Abstract
Nicotine modulation of learning may contribute to its abuse liability. The role of hippocampal nicotinic acetylcholine receptors (nAChRs) in the effects of acute, chronic and withdrawal from chronic nicotine on learning was assessed via intrahippocampal drug infusion in mice. Acute dorsal hippocampal nicotine infusion enhanced contextual fear conditioning. Conversely, chronic intrahippocampal infusion of a matched dose had no effect, and withdrawal from chronic infusion impaired learning. Thus, hippocampal functional adaptation, evidenced by learning deficits during abstinence, occurs with the transition from acute to chronic nicotine exposure. To investigate which hippocampal nAChRs mediate these adaptations, C57BL/6, β2 nAChR subunit knockout (KO), and wildtype (WT) mice treated chronically with systemic nicotine received intrahippocampal dihydro-β-erythroidine (a high affinity nAChR antagonist). Intrahippocampal dihydro-β-erythroidine precipitated learning deficits in all but the KO mice. Therefore, the action of nicotine at hippocampal β2* nAChRs mediates adaptations in hippocampal function that underlie withdrawal deficits in contextual fear conditioning.
Keywords: nicotine, withdrawal, hippocampus, learning, nicotinic acetylcholine receptors, dihydro-β-erythroidine, addiction, cognition
High rates of smoking despite known negative health and social consequences exemplify the fact that nicotine is addictive (Centers for Disease Control and Prevention, 2007; Rehm et al., 2006). Recent studies (see LeFoll and Goldberg, 2005; Stolerman and Jarvis, 1995 for reviews) suggest that, in addition to reward processes, other processes may contribute to nicotine addiction. Research indicating that nicotine addiction and learning share common neural and cellular substrates (see Hyman, 2005; Hyman and Malenka, 2001; Hyman et al., 2006; Kelley, 2004; Tinsley et al., 2004 for reviews) suggests that one process that may contribute to nicotine use is altered learning. Support for this contention comes from three lines of evidence: 1) Nicotine administration is associated with direct effects on learning and memory (see Davis and Gould, 2008; Gould, 2006; Levin, 2002; Levin and Simon, 1998; Rezvani and Levin, 2001 for reviews), 2) Environmental stimuli associated with nicotine use and/or the effects of nicotine can maintain and reinstate nicotine use (Caggiula et al., 2001; Goldberg et al., 1981 and see LeFoll and Goldberg, 2006 for review), 3) Nicotine withdrawal produces cognitive deficits including disrupted learning (Bell et al., 1999; Blake and Smith, 1999; Davis et al., 2005; Hughes et al., 1989; Jacobsen et al., 2005; Xu et al., 2006). Although some work has been done to assess the neural substrates of the effects of nicotine on learning (see Davis and Gould, 2008; Levin et al., 2006 for reviews), few animal studies have examined the neural substrates of nicotine withdrawal-associated deficits in learning-related processes. This is surprising in light of research suggesting that changes in cognition during abstinence predict relapse (Rukstalis et al., 2005).
One paradigm that has been utilized to examine the effects of nicotine on learning is fear conditioning (see Davis and Gould, 2008; Gould, 2006 for reviews). In fear conditioning, paired presentations of a discrete conditioned stimulus (CS) with an unconditioned stimulus (US) result in the formation of associations between the CS and the US (i.e. cued fear conditioning) and between the training context and the US (i.e. contextual fear conditioning). Previous work has demonstrated that withdrawal from chronic nicotine treatment selectively disrupts contextual but not cued fear conditioning (Andre et al., 2008; Davis and Gould, 2007; Davis et al., 2005; Gulick and Gould, 2008; Portugal and Gould, 2007; Portugal et al., 2008; Raybuck et al., 2008). Since only the former requires the hippocampus (Kim et al., 1993; Logue et al., 1997; Phillips and LeDoux, 1992), this suggests that nicotine withdrawal disrupts hippocampal function either directly or indirectly via functional alterations in afferent and/or efferent areas. Studies have demonstrated that withdrawal from chronic systemic nicotine alters neural processes in discrete brain regions (Bruijnzeel and Markou, 2004; Liu and Jin, 2004; Marttila et al., 2006; Panagis et al., 2000; Paterson et al., 2007; Rada et al., 2001) but these studies cannot discriminate if the changes are due to direct effects of nicotine in those areas or effects of nicotine in afferent areas.
The present studies determined if withdrawal from chronic infusion of nicotine into the hippocampus is sufficient to produce withdrawal-related deficits in contextual fear conditioning. As a control, separate groups of mice were withdrawn from chronic infusions of nicotine into the cortex above the hippocampus or the thalamus below the hippocampus. In addition, the effects of acute, chronic, and withdrawal from chronic infusion of nicotine into the hippocampus on contextual fear conditioning were compared. Finally, the ability of intrahippocampal infusions of the high affinity nicotinic acetylcholinergic receptor antagonist (nAChR) dihydro-beta-erythroidine (DHβE) to alter contextual fear conditioning in C57BL/6 mice, β2 nAChR subunit knockout (KO) mice, and wildtype (WT) mice treated chronically with systemic nicotine was measured. These experiments determined that the effects of nicotine at high affinity nAChRs in the hippocampus were sufficient to induce withdrawal deficits in learning.
