Abstract
Effective, one-pot syntheses of 2,3-disubstituted furans and thiophenes, exploiting 2-t-butyldimethylsilyl-3-formyl furan and thiophene as the respective bifunctional linchpins, have been developed. The synthetic protocol involves multicomponent Type II Anion Relay Chemistry (ARC) mediated by a solvent-controlled C(sp2)→ O 1,4-Brook Rearrangement. Simple organolithiums and α-disubstituted ester enolates prove effective as the initiating nucleophiles.
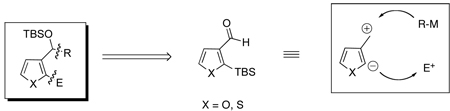
Functionalized furans and thiophenes comprise important synthetic targets given their application in natural product total synthesis, drug discovery, and materials science.1,2 Thus methods to assemble quickly such diverse substituted heterocycles are of considerable importance. Although a few one-step strategies have been recently reported,3 most methods to access poly-substituted furans and thiophenes rely upon multiple-step manipulations to install each substituent sequentially.4 Given the potential utility of multicomponent Anion Relay Chemistry (ARC),5 we anticipated that this tactic would constitute an effective protocol to access such heterocycles. Precedent for this approach derives from our recent one-pot ARC constructions of both 2,3-disubstituted thiophenes exploiting 3-bromo-2-silyl-thiophene (1b)6 and ortho disubstituted benzene derivatives employing ortho-trialkylsilyl benzaldehyde (Scheme 1). 7
