Abstract
Study Objective
To determine whether changes in serum glucose, serum potassium, and plasma insulin levels are correlated in a cohort of hypertensive patients.
Design
Prespecified subgroup analysis of results from a prospective, multicenter, randomized, open-label, parallel-group study.
Setting
Primary care clinics at three tertiary care medical centers.
Patients
Community-based ambulatory population of 202 patients (age range 17–65 yrs) with a new diagnosis of hypertension, untreated hypertension, or known hypertension, who were previously treated with fewer than three antihypertensive drugs and had no evidence of cardiovascular disease or diabetes mellitus.
Intervention
Monotherapy with oral hydrochlorothiazide 12.5 or 25 mg/day for 9 weeks.
Measurements and Main Results
Fasting serum glucose, serum potassium, and plasma insulin levels were obtained at baseline (before hydrochlorothiazide therapy was started) and after 9 weeks of therapy. Significant elevations were noted in fasting serum glucose (mean ± SD 3.42 ± 10.38 mg/dl, p<0.0001) and plasma insulin (2.35 ± 9.47 μIU/ml, p<0.0001) levels, and a significant reduction in serum potassium level (0.30 ± 0.44 mEq/L, p<0.0001) was noted. No significant correlation was observed between changes in fasting serum glucose and potassium levels (r = 0.022, 95% confidence interval (CI) −0.120–0.164, p=0.757) or between changes in serum potassium and plasma insulin levels (r = −0.112, 95% CI −0.256–0.037, p=0.140). Changes in serum glucose levels did not differ significantly between patients maintaining serum potassium levels of 4.0 mEq/L or greater and those with levels below 4.0 mEq/L.
Conclusion
Changes in serum potassium and serum glucose levels were not correlated in individuals receiving hydrochlorothiazide monotherapy; thus maintenance of normal potassium levels may not attenuate the risk of thiazide diuretic–induced hyperglycemia.
Keywords: thiazide diuretics, hyperglycemia, hypokalemia, thiazide-induced hyperglycemia, hydrochlorothiazide.
Hypertension is the leading underlying cause of mortality worldwide and is a major risk factor for the development of coronary heart disease and stroke.1 It is estimated that hypertension affects one quarter of the world's adult population, with many more exhibiting prehypertension.2 Furthermore, only 40% of treated hypertensive patients are adequately controlled.3, 4 Thiazide-type diuretics are recommended as first-line therapy for the treatment of hypertension.5 This recommendation originates from large, randomized, controlled trials, the most notable being the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) and the Systolic Hypertension in the Elderly Program (SHEP).6, 7
Although numerous trials and a recent large meta-analysis have demonstrated thiazide diuretics to be an optimal treatment option for most hypertensive patients,6–8 adverse metabolic consequences, including hyperglycemia, have limited their use. Glucose intolerance and reduced insulin sensitivity have long been attributed to diuretic therapy, but the underlying mechanisms are not well understood. One prevailing theory is that thiazide-induced potassium depletion is responsible for decreased insulin secretion and/or reduced insulin sensitivity, leading to impaired glucose tolerance and hyperglycemia.9, 10 This association was first observed in small prospective studies; however, more recent evidence linking these metabolic parameters comes from meta-analyses and retrospective observational studies that observed aggregate data from individual studies rather than individual patients. This theory has been reinforced by a recent post hoc analysis of SHEP trial data that observed a 45% increase in the risk of new-onset diabetes with every 0.5-mEq/L decrease in serum potassium level.11
Potassium is thought to play a key role in glucose homeostasis. Potassium is involved in the release of insulin as well as insulin-mediated glucose uptake into skeletal muscle.9, 12 Both serum potassium depletion and glucose level elevation are common in patients treated with thiazide-type diuretics. Several smaller studies have not observed a correlation between serum potassium and serum glucose level changes or insulin sensitivity after thiazide-type diuretic treatment, even though the mean serum potassium level decreased and fasting blood glucose level increased.10, 13–16 However, these findings contrast with early case reports and small prospective studies that have suggested an association between serum potassium and glucose levels.17–20 In addition, a recent quantitative review correlated mean serum potassium level changes with mean fasting serum glucose level changes.12 The authors found a significant negative correlation between serum potassium and serum glucose levels (r = −0.28, 95% confidence interval [CI] −0.47 to −0.07, p<0.01). They further suggested that maintaining serum potassium levels above 4 mEq/L may attenuate the hyperglycemic response to thiazide-type diuretic treatment. This recommendation has recently been endorsed by others.11, 21 However, the correlation from the above-mentioned meta-analysis12 was derived from aggregate pooled (mean) changes from multiple studies and not individualized patient data. In addition, a recent cross-sectional study suggested that abdominal obesity may predispose patients to thiazide-induced hyperglycemia in association with potassium depletion.22
To better define the potassium-glucose paradigm, we sought to determine if serum potassium level changes correlate with fasting serum glucose level changes in hypertensive patients treated with hydrochlorothiazide monotherapy for 9 weeks.