Methods
Subjects
Subjects were male C57BL/6J mice (aged 8 – 12 weeks; Jackson Laboratories) for all studies except the knockout study. Since previous work has not demonstrated a sex difference for the effects of nicotine on fear conditioning (Gould, 2003), and since KO mice are an expensive resource, male and female β2 nAChR subunit KO mice were compared to their male and female WT littermates (aged 8 – 12 weeks) for the knockout study. The original line of β2 nAChR subunit KO mice was generated as described by Xu et al. (1999) and backcrossed to the C57BL/6 strain for at least seven generations. Animals were genotyped using procedures described previously (Xu et al., 1999). Mice were group housed (2 - 4 same sex per cage) prior to surgery and singly housed following surgery with ad libitum access to food and water. Mice were maintained on a 12:12 light/dark cycle (lights on 7:00 am). Training and testing occurred during the light phase. The Temple University Institutional Animal Care and Use Committee approved all procedures.
Surgical Procedures
For acute intrahippocampal infusions of drug or vehicle, double guide cannulae (C232G, 22 gauge, Plastics One, Roanoke, VA) were implanted in the hippocampus (−1.70 mm posterior to bregma, +/− 1.50 mm lateral to the midline, final injection depth −2.30 mm ventral to the skull surface) of mice under isoflourane anesthesia (5% induction, 2% maintenance). Cannulae were fixed to the skull with dental cement and fitted with double dummy cannulae (C232DC, Plastics One, Roanoke, VA) to prevent clogging. Mice in the acute treatment groups received sham pump implantations during the initial surgery and sham pump removals one day before training to match surgeries in the chronic and withdrawal from chronic intrahippocampal nicotine conditions.
Mice treated chronically and mice withdrawn from chronic intrahippocampal treatment had two nicotine or saline-filled Alzet Mini-Osmotic pumps (1002, Alzet, Cupertino, CA) implanted subcutaneously between the shoulder blades. Pumps were connected, via PE50 tubing (Plastics One, Roanoke, VA), to bilateral chronic indwelling cannulae (3280PD, osmotic pump connect, 28 gauge, Plastics One, Roanoke, VA) aimed at the dorsal hippocampi (−1.70 mm posterior to bregma, +/− 1.50 mm lateral to the midline, and −2.30 mm ventral to the skull surface), above the dorsal hippocampi (−0.85 mm ventral to the skull surface) or below the dorsal hippocampi (−3.30 mm ventral to the skull surface). Pump removal (for mice in the withdrawal treatment groups) or sham pump removal surgery (for the other groups) was performed 24 hours before training.
For experiments that examined the effects of intrahippocampal DHβE on contextual fear conditioning in mice treated systemically with chronic nicotine or saline, minipumps were implanted 13 days and guide cannulae surgeries were performed at least 5 days before training procedures. Surgeries were performed as described in the previous section. Buprenex (0.03 mg/kg) or Ketoprofen (2.0 mg/kg) was administered subcutaneously following all surgical procedures to control for post-operative pain.
Drugs and Infusions
Nicotine hydrogen tartrate salt (doses reported as freebase) and DHβE (Sigma Co., St. Louis, MO) were dissolved in physiological saline. Previous work (see Matta et al., 2007) indicates that neutralized nicotine solutions are unstable and will degrade by up to 50% in an osmotic minipump over 10 days. Therefore, following recommendations of Matta and colleagues (2007), nicotine solutions were not neutralized for the present studies; all nicotine solutions had a pH ~ 3.2.
For acute intrahippocampal nicotine (0.35 μg/side) or saline infusions, mice were gently restrained, and dummy cannulae were removed and replaced with 22 gauge infusion cannulae that extended 0.80 mm beyond the tip of the guide cannulae. Nicotine or saline (0.50 μl/side) was infused over 1 minute, 2 – 4 minutes before training and testing (dose based on Davis et al., 2007). Injection cannulae were left in place for 1 minute following the infusion to allow for diffusion away from the infusion cannula tip.
For chronic intrahippocampal administration, minipumps that administered solution at a rate of 0.25 μl/hour were filled with saline or with a concentration of nicotine (0.35 μg/0.50 μl) that matched the concentration used in the acute nicotine group. Chronic intrahippocampal nicotine administration occurred over 14 days for mice in the chronic treatment groups (i.e. intrahippocampal infusion of saline or nicotine occurred continuously over training on day 13 and testing on day 14) or over 12 days for mice in the withdrawal groups. For mice in the withdrawal treatment groups, nicotine administration was discontinued 24 hours before fear conditioning training (day 13).
For chronic systemic administration, minipumps administered nicotine (6.3 mg/kg/day) or saline at a rate of 0.25 μL/hour for 14 days. As reported by Davis et al. (2005), this chronic dose of nicotine produces plasma nicotine levels that are in the range reported for smokers (Benowitz et al., 1989; Henningfield and Keenan, 1993). DHβE (18.0 μg/0.50 μl/side over 1 minute; based on Davis et al., 2007; Levin et al., 2002) or saline infusions into the dorsal hippocampus were administered 15 minutes before training. Mice were trained on day 13 and tested on day 14. Chronic systemic infusions of saline or nicotine were continued through both training and testing.
Apparatus
Mice were trained and tested for contextual fear conditioning in four identical conditioning chambers (17.78 × 19.05 × 38.10 cm) housed in sound attenuating boxes (Med Associates, St. Albans, VT). Each chamber was constructed of clear Plexi-glas walls in the front, back, and top and stainless steel on the sides. The chamber floors, which were connected to a shock generator and scrambler, were comprised of 18 metal rods spaced 0.60 cm. apart. Speakers for administering the CS were located on the right wall of each chamber; and ventilation fans, which provided air exchange and background noise (69 dB), were located on the right wall of each sound attenuating box. A 28V bulb located at the top of the left wall of each chamber provided illumination. A computer running MED-PC software controlled stimuli administration.