Scheme 1.
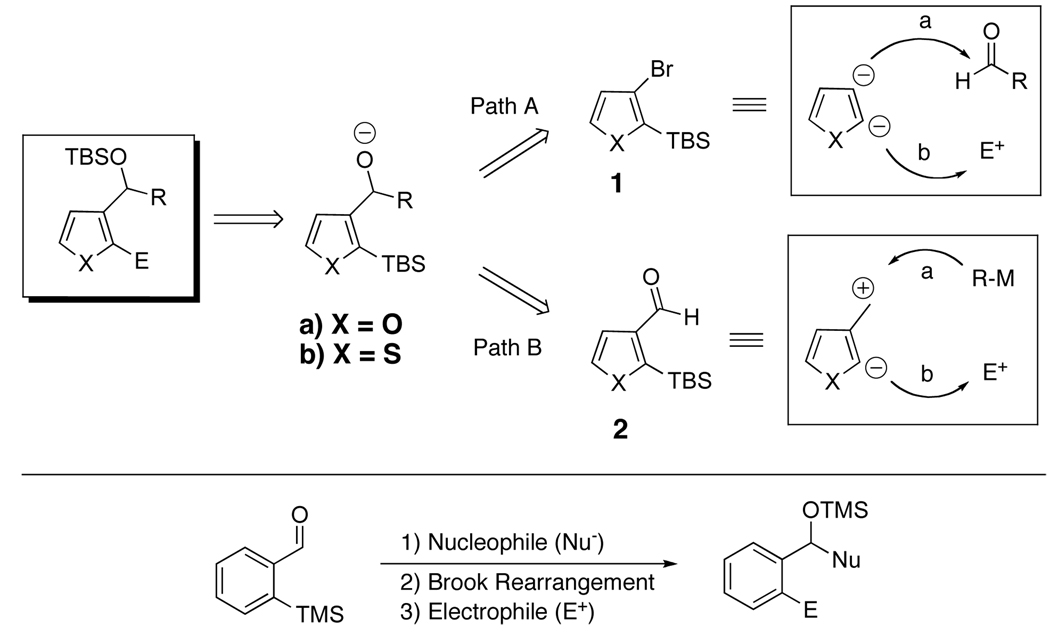
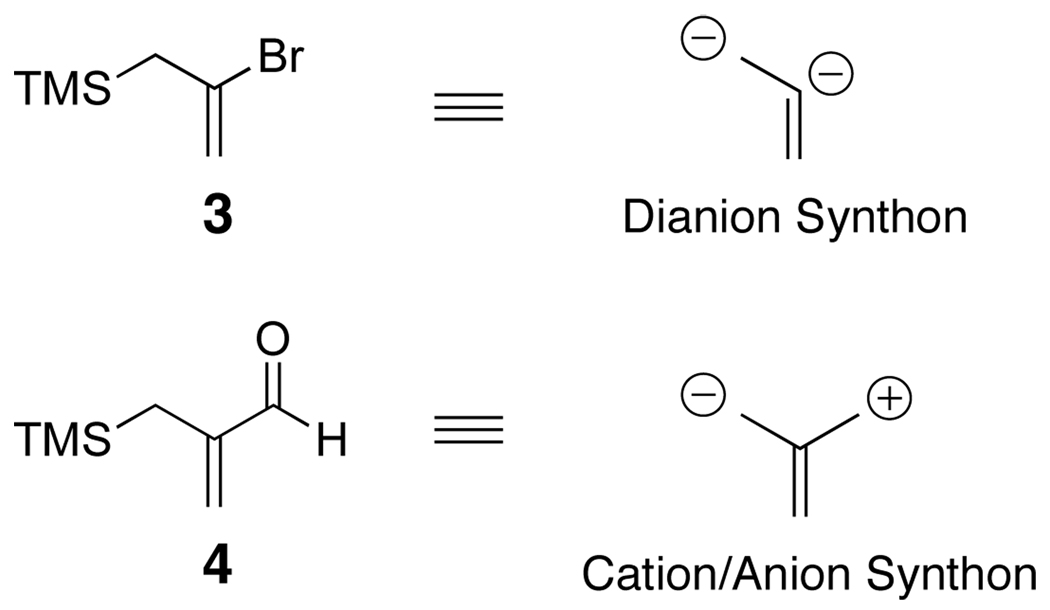
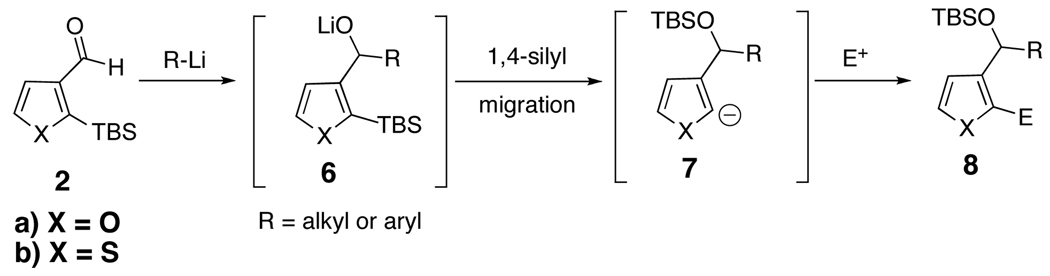
We reasoned that the same 2-trialkylsilyl-3-alkoxy modular anion derived from 1, generated via lithiation and reaction with an aldehyde or ketone (Scheme 1, Path A), could also arise upon addition of various nucleophiles to 2-trialkylsilyl-3-formyl- furans (2a) and thiophenes (2b) (Path B). From the perspective of synthetic planning, it is important to recognize that these linchpins (1 and 2) are fundamentally different; linchpin 1 comprises a dianion synthon, while 2 entails a synthon possessing an electrophilic and nucleophilic site. Recognition of this difference, also apparent in linchpins 35a and 4,5d foreshadows significant additional chemical versatility of the multicomponent Type II ARC tactic (Figure 1). With these considerations in mind, we report here the use of organolithiums and lithium enolates derived from α-disubstituted esters as nucleophiles to generate, in a single flask, a series of 2,3-disubstituted furans and thiophenes via the multicomponent Type II ARC tactic.
Figure 1.