Methods
We conducted a prespecified subgroup analysis of metabolic parameter data of patients participating in the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study, a prospective, multicenter, randomized, open-label, parallel-group study with a primary focus on identifying the genetic determinants of antihypertensive and adverse metabolic responses to thiazide diuretics and β-blockers.23 In the PEAR study, patients were randomized to receive monotherapy with either hydrochlorothiazide or atenolol; if after 9 weeks of treatment their blood pressure was above 120/70 mm Hg, then the other drug was added to their regimen. Fasting blood samples were collected at baseline (before either treatment), before the additional drug was added, and after 9 weeks of combination therapy. Complete details of the PEAR trial design and purpose have been previously published.23 Patients in the PEAR study (and thus in this subgroup analysis) were treated at the University of Florida (Gainesville, FL), Emory University (Atlanta, GA), or the Mayo Clinic (Rochester, MN). All participants provided written informed consent, and the institutional review boards of participating study centers approved the study protocol.
Study Participants
Males and females with mild-to-moderate essential hypertension, of any race-ethnicity, and aged 17–65 years were eligible for participation. Study participants were those with newly diagnosed hypertension, untreated hypertension, or known hypertension previously treated with fewer than three antihypertensive drugs (potassium-sparing diuretics were not considered an antihypertensive drug). After a washout period of at least 18 days, participants were screened for inclusion based on home and clinic blood pressure measurements. Eligible participants were those with an average seated diastolic blood pressure measurement above 85 mm Hg and systolic blood pressure measurement below 180 mm Hg, measured at home over 1 week, and clinic measurements of seated diastolic blood pressure of 90–110 mm Hg and systolic blood pressure less than 180 mm Hg.
Patients were excluded from the study if they had been treated with antihypertensive drugs but still had a clinic systolic blood pressure above 170 mm Hg. Other exclusion criteria were secondary forms of hypertension, isolated systolic hypertension, other diseases requiring treatment with blood pressure–lowering drugs, heart rate less than 55 beats/minute, known cardiovascular disease, diabetes mellitus (type 1 or 2), renal insufficiency (defined as serum creatinine concentration > 1.5 mg/dl in men and 1.4 mg/dl in women), pregnancy or lactation, history of Raynaud's syndrome, long-term treatment with drugs known to elevate blood pressure (e.g., nonsteroidal antiinflammatory drugs, oral contraceptives), active alcoholism, or elevated liver enzyme levels.
Study Procedures
Patients received hydrochlorothiazide 12.5 mg/day titrated to 25 mg/day after 2 weeks if blood pressure remained above 120/70 mm Hg. Patients were included in the substudy only if they had completed at least 9 weeks of therapy with hydrochlorothiazide monotherapy. Compliance was assessed based on pill counts from study drug blisterpacks. Use of potassium-sparing diuretic agents was not permitted. Potassium supplements were allowed at the discretion of the study physician for any serum potassium level and mandated for any patient with a serum potassium concentration below 3.2 mEq/L. Study participants who received any form of potassium supplementation at any time during the study were included in the primary analysis.
Fasting serum glucose, serum potassium, serum uric acid, and plasma insulin levels were obtained at baseline (before hydrochlorothiazide therapy was started) and after 9 weeks of therapy.