Testing for cued fear conditioning took place in four chambers (20.30 × 22.90 × 17.80 cm) housed in sound attenuating boxes. These cued fear conditioning testing chambers were located in a different room and were distinct from those used for training. The chambers consisted of Plexi-glas front and back walls, metal side walls with visual stimuli distinct from the training chambers, and grid floors covered by opaque white plastic. Speakers for delivering the CS and a 28 V bulb were mounted on the left wall of each chamber. The walls of the sound attenuating boxes that housed these chambers differed in color from those that housed the training chambers. Background noise and air exchange were provided by fans, which were mounted on the left wall of the sound attenuating boxes. A novel olfactory cue, vanilla extract, was applied to a paper towel and placed under each chamber to further distinguish the chambers from those used for training.
Behavioral Procedures
Mice were trained and tested in contextual fear conditioning according to previously described procedures (Gould and Wehner, 1999). Mice were placed in conditioning chambers for 330 seconds and trained using two co-terminating CS (30 second, 85 dB white noise) – US (2 second, 0.57 mA footshock) presentations separated by a 120 second intertrial interval. Freezing, defined as the absence of movement except for respiration (Blanchard and Blanchard, 1969), served as the dependent measure and was scored by an experimenter during each session. Each animal’s behavior was assessed for one second every 10 seconds. Baseline freezing was assessed during the first 120 seconds after the start of the training session, and the first CS – US presentation occurred immediately after this baseline period. Twenty-four hours later mice were tested for freezing to the training context. Freezing behavior was assessed over 300 seconds. Chambers were cleaned with a 70% ethanol solution following all behavioral procedures.
Any observed effects of intrahippocampal nicotine administration on contextual fear conditioning could reflect alterations in arousal, locomotor activity, or attentional processes rather than alterations in hippocampus-dependent learning. To assess this possibility, separate groups of mice were trained and tested in cued fear conditioning, a task that does not require the hippocampus, and contextual fear conditioning. A modified fear conditioning training procedure was utilized (Gould et al., 2004) in order to reduce freezing in response to the CS thereby potentially increasing sensitivity to withdrawal-related changes in cued fear conditioning. Mice were trained using one CS (85 dB white noise, 15 seconds) − US (0.57 mA footshock, 1 second) pairing. Mice were returned to the training context 24 hours later, and freezing in response to the context was assessed for 300 seconds. One hour after contextual fear conditioning testing, mice were placed in an altered context for 360 seconds. Freezing in response to the altered context (PreCS) was assessed for the first 180 seconds, and freezing to the CS was assessed for the second 180 seconds.
Histology
Upon completion of behavioral testing, brains were stored in a 10% formalin solution until sectioning. Brains were sectioned using a cryostat maintained at −18° C; 60 μm coronal sections were taken proximal to cannulae tracts. Sections were mounted on microscope slides and stained with cresyl violet. Cannulae placements were determined using a light microscope and recorded on schematics of the mouse brain (Paxinos and Franklin, 2001). The infusion sites in the dorsal hippocampus (Figures 1D-F, 3B), above the dorsal hippocampus (2C) and below the dorsal hippocampus (2D) were assessed. Figures 4B and 5B depict infusion sites in the dorsal hippocampi for DHβE experiments. The data from mice with incorrect placements (less than 5% of placements) were excluded from analyses.
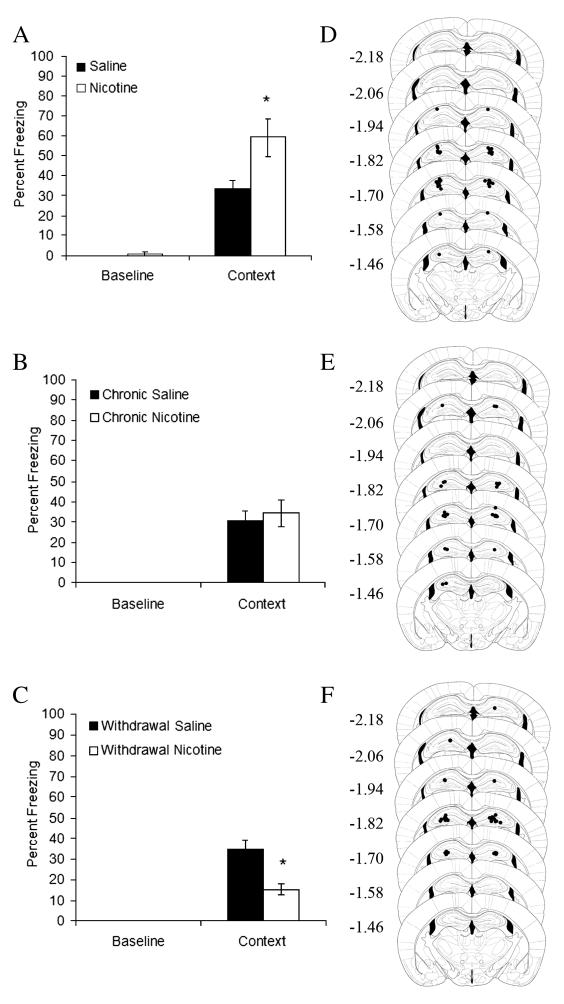
Figure 1.