Linchpin Synthon
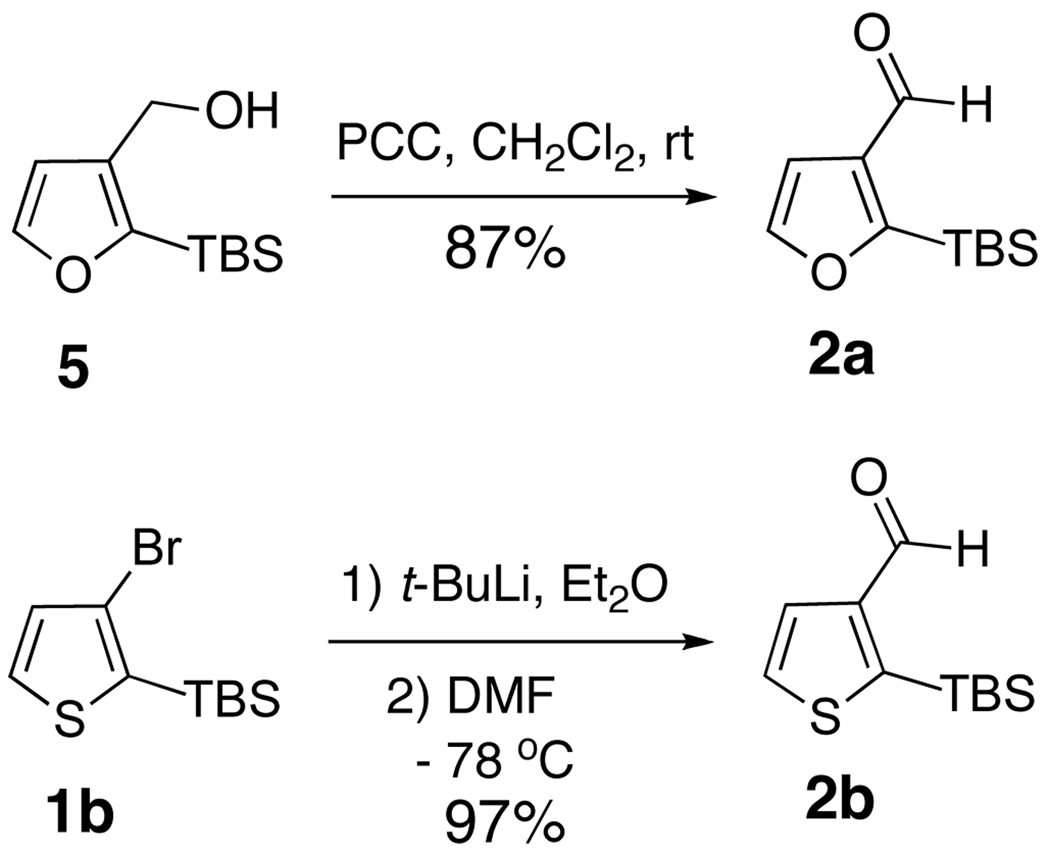
The requisite linchpins 2-TBS-3-formyl-furan (2a) and thiophene (2b) are readily prepared in excellent yield (Scheme 2): linchpin 2a via PCC oxidation8 of 2-TBS-3-(hydroxymethyl)-furan 59 and linchpin 2b via formylation of 2-TBS-3-bromothiophene (1b),6 involving t-butyllithium metalation followed by reaction with DMF.
Scheme 2.
Based on our earlier studies,6,7 we reasoned that the reaction of 2a or 2b with alkyllithium would form alkoxide intermediate 6 (Scheme 3), which upon addition of either DMPU or HMPA would trigger a C(sp2)→O 1,4-silyl migration to furnish carbanion 7, which in turn could be captured by various electrophiles to provide disubstituted furans and thiophenes (cf., 8). Earlier studies by Keay and coworkers9 demonstrated the viability of C(sp2)→O 1,4-silyl migration with a series of silyl furan and thiophene substrates when sodium or potassium bases were used. However, no attempts to extend these reactions to a solvent-controlled multi-component union have been reported.
Scheme 3.