Laboratory Assessments
Fasting serum glucose, potassium, and uric acid levels were determined by using an Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). Fasting plasma insulin concentrations were measured by using the Access Ultrasensitive Insulin Immunoassay System (Beckman Instruments, Chaska, MN). All laboratory parameters were determined in a central laboratory at the Mayo Clinic. Insulin resistance was assessed based on the homeostatic assessment model of insulin resistance (HOMA-IR), which incorporates fasting glucose and fasting insulin levels to arrive at a measure of insulin sensitivity.24
Statistical Analysis
Descriptive statistics were used to represent demographic information and laboratory measurements. Sampling distribution of laboratory parameters was checked by Kolmogorov-Smirnovand test and QQ plot. The Kolmogorov-Smirnovand test of p value of 0.02 for nontransformed data showed the skewness from normal distribution. Thus, nonparametric tests were used for comparisons.
We compared baseline and posttreatment values by using the Wilcoxon signed-rank test. The Spearman rank correlation coefficient was calculated to assess correlations among plasma insulin level, serum glucose level, serum potassium level, and HOMA-IR before treatment and 9 weeks after starting treatment; and correlations among the changes in these parameters. In addition, we estimated the partial correlations among these parameters with covariate adjustments for age, sex, race-ethnicity, body mass index, and fasting serum uric acid levels. We used the Fisher z transformation to derive correlation confidence intervals (CIs) and p values for estimates of correlation and partial correlation.
Statistical significance was defined a priori as a p value less than 0.05. All statistical analyses were performed with SAS 9.1.3 (SAS Institute Inc., Cary, NC) or SPSS 11.0 (SPSS Inc., Chicago, IL) statistical software.
Results
Serum glucose, serum potassium, uric acid, and plasma insulin levels were collected for 202 patients enrolled in the PEAR study from October 2005–October 2008 who were receiving hydrochlorothiazide monotherapy for at least 9 weeks. Baseline demographics for these individuals are summarized in Table 1. Among the patients, 54% were female with the majority (56%) being Caucasian and middle-aged (mean age 50.5 yrs). Of the 202 patients, 198 (98%) were evaluated with the maximum allowed dose of 25 mg/day, whereas the remaining 4 (2%) were evaluated with the 12.5-mg/day regimen. One hundred ninety-one patients (95%) were fully adherent to therapy at the completion of the hydrochlorothiazide monotherapy phase. In the week before completion of this phase, 95% (192 patients) missed no doses, 3% (six patients) missed one dose, and 1% (two patients) missed two or more doses (adherence data were missing for 1% [two patients]).
Table 1.
Baseline Demographics of the 202 Study Patients
| Characteristic | Value |
|---|---|
| Age, yrs, mean (range) | 50.5 (26–65) |
| Body mass index, kg/m2, mean ± SD (range) |
30.7 ± 4.9 (20.2–45.5) |
| |
No. (%) of Patients |
| Female | 109 (54) |
| Race-ethnicity | |
| Caucasian | 114 (56) |
| African-American | 81 (40) |
| Other | 7 (4) |
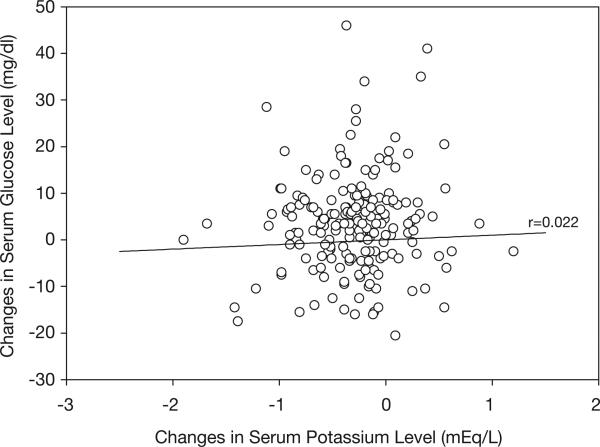
Baseline and posttreatment mean fasting values for metabolic study parameters are summarized in Table 2. After 9 weeks of treatment with hydrochlorothiazide monotherapy, significant changes were noted in fasting serum glucose, serum potassium, plasma insulin, and uric acid levels, and HOMA-IR. However, no significant correlation was seen between changes in serum potassium and serum glucose levels (r=0.022, 95% CI −0.120–0.164, p=0.757; Table 3, Figure 1). Similar results were found after controlling for age, sex, race-ethnicity, body mass index, and uric acid (r=0.033, p=0.68). Furthermore, no statistically significant correlation was observed between 9-week treatment levels of fasting serum glucose and serum potassium. Likewise, no significant correlation was observed between changes in serum potassium and plasma insulin levels before or after controlling for covariates. A significant positive correlation was observed between changes in plasma insulin and serum glucose (r=0.377, p<0.0001) that remained unchanged after covariate adjustment. An additional correlation analyses in which study patients were stratified by the presence or absence of abdominal obesity did not alter the primary study findings (Table 4).