The effects of acute intrahippocampal nicotine, chronic intrahippocampal nicotine, and withdrawal from chronic intrahippocampal nicotine were examined. A) Acute intrahippocampal nicotine enhanced contextual fear conditioning (n = 9); * p < 0.05 compared to saline. B) Chronic intrahippocampal nicotine had no effect on contextual fear conditioning (n = 7). C) Withdrawal from chronic intrahippocampal nicotine impaired contextual fear conditioning (n = 8) ; * p < 0.05 compared to saline. Error bars represent standard error of the mean. Representation of cannulae placements for mice in D) acute treatment groups, E) chronic treatment groups and F) withdrawal treatment groups. Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma (picture modified from Paxinos and Franklin, 2001).
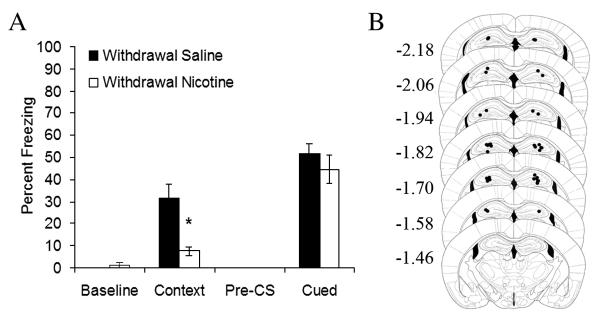
Figure 3.
Withdrawal from chronic intrahippocampal nicotine administration impaired contextual fear conditioning and had no effect on cued fear conditioning (A, n = 7); p < 0.05 compared to saline. Error bars represent standard error of the mean. B) Representation of cannulae placements for mice withdrawn from chronic nicotine infused into the dorsal hippocampus. Mice were trained using one CS (85 dB white noise, 15 seconds) − US (0.57 mA footshock, 1 second) pairing. Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma (pictures modified from Paxinos and Franklin, 2001).
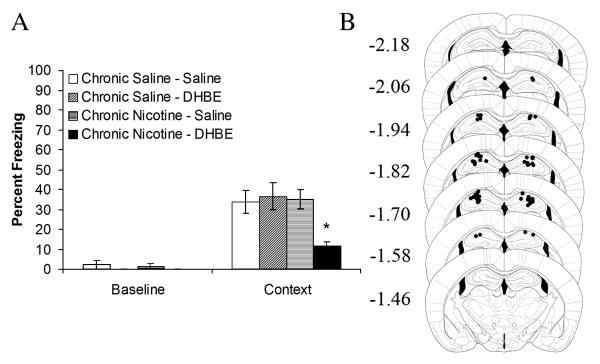
Figure 4.
A) Intrahippocampal administration of the nicotinic acetylcholine receptor antagonist, dihydro-β-erythroidine, precipitated deficits in contextual fear conditioning in C57BL/6 mice treated chronically with systemic nicotine. There was no effect of intrahippocampal dihydro-β-erythroidine in saline treated mice (n = 7 − 8). *p < 0.05 compared to all other groups. Error bars represent standard error of the mean. B) Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma (pictures modified from Paxinos and Franklin, 2001).
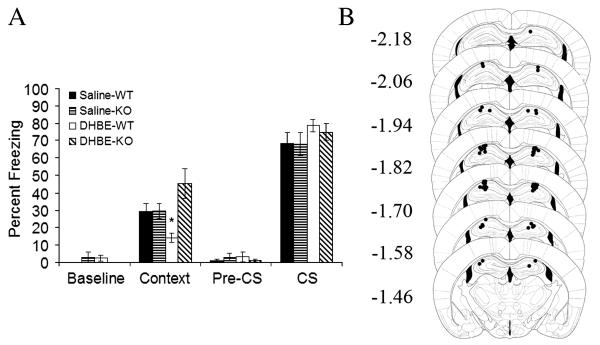
Figure 5.
A) Intrahippocampal dihydro-β-erythroidine administration precipitated deficits in contextual fear conditioning in WT but not β2 KO mice treated chronically with systemic nicotine (n = 7 − 8). All mice were treated chronically with systemic nicotine. * p < 0.05 compared to all other groups. Error bars represent standard error of the mean. B) Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma (pictures modified from Paxinos and Franklin, 2001).
Statistical Analysis
Independent samples t-tests were performed on percent freezing data from acute, chronic, and withdrawal from chronic intrahippocampal nicotine infusion experiments. Levene’s tests were carried out to determine if group variances were equal. If variances were unequal, adjusted independent samples t-tests were utilized. Data from the DHβE infusion experiment that utilized C57BL/6 mice were analyzed using 2 (chronic treatment) × 2 (acute infusion) ANOVAs. Initial analyses of data from the DHβE infusion experiment that utilized β2 KO and WT mice using 2 (sex) × 2 (genotype) × 2 (infusion) ANOVAs revealed no significant interactions between sex and the other variables. Thus, data from male and female mice were collapsed and analyzed using 2 (genotype) × 2 (infusion) ANOVAs. Tukey HSD (equal variances) or Games-Howell (unequal variances) post-hoc analyses were carried out to examine pair-wise differences in percent freezing data. Analyses were performed with SPSS version 11.0.