We began with linchpin 2a employing the solvent conditions of DMPU and THF (1:4) which were previously effective at triggering 1,4-silyl migration when applied to 3-bromo-2-silyl-thiophene (1b; Scheme 1, Path A).6 Surprisingly, silyl migration proved problematic, furnishing only trace amounts of the desired product. However, when the electrophile was added in a mixture of HMPA and Et2O (1:2) to the alkoxy anion initially derived at −78 °C, the desired 1,4-Brook process ensued to furnish 2,3-disubstituted furans in moderate to good yield. A series organolithium reagents (i.e., n-BuLi, PhLi, and 3-thienyllithium) served to initiate the multicomponent ARC reaction (Table 1). Aldehydes and ketones proved effective electrophiles for the Type II ARC process, providing the 2,3-disubstituted furans (9a–9g) in yields ranging from 62 to 83%. When isobutyraldehyde was employed as the electrophile, the Brook rearrangement took place to afford 9h without capture of the aldehyde (Entry 8). Presumably, the basicity of the C(2) furan anion in the presence of HMPA, the latter known to increase the basicity of organolithium agents,10 leads to aldehyde deprotonation faster than reaction with the carbonyl group. Also, of interest, use of reactive alkyl halides such as methyl iodide furnished only oxygen alkylation 9i (Entry 9).
Table 1.
One-pot synthesis of 2,3-disubstituted furans from 2-TBS-3-formylfuran 2a.
| Entry | R-M | E | Product(yield) | Entry | R-M | E | Product(yield) |
|---|---|---|---|---|---|---|---|
| 1 | n-BuLi | t-Bu-CHO | 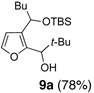 |
6 | Ph-CHO | 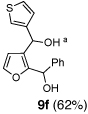 |
|
| 2 | n-BuLi | Ph-CHO | 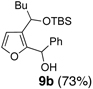 |
7 | t-Bu-CHO | 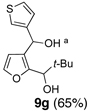 |
|
| 3 | n-BuLi | (Ph)2CO | 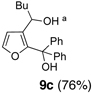 |
8 | n-BuLi | i-Pr-CHO | 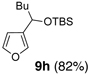 |
| 4 | Ph-Li | t-Bu-CHO | 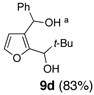 |
9 | n-BuLi | MeI | 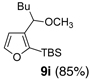 |
| 5 | Ph-Li | Ph-CHO | 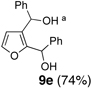 |
Condition: Step 1: 2a (1.0 eq.), Nucleophile (1.1 eq.), Et2O, −78 °C, 30 min;
Step 2: HMPA/Et2O = (1/1), Electrophile (1.5 eq.), −78 °C to RT, 3 h
TBS group was removed by TBAF in THF for 30 min to facilitate purification.