Table 2.
Metabolic Parameters for the 202 Study Patients
| Parameter | Baselinea | After 9 Weeks of HCTZ Therapy | Mean Change | p Value |
|---|---|---|---|---|
| Fasting serum glucose (mg/dl) | 91.67 ± 10.08 | 95.03 ± 12.55 | 3.42 ± 10.38 | <0.0001 |
| Serum potassium (mEq/L) | 4.27 ± 0.47 | 3.96 ± 0.48 | −0.30 ± 0.44 | <0.0001 |
| Plasma insulin (μIU/ml) | 9.52 ± 8.21 | 11.92 ± 13.48 | 2.35 ± 9.47 | <0.0001 |
| HOMA-IRb | 2.23 ± 2.09 | 2.93 ± 3.68 | 0.69 ± 2.78 | <0.0001 |
| Uric acid (mg/dl) | 5.69 ± 1.53 | 6.78 ± 1.86 | 1.07 ± 0.88 | <0.0001 |
Data are mean ± SD.
HCTZ = hydrochlorothiazide; HOMA-IR = homeostatic model assessment of insulin resistance.
Baseline values after washout period (2–6 wks).
HOMA-IR was calculated by using the following equation: [glucose (mg/dl) × insulin (μIU/ml)]/405.24
Table 3.
Correlations Among Changes in Serum Glucose, Serum Potassium, and Plasma Insulin Levels
| Without Covariate Adjustment |
With Covariate Adjustmenta |
||||||
|---|---|---|---|---|---|---|---|
| Variable 1 | Variable 2 | r Value | 95% CI | p Value | r Value | 95% CI | p Value |
| Glucose | Potassium | 0.022 | −0.120–0.164 | 0.757 | 0.033 | −0.124–0.189 | 0.680 |
| Insulin | Glucose | 0.377 | 0.241–0.496 | <0.001 | 0.377 | 0.233–0.503 | <0.001 |
| Insulin | Potassium | −0.112 | −0.256–0.037 | 0.140 | −0.122 | −0.273–0.036 | 0.128 |
| HOMA-IR | Potassium | −0.092 | −0.237–0.057 | 0.225 | −0.099 | −0.252–0.058 | 0.214 |
| Uric Acid | Potassium | −0.095 | −0.223–0.047 | 0.189 | −0.080 | −0.237–0.075 | 0.301 |
CI = confidence interval; HOMA-IR = homeostatic model assessment of insulin resistance.
Adjustment for age, sex, race-ethnicity, and body mass index.
Figure 1.
No significant correlation was found between changes in fasting serum glucose and serum potassium levels after 9 weeks of hydrochlorothiazide monotherapy in the 202 study patients.
Table 4.
Correlation Analysis Between Changes in Fasting Serum Glucose and Potassium Levels
| Abdominal Obesitya | No. of Patientsb | Without Covariate Adjustment |
With Covariate Adjustmentb |
||||
|---|---|---|---|---|---|---|---|
| r Value | 95% CI | p Value | r Value | 95% CI | p Value | ||
| No | 78 | 0.057 | −0.168–0.275 | 0.624 | 0.033 | −0.201–0.262 | 0.786 |
| Yes | 113 | −0.001 | −0.186–0.183 | 0.990 | 0.074 | −0.118–0.261 | 0.447 |
CI = confidence interval.