Results
The effects of acute, chronic, and withdrawal from chronic intrahippocampal nicotine
Mice receiving acute intrahippocampal nicotine demonstrated significantly higher levels of contextual fear conditioning than their saline-treated counterparts (n’s = 9; t(11.68) = 2.60, p = 0.02; Figure 1A). Acute intrahippocampal nicotine most likely had no effect on baseline locomotor activity because throughout the series of experiments, no changes were seen in baseline activity. Furthermore, previous research indicates that intrahippocampal nicotine has no effect on cued fear conditioning (Davis et al., 2007); if acute intrahippocampal nicotine altered locomotor activity or acted as an interoceptive cue for the potential shock at testing, then changes in baseline activity, pre-CS activity, and CS-related activity would be seen. Rather, acute intrahippocampal nicotine enhances contextual learning processes.
No significant differences existed between mice treated chronically with nicotine and mice treated chronically with saline (n’s = 7; t(12) = 1.00, p = 0.61; Figure 1B), suggesting that tolerance to the effects of nicotine on contextual fear conditioning may develop with chronic intrahippocampal treatment. In contrast, mice withdrawn from chronic intrahippocampal nicotine administration 24 hours prior to training demonstrated significantly lower levels of contextual fear conditioning than their saline-treated counterparts (n’s = 8; t(14) = 4.40, p = 0.001; Figure 1C). For both mice treated chronically with nicotine and for mice withdrawn from chronic nicotine treatment, no significant differences in baseline freezing existed. Therefore, chronic intrahippocampal nicotine and withdrawal from intrahippocampal nicotine did not alter baseline locomotor activity.
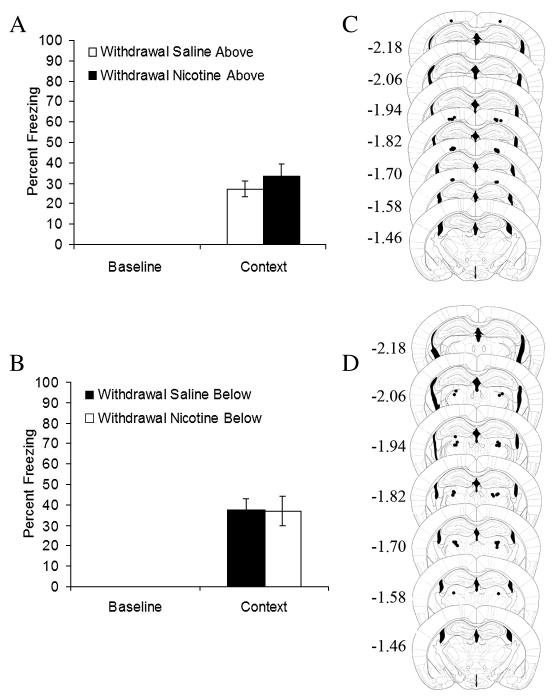
It is possible that the withdrawal-associated deficit in contextual fear conditioning was due to drug diffusion into cortical regions above and/or thalamic regions below the hippocampus. This possibility was assessed in separate groups of mice. Independent samples t-tests revealed no significant differences between nicotine-withdrawn mice and saline-withdrawn mice in contextual fear conditioning when nicotine or saline was chronically administered above (n’s = 7; Figure 2A) or below (n’s = 8; Figure 2B) the hippocampus prior to withdrawal. In addition, there were no significant differences in baseline freezing for either experiment. Thus, the nicotine withdrawal-associated deficit in contextual fear conditioning reflects neural alterations due to direct effects of nicotine in the hippocampus.
Figure 2.
Withdrawal from chronic infusion of nicotine A) above the dorsal hippocampus (n = 7) and B) below the dorsal hippocampus (n = 8) had no effect on contextual fear conditioning. Error bars represent standard error of the mean. Representation of cannulae placements for mice C) withdrawn from chronic nicotine infused above the dorsal hippocampus (from A), and D) below the dorsal hippocampus (from B). Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma (pictures modified from Paxinos and Franklin, 2001).
Withdrawal from chronic intrahippocampal nicotine could impair contextual fear conditioning via alterations in associative processes or via alterations in nonassociative processes such as arousal and attention. If the withdrawal-associated deficit reflects alterations in nonassociative processes, then other types of learning should be impaired by intrahippocampal nicotine withdrawal. To examine this possibility, the effects of withdrawal from chronic intrahippocampal nicotine on both contextual and cued fear conditioning were examined (Figure 3A). Independent samples t-tests revealed that mice withdrawn from chronic intrahippocampal nicotine administration demonstrated significantly lower levels of contextual fear conditioning than their saline-treated counterparts (n’s = 7; t(8.17) = 2.96, p = 0.02). There were no significant effects of withdrawal from chronic intrahippocampal nicotine treatment on baseline freezing, pre-CS freezing, or freezing in response to the CS. Thus, the impairing effect of withdrawal from chronic intrahippocampal nicotine on contextual fear conditioning likely reflects alterations in associative processes specific to the task.