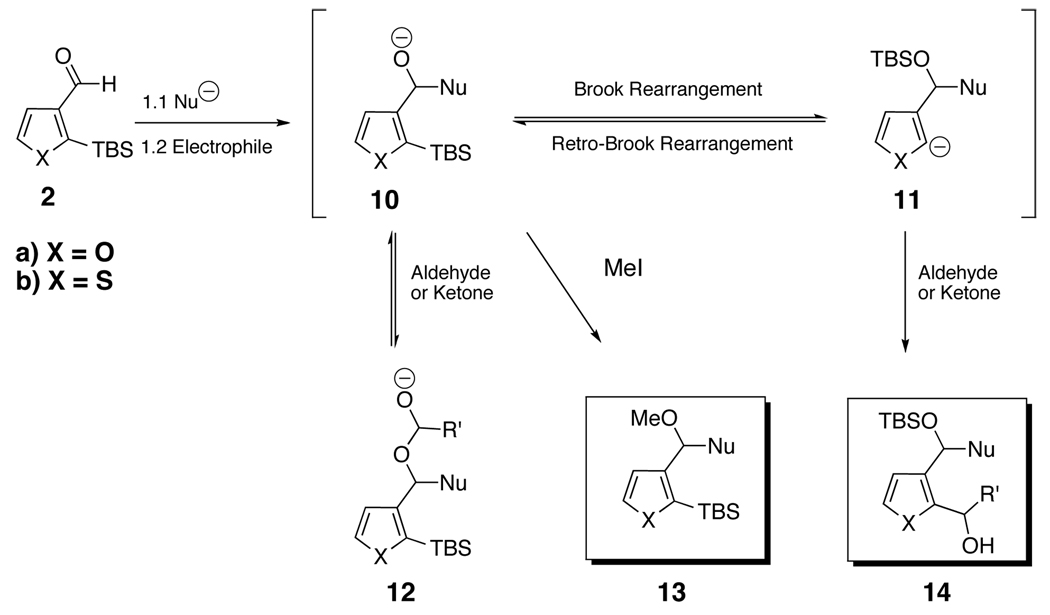
Brook and retro-Brook rearrangements are known to be reversible processes proceeding via a pentacoordinate silicon intermediate (Scheme 4).11 Given such an equilibrium exists between alkoxide 10 and the Brook rearranged organolithium 11, product formation would be based on the relative rate of the 1,4-Brook/retro-Brook rearrangement in conjunction with the stability of two anions (oxy anion vs carbanion). When MeI was employed as an electrophile, the result of irreversible O-methylation 13 is faster than Brook rearrangement in this system. On the other hand, when either an aldehyde or ketone is used, 14 was obtained. In essence, 12 serves as reservoir of the carbonyl electrophile.
Scheme 4.
Encouraged by the viability of the Type II ARC process employing 2-TBS-3-formyl-furan 2a, we turned to the use of 2-TBS-3-formyl thiophene 2b as the bifunctional linchpin (Table 2). In this case, when DMPU and THF (1:4)6 was employed as the solvent system, 2b provided the 2,3-disubstituted thiophenes in moderate to good yield. Aldehydes, non-enolizable ketones, and in this case methyl iodide proved competent as electrophiles for the 1,4-Brook rearranged anion, with isolated yields of 15a–15h ranging from 45 to 87%. Unlike the reaction with 2a, enolizable aldehydes (cf., isobutyraldehyde) furnished 15a and 15g in moderated yield (Entries 1 and 7), presumably due to the decreased basicity of the thiophene anion. Interestingly, use of HMPA was not an effective agent to trigger the 1,4-Brook process with 2b.6
Table 2.
One-pot synthesis of 2,3-disubstituted thiophenes from 2-TBS-3-formylthiophene 2b.
| Entry | R-M | E | Product(yield) | Entry | R-M | E | Product(yield) |
|---|---|---|---|---|---|---|---|
| 1 | n-BuLi | i-Pr-CHO | 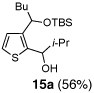 |
5 | Ph-Li | Ph-CHO | 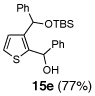 |
| 2 | n-BuLi | Ph-CHO | 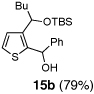 |
6 | Ph-CHO | 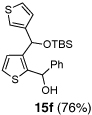 |
|
| 3 | n-BuLi | (Ph)2CO | 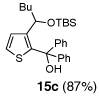 |
7 | i-Pr-CHO | 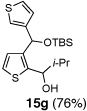 |
|
| 4 | Ph-Li | MeI | 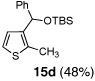 |
8 | n-BuLi | MeI | 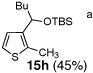 |
Condition: Step 1: 2b (1.0 eq.), Nucleophile (1.1 eq.), THF, −78 °C, 30 min;
