Abstract
Rationale
The adult heart possesses a pool of progenitor cells stored in myocardial niches but the mechanisms involved in the activation of this cell compartment are currently unknown.
Objective
Ca2+ promotes cell growth raising the possibility that changes in intracellular Ca2+ initiate division of c-kit-positive human cardiac progenitor cells (hCPCs) and determine their fate.
Methods and Results
Ca2+ oscillations were identified in hCPCs and these events occurred independently from coupling with cardiomyocytes or the presence of extracellular Ca2+. These findings were confirmed in the heart of transgenic mice in which EGFP was under the control of the c-kit-promoter. Ca2+ oscillations in hCPCs were regulated by the release of Ca2+ from the ER through activation of inositol 1,4,5-triphosphate receptors (IP3Rs) and the re-uptake of Ca2+ by the sarco/endoplasmic reticulum Ca2+ pump (SERCA). IP3Rs and SERCA were highly expressed in hCPCs while ryanodine receptors were not detected. Although Na+-Ca2+ exchanger, store-operated Ca2+-channels and plasma membrane Ca2+-pump were present and functional in hCPCs, they had no direct effects on Ca2+ oscillations. Conversely, Ca2+ oscillations and their frequency markedly increased with ATP and histamine which activated purinoceptors and histamine-1 receptors highly expressed in hCPCs. Importantly, Ca2+ oscillations in hCPCs were coupled with the entry of cells into the cell cycle and BrdUrd incorporation. Induction of Ca2+ oscillations in hCPCs prior to their intramyocardial delivery to infarcted hearts was associated with enhanced engraftment and expansion of these cells promoting the generation of a large myocyte progeny.
Conclusion
IP3R-mediated Ca2+ mobilization control hCPC growth and their regenerative potential.
Keywords: human cardiac progenitor cells, calcium oscillations, cell growth
The recognition that the adult heart in animals and humans possesses a pool of stem/progenitor cells1-3 has raised the critical question concerning the mechanisms involved in the activation of this cell compartment and the modulation of cardiac homeostasis and repair. During the course of life before the manifestations of myocardial aging become apparent,4 dying parenchymal cells are continuously replaced by newly formed myocytes5,6 through activation and commitment of quiescent cardiac progenitor cells (CPCs) stored in myocardial niches.7,8 However, the signals responsible for the initiation of the cell cycle in CPCs, cardiomyocyte generation and preservation of the steady state of the organ are currently unknown. Calcium has two fundamental functions in the heart: it activates growth processes9,10 and modulates the mechanical behavior of cardiomyocytes.11,12 Critical for understanding physiological cell turnover and myocardial regeneration following injury is the identification of the mechanisms by which CPCs divide and acquire the myocyte phenotype. Changes of calcium levels in CPCs may occur and trigger a cascade of events that dictate their ultimate fate. Therefore, the objectives of the current study were: 1. to determine the pathways that regulate intracellular Ca2+ in human CPCs (hCPCs); 2. to establish whether Ca2+ oscillations in hCPCs condition cell replication; and 3. to assess whether Ca2+ oscillations are intrinsic to the cells or are triggered by interaction of hCPCs with cardiomyocytes. This cell-to-cell communication may favor the translocation of Ca2+ from myocytes to quiescent hCPCs initiating a cellular growth response. Moreover, transmembrane Ca2+ fluxes may contribute to rapid and transient rise in cytosolic Ca2+ promoting the entry of hCPCs into the cell cycle. These variables include a functional endoplasmic reticulum (ER) where Ca2+ is stored, the activity of ER channels that promote Ca2+ release, and the membrane systems modulating the exchange of Ca2+ with the extracellular compartment.
Methods
hCPCs were isolated from myocardial specimens obtained from patients who underwent cardiac surgery. Cytosolic Ca2+ levels in cultured hCPCs were measured utilizing the Ca2+ indicator Fluo-3 and two-photon microscopy. Cell proliferation in vivo and in vitro was evaluated by BrdUrd incorporation. Results are shown as mean±SEM. An expanded Materials and Methods section can be found in the online data supplement available at http://circres.ahajournals.org.
Results
Intracellular Ca2+ in hCPCs
Changes in [Ca2+]i occur in excitable and non-excitable cells raising the possibility that a similar phenomenon is present in hCPCs and may have a functional role. Thus, hCPCs were loaded with the Ca2+ sensitive dye Fluo-3 and the intensity of the fluorescent signal was monitored over a period of ~30 minutes. During this interval, 79% hCPCs maintained stable levels of [Ca2+]i while 21% displayed one or more consecutive Ca2+ oscillations. Repetitive events were restricted to a small percentage of cells and were comparable in amplitude and duration (Figure 1A through 1C). The fraction of hCPCs displaying Ca2+ oscillations increased with time up to 2 hours although the frequency of these episodes remained low (Figure 1D). These cells were all positive for the stem cell antigen c-kit (Supplemental Figure I). Ca2+ oscillations increased in hCPCs at the G1-S phase transition but decreased at G2-M (Figure 1 E and Supplemental Figure II).
Figure 1.
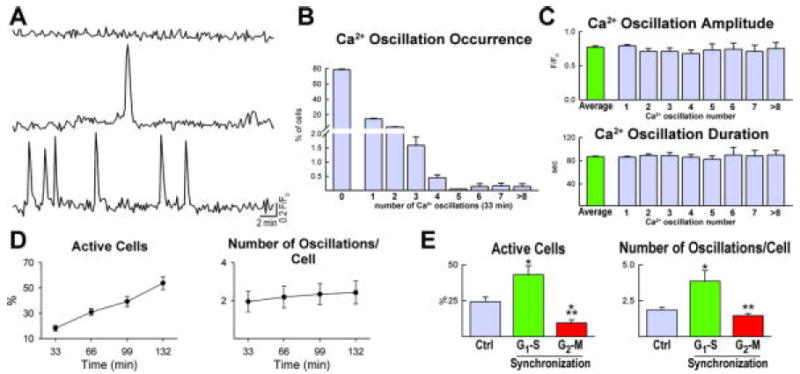
Intracellular Ca2+ in hCPCs. A, Cytosolic Ca2+ levels in a quiescent hCPC (upper trace) and in two hCPCs showing single (middle trace) and multiple (lower trace) Ca2+ oscillations. B, Distribution of Ca2+ oscillations, from 1 to more than 8, in hCPCs over a period of 33 min. C, Amplitude and duration of Ca2+ events in hCPCs. D, Ca2+ oscillations in hCPCs (Active Cells) analyzed for a period of 132 minutes. E, Ca2+ oscillations in hCPCs in control condition (Ctrl) and at the G1-S and G2-M transition.