Intrahippocampal DHβE infusion in chronic systemic nicotine-treated mice
To confirm hippocampal involvement and to investigate the role of high-affinity nAChRs in the effects of withdrawal from chronic systemic nicotine on contextual fear conditioning, C57BL/6 mice treated chronically with systemic nicotine or saline received an acute intrahippocampal infusion of DHβE or saline. Analysis of the contextual fear conditioning data using a 2 × 2 ANOVA revealed significant main effects of chronic treatment (F(1, 27) = 5.78, p = 0.02) and acute infusion (F(1, 27) = 4.46, p = 0.04) and a significant interaction between the chronic treatment and the acute infusion variables (n’s = 7 − 8; F (1, 27) = 7.34, p = 0.01; Figure 4A). Follow-up, Tukey HSD analyses revealed that chronic nicotine-treated mice that received intrahippocampal DHβE demonstrated significantly lower levels of contextual fear conditioning than chronic saline-treated mice that received intrahippocampal saline (t(27) = 3.25, p = 0.02), chronic saline-treated mice that received intrahippocampal DHβE (t(27) = 3.68, p = 0.01), and chronic nicotine-treated mice that received intrahippocampal saline (t(27) = 3.35, p = 0.01). There were no other pair-wise differences. No significant differences were seen for baseline freezing. These data suggest that alterations in high affinity hippocampal nAChRs, or downstream processes, may underlie nicotine withdrawal-associated deficits in the acquisition of contextual fear conditioning.
Intrahippocampal DHβE infusion in chronic systemic nicotine-treated β2 KO mice
DHβE acts at a variety of nAChRs that bind nicotine with high affinity, including α4β2, α4β4, α3β2, α2β2, and α2β4 nAChRs (Harvey et al., 1996; Khiroug et al., 2004). Thus, impaired contextual fear conditioning demonstrated by C57BL/6 mice treated with intrahippocampal DHβE and chronic systemic nicotine may reflect the action of the nAChR antagonist at one or more of these nAChR subtypes. Prior research demonstrated that chronic nicotine does not alter contextual fear conditioning (Davis et al., 2005). To further examine which nAChR subtypes mediate intrahippocampal DHβE-precipitated deficits in contextual fear conditioning, β2 nAChR subunit KO mice and their WT littermates were treated chronically with systemic nicotine and received an acute intrahippocampal infusion of either DHβE or saline 15 minutes before training (Figure 5A). Thus, the following treatment groups existed: 1) WT mice receiving chronic systemic nicotine and acute intrahippocampal saline prior to training, 2) WT mice receiving chronic systemic nicotine and acute intrahippocampal DHβE prior to training, 3) β2 nAChR subunit KO mice receiving chronic systemic nicotine and acute intrahippocampal saline prior to training, 4) β2 nAChR subunit KO mice receiving chronic systemic nicotine and acute intrahippocampal DHβE prior to training.
Analyses of the contextual fear conditioning data revealed no main effect of hippocampal infusion, a significant main effect of genotype (n’s = 7 − 8 ;F(1, 31) = 7.47, p = 0.01) and a significant interaction between the genotype and the hippocampal infusion variables (F(1, 31) = 7.13, p = 0.01). Games-Howell comparisons indicated that WT mice that received chronic systemic nicotine and intrahippocampal DHβE prior to training demonstrated significantly lower levels of contextual fear conditioning than all other groups (t(31) = 3.02, p = 0.05; t(31) = 3.25, p = 0.03; t(31) = 3.71, p = 0.02 versus WT mice treated with intrahippocampal saline, KO mice receiving intrahippocampal saline, and KO mice receiving intrahippocampal DHBE, respectively). In addition, contextual fear conditioning levels demonstrated by WT and β2 KO mice treated chronically with systemic nicotine and receiving intrahippocampal saline were similar suggesting that WT and β2 KO mice responded similarly to the stress associated with the infusion procedures. Thus, DHβE acts at β2* nAChRs (* designates contains other subunits, e.g., α4β2 nAChRs) in the dorsal hippocampus to precipitate deficits in contextual fear conditioning. No significant effects were seen for baseline freezing, preCS freezing, and freezing in response to the CS.
Discussion
The present study is the first to directly compare the effects of acute, chronic, and withdrawal from chronic infusion of nicotine into the hippocampus on learning and the first to demonstrate that the effects of chronic nicotine in the hippocampus are sufficient to induce withdrawal-related changes in hippocampus-dependent learning. Acute intrahippocampal infusion of nicotine enhanced contextual fear conditioning. In contrast, chronic infusion of nicotine had no effect on contextual fear conditioning, and mice trained after cessation of chronic nicotine infusion demonstrated deficits in contextual fear conditioning. No deficits were seen in cued fear conditioning indicating that withdrawal from chronic intrahippocampal nicotine did not alter processes that affect both contextual and cued fear conditioning such as locomotor activity or anxiety. In addition, no change in contextual fear conditioning was seen in mice withdrawn from chronic infusion of nicotine into the cortex above or the thalamus below the hippocampus, suggesting that the withdrawal-associated impairment in contextual fear conditioning was not due to diffusion of the drug into regions surrounding the dorsal hippocampus. These results suggest that nicotine acts in the hippocampus to alter contextual learning. Furthermore, with the switch from acute to chronic administration, adaptation in hippocampal function occurs resulting in learning deficits during abstinence.