Step 2: DMPU/THF = (1/1), Electrophile (1.5 eq.), −78 °C to RT, 3 h
We also obtained O-methylation product (15 i) in 41%
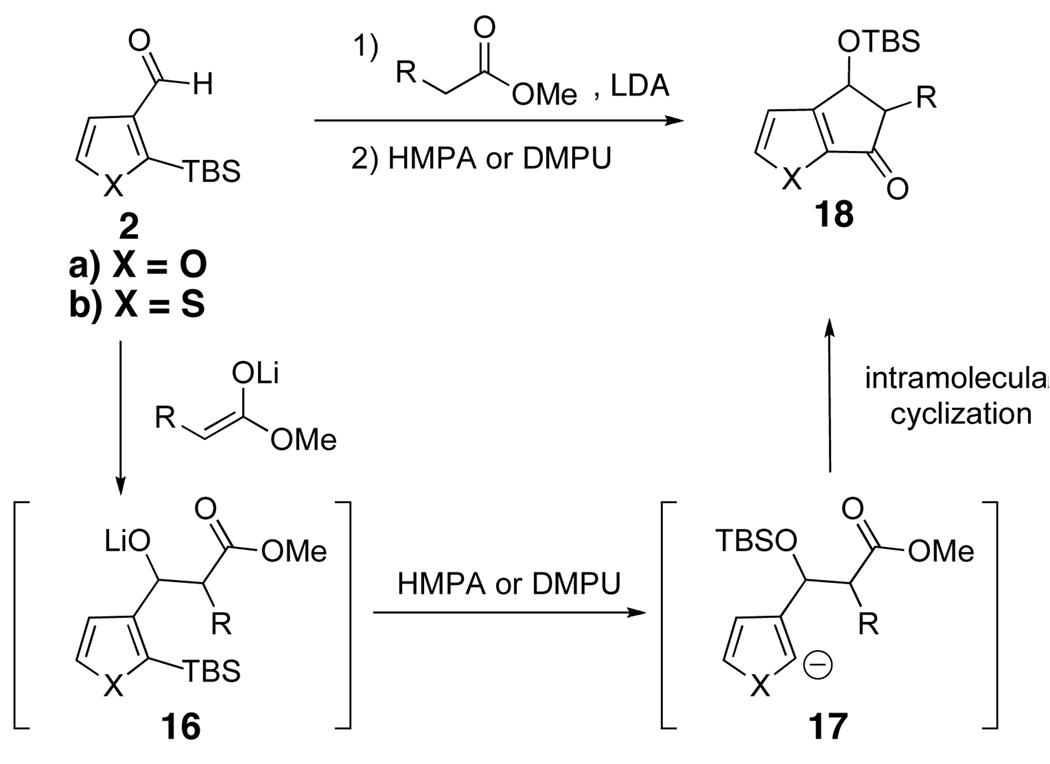
We next turned to the possibility of using ester enolates as the initiating nucleophile in the ARC process (Scheme 5). We reasoned that intramolecular capture after silyl migration might occur to form 5-membered cyclic products (cf., 18).
Scheme 5.
As illustrated in Table 3, enolates derived from a series of methyl esters, generated upon treatment with LDA were reacted with linchpins 2a and 2b. After initial generation of the intermediate 16, addition of either HMPA or DMPU triggered the C(sp2)→O 1,4-Brook rearrangement. In the case of the thiophene linchpin (2b), cyclization occurred. Good to excellent yields of 18a–18c (Entries 1–3) were obtained employing esters possessing α-disubstitution. However, only trace amounts of cyclized products were observed when enolates derived from α-unsubstituted esters were employed as the initiating nucleophiles (Entry 4). Again, acidic protons present on the initially derived adducts are presumably sufficiently acidic to be protonated by the intermediate carbanion. In the case of the enolate derived from isopropyl acetate, β-elimination product 18d was isolated in moderate yield. Also of interest, when 2-TBS-3-formyl-furan 2a was used as the linchpin, 1,4-Brook rearranged products (19a–19c) not having gone intramolecular cyclization were obtained as the major product (Entries 1–3). The latter results presumably derived either from the reactivity (i.e., geometry) and/or basicity differences of the C(2) anion of the furan and thiophene.12
Table 3.
Synthesis of 3- and 2,3-disubstituted furans and thiophenes from 2a and 2b employing ester enolates.
| Entry | Enolate | Product(yield) |
|---|---|---|
| 1 | 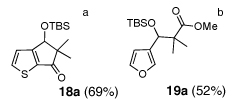 |
|
| 2 | 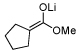 |
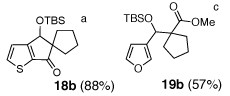 |
| 3 | 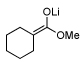 |
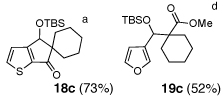 |
| 4 | 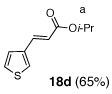 |
Condition A: Step 1: 2b (1.0 eq.), ester enolate (1.1 eq.), THF, −78 °C, 25 min;
Step 2: DMPU, −78 °C to RT, 19 h
Condition B: Step 1: 2a (1.0 eq.), ester enolate (1.2 eq.), Et2O, −78 °C, 30 min;
Step 2: HMPA, −78 °C to RT, 19 h.