Abdominal obesity defined as waist circumference > 35 inches (women) or > 40 inches (men) according to Adult Treatment Panel III guidelines.25
Data were not available for 11 patients.
cAdjustment for age, sex, and race-ethnicity.
No significant inverse correlations were observed between changes in fasting serum glucose and serum potassium levels when study patients were stratified into quartiles according to serum potassium level changes with (Table 5) or without covariate adjustment (data not shown). Stratification of patients according to potassium levels before and after 9 weeks of treatment with hydrochlorothiazide was not associated with an attenuation of glucose level elevations. Glucose levels increased by a mean ± SD of 3.68 ± 8.43 mg/dl for the 101 participants who maintained serum potassium levels less than 4.0 mEq/L at all times during the study, whereas the increase in glucose level for the 73 participants who maintained a serum potassium level of 4.0 mEq/L or higher at all times during the study was 3.33 ± 11.84 mg/dl (p=0.57 for the comparison of glucose level changes). Likewise, no significant correlation was observed between fasting serum glucose and serum potassium levels after stratifying patients according to baseline glucose levels (Table 6).
Table 5.
Unadjusted Correlation Analysis Among Changes in Fasting Serum Glucose and Potassium Levels Stratified by Change in Potassium Level
| Reduction on Potassium Level | No. of Patientsa | Sample Correlation | 95% CI | p Value |
|---|---|---|---|---|
| < 0.07 mEq/L (quartile 1)b | 50 | −0.033 | −0.308–0.248 | 0.821 |
| 0.07 to < 0.27 mEq/L (quartile 2) | 47 | −0.14 | −0.413–0.150 | 0.334 |
| 0.27 to < 0.58 mEq/L (quartile 3) | 50 | 0.28 | 0.00015–0.517 | 0.048 |
| ≥ 0.58 mEq/L (quartile 4) | 46 | 0.11 | −0.187–0.387 | 0.469 |
Data were not available for nine patients.
Quartile 1 includes patients with a reduction in serum potassium of < 0.07 mEq/L, no change in potassium, or an increase in potassium from baseline.
Table 6.
Correlation Analysis Among Changes in Fasting Serum Glucose and Potassium Levels
| Baseline Serum Glucose Level | Covariate Adjustment | No. of Patients | Sample Correlation | 95% CI | p Value |
|---|---|---|---|---|---|
| < 100 mg/dl | Unadjusted | 164 | 0.0964 | −0.0582–0.246 | 0.219 |
| Adjusteda | 158 | 0.119 | −0.0392–0.273 | 0.138 | |
| ≥ 100 mg/dl | Unadjusted | 36 | −0.299 | −0.571–0.0329 | 0.073 |
| Adjusteda | 33 | −0.215 | −0.539–0.164 | 0.257 |
Adjustment for age, sex, race-ethnicity, and body mass index.
As a sensitivity analysis, we excluded five participants who received potassium supplementation or dietary potassium recommendations. All but one of these participants were normokalemic at the end of 9 weeks. Exclusion of these patients from the primary analysis did not alter study findings.
Finally, we performed a secondary analysis on 206 patients from the PEAR study who were randomized to receive atenolol with subsequent addition of hydrochlorothiazide. After 9 weeks of atenolol and hydrochlorothiazide combination therapy, changes in levels of serum potassium, fasting serum glucose, and plasma insulin were a mean ± SD of −0.32 ± 0.45 mEq/L (p<0.0001), 3.71 ± 10.21 mg/dl (p<0.0001), and 1.92 ± 10.47 μIU/ml (p=0.0024), respectively, from the end of atenolol monotherapy to end of atenolol-hydrochlorothiazide combination therapy. No significant correlation was found between changes in serum potassium and fasting serum glucose levels after the addition of hydrochlorothiazide to atenolol (r = −0.113, 95% CI −0.255–0.034, p=0.131). Likewise, there was no significant correlation between changes in serum potassium and plasma insulin concentrations (r = −0.083, 95% CI −0.235–0.072, p=0.292).