The present results also suggest that deficits in contextual fear conditioning following cessation of chronic nicotine administration are mediated by hippocampus nAChRs that bind nicotine with high affinity (i.e. DHβE-sensitive nAChRs). Intrahippocampal infusion of DHβE, an antagonist of α4β2, α4β4, α3β2, α2β2, and α2β4 nAChRs (Harvey et al., 1996; Khiroug et al, 2004), precipitated deficits in contextual fear conditioning in C57BL/6 mice and WT mice treated chronically with nicotine. Intrahippocampal DHβE failed to alter contextual fear conditioning in chronic nicotine-treated β2 nAChR subunit KO mice, suggesting that β2* nAChRs are critically involved in the withdrawal-associated deficit. Furthermore, data indicating that intrahippocampal infusion of DHβE had no effect in control animals suggest that chronic nicotine treatment may alter the function of β2* nAChRs, including α4β2 nAChRs, and/or down stream cell-signaling processes in the hippocampus. In support, chronic nicotine administration is associated with the desensitization of nAChRs, an increase in the density of nAChRs (see Gentry and Lukas, 2002; Marks, 1998 for reviews), and with alterations in the signaling of second messengers and transcription factors that are critically involved in learning and memory (see Davis and Gould, 2008; Gould, 2006; Zhai et al., 2008 for reviews).
β2* nAChRs appear to mediate many behaviors implicated in nicotine addiction. For instance, β2* nAChRs are involved in nicotine self-administration (Besson et al., 2006; Picciotto et al., 1998) and in the formation of nicotine-context associations that are measured as conditioned place preferences (Grabus et al., 2005; Walters et al., 2006). Likewise, β2* nAChRs are critically involved in nicotine withdrawal-related changes in anxiety (Damaj et al., 2003; Jackson et al., 2008), and DHβE-sensitive nAChRs in the ventral tegmental area mediate withdrawal-associated decreases in reward function (Bruijnzeel and Markou, 2004; Stoker et al., 2008). The present results provide additional evidence for the involvement of β2* nAChRs in behavior that contributes to/supports nicotine addiction. Furthermore, the data identify hippocampal β2* nAChRs as the critical population of nAChRs through which the effect of nicotine withdrawal on contextual conditioning is mediated. Future work to identify if other effects of nicotine are mediated by β2* nAChRs in specific brain regions will greatly enhance current understanding of the role of β2* nAChRs in nicotine addiction.
Not all symptoms of nicotine withdrawal are mediated by β2* nAChRs. Previous research demonstrated (Damaj et al., 2003) that DHβE precipitated somatic signs of withdrawal in mice treated chronically with a higher dose of nicotine than used in the current study (24 mg/kg/day for 15 days), suggesting a potential role for high affinity nAChRs in the somatic effects of nicotine withdrawal. However, it is unclear which subclass(es) of DHβE-sensitive nAChRs, β2* nAChRs or β4* nAChRs (Harvey et al., 1996; Khiroug et al., 2004), mediated this effect. Work with KO mice (Besson et al., 2006; Salas et al., 2004) has elucidated this issue and suggested that β2* nAChRs are not critically involved in somatic signs of nicotine withdrawal; β2* KO mice and their WT counterparts that received chronic nicotine both demonstrated somatic signs of withdrawal following administration of the broad spectrum nAChR antagonist, mecamylamine (Besson et al., 2006). In contrast, β4* KO mice receiving chronic nicotine exhibited significantly reduced somatic signs of nicotine withdrawal compared to their WT counterparts following administration of mecamylamine suggesting a critical role for β4* nAChRs in somatic signs of nicotine withdrawal.
Another subclass of nAChRs, α7 nAChRs, has also been implicated in a number of behaviors that may contribute to nicotine addiction including withdrawal-associated hyperanalgesia and somatic symptoms of nicotine withdrawal (Grabus et al., 2005; Jackson et al., 2008; Salas et al., 2007). A previous study (Portugal et al., 2007), however, failed to demonstrate a role for α7 nAChRs in nicotine withdrawal-associated deficits in contextual fear conditioning: α7 nAChR KO mice demonstrated deficits in contextual fear conditioning following withdrawal from chronic systemic nicotine administration. Similarly, there is little evidence for a role of α7 nAChRs in the effects of acute nicotine on contextual fear conditioning (Davis et al., 2007; Davis and Gould, 2007; Davis and Gould 2006; Wehner et al., 2007). Thus, the present studies did not assess the role of dorsal hippocampal α7 nAChRs in nicotine withdrawal-associated deficits in contextual fear conditioning.
In the present studies, intrahippocampal administration of DHβE at training precipitated contextual fear conditioning deficits in mice treated chronically with nicotine. These data suggest that nicotine withdrawal-associated deficits in contextual learning reflect a disruption of acquisition or consolidation processes rather than retrieval processes. Additional support for this contention comes from a recent study (Kenney and Gould, 2008), which demonstrated that context-nicotine associations formed before the initiation of chronic nicotine administration remained intact following nicotine withdrawal. New contextual learning, however, was disrupted. In addition, a previous study (Portugal et al., 2008) demonstrated that systemic administration of DHβE prior to training alone or prior to both training and testing precipitated deficits in contextual fear conditioning in chronic nicotine-treated mice, while pre-testing administration of the antagonist had no effect. Importantly, Portugal and colleagues’ (2008) findings argue against an alternative interpretation of the present findings, which would postulate that nicotine and DHBE administered together at training produce a state that differs from the state at testing when only nicotine is present; according to this interpretation, this shift in states is responsible for the deficit.