Condition C: Same as the condition A except for the amount of DMPU.
Condition D: Step 1: silyl enol ether (1.36 eq.), MeLi (1.28 eq.), Et2O, RT, 1 h;
Step 2: 2a (1.0 eq.), Et2O, −78 °C, 30 min;
Step 3: HMPA, −78 °C to RT, 19 h.
In summary, a multicomponent one-pot synthesis of 2,3-disubstituted furans and thiophenes exploiting Type II Anion Relay Chemistry (ARC) has been developed employing 2-TBS-3-formyl-furan and thiophene as the bifunctional linchpins. Central to this process is a solvent mediated C(sp2)→O 1,4-Brook rearrangement. As such, this study foreshadows the use of other nucleophiles (cf., organozincates, organocuprates, etc.) as well as the use of cross-coupling reactions as recently demonstrated with ortho-TMS benzaldehyde,7 to access a wide variety of diverse polyfunctionalized heterocycles. Progress towards this end will be reported in due course.
Supplementary Material
Acknowledgment
This work has been supported by the Washington State University and at the University of Pennsylvania through grant CA-19033. We are also grateful to Cephalon, Inc for a Dr. Horst Witzel fellowship awarded to Won-Suk Kim.
Footnotes
Supporting Information Available: Experimental procedures and characterization data of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Dunlop AP, Peters FN. Reinhold. New York: 1953. Reinhold, ‘The Furans’ (A.C.S. Monograph No. 119) [Google Scholar]; (b) Hau XL, Yang Z, Wong HNC. In: Progress in Heterocyclic Chemistry. Gribble GW, Gilchrist TL, editors. Vol. 15. Oxford: Pergamon; 2003. p. 167. [Google Scholar]; (c) Keay BA, Dibble PW. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 2. Oxford: Elsevier; 1997. p. 395. [Google Scholar]; (d) Montagnon T, Tofi M, Vassilikogiannakis G. Acc. Chem. Res. 2008;41:1001. doi: 10.1021/ar800023v. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bohlmann F, Zdero C. In: Thiophenes and its Derivates. Gronowitz S, editor. Vol. 44. New York: John Wiley & Sons; 1985. p. 261. The Chemistry of Heterocyclic Compounds, Part 1. [Google Scholar]; (b) Press JB. In: Thiophenes and Its Derivates. Gronowitz S, editor. Vol. 44. New York: John Wiley & Sons; 1991. p. 397. The Chemistry of Heterocyclic Compounds, Part 4. [Google Scholar]; (c) Roncali J. Chem. Rev. 1992;92:711. [Google Scholar]; (d) Schopf G, Komehl G. In: Advances in Polymer Sciences: Polythiophenes-Electrically Conductive Polymers. Abel A, editor. Vol. 129. Berlin, Heidelberg, New York: Springer-Verlag; 1997. p. 1. [Google Scholar]
- 3.For selected examples, see: For Furans:Barluenga J, Riesgo L, Vicente R, Lopez LA, Tomas M. J. Am. Chem. Soc. 2008;130:13528. doi: 10.1021/ja8058342.Babudri F, Cicco SR, Farinoia GM, Lopez LC, Naso F, Pinto V. Chem. Commun. 2007:3756. doi: 10.1039/b705257j.Liu Z, Yu W, Yang L, Liu Z-L. Tetrahedron Lett. 2007;48:5321.Garcon S, Vassiliou S, Cavicchioli M, Hartmann B, Monteiro N, Balme G. J. Org. Chem. 2001;66:4069. doi: 10.1021/jo0017990.For Thiophenes:Mitsudo K, Thansandote P, Wilhelm T, Mariampillai B, Lautens M. Org. Lett. 2006;8:3939. doi: 10.1021/ol061373t.Ye XS, Li WK, Wong HNC. J. Am. Chem. Soc. 1996;118:2511.Fattuoni C, Usai MG, Cadoni E, De Montis S, Cabiddu S. Synthesis. 2006:3855.Fattuoni C, Usai M, Cabiddu M, Cadoni E, De Montis S, Cabiddu S. Synthesis. 2006:3855.Wang Y, Dong D, Yang Y, Huang J, Ouyang Y, Liu Q. Tetrahedron. 2007;63:2724.
