of zebrafish embryos, we also increased this value to 0.15. The risk of obtaining a false-positive (observing a difference in survival when in fact there is none) will not significantly affect the usability of our device. To further confirm these results, one-way ANOVA was performed on both survival rate versus location and survival rate versus treatment. We also analyzed the probability that orientation of embryo immobilization will allow exposure to growth factors by calculating averages and standard deviations for three experiments.
Results
Microfluidic device designs
Several designs, including a peg design, differential pressure design, and Y-channel design, were formulated, modeled (using the COMSOL program), and rejected based either on modeling alone or on a combination of modeling and empirical testing. Additional features that were modeled in each design were ease of growth factor delivery and its ability to reach the embryo and difficulty of device fabrication. For example, the peg design delivers spatially localized growth factors through hypodermic needles, which were also used to immobilize the viable embryo in a single channel. However, based on the modeling experiments, growth factors were not expected to reach the embryo and the device was difficult to fabricate and therefore abandoned.
Funnel design selected
We selected a design for the final device that immobilizes a zebrafish embryo in the middle of a funnel-shaped hole within the central membrane of a two-layer microfluidic chip (Fig. 2).
The funnel layer
The top layer of the microfluidic chip, made of PDMS, contains a funnel-shaped hole that immobilizes an embryo by gravity. This embryo acts as a barrier between the fluid in the microchannel below and the fish water in the Petri dish above the embryo. The upper end of the funnel-hole is exposed to fish water in the Petri dish, and narrows to the lower opening at the microchannel, through which growth factor enhanced fish water flows. The funnel has an upper diameter of 1 mm and tapers down to a lower diameter of 0.3 mm. The upper diameter is large enough to allow a 750-μm-diameter embryo to enter the top of the funnel and the tip diameter is small enough to prevent the embryo from passing into the micro-channel containing the moving growth factor fluid stream. When the embryo is placed in the funnel, it creates a seal that separates the two layers of fluid. The small tip diameter exposes only a small surface area of the embryo to the GF-containing fluid below, as the majority of the embryo is either pressed against the side of the funnel or exposed to the fish water from the Petri dish. The majority of the embryo surface area is shielded from the moving fluid in the microchannel, which minimizes shear stress. This device offers many advantages, including the ability to maintain embryo viability and orientation as well as localized delivery of growth factors, and very few disadvantages, although one common to all the devices is control over embryo orientation.
A gravity-driven pump (a 100 mL beaker) delivers fish water containing growth factor through the microchannel of the chip, which is collected in a similar beaker placed below the device. A length of silicone tubing (30 cm), with an inner diameter of 0.4 mm, is connected to the channel layer of the microfluidic chip. This setup allows fluid velocities of up to 5 cm/s. Gravity flow was chosen because it provides a less abrupt and more gradual and continuous flow than peristaltic or syringe pumps while also requiring the least equipment. For a task in which exact control of flow speed (or complex flow patterns) was not a priority, and for which more gentle flow changes were preferable, we believe gravity flow was the best choice.
Both simulations with ball bearing (Fig. 2) and pearl tapioca embryo surrogates and experiments with living embryos in their chorions and dechorionated embryos indicated that the embryo did not rotate or become displaced during the course of the experiment as monitored by dissecting microscope observation. The funnel device is also easy to fabricate and simple to operate. It allows insertion and removal of an intact dechorionated embryo and is able to be modified into a high-throughput device, with serial channels underlying 20 or more wells delivering the same growth factor or cytokine to the embryo exterior. However, no device, including this device, allows researchers to control the specific region of the embryo that is exposed to growth factors; embryo orientation is random. In the high-throughput device (100–200 embryos), more embryos will have similar regions of the dorsal surface exposed to growth factors by chance (exactly which region will be determined retrospectively through BDC labeling).
Figure 2 provides a representation of the assembled device, and illustrates the functional relationships between the various components of the device.