Taken together, the present results along with previous findings suggest that contextual learning processes and the direct effects of nicotine on these processes can contribute to nicotine addiction in a variety of ways. Acute nicotine enhances contextual learning via alterations in hippocampal function; enhanced learning may positively reinforce smoking. Additionally, acute nicotine could facilitate the formation of drug-context associations, which may contribute to context-evoked cravings (see Caggiula et al., 2002 for review). As nicotine administration continues, neural adaptation in the hippocampus mediated by high-affinity nAChRs leads to tolerance and deficits in learning when nicotine treatment ceases. Thus, during withdrawal smokers may have deficits in learning processes. Prior maladaptive drug-context associations, however, may remain intact thereby contributing to cravings that, along with the withdrawal deficits, could facilitate relapse. In addition, cognitive/learning deficits during quit attempts could impede the learning of adaptive behaviors that could facilitate abstinence (Gutkin et al., 2006). Because the hippocampus is involved in the declarative learning and memory processes that define who we are and anchor us to past events, places, and experiences (Eichenbaum, 1999), diseases and drugs that alter the hippocampus may have a particularly insidious effect and contribute to the difficulty in successfully treating diseases such as nicotine addiction. This is supported by human imaging studies that show changes in hippocampal activity with change in abstinence states (Due et al., 2002; Wang et al., 2007; Zubieta et al., 2005).
Acknowledgments
Role of Funding Source: The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG; DA024787 TG). Jennifer A. Davis was supported by a National Institute on Drug Abuse predoctoral fellowship (DA021949). The National Institute on Drug Abuse had no further role in this research.
Footnotes
Conflicts of Interest: None
References
- Andre JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behav. Brain Res. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob. Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog. Brain Res. 1989;79:279–287. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, Granon S. Genetic dissociation of two behaviors associated with nicotine addiction: beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology. 2006;187:189–199. doi: 10.1007/s00213-006-0418-z. [DOI] [PubMed] [Google Scholar]
- Blake J, Smith A. Effect of smoking and smoking deprivation on the articulatory loop of working memory. Hum. Psychopharmacology Clin. Exp. 1999;12:259–264. [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J. Comp. Physiol. Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in tats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol. Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol. Biochem. Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Morbidity and Mortality Weekly Report. Vol. 56. Centers for Disease Control and Prevention; [Retrieved June 20, 2008]. 2007. Cigarette smoking among adults—United States, 2006; pp. 1157–1161. Web site: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J. Pharmacol. Exp. Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Associative learning, the hippocampus, and nicotine addiction. Curr. Drug Abuse Rev. 2008;1:9–19. doi: 10.2174/1874473710801010009. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology. 2007;32:2011–2019. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology. 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Seigel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J. Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imagine. Am. J. Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Conscious awareness, memory and the hippocampus. Nature Neurosci. 1999;2:775–776. doi: 10.1038/12137. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr. Drug Targets CNS Neurol. Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr. Physiol. Behav. Sci. 2003;38:124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol. Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behav. Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Damaj MI. Nicotine physical dependence in the mouse: involvement of the α7 nicotinic receptor subtype. Eur. J. Pharmacol. 2005;515:90–93. doi: 10.1016/j.ejphar.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology. 2008;196:483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkin BS, Dehaene S, Changeux JP. A neurocomputational hypothesis for nicotine addiction. Proc Natl Acad Sci U S A. 2006;103:1106–1111. doi: 10.1073/pnas.0510220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determents of dihydro-ß-erythroidine sensitivity on rat neuronal nicotinic receptor subunits. J. Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keenan RM, Yellin A. Effect of tobacco withdrawal on sustained attention. Addict. Behav. 1989;14:577–580. doi: 10.1016/0306-4603(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am. J. Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and persistence. Nature Rev. Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav. Neurosci. 2008;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Khiroug L, Yakel JL. Rat nicotinic acetylcholine receptor α2ß2 channels: comparison of functional properties with α4ß2 channels in Xenopus oocytes. Neurosci. 2004;124:817–822. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav. Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- LeFoll B, Goldberg SR. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol. Sci. 2005;26:287–293. doi: 10.1016/j.tips.2005.04.005. [DOI] [PubMed] [Google Scholar]
- LeFoll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–81. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J. Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neurosci. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav. Neurosci. 1997;11:104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Jin WQ. Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. Neuroreport. 2004;15:1479–1481. doi: 10.1097/01.wnr.0000126218.25235.b6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Marks MJ. Desensitization and the regulation of neuronal nicotinic receptors. In: Arneric SP, Brioni JD, editors. Neuronal Nicotinic Receptors: Pharmacology and Therapeutic Opportunities. Wiley Liss; New York: 1998. pp. 65–79. [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal FosB/DeltaFosB and c-Fos expression in rats and mice. Neuropharmacology. 2006;51:44–51. doi: 10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Panagis G, Hildebrand BE, Svensson TH, Nomikos GG. Selective c-fos induction and decreased dopamine release in the central nucleus of amygdala in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Synapse. 2000;35:15–25. doi: 10.1002/(SICI)1098-2396(200001)35:1<15::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. European J. Neurosci. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic; San Diego: 2001. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol. Biochem. Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol. Learn. Memory. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetycholin in the rat nucleua accumbens. Psychopharmacology. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav. Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Taylor B, Room R. Global burden of disease from alcohol, illicit drugs and tobacco. Drug Alcohol Rev. 2006;25:503–513. doi: 10.1080/09595230600944453. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol. Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J. Subst. Abuse Treat. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha 7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J. Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57Bl/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Tinsley MR, Quinn JJ, Fanselow MS. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing Pavlovian fear conditioning and inhibitory avoidance. Learn. Memory. 2004;11:35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre J, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J. Neurosci. 2007;27:14030–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-back task: a preliminary study. Psychiatry Res. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cellular and Mol. Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, Domino EF. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am. J. Psychiatry. 2005;162:567–77. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]