Cell-to-Cell Interaction and Ca2+ Oscillations in hCPCs
The next objective was to establish whether Ca2+ oscillations in hCPCs are modulated at the single cell level or are mediated by adjacent cells. hCPCs are nested in myocardial niches and connexins are found between hCPCs and myocytes which operate as supporting cells.3,7 These intercellular communications may account for the generation of Ca2+ oscillations in hCPCs, a process that is commonly observed in cardiomyocytes (Supplemental Figure III and Movie 1 in the online data supplement). Therefore, to identify the origin of Ca2+ oscillations in hCPCs, these cells were cultured alone or together with neonatal cardiomyocytes. Initially, dye transfer assays were performed3,7,13 to document the formation of functional gap junctions between hCPCs. The fluorescent dye cascade blue was microinjected in individual hCPCs and found to rapidly migrate to neighboring cells through gap junction channels expressing connexin 43 (Figure 2A through 2E). However, the high molecular weight rhodamine-labeled dextran, injected simultaneously with cascade blue, failed to translocate to adjacent hCPCs. Additionally, DiI labeled hCPCs loaded with calcein were cultured with untreated cells. After ~12 hours, calcein was detected in unlabeled hCPCs structurally connected to DiI-calcein-positive cells (Supplemental Figure IV). Intracellular Ca2+ was then measured before and after exposure of hCPCs to the connexin hemi-gap-junction channel blocker octanol. Octanol did not affect the frequency and properties of Ca2+ oscillations in hCPCs (Figure 2F and 2G).
Figure 2.
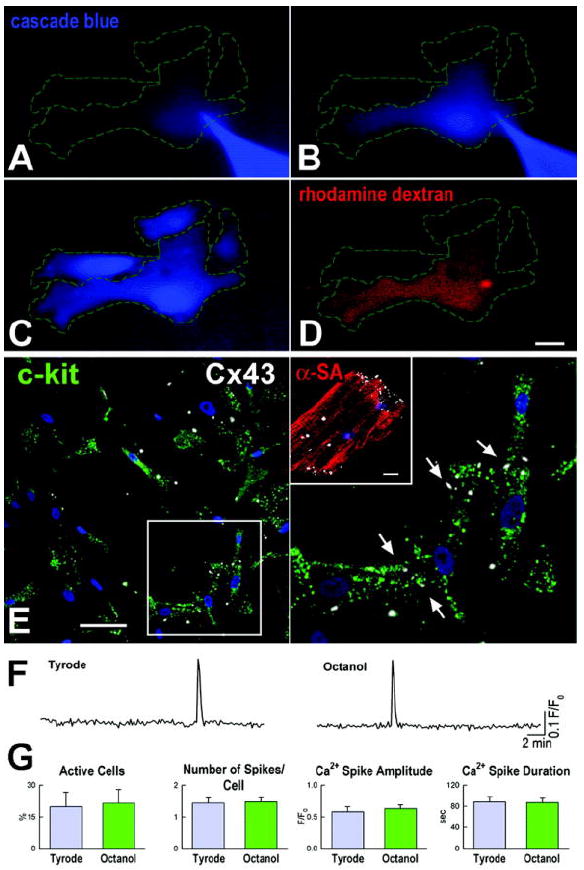
Cell-to-cell interaction and Ca2+ oscillations. A-C, Cascade blue (blue) microinjected in a single hCPC (A) translocated spontaneously to adjacent cells (B and C). D, Rhodamine-labeled dextran (red), delivered simultaneously with cascade blue, remained confined to the injected cell. Scale bar: 20 μm. Cascade blue translocation was detected in 6 experiments. E, Connexin 43 (Cx43, white) is present between hCPCs (c-kit, green). Nuclei are stained by DAPI (blue). Scale bar: 20 μm. A group of cells is shown at higher magnification on the right panel. The inset shows Cx43 labeling in a myocyte [α-sarcomeric actin, (α-SA) red]. Scale bar: 10 μm. F, Intracellular Ca2+ in hCPCs before (left trace) and after exposure to octanol (right trace). G, Effects of uncoupling on Ca2+ oscillations.
Subsequently, the effect of Ca2+ cycling in myocytes on hCPC function was assessed by plating together DiI-labeled hCPCs and unlabeled cardiomyocytes. In spite of the presence of functional gap junctions between these two cell populations (Figure 3A and Supplemental Figure V), spontaneous or electrically stimulated Ca2+ transients in myocytes had no detectable consequence on [Ca2+]i of hCPCs (Figure 3B and 3C). In fact, Ca2+ oscillations in hCPCs persisted in the presence of cadmium which abolished Ca2+ transients in myocytes.
Figure 3.