Microchannel layer
The bottom layer of the PDMS microfluidic chip is the microchannel layer, which allows flow of growth factor fluid past the immobilized embryo in the funnel layer. The micro-channel is directly below the tip of the funnel layer. This creates a continuous space for the fish water in the Petri dish to flow into the channel. When the embryo is inserted, it separates the two fluids. The microchannel layer of the chip contains a 20-mm-long channel with a 2×0.1 mm cross section. This minimizes the required amount of fluid and conserves expensive or difficult to obtain growth factors; these are the smallest dimensions we can construct with our available fabrication methods. Additionally, the channel must be at least 0.3 mm wide to expose the entire funnel tip to growth factors. The channel runs along the length of the microfluidic chip to allow simple fabrication, and must be at least 16 mm in length to achieve steady flow past the embryo. Concatenation of additional embryo-containing funnels serially to this channel is possible in a high-throughput device.
The PDMS components that make the multi-layered chip are sealed together by plasma oxidation.16,17 The chip is approximately the size of a microscope slide, with a length of 40 mm, width of 24 mm, and thickness of 8 mm. This allows observation of an embryo placed in the device by a dissecting microscope. Additionally, the device is large enough to allow easy handling by the user, eliminating the need for specialized tools or training to set up and operate the device. The entire microfluidic chip is submerged in a Petri dish filled with fish water, allowing the top portion of the embryo to be exposed to the surrounding fish water, which simulates the normal fish tank environment.
Device operation
Because, once the embryo is manually inserted into the microfluidic funnel, fluid moves by gravitational potential energy, device operation requires little user input during the course of the experiment, making it relatively simple. A typical operation begins by adding fish water to the pump and flushing the entire device (all components) with the fluid. After all visible bubbles have been flushed out of the device, a carefully dechorionated embryo is pipetted into the submerged funnel hole under the microscope in an effort to maintain the dorsal side to the bottom of the funnel hole. Concentrated growth factor is added to the pump and mixed to produce a diluted solution of the desired final concentration. The pump is monitored regularly (every 15 min) and refilled when necessary so that flow rate remains constant. The dorsal axis of the embryo is exposed to the growth factor solution for 3–24 h. The fluid enters the chip from the gravity pump tubing on one side and exits through tubing into the collection reservoir.
Embryo viability is recorded at regular intervals during the operation of the device, and morphological assessment (behavioral and molecular) is conducted in detail once testing is complete for up to 5 days postfertilizatio. Once GF exposure is complete, the embryo is extracted from the funnel hole with a wide-mouthed fire-polished siliconized Pasteur pipet, and the device is flushed with plain fish water. The embryo is then allowed to develop in a well of a 24-well plate filled with fish water at 28.5°C until it reaches the desired stage of development. Any changes in zebrafish development associated with exposure to noggin, MIF, aT-RA, or other growth factors is then recorded in comparison to embryos exposed only to fish water.
Failure risk analysis
The original design failure mode and effects analysis identified a number of areas that posed problems for the effective operation of the device, including inconsistencies in growth factor delivery due to bubble formation, evaporation through the chip, drying of the chip, and leakage of growth factor media at the tubing/chip interface. Initial dye studies indicated that the chance of design failure could be diminished with only minor procedural alterations. Because bubble formation within the channel and/or funnel occurs only during initial device setup, proper precautions taken during the system flush should dislodge bubbles in the microchannel or funnel during the experiment. Hence, no design modifications were required. Both evaporation through the chip and drying of the chip were found to be inconsequential, because the entire microfluidic chip is submerged in fish water, effectively eliminating this risk. Finally, leakage was initially observed at the tubing–chip interface when the system was disturbed due to poor connections between the tubing connectors and the chip. However, removing the tubing connectors, and inserting the tubing directly into the chip solved this problem. This reduced the mechanical stress on the chip and created a more effective seal, effectively reducing the chance of failure. An extensive failure analysis diagram (the final one for the course) is shown in Supplemental Fig. S1.
The most significant failure modes involved funnel size (i.e., funnel was too large to adequately sequester the embryo), difficulty in embryo removal, and flow rates that would cause device operation to be very expensive due to a requirement for large volumes of required growth factors (Table 1).The final device prototype does not exhibit any currently identified high-priority failure modes, and operation has been shown to be reliable and repeatable through multiple iterations of the experiments. A redesign of the funnel mold, a reduction in the microchannel thickness, a reduction in the size and setup of the gravity pump, and the development of a new embryo removal technique solved the initial problems.