Discussion
After 9 weeks of hydrochlorothiazide monotherapy, there were significant increases in fasting serum glucose levels and significant reductions in serum potassium concentrations. These changes are consistent with previously reported results in which metabolic consequences of thiazide and thiazide-like diuretic therapy were assessed. However, there was no correlation found between changes in fasting serum glucose and serum potassium concentrations in the primary analysis. Our findings are in contrast to those of the recent meta-analysis that suggested a significant inverse correlation (r = −0.28) between changes in serum potassium concentrations and serum glucose levels.12 In addition, the correlation coefficient observed in the meta-analysis is not within the 95% CI (−0.120–0.164) demonstrated in our study. This disparity may be attributed to different study methods. The authors of the meta-analysis retrospectively compared aggregate mean changes from multiple studies.12 In our study, we prospectively assessed individual patient changes. In addition, studies using various thiazide and thiazide-like diuretics (including hydrochlorothiazide, chlorthalidone, trichlormethiazide, bendrofluazide, metolazone, and amiloride-hydrochlorothiazide) were included in the meta-analysis, whereas only hydrochlorothiazide was used in our study. Although the scarcity of comparative data makes such comparisons difficult, it is unknown whether there are clinically significant differences in serum potassium depletion potential between agents. Many references commonly report therapeutic equivalence of hydrochlorothiazide and chlorthalidone on a milligram-to-milligram basis, but evidence supporting the superior potency of chlorthalidone (and thus, potentially more significant adverse metabolic consequences) to an equivalent hydrochlorothiazide dose has been published.26, 27 Taken together, these differences may account for the contrasting results. However, the change in serum potassium level observed in the meta-analysis weighted by sample size (−0.34 mEq/L) was similar to the serum potassium level changes seen in our study (−0.30 mEq/L).
In addition, in a recent analysis of SHEP trial data, the authors observed a doubling in the risk of incident diabetes mellitus in patients with pronounced potassium depletion (> 0.5 mEq/L), suggesting that the association may be dependent on the magnitude of potassium loss.11 However, our results indicate no correlation between fasting serum glucose level elevations and potassium depletion in the quartile of patients with 0.58 mEq/L or greater potassium loss. Furthermore, the authors observed a significantly increased risk of incident diabetes (hazard ratio 3.23/10 mg/dl, p<0.001) in those subjects whose baseline glucose level was above 100 mg/dl.11 We performed a similar analysis stratifying subjects by baseline glucose level that revealed no significant correlation between serum potassium and fasting serum glucose levels in those subjects with baseline glucose values of 100 mg/dl or greater. Although the unadjusted correlation value approached statistical significance in those with higher baseline glucose values, covariate adjustment drove the correlation analysis considerably further from statistical significance.
Of the 59 studies reviewed in the previously mentioned meta-analysis,12 none reported a correlation analysis between changes in serum potassium concentration and fasting serum glucose level. However, the vast majority of these studies reported significant serum potassium depletion with significant elevations in fasting serum glucose concentration. When viewed together, it is not surprising that a significant negative correlation was observed in the meta-analysis. Based on our study results, we cannot confirm the previous conclusions of small prospective, observational, and meta-analysis studies that a true inverse correlation exists between individual level reductions in serum potassium and elevations in fasting serum glucose concentrations. The results of our study may be further strengthened by the absence of any correlation between serum potassium and fasting serum glucose levels in patients prescribed dual therapy with atenolol and hydrochlorothiazide, given the well-known hyperglycemic effects of β-blockers. Furthermore, our results do not suggest that abdominal obesity is associated with correlations among serum potassium and fasting serum glucose level changes, as has been previously described.22
Changes in plasma insulin levels have been suggested as the clinical connection between decreased serum potassium concentrations and increases in fasting serum glucose levels.9 Hyperkalemia has been shown to stimulate insulin secretion and promote intracellular movement of potassium. However, there is insufficient and conflicting evidence that hypokalemia substantially impairs insulin secretion and glucose uptake. Our analysis found no correlation between changes in serum potassium concentration and plasma insulin levels before or after 9 weeks of treatment with hydrochlorothiazide. This finding may be reflective of insufficient serum potassium lowering to cause a significant effect or the absence of any true correlation. Alternatively, changes in insulin release may be associated with changes in intracellular potassium levels rather than serum levels.