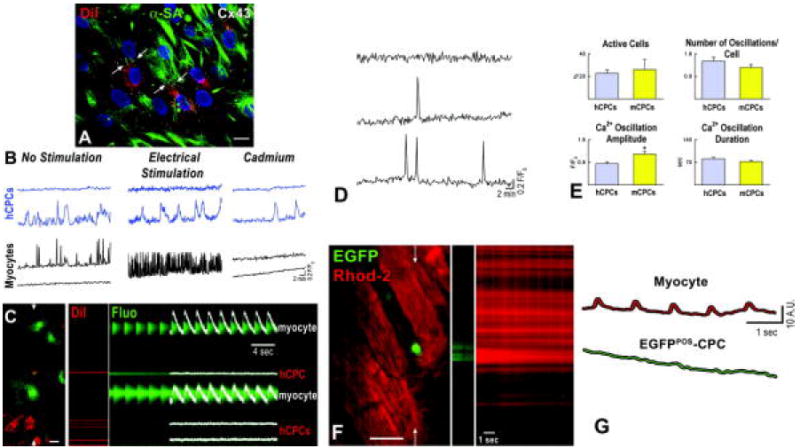
Ca2+ cycling in myocytes and CPCs. A, Cx43 (white) between DiI-labeled hCPCs (DiI, red) and myocytes (α-SA, green). Nuclei are identified by DAPI. Scale bars: 10 μm. B, Intracellular Ca2+ in hCPCs (blue traces) and adjacent co-cultured neonatal myocytes (black traces). The effects of electrical stimulation and cadmium chloride are also shown. Different cells were used in the three conditions. C, Intracellular Ca2+ in hCPCs and neonatal myocytes in line scan mode. Red identifies hCPCs loaded with DiI and green corresponds to Fluo-3; scale bar: 20 μm. D, Cytosolic Ca2+ in a quiescent mCPC (upper trace) and two mCPCs showing a single (middle trace) and multiple (lower trace) Ca2+ oscillations. E, Properties of Ca2+ oscillations in hCPCs and mCPCs. E, c-kit-EGFP mouse heart loaded with Rhod-2 (red), stimulated at 1 Hz and analyzed in line-scan mode (arrows). CPCs were identified by EGFP (green). G, Ca2+ transients in myocytes (red trace) did not affect Ca2+ levels in the neighboring EGFP-positive CPC (green trace). Identical results were obtained in 7 other experiments. Scale bars: 20 μm.
Cell-to-Cell Interaction and Ca2+ Oscillations in Mouse CPCs
To strengthen these in vitro results, a transgenic mouse model in which EGFP was under the control of the c-kit promoter14 was employed to test whether Ca2+ cycling in myocytes triggers Ca2+ oscillations in CPCs in situ within the myocardium. Preliminary studies were conducted to evaluate whether EGFP-positive mouse CPCs (mCPCs) in vitro showed spontaneous Ca2+ oscillations, mimicking the behavior of hCPCs. The percentage of mCPCs exhibiting Ca2+ oscillation was comparable to that measured in human cells. Similarly, the rate of these events and their duration did not differ in these two cell classes while the amplitude was larger in mCPCs (Figure 3D and 3E).
The heart of these transgenic mice was then examined ex vivo by two-photon microscopy3,13 following perfusion of the coronary circulation with the Ca2+ indicator Rhod-2. The possibility that EGFP may interfere with the detection of Rhod-2 was excluded in preliminary studies conducted in EGFP-positive mouse myocytes (Supplemental Figure VI). Based on these observations, the mouse heart was stimulated at 1 Hz and the Ca2+ levels in EGFP-positive mCPCs were found not to be affected by the changes in Ca2+ transients in neighboring cardiomyocytes (Figure 3F and 3G). Thus, human and mouse CPCs appear to possess an intracellular Ca2+ regulatory system which is independent from that of terminally-differentiated parenchymal cells.
Intracellular Ca2+ Control in hCPCs
We then determined whether activation of inositol 1,4,5-triphosphate receptors (IP3Rs) and/or ryanodine receptors (RyRs) resulted in the release of Ca2+ from the ER and Ca2+ oscillatory events. Moreover, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) is responsible for the re-uptake of Ca2+ into the ER and restoration of Ca2+ stores.10,11 Before conducting the functional studies, quantitative RT-PCR and immunolabeling were employed to document in hCPCs the presence of transcripts and proteins for IP3Rs and SERCA. Both IP3Rs and SERCA were highly expressed in hCPCs. However, RyRs were not identified in these cells (Figure 4A through 4D).
Figure 4.
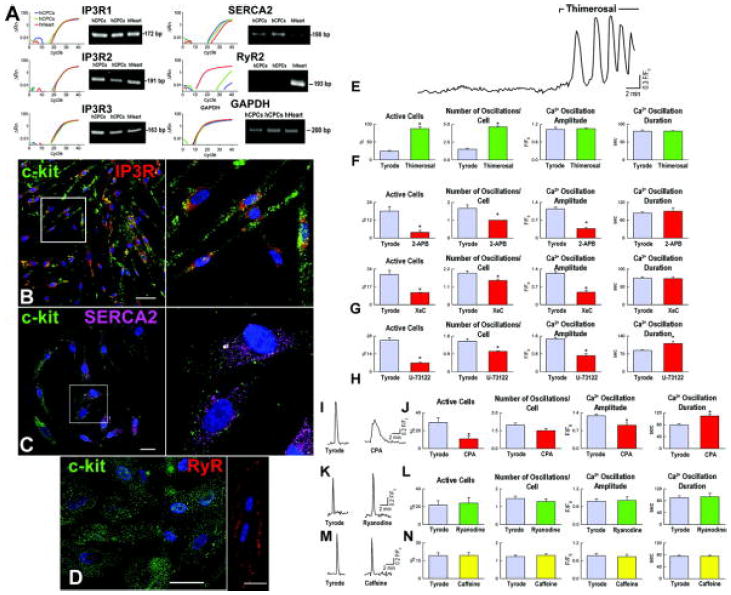
Ca2+ regulatory proteins in hCPCs. A-D, Expression at the mRNA (A) and protein (B-D) levels of the components of the ER that are implicated in Ca2+ homeostasis. Myocytes were used as positive control for RyRs. Human heart (hHeart) was used as positive control. Scale bars: 20 μm. E, Repetitive Ca2+ oscillations in hCPCs in the presence of IP3R agonist. F-H, Ca2+ oscillations in hCPCs at baseline (Tyrode) and in the presence of activation (F) and inhibition (G and H) of IP3R function. Xestopsongin-C, XeC. I-N, Ca2+ in hCPCs in the presence of modulators of SERCA (I and J) and RyRs (K-N). *P<0.05 vs. Tyrode.