Table 1.
Possible Failure Modes
| Component | Problem | Cause | Action taken |
|---|---|---|---|
| Microfluidic chip | Funnel too large for embryos | Unsatisfactory molding choice | Reduce funnel size |
| Embryo removal difficult | Embryo too fragile | Change removal technique | |
| Allow embryo to develop further | |||
| Growth factor delivery | Bubble formation in channel/funnel | None: Re-flush system | |
| Tubing | Leakage | Poor seal between tubing and chip | Insert tubing directly into chip |
| Gravity pump | Flow rate too rapid | Microchannel too large | Reduce channel thickness |
| Gravity pump too large | Reduce size/height differential |
Dye studies
In the initial studies, we used either a small metal bead or pearl tapioca swollen to the size of a zebrafish embryo to serve as surrogates for embryos with chorions or dechorionated embryos. In these tests neither the tapioca bead nor the metal bead was displaced, and we observed that the red dye from the channel traveled up the funnel hole and exposed the bottom portion of the bead or tapioca pearl to the neutral red dye solution. The shear rate (the ratio of flow speed and distance between parallel faces experiencing shear) was 20/s, which is well below our specification limit of 70/s (which is a value the students arrived at from literature searches of previous related studies39). Shear rates are measured in reciprocal seconds; velocity is measured in meters per second. Therefore, a shear rate of 20/s is 20 m/s.
We then immobilized dechorionated embryos in our final funnel prototype and delivered two types of dye (fast green and neutral red) through the microchannels to quantitatively measure mixing between fluid in the microchannel and the surrounding Petri dish with an embryo in place. We also ran clear fish water through microchannels of control devices for this dye study and tested embryo viability under all conditions. This experiment used devices with funnels of 0.3-mm tip diameter to immobilize dechorionated embryos. Embryos were exposed to dye for 3 h, and then the absorbance of the dyes in fish water was sampled both directly above the funnel of a device and at the perimeter of the Petri dish containing the devices, fish water from the no-dye devices, and the diluted green or red dye solutions themselves. These experiments (three experiments, n = 16 embryos total) demonstrated that no leaks had occurred, because the spectrophotometric analysis of fish water above the embryo and at the perimeter was identical to that of fish water alone. Exposure to fast green dye, however, was toxic to the embryos, and was abandoned in favor of neutral red.
The final device separated the fluids in the microchannel and the Petri dish and kept embryos alive for at least 3 h. Also, we were able to remove all of the embryos from the device after exposure to neutral red-containing microchannel streams. During the 3 h, the embryos developed normally and remained viable during removal from the devices and subsequently for up to 6 days postfertilization (n = 21/21 compared to embryos exposed only to fish water in both channels n = 13/13).
Test of embryos with growth factors/cytokines
To test embryo viability and orientation of immobilization, we placed dechorionated embryos in devices and delivered the cytokine MIF, the BMP4 antagonist, noggin, and the growth factor, all-trans retinoic acid (aT-RA), through the microchannels in a variety of concentrations and for times from 3 to 24 h. We also tested and monitored pH variability46 in the fluid surrounding the embryo. To quantify the impact of our device on zebrafish embryo survival and development, we statistically analyzed embryo survival data over 24 h to 7 days postfertilization across all experiments (see viability studies).
Flow by gravity-driven pump
The mean fluid velocity in the microchannel was discovered experimentally to be on the order of 1–3 cm/s depending on height differences between inlet and outlet reservoirs.
Embryo immobilization
To expose isolated regions of a zebrafish embryo to two distinct fluid streams, the embryo must be immobilized. This can be broken down into two sub-problems: embryo displacement and embryo rotation. If the embryo is displaced, it can wash out with the fluid streams or cause disturbances in stream uniformity. If the embryo rotates, a larger region of the embryo than anticipated will be exposed to growth factors, rendering the experimental results inconclusive. The addition of BDC to the fluid allows us to determine retrospectively which areas of the embryo's external surface were exposed to the growth factors.