It has been suggested that maintenance of serum potassium level above 4.0 mEq/L may attenuate the hyperglycemic response in patients taking thiazide diuretics.11, 12, 21 This recommendation has been based on long-held assumptions from observational studies that serum potassium depletion is responsible for elevations in glucose levels. However, given the lack of any significant difference in glucose level elevations among patients who maintained a serum potassium level of 4.0 mEq/L or higher compared with those in whom the level remained below 4.0 mEq/L, it seems unlikely that this target will have a significant effect on suppressing elevations in fasting serum glucose levels. Moreover, stratifying patients into quartiles based on serum potassium levels revealed no significant differences in glucose level elevations. Potassium supplementation may still be necessary for hypokalemic patients in order to prevent other complications, but our data do not provide evidence that would suggest such therapy would alter the risk of hyperglycemia. In light of the paucity of evidence and the potential implications of thiazide-induced diabetes, controlled trials assessing the benefits of this recommendation are needed.
Recently proposed alternative mechanisms for thiazide-induced dysglycemia include visceral fat redistribution,16 persistent sympathetic nervous system activation,28 and reduced perfusion of skeletal muscle mass.29 The mechanisms may be independent of serum potassium level; however, further study is needed to elucidate their impact on thiazide-induced dysglycemia.
There are some limitations to our study. First, the dose of hydrochlorothiazide was lower than that used in many previous thiazide diuretic studies. However, the dose in this study reflects current prescribing patterns of hydrochlorothiazide, as well as what is recommended in current practice guidelines.5 Higher doses may be prone to significantly greater adverse metabolic consequences, including more hypokalemia and hyperglycemia. Second, although we found no evidence that maintaining serum potassium levels of 4.0 mEq/L or greater would alter the risk of hyperglycemia, our study was not designed to prospectively address this issue. Third, participants were treated with hydrochlorothiazide for 9 weeks. Although some metabolic alterations may occur after this time frame, we believe that most changes occur within the first 4 weeks of therapy.
Conclusion
We found no correlation between thiazide diuretic–induced changes in potassium and fasting serum glucose levels. Although it is apparent that thiazide diuretics have the potential to induce glucose level changes and ostensibly new-onset diabetes, the mechanism remains unclear. Previously accepted assumptions on the mechanism of this metabolic effect have been based primarily on observational studies and meta-analysis. Our prespecified subgroup analysis does not support this long-held assumption. Potassium supplementation and the use of potassium-sparing diuretics in combination with thiazide diuretics remain important treatment strategies for combating hypokalemia, but may not be expected to lower the risk of hyperglycemia. This study reinforces the importance of primary patient-level research, suggesting that such data must be balanced with the findings of large-scale pooled analyses and retrospective observational data.
Acknowledgments
We acknowledge and thank the valuable contributions of the study participants, support staff, and study physicians: Drs. George Baramidze, Carmen Bray, Kendall Campbell, R. Whit Curry, Frederic Rabari-Oskoui, Dan Rubin, and Seigfried Schmidt.
Supported by a grant (U01 GM074492) from the National Institutes of Health (NIH) as part of the Pharmacogenetics Research Network. In addition, grants from the NIH National Center for Research Resources were provided to the University of Florida (M01 RR00082), Emory University (UL1 RR025008 and M01 RR00039), and Mayo Clinic (UL1 RR024150). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, its final contents, and the decision to submit for publication.
Footnotes
Presented in part at the annual meeting of the American College of Clinical Pharmacy, Louisville, Kentucky, October 18–22, 2008.
References
- 1.Ezzati M, Lopez AD, Rogers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Stafford RS. Screening, treatment, and control of hypertension in U.S. private physician offices, 2003–2004. Hypertension. 2008;51:1275–81. doi: 10.1161/HYPERTENSIONAHA.107.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Group Members for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2008 update [online exclusive article]. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 7.The SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the systolic hypertension in the elderly program (SHEP). JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 8.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–44. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 9.Carter BL, Enhorn PT, Brands M, et al. Thiazide-induced dysglycemia: call for research from a working group from the National Heart, Lung, and Blood Institute. Hypertension. 2008;52:30–6. doi: 10.1161/HYPERTENSIONAHA