Subsequently, IP3 binding to IP3Rs was enhanced by thimerosal and this intervention markedly increased the number of hCPCs displaying Ca2+ oscillations and the frequency of Ca2+ oscillatory episodes per cell (Figure 4E and 4F). An opposite response was observed by inhibition of IP3R function with 2-amino-ethoxydiphenyl borate (2-APB) or xestospongin-C or attenuation of IP3 formation by phospholipase-C (PLC) blockade with U-73122 (Figure 4G and 4H). Similarly, inhibition of SERCA with cyclopiazonic acid (CPA) reduced the fraction of activated hCPCs (Figure 4I and 4J). Additionally, Ca2+ oscillations were smaller in amplitude and prolonged in duration, pointing to SERCA as a relevant component of hCPC function. Consistent with the lack of identify RyRs in hCPCs, their agonists ryanodine and caffeine had no effects on Ca2+ oscillations; the number of active cells and the frequency, amplitude and duration of Ca2+ events did not differ from baseline (Figure 4K through 4N). These data point to the IP3-IP3R system and SERCA as the predominant modulators of Ca2+ in hCPCs.
ATP-specific P2-purinoceptors (P2Y2) and histamine (H1) receptors are Gq-protein coupled receptors that are implicated in the release of intracellular Ca2+ from the ER by activation of PLC, IP3 synthesis and, ultimately, IP3R binding.15,16 P2Y2 and H1 receptors were expressed at the mRNA and protein level in hCPCs (Figure 5A through 5C), suggesting that they may be implicated in Ca2+ cycling of these progenitor cells. In the presence of ATP or histamine, a more than 3-fold increase in the number of hCPCs exhibiting oscillations was detected. Similarly, the number of events per cell markedly increased while the amplitude and duration of Ca2+ elevations did not change (Figure 5D through 5G). Inhibition of IP3Rs or IP3 formation prevented the effects of ATP and histamine on Ca2+ mobilization (Supplemental Figure VII).
Figure 5.
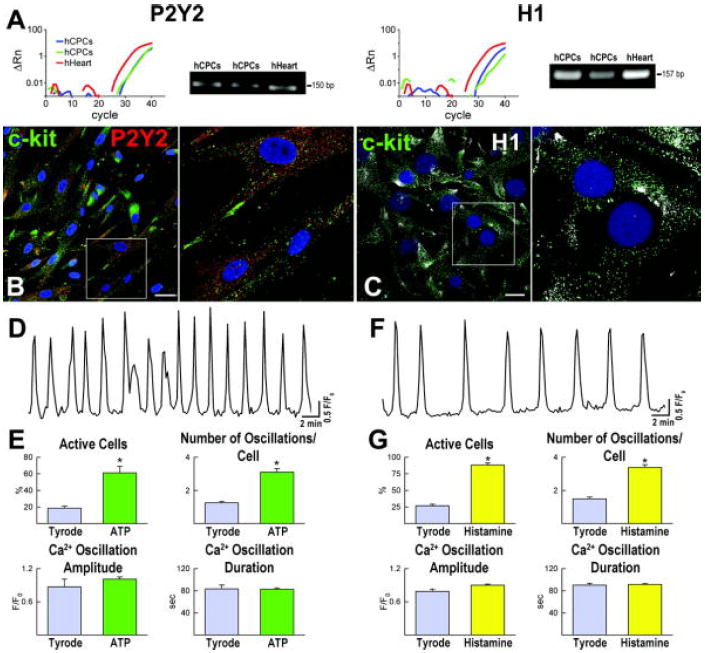
Gq-protein coupled receptors and intracellular Ca2+ in hCPCs. A-C, Expression at the mRNA (A) and protein (B and C) levels of P2Y2 and H1 receptors in hCPCs. Human heart (hHeart) was used as positive control. Scale bars: 10 μm. D-G, Ca2+ oscillations in hCPCs in the presence of ATP (D and E) or histamine (F and G). *P<0.05 vs. Tyrode.
Since PLC-β3 may be modulated by P2Y2 receptor activation,17 the expression of PLC-β subunits was evaluated in hCPCs. Additionally, the functional role of PLC-β3 in mediating intracellular Ca2+ mobilization upon ATP stimulation was established. PLC-β3 mRNA in hCPCs was significantly higher than that of the other subunits (Supplemental Figure VIII, A through C). Thus, siRNA strategy was employed to downregulate PLC-β3 in hCPCs prior to their stimulation with ATP. This intervention markedly attenuated the ability of ATP to dramatically increase the pool of hCPCs displaying Ca2+ oscillations (Supplemental Figure VIII, D and E).
Although IP3R-mediated Ca2+ mobilization from the ER is responsible for the generation of Ca2+oscillations in hCPCs, transmembrane Ca2+ fluxes maybe operative contributing to intracellular calcium cycling. Exposure of hCPCs to Ca2+ free medium did not alter the frequency and characteristics of Ca2+ oscillations (Supplemental Figure IX), strengthening the role of intracellular stores as the source of Ca2+ for oscillatory events. Immunolabeling and PCR data, together with patch clamp and cytosolic Ca2+ imaging (Supplemental Figures X and XI), revealed that store operated channels (SOC), Na+-Ca2+ exchanger (NCX) and plasma membrane Ca2+ pump (PMCA) were functional in hCPCs. However, these systems did not appear to participate in the generation of Ca2+ oscillations in these primitive cells.