Maintaining viability of the zebrafish embryo
Control and experimental dechorionated embryos, which are only subjected to normal growth media and no experimental growth factors, must develop at least to the 50% epiboly44,45 (gastrulation of the embryo begins at this time in development) stage when placed in the device. Experimental and Control fish must also develop into viable larvae, indicated by survival of at least two days after transfer to Petri dishes after removal from the device. Embryo viability is affected by changes in the microenvironment, so a subfunction of the device is to maintain the required growth conditions for zebrafish embryos that are provided in the literature.44–46 Specifically, pH must be maintained at 7.2 ± 1.46 Because solutions will be used at room temperature, which is maintained at 28.5°C, this aspect of the microenvironment did not need to be regulated in our controlled temperature procedure rooms. Shear stress is another factor that will impact viability; it can cause abnormal development or embryo lysis. Therefore, a subfunction of the device is to prevent fatal shear rates. Based on literature research, shear rates below 70s−1 are acceptable,39 and the calculated shear rates in the modeling experiments were substantially below this.
Controlled delivery of growth factors
Because the device must enable delivery of growth factors to distinct regions of an embryo for developmental study, the device must allow fluid flow to a specific region of a zebrafish embryo. In initial experiments, we attempted delivery of growth factors to the dorsal side of an embryo because the ventral side has a yolk sac.
The device is required to deliver uniform fluid over the entire isolated region of the embryo in all the experiments. This is necessary to obtain reliable experimental results. This intuitively translates to ensuring that uniformity is present in the microchannel holding the embryo.39 It is related to the second sub-function, which is to maintain constant concentrations.
Noggin exposure of embryos
Noggin was produced from CHO cells as described in our earlier reports and was determined by ELISA23 to be present at a concentration of 20 ng/mL. Undiluted noggin solution exposure to whole embryos (with chorion intact or dechorionated) in emersion experiments killed the embryos (all embryos exposed for even a short period of time [5 h]; n = 42 died). However, similar concentrations in streams in the microfluidic device resulted in relatively little embryo death during the course of the experiment and subsequently during the next few days, compared to control embryos. By 144 h, 87% of the embryos exposed in the microfluidic device for this period of time were viable (50/58), and none showed any developmental abnormalities. None of the embryos exposed to noggin in the microfluidic device had adverse behavioral or developmental effects from this exposure during the entire period of time of the experiment. This experiment suggests that the bioreactor is indeed restricting the delivery of noggin only to a selected region of the embryo.
It should be noted that of the 58 embryos examined at the start of the experiment, the number of embryos that were in the same orientation in the device with the same relative areas (cells) exposed to the microfluidic stream was very small. Therefore, all results have been added together. It is clear from these experiments that, in subsequent experiments, whole embryos should be exposed to lower concentrations of noggin and that, if possible, higher concentrations of noggin should be used in the fluid streams in the microfluidic devices, with special care taken to expose larger numbers of embryos and to use the BDC marking mechanism to mark all embryos for retrospective study after exposure in the high-throughput device.
Retinoic acid (RA) treatment and its effect on viability of embryos with and without the chorion in plastic Petri dishes, on PDMS, or in the microfluidic device
Here we report the effects on dechorionated embryo viability over time in the microfluidic device, after immersion in the same concentration of aT-RA (20 nM) as in the micro-fluidic stream in a Petri dish and exposure to aT-RA on a PDMS-coated Petri dish. We found that aT-RA reduced the number of viable embryos compared to embryos in the device that were exposed to fish water alone. However, this was only a 10% reduction in viability and may be only a trend and not statistically significant, due to the small number of embryos we were able to recover from the device (103). We also found that three of these embryos were abnormal in their nervous systems (smaller brains) although this was a very small number compared to the number of embryos that appeared to be normal (100). One additional embryo appeared to have effects on its swimming behavior (it only swam in response to touch and its swimming ability was limited). However, although this might indicate that there were effects of the device, the numbers were insufficiently large to draw any conclusions. However, because few embryos in these experiments were oriented in an identical manner in the device, many more embryos in a high-throughput version need to be tested and retrospectively identified through BDC labeling.