- 4.For selected reviews, see: For Furans:Luh T. Pure Appl. Chem. 2005;77:1213.Keay BA. Chem. Soc. Rev. 1999;28:209.Hou XL, Cheung HY, Hon TY, Kwan PL, Lo TH, Tong SY, Wong HNC. Tetrahedron. 1998;54:1955.Wong HNC. Pure Appl. Chem. 1996;68:335.For Thiophenes: Ila H, Baron O, Wagner AJ, Knochel P. Chem. Commun. 2006:583. doi: 10.1039/b510866g.Guernion NJL, Hayes W. Curr. Org. Chem. 2004;8:637.Weissberger A, Taylor EC. In: Thiophenes and Its Derivates. Gronowitz S, editor. Vol. 44. New York: John Wiley; 1985. p. 1. The Chemistry of Heterocyclic Compounds, Part 1.
- 5.Anion Relay Chemistry (ARC) involves the migration of a negative charge within a molecular system (i.e., through bond or through space, the latter employing a transfer agent such as a trisubstituted silyl group). Two types of through space ARC have been identified. In Type I ARC the anion after initial migration returns to the site of origin, whereas in Type II ARC the anion is relayed to a new remote site. For an account on the evolution of Type I and Type II Anion Relay Chemistry, see ref. 5e. For earlier examples of Anion Relay Chemistry developed by Smith and co-workers, see references 5a–d:Smith AB, III, Duffey MO. Synlett. 2004:1363.Smith AB, III, Xian M. J. Am. Chem. Soc. 2006;128:66. doi: 10.1021/ja057059w.Smith AB, III, Xian M, Kim W-S, Kim D-S. J. Am. Chem. Soc. 2006;128:12368. doi: 10.1021/ja065033e.Smith AB, III, Kim D-S, Xian M. Org. Lett. 2007;9:3307. doi: 10.1021/ol071281j.Smith AB, III, Wuest WM. Chem. Commun. 2008:5883. doi: 10.1039/b810394a.
- 6.Devarie-Baez NO, Shuhler BJ, Wang H, Xian M. Org. Lett. 2007;9:4655. doi: 10.1021/ol702149c. [DOI] [PubMed] [Google Scholar]
- 7.Smith AB, III, Kim W-S, Wuest WM. Angew. Chem. Int. Ed. 2008;47:7082. doi: 10.1002/anie.200802301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey EJ, Suggs W. Tetrahedron Lett. 1975;16:2647. [Google Scholar]
- 9.(a) Bures E, Spinazze PG, Beese G, Hunt IR, Rogers C, Keay BA. J. Org. Chem. 1997;62:8741. [Google Scholar]; (b) Spinazze PG, Keay BA. Tetrahedron Lett. 1989;30:1765. [Google Scholar]
- 10.Clayden J, Yasin SA. New J. Chem. 2002;26:191. [Google Scholar]
- 11.(a) Jiang X, Bailey WF. Organometallics. 1995;14:5704. [Google Scholar]; (b) Kawashima T, Naganuma K, Okazaki R. Organometallics. 1998;17:367. [Google Scholar]; (c) Naganuma K, Kawashima T, Okazaki R. Chem. Lett. 1999:1139. [Google Scholar]
- 12.Fraser RR, Mansour TS, Savard S. Can. J. Chem. 1985;63:3505. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.