Ca2+ Oscillations and hCPC Growth
To evaluate the functional import of Ca2+ oscillations in hCPC replication, these cells were cultured in serum free medium for 24 hours and were exposed to either ATP or histamine to induce Ca2+ release from the ER and oscillatory events. BrdUrd was added to the medium and its incorporation in hCPCs was measured 24 hours later. To exclude the potential confounding effect of cell death on the evaluation of cell proliferation, apoptosis was also determined. With either ATP or histamine, a nearly 2-fold increase in BrdUrd labeling of hCPCs was detected (Figure 6A and 6B). Conversely, inhibition of Ca2+ release from the ER led to a decrease in BrdUrd incorporation in hCPCs to values lower than those seen at baseline (Figure 6A through 6C). Moreover, purinergic stimulation failed to promote proliferation of hCPCs when PLC-β3 was downregulated and the P2Y2-IP3R axis was disrupted (Supplemental Figure XII). ATP and histamine had no influence on hCPC apoptosis (Figure 6D and 6E), suggesting that increases in Ca2+ oscillations were not coupled with the activation of the cell death program.
Figure 6.
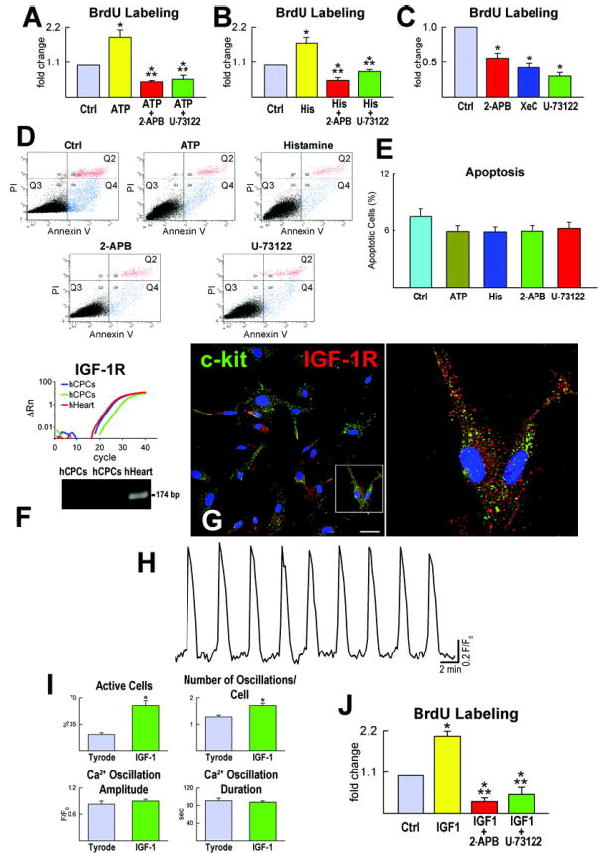
hCPC growth and apoptosis. A-C, ATP and histamine increase Ca2+ oscillations and proliferation of hCPCs. Inhibitors of Ca2+ oscillations prevent the effects of ATP and histamine. Control, Ctrl; histamine, His. *P<0.05 vs. Ctrl, **P<0.05 vs. agonist. D and E, Apoptosis of hCPCs measured by Annexin V labeling and FACS. PI, propidium iodide. Q2, late apoptotic or necrotic cells; Q3, alive cells; Q4, cells undergoing apoptosis. F and G, IGF-1R transcript and protein in hCPCs and hHeart. Scale bar: 20 μm. Right panel in G illustrates selected cells at higher magnification. H and I, Intracellular Ca2+ in hCPCs exposed to IGF-1. *P<0.05 vs. Tyrode. J, Proliferation of hCPCs in the presence of IGF-1 alone or in combination with inhibitors of Ca2+ oscillations. *P<0.05 vs. Ctrl, ** P<0.05 vs. IGF-1.
To strengthen the possibility of a cause and effect relationship between Ca2+ oscillations and cell cycle activation in hCPCs, the impact of a well-established activator of progenitor cell division, insulin-like growth factor-1 (IGF-1) was determined. Resident cardiac progenitors express IGF-1 receptors (Figure 6F and 6G) and synthesize and secrete the ligand.6 IGF-1 increased the percentage of hCPCs showing Ca2+ oscillations by nearly 3-fold (Figure 6H and 6I). Similarly, there was an increase in the number of Ca2+ oscillations per cell while the amplitude and duration of Ca2+ events remained essentially constant. When Ca2+ oscillations were blocked, the growth promoting effects of IGF-1 on hCPCs were completely prevented. IGF-1 increased hCPC proliferation by ~2-fold and blockade of Ca2+ release from the ER decreased cell replication to levels below baseline values (Figure 6J). Similarly, Ca2+ oscillations mediated by IGF-1 were abrogated by PLC and IP3R antagonists (supplemental Figure XIII).
To establish whether Ca2+ oscillations in hCPCs favor the acquisition of the myocyte lineage, these cells were cultured in differentiating medium3 in the absence or presence of ATP or histamine. After one week, the fraction of primitive cells expressing α-sarcomeric actin was comparable in stimulated and non-stimulated hCPCs (Supplemental Figure XIV), suggesting that these agents did not impact on the differentiation of hCPCs into the myocyte phenotype.
Ca2+ Oscillations in hCPCs and Myocardial Regeneration
In both animals and humans, shortly after ischemic myocardial injury, there is an increase of resident progenitors mostly restricted to the border zone of the infarcted heart.1 These cells rapidly acquire the myocyte lineage and result in small foci of cardiac repair.18 The possibility that factors naturally released in the damaged area facilitate this process was confirmed here in our transgenic mouse model in which EGFP-labeling made rather easy the identification of c-kit-positive CPCs. Myocardial infarction at 2 days led to a 15-fold and 5-fold increase in CPCs in the region bordering and remote from the infarction, respectively (Supplemental Figure XV).