Experiments with the cytokine MIF
To test embryo viability in the presence of a cytokine that is known to affect the embryonic neural axis, we placed dechorionated embryos in devices and delivered MIF, an inflammatory immune cytokine growth factor, through the microchannel to determine if any developmental changes occurred. We immobilized dechorionated embryos in four different devices and observed their orientation at 50× magnification. We connected the devices to a gravity-driven pump with 30 mL of 30 ng/mL MIF. We incubated the embryos (both within the chorion and dechorionated) in Petri dishes with either the same concentration of MIF or in standard fish water at 28.5°C. We allowed MIF to flow through the microchannels in the device for 3 h, and if the embryo was left in the device, we then administered fish water through the device for the remaining 21 h, and then disconnected the tubing and gravity-driven pump. We observed the embryos after 3 h and 24 h in the device. After 24 h, we removed all the embryos from the devices to Petri dishes.
None of the 53 embryos exposed to 30 ng/mL MIF in a restricted region of the embryo exterior exhibited any developmental changes. In the control Petri dish of the four embryos exposed (whole body) to 30 nM MIF; however, two embryos developed abnormally (one was missing a head, and the other had a misshapen tail), and two were dead. In the standard control without MIF, one embryo was alive and developing normally, and the other three were dead. The pH in both Petri dishes at the beginning of the experiment was 6.6. After 3 h, the pH in one Petri dish was 6.59, and 6.65 in the second Petri dish. All of these experiments need to be repeated in more detail in a large throughput device at additional concentrations. Closer examination of embryos in larger numbers is certainly desirable.
We also determined that embryos were immobilized in an orientation that exposes the cell mass to growth factors in approximately half of the instances. For 53 embryos across 5 trials, the zebrafish embryos were immobilized in the preferred sideways orientation 45.2 ± 15.5% of the time.
Legacies of the course
This course provided an opportunity for students to employ and learn both engineering and developmental biology skill sets and to mesh them. Two postdoctoral fellows received a great deal of hands-on teaching experience, and through joint meetings of the postdocs with the design teams and faculty, each postdoctoral fellow also acquired quite a bit of knowledge about the other's field.
Discussion
During the course of the semester, we designed, fabricated, and validated the first known microfluidic immobilizer for the investigation of zebrafish embryonic development.5 This PDMS device, which will be described more fully in a detailed techniques paper (Shen et al., unpublished observations) and which is relatively nontoxic to the environment even in its breakdown products,47 immobilizes an embryo in a funnel within a membrane, separating fish water that contains growth factors in an underlying microchannel from fish water surrounding the rest of the embryo in a microfluidic chip in a Petri dish where oxygenation and temperature can also be controlled.
The advantages of the funnel design included maintenance of embryo viability, immobilization of the embryo, localized growth factor delivery, ease of fabrication, ability to remove an intact embryo, reusability, ease of use by relatively untrained undergraduate students, and the ability to modify the design into a high-throughput device for 100–200 embryos (see the failure analysis of the overall report by the students given in Supplemental Fig. S1). Simulations indicated that the funnel design was the optimal configuration. It shields the embryo from shear stress, which helps significantly to maintain embryo viability. These simulations also showed that the funnel design successfully immobilized the embryo as well as delivered localized growth factors. Embryo immobilization has previously been reported.38 Further, the funnel is easy to fabricate because it is a single channel covered by two membranes with single holes placed in them. Embryos can be inserted and removed easily using a siliconized pipet without damaging the embryo or device. Because of this, the device can be reused. After embryo insertion, the device required minimal user maintenance or supervision. Finally, this device could be modified to be high throughput because the growth factors can be used in different channels and spatially separated. Although in a high-throughput device there will be more chance of an individual well either leaking or the embryo becoming dislodged from the well, the fact that the microfluidic stream carrying the growth factors is in constant flow minimizes the chances that the two will mix, even after embryo loss from the device. The fish water could also be circulated and streamed to minimize mixing.
Further modifications to our device should include production of a high-throughput device, in which many embryos can be simultaneously assessed, and fabrication that does not include plasma oxidation because of cost of use of the oxidation facility. Instead, we found that a thin metal bar could be used to make the channel, by simply pouring PDMS around it and pulling the bar out after PDMS was cured. These modifications minimized both the time required for device fabrication and its cost.
Future growth factor experiments will vary concentration of the growth factors; combinations of growth factors and combinations of growth factors and antagonists will be explored. It has been demonstrated that aT-RA is upstream of MIF in the neural development of embryonic stem cells.48 Therefore, there is good reason to believe that there may be synergistic interactions among the growth factors being tested in these experiments.
The students in the course learned a great deal about both microfluidics and developmental biology. They wrote a detailed almost 100-page formal final report with 13 appendices detailing the modeling, testing, and experiments done with the device. They gave three PowerPoint presentations to the whole class, produced a final poster that was displayed in a poster session in the class and modified to the version presented in this paper, which was also presented at the 8th International conference on Zebrafish Development and Genetics meeting in the summer of 2008. All the students in the course invested more than 20 h a week in the course. Some students invested as many as 50 h a week, particularly those who worked with the zebrafish. All students received an A+ in the course. The postdocs and faculty commented that they enjoyed teaching this hard-working, interactive, and collaborative enthusiastic team of students. A great deal of credit for the smooth working of the course was due to the postdocs, to the course director, and to the students themselves, who were both organized and disciplined.
Supplementary Material
Acknowledgment
Thanks to Peter Hitchcock, University of Michigan Departments of Cell and Developmental Biology and Ophthalmology and the Neuroscience Program, for critical comments on the manuscript and helpful suggestions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Warner SJ. Hutson MH. Oh S-H. Gerlach-Bank LM. Lomax MI. Barald KF. Expression of ZIC genes in the development of the chick inner ear and nervous system. Dev Dyn. 2003;226:702–712. doi: 10.1002/dvdy.10262. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y-C. Thompson DL. Chong AS-C. Wu K. Linn SA. Barald KF. Involvement of the MIF pathway in the development of zebrafish neural and sensory systems. 8th International conference on Zebrafish Development and Genetics; 2008. p. 741. (Abstracts.) [Google Scholar]
- 3.Doudou E. Barald KF. Postlethwait JH. Over-expression of Zic2a rescues ventralized zebrafish embryos. Zebrafish. 2004;1:239–257. doi: 10.1089/zeb.2004.1.239. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi LM. Daruwalla Z. Richards A-L. Roth TM. Attia N. Lukacs NW, et al. Immortalized mouse inner ear cell lines demonstrate a role for chemokines in promoting the growth of developing statoacoustic ganglion neurons. JARO. 2005;6:355–367. doi: 10.1007/s10162-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y-C. Bersano-Begey T. Ali S. Flak B. Li D. Perrin C, et al. A microfluidic approach to Mif pathway studies in the zebrafish. 8th International conference on Zebrafish Development and Genetics; 2008. p. 308. (Abstracts.) [Google Scholar]
- 6.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 7.Bolin LM. Murray R. Lukacs NW. Strieter RM. Kunkel SL. Schall TJ, et al. Primary sensory neurons migrate in response to the chemokine RANTES. J Neuroimmunol. 1998;81:49–57. doi: 10.1016/s0165-5728(97)00158-6. [DOI] [PubMed] [Google Scholar]