However, spontaneous regeneration is severely limited and only a minimal fraction of a variety of progenitor cells delivered to the infarcted myocardium survives and integrates in the unfavorable environment of the necrotic tissue.13,19 Activation of hCPCs with ATP and histamine may enhance their engraftment, growth and formation of a myocyte progeny. EGFP-labeled hCPCs were exposed to ATP or histamine 30 min prior to their injection in the area bordering an acute infarct in immunosuppressed mice. Untreated hCPCs were used as control. All cell preparations were serum-starved for 24 hours before ATP or histamine exposure. Animals were examined 2 days later when cell engraftment is completed, cell death is markedly attenuated and the number of cells available for cardiac repair is established.13,20 Mice were exposed to BrdUrd to obtain cumulative values of cell regeneration. Additional groups of mice were sacrificed at 7 days to asses the impact of this protocol on myocardial regeneration and cardiac function.
Of the 60 × 103 hCPCs injected in each heart, 7,000 EGFP-positive cells were found in control hearts while nearly 19,000 cells (P<0.002) were detected in hearts in which hCPCs were treated with ATP or histamine. Cell engraftment was confirmed by the detection of connexin 43 and N-cadherin at the interface of hCPCs and spared myocytes (Supplemental Figure XVI). Moreover, the number of EGFP-positive cells labeled by BrdUrd was 10-12-fold higher in infarcts injected with ATP or histamine activated hCPCs (Figure 7A through 7C). Most importantly, the aggregate number of EGFP-positive cells expressing the myocyte transcription factors Nkx2.5 was ~4-fold larger in hearts treated with cells exposed to ATP or histamine.
Figure 7.
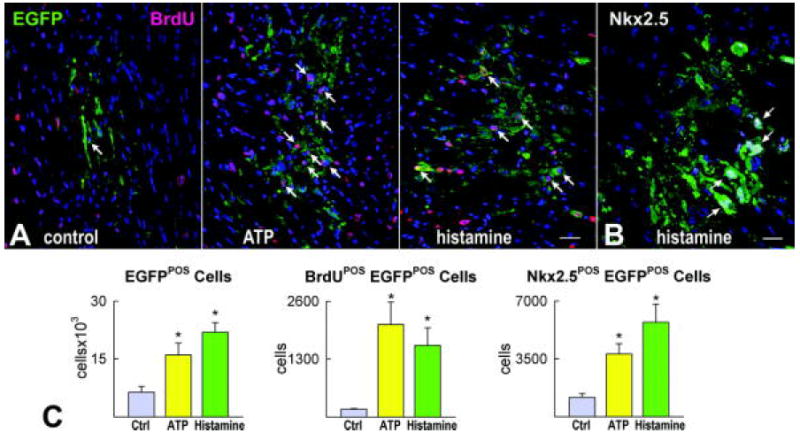
Ca2+ oscillations and growth of hCPCs in vivo. A, EGFP-positive hCPCs 48 hours after implantation in the infarcted mouse heart under control conditions and following activation of hCPCs with ATP or histamine. Proliferation of EGFP-positive cells (green) is documented by BrdU labeling (magenta, arrows). B, Nkx2.5 (white) is present in several EGFP-positive cells (arrows). Scale bars: 20 μm. C, Results are shown as mean±SEM. *P<0.05 vs. Ctrl.
Seven days after coronary artery occlusion and cell implantation, the extent of myocardial regeneration associated with the delivery of activated hCPCs was markedly superior to that obtained with untreated cells. The number of newly formed cardiomyocytes with ATP-histamine treatment was ~3-fold greater than in controls. However, the volume of cardiomyocytes was similar in the two groups (Figure 8A and 8B). Importantly, LV hemodynamics revealed that pre-treatment of hCPCs with ATP or histamine enhanced the effects of cell transplantation and positively interfered with the deterioration of cardiac function following myocardial injury (Figure 8 C).
Figure 8.
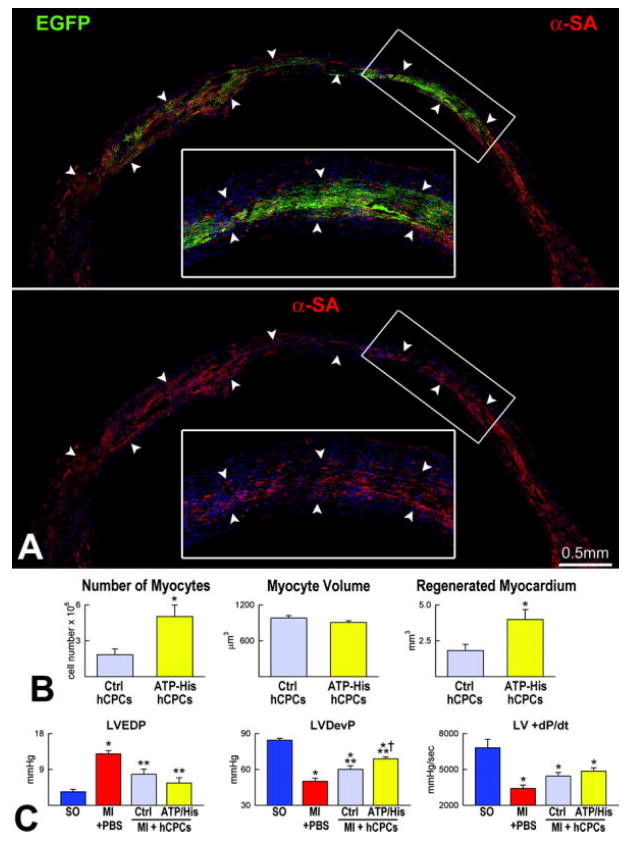
Myocardial regeneration by activated hCPC. A, Mouse heart treated with histamine-stimulated hCPCs. The mid-portion of the infarct is replaced by EGFP-positive (upper panel, green) α-SA-positive cardiomyocytes (lower panel, red). The area in the rectangle is shown at higher magnification in the inset. B, Extent of regeneration mediated by hCPCs non activated (Ctrl hCPCs) or exposed to ATP or histamine (ATP-His hCPCs). C, LV function in sham operated (SO), infarcted untreated (MI + PBS) and hCPC-treated (MI + hSCPs) mice 7 days after coronary ligation. Ctrl, ATP and His identify non-stimulated, ATP-stimulated and histamine-stimulated hCPCs, respectively. LVEDP, LV end-diastolic pressure; LVDevP and +dP/dt. *P<0.05 vs. SO, **P<0.05 vs. MI + PBS, † P<0.05 vs. MI injected with untreated hCPCs.