- 8.Bajetto A. Bonavia R. Barbero S. Florio T. Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–184. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 9.Bajetto A. Bonavia R. Barbero S. Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M. Takamura Y. Maeno M. Tochinai S. Iyaguchi D. Tanaka I, et al. Xenopus laevis macrophage migration inhibitory factor is essential for axis formation and neural development. J Biol Chem. 2004;279:21406–21414. doi: 10.1074/jbc.M311416200. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S. Satomura K. Levsky JM. Sreenath T. Wistow GJ. Semba I, et al. Expression pattern of macrophage migration inhibitory factor during embryogenesis. Mech Dev. 1999;84:153–156. doi: 10.1016/s0925-4773(99)00057-x. [DOI] [PubMed] [Google Scholar]
- 12.Ito K. Yoshiura Y. Ototake M. Nakanishi T. Macrophage migration inhibitory factor (MIF) is essential for development of zebrafish, Danio rerio. Dev Comp Immunol. 2008;32:664–672. doi: 10.1016/j.dci.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Koda M. Nishio Y. Hashimoto M. Kamada T. Koshizuka S. Yoshinaga K, et al. Up-regulation of macrophage migration-inhibitory factor expression after compression-induced spinal cord injury in rats. Acta Neuropathol (Berl) 2004;108:31–36. doi: 10.1007/s00401-004-0853-z. [DOI] [PubMed] [Google Scholar]
- 14.Tofaris GK. Patterson PH. Jessen KR. Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heo YS. Cabrera LM. Song JW. Futai N. Tung Y-C. Smith GD. Takayama S. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal Chem. 2007;79:1126–1134. doi: 10.1021/ac061990v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddings MA. Johnson MA. Gale BK. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J Micromech Microeng. 2008;18:067001.1–067001.4. [Google Scholar]
- 17.Duffy DC. McDonald JC. Schueller OJ. A whitesides GM rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 18.Smith WC. Knecht AK. Wu M. Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman LB. De LB. Jesus-Escobar JM. Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 20.Smith WC. Harland RM. Expression cloning of noggin. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 21.Smith WC. McKendry RM. Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 22.Knecht AK. Good PJ. Dawid IB. Harland RM. Dorsal–ventral patterning and differentiation of noggin. Development. 1995;121:1927–1936. doi: 10.1242/dev.121.6.1927. [DOI] [PubMed] [Google Scholar]
- 23.Gerlach LM. Hutson MR. Germiller JA. Nguyen-Luu D. Barald KF. Addition of the BMP4 antagonist, noggin disrupts avian inner ear development. Development. 2000;127:45–54. doi: 10.1242/dev.127.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Fjose A. Weber U. Mlodzik M. A novel vertebrate svp-related nuclear receptor is expressed as a step gradient in developing rhombomeres and is affected by retinoic acid. Mech Dev. 1995;52:33–246. doi: 10.1016/0925-4773(95)00404-o. [DOI] [PubMed] [Google Scholar]
- 25.Thompson D. Gerlach-Bank LM. Barald KF. Koenig RJ. Retinoic acid repression of BMP4 in inner ear development. Mol Cell Biol. 2003;23:2277–2286. doi: 10.1128/MCB.23.7.2277-2286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moody SA. Encyclopedia of Life Sciences. Wiley and Sons; 2001. Xenopus embryo: neural induction. [Google Scholar]
- 27.Spemann H. Uber den Anteil von Implantat und Wirtskeim an der Orientierung and Beschaffenheit der induzierten Embryonalanlage, Roux' Arch. Entwicklungsmech. 1931;123:389–517. doi: 10.1007/BF01380646. [DOI] [PubMed] [Google Scholar]
- 28.Spemann H. Mangold H. Uber induktion van Embryonalanlagen durch Implantation artfremder Organisatoren. Arch Mikrosk Anat Entwicklungsmech. 1924;100:599–638. [Google Scholar]
- 29.Cho KW. Blumberg B. Steinbeisser H. De Robertis EM. Molecular nature of Spemann's organizer: the role of the Xenopus goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoltewicz JS. Gerhart J. The Spemann organizer of Xenopus is patterned along its anteroposterior axis at the earliest gastrula stage. Dev Biol. 1997;192:482–491. doi: 10.1006/dbio.1997.8774. [DOI] [PubMed] [Google Scholar]
- 31.Gerlach-Bank LM. Ellis AD. Noonen B. Barald KF. Cloning and expression analysis of the chick DAN gene, an antagonist of the BMP family of growth factors. Dev Dyn. 2002;224:109–115. doi: 10.1002/dvdy.10079. [DOI] [PubMed] [Google Scholar]
- 32.Gerlach-Bank LM. Cleveland AR. Barald KF. DAN directs endolymphatic sac and duct outgrowth in the avian inner ear. Dev Dyn. 2004;229:219–230. doi: 10.1002/dvdy.10414. [DOI] [PubMed] [Google Scholar]
- 33.Shen YC. Jeyabalan AK. Wu KL. Hunker KL. Kohrman DC. Thompson DL, et al. The transmembrane inner ear (tmie) gene contributes to vestibular and lateral line development and function in the zebrafish (Danio rerio) Dev Dyn. 2008;237:941–952. doi: 10.1002/dvdy.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babb-Clendenon S. Shen Y-C. Liu Q. Turner KE. Mills MS. Cook GW, et al. Cadherin-2 function in the morphogenenesis of the zebrafish inner ear. J Cell Sci. 2007;119:5169–5177. doi: 10.1242/jcs.03299. [DOI] [PubMed] [Google Scholar]
- 35.Wilson AL. Shen YC. Babb-Clendenon SG. Rostedt J. Liu B. Barald KF, et al. Adherin-4 plays a role in the development of zebrafish cranial ganglia and lateral line system. Dev Dyn. 2007;236:893–902. doi: 10.1002/dvdy.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper MS. D'Amico LA. Henry CA. Analyzing morphogenetic cell behavior in vitally stained zebrafish embryos. Methods Mol Biol. 1998;122:185–204. doi: 10.1385/1-59259-722-x:185. [DOI] [PubMed] [Google Scholar]
- 37.Torisawa Y-S. Chueh B-H. Huh D. Ramamurthy P. Roth TM. Barald KF. Takayama S. Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab Chip. 2007;7:770–776. doi: 10.1039/b618439a. [DOI] [PubMed] [Google Scholar]
- 38.Raty S. Walters EM. Davis J. Zeringue H. Beebe DJ. Rodriguez-Zas SL. Wheeler MB. Embryonic development in the mouse enhanced by via microchannel culture. Lab Chip. 2004;4:186–190. doi: 10.1039/b316437c. [DOI] [PubMed] [Google Scholar]
- 39.Lucchetta EM. Lee JH. Fu LA. Patel NH. Ismagilov RF. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature. 2005;434:1134–1138. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagani GT. Monzo K. Fakhoury JR. Chen CC. Sisson JC. Zhang X. Microfluidic self-assembly of live Drosophila embryos for versatile high-throughput analysis of embryonic morphogenesis. Biomed Microdevices. 2007;9:681–694. doi: 10.1007/s10544-007-9077-z. [DOI] [PubMed] [Google Scholar]
- 41.Barald KF. Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- 42.Germiller JA. Smiley EC. Ellis AD. Hoff JS. Deshmukh I. Allen SJ. Barald KF. Molecular characterization of conditionally-immortalized cell lines derived from early mouse embryonic inner ear. Dev Dyn. 2004;231:815–827. doi: 10.1002/dvdy.20186. [DOI] [PubMed] [Google Scholar]
- 43.Blauwkamp MN. Beyer LA. Kabara L. Takemura K. Buck T. King WM. The role of bone morphogenetic protein 4 in inner ear development and function. Hear Res. 2007;225:71–79. doi: 10.1016/j.heares.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westerfield M. The Zebrafish Book. A guide for the laboratory use of zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2002. [Google Scholar]
- 45.Kimmel CB. Ballard WW. Kimmel SR. Ullmann B. Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 46.Dave G. Effect of pH on pentachlorophenol toxicity to embryos and larvae of zebrafish (Brachydanio rerio) Bull Environ Contam Toxicol. 1984;33:621–630. doi: 10.1007/BF01625593. [DOI] [PubMed] [Google Scholar]
- 47.Lehmann RG. Miller JR. Volatilization and sorption of dimethylsilanediol in soil. Environ Toxicol Chem. 1996;15:1455–1460. [Google Scholar]
- 48.Sarkar SA. Sharma RP. Expression of selected apoptosis related genes, MIF, IGIF and TNF alpha, during retinoic acid-induced neural differentiation in murine embryonic stem cells. Cell Struct Funct. 2002;27:99–107. doi: 10.1247/csf.27.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.