Discussion
The results of the current study indicate that hCPCs display spontaneous elevations in intracellular Ca2+ due to IP3R-mediated Ca2+ release from the ER. Re-uptake of Ca2+ into the ER is accomplished by SERCA which replenishes the Ca2+ stores allowing repetitive oscillations with preserved amplitude and duration. The Ca2+ handling molecules NCX, PMCA and SOC are functional and contribute to Ca2+ homeostasis in hCPCs, but are not implicated in the initiation and incidence of Ca2+ oscillations in these undifferentiated cells. Agonists of Gq-protein coupled receptors and histamine and ATP stimulate PLC and IP3 formation leading to an increase in the number of activated hCPCs and frequency of Ca2+ oscillatory episodes per cell in vitro. These Ca2+ oscillations promote hCPC proliferation, documenting that cytosolic Ca2+ plays a primary role in hCPC growth. Induction of Ca2+ oscillatory events in hCPCs prior to their intramyocardial delivery in vivo was coupled with enhanced the engraftment of these cells within the infarcted heart, their expansion in the unfavorable environment of the necrotic tissue and the generation of a myocyte progeny.
Origin of Ca2+ Oscillations in hCPCs
In the present study, a fundamental issue in need of resolution involved the recognition whether Ca2+ oscillations in hCPCs represent an intrinsic property of these primitive cells or the consequence of Ca2+ entry from cardiomyocytes and/or the extracellular compartment. Collectively, our results on the regulation of Ca2+ in hCPCs in vitro and in mouse CPCs within the myocardium in ex vivo preparations, suggest that cell-to-cell communication and the interstitial milieu are not implicated in the rapid and transient elevations of Ca2+ in CPCs. Under our experimental conditions, Ca2+ cycling in myocytes appears to have no influence on Ca2+ oscillations in CPCs. However, changes in the rate and amplitude of Ca2+ transients in myocytes may affect Ca2+ loading in CPCs. Cardiomyocytes function as supporting cells in myocardial niches7 and are connected by gap and adherens junctions to CPCs making them the ideal candidate for the translocation of Ca2+ and the initiation of oscillatory processes in CPCs. Although this was not found to be the case, this phenomenon may occur later during differentiation; the intercellular passage of Ca2+ may activate in lineage committed CPCs the release of Ca2+ from the ER conditioning the acquisition of the adult cardiomyocyte phenotype and contractile function.
The most significant regulator of IP3R-mediated Ca2+ release from the ER is Ca2+ itself. IP3R open channel probability is stimulated at low [Ca2+]i while high [Ca2+]i exerts an inhibitor effect.16 Accordingly, changes in [Ca2+]i may initiate and end Ca2+ oscillations in hCPCs. IP3 regulates IP3R channels mainly by enhancing their sensitivity to Ca2+. Two receptor ligand systems, P2Y2-ATP and H1-histamine, were identified in hCPCs and their importance in the modulation of intracellular Ca2+ and progenitor cell growth was defined in vitro and in vivo to characterize their potential function in cardiac homeostasis and regeneration. Importantly, the doses of ATP and histamine employed here have previously been shown to exert a powerful effect on Ca2+ mobilization in other cell systems.21,22
In physiological conditions, cell loss by normal wear and tear may result in an increase in the local level of ATP15 leading to Ca2+ oscillations in neighboring hCPCs which in turn activate cell replication and expansion. Additionally, the release of ATP from synaptic vesicles in terminal nerves15 may produce a comparable role in the hCPC compartment. These two mechanisms of ATP accumulation in the interstitial space are enhanced by prolonged myocardial ischemia and myocyte death.15,23 Cardiac pathology potentiates the load on the spared myocardium and mechanical stretch further enhances exocytosis of ATP from cardiomyocytes.15 In humans and animals, myocardial infarction is associated with CPC division and the generation of functionally competent cardiomyocytes,1 supporting the notion that ATP-mediated CPC growth may be critical for cardiac repair.
Mast cells are the predominant source of histamine in the myocardium.24 The number of mast cells and CPCs is comparable in the rodent and human heart.3,7,25,26 Additionally, tissue damage and inflammation recruit mast cells and CPCs, suggesting that histamine released from mast cells may be implicated in the activation of cardiac progenitors and the creation of myocytes. ATP is formed largely by cardiomyocytes that represent ~85% of the myocardium while histamine is synthesized by a very small number of mast cells, ~2-3/mm2 of tissue.15,25,26 However, extracellular ATP is rapidly degraded to inactive ADP. Conversely, histamine has a longer half-life time,15,27 indicating that these two molecules may have complementary function in the modulation of hCPC function.
As shown here for hCPCs, IP3R-mediated Ca2+ mobilization from the ER has been reported in human mesenchymal stem cells28 and mouse embryonic stem cells.29,30 In both cases, the increases in intracellular Ca2+ have been linked to cell growth and lineage specification. Embryonic stem cells and hCPCs differentiate into cardiomyocytes and the characteristics of Ca2+ cycling in these cells is regulated by the Ca2+-induced Ca2+-release mechanism that controls myocyte mechanics and ventricular function in vivo.11 Whether mesenchymal stem cells have a similar capacity is currently debatable.
IGF-1 and Ca2+ Oscillations in hCPCs
The function of IGF-1 is largely mediated by binding to the receptor tyrosine kinase IGF-1R. Phosphorylation of IGF-1R leads to recruitment of the insulin receptor substrate protein 1 (IRS-1) that modulates the effects of IGF-1R on cellular responses in the heart. The recruitment of IRS-1 upregulates PI3 kinase which phosphorylates Akt; Akt activation favors cell differentiation, hypertrophy or proliferation.31,32 IRS-1 also promotes the interaction of Ras, Raf and ERK which may lead to cellular hypertrophy or division.33 Surprisingly, in the current study, IGF-1 induced Ca2+ oscillatory episodes in hCPCs through the activation of IP3Rs and the release of Ca2+ from the ER. Whether this was a direct effect or was mediated by the generation of IP3 is currently unclear. However, the mitogenic properties of IGF-1 appear to be mediated, at least in part, by the release of Ca2+ from the ER, strengthening the notion that Ca2+ mobilization via IP3R is involved in cell cycle progression and growth of hCPCs.
Ca2+ Oscillations and hCPC In Vivo
A critical component of cell therapy is related to the recognition of the variables implicated in the engraftment and expansion of the delivered cells within the damaged myocardium.34 The function of progenitor cells is determined by causes inherent to the cells and risk factors for cardiovascular diseases. The former includes the telomere-telomerase axis, DNA damage and the expression of genes implicated in the forced entry of cells into in an irreversible quiescent state and/or activation of the endogenous cell death program.6,35 The latter involves several pathologic states such as diabetes, hypertension, coronary artery disease, valvular defects, dilated cardiomyopathy and myocardial aging.34
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by NIH grants and BWH-BRI. JFM. was supported by the Ministry of Science of Portugal and DT is the recipient of a Sarnoff fellowship.
Non-standard Abbreviations and Acronyms
- CPA
cyclopiazonic acid
- CPCs
cardiac progenitor cells
- H1
histamine receptor-1
- hCPCs
human cardiac progenitor cells
- His
histamine
- IP3
inositol 1,4,5-triphosphate
- IP3Rs
inositol 1,4,5-triphosphate receptors
- NCX
Na+-Ca2+ exchanger
- PMCA
plasma membrane Ca2+ pump
- P2Y2
P2-purinoceptors
- RyRs
ryanodine receptors
- SERCA
sarco/endoplasmic reticulum Ca2+ pump
- SOC
store operated channels
- XeC
xestospongin-C
- 2-APB
2-aminoethyl diphenylborinate
- BrdUrd
5-bromodeoxyuridine
- CPC
cardiac progenitor cell
- Dil
1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine
- EGFP
enhanced green fluorescent protein
- ER
endoplasmic reticulum
- IGF
insulin-like growth factor
- IGF-1R
insulin-like growth factor-1 receptor
- IRS
insulin receptor substrate protein
- LV
left ventricular
- mCPC
mouse cardiac progenitor cell
- PLC
phosphohpase-C
Footnotes
Disclosures None
References
- 1.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 2.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 3.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 5.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 7.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 11.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 12.Houser SR, Molkentin JD. Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci Signal. 2008;1:pe31. doi: 10.1126/scisignal.125pe31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns LA, Moroni E, Levantini E, Giorgetti A, Klinger FG, Ronzoni S, Tatangelo L, Tiveron C, De Felici M, Dolci S, Magli MC, Giglioni B, Ottolenghi S. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood. 2003;102:3954–3962. doi: 10.1182/blood-2003-04-1296. [DOI] [PubMed] [Google Scholar]
- 15.Vassort G. Adenosine 5’-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 16.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strassheim D, Williams CL. P2Y2 purinergic and M3 muscarinic acetylcholine receptors activate different phospholipase C-beta isoforms that are uniquely susceptible to protein kinase C-dependent phosphorylation and inactivation. JBiol Chem. 2000;275:39767–39772. doi: 10.1074/jbc.M007775200. [DOI] [PubMed] [Google Scholar]
- 18.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–1202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 20.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob R, Merritt JE, Hallam TJ, Rink TJ. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988;335:40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- 22.Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y2 purinoceptor inhibits the activity of the Na+/K+-ATPase in HeLa cells. Cell Signal. 2003;15:115–121. doi: 10.1016/s0898-6568(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 23.Vial C, Owen P, Opie LH, Posel D. Significance of release of adenosine triphosphate and adenosine induced by hypoxia or adrenaline in perfused rat heart. J Mol Cell Cardiol. 1987;19:187–197. doi: 10.1016/s0022-2828(87)80561-8. [DOI] [PubMed] [Google Scholar]
- 24.Wolff AA, Levi R. Histamine and cardiac arrhythmias. Circ Res. 1986;58:1–16. doi: 10.1161/01.res.58.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Olivetti G, Lagrasta C, Ricci R, Sonnenblick EH, Capasso JM, Anversa P. Long-term pressure-induced cardiac hypertrophy: capillary and mast cell proliferation. Am J Physiol. 1989;257:H1766–H1772. doi: 10.1152/ajpheart.1989.257.6.H1766. [DOI] [PubMed] [Google Scholar]
- 26.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasche P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Church MK, Caulfield JP. Mast cell and basophil function. In: Holgate ST, Church MK, editors. Allergy. New York, NY: Raven Press Ltd; 1993. [Google Scholar]
- 28.Kawano S, Shoji S, Ichinose S, Yamagata K, Tagami M, Hiraoka M. Characterization of Ca(2+) signaling pathways in human mesenchymal stem cells. Cell Calcium. 2002;32:165–174. doi: 10.1016/s0143416002001240. [DOI] [PubMed] [Google Scholar]
- 29.Kapur N, Mignery GA, Banach K. Cell cycle-dependent calcium oscillations in mouse embryonic stem cells. Am J Physiol Cell Physiol. 2007;292:C1510–C1518. doi: 10.1152/ajpcell.00181.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kapur N, Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol. 2007;581:1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 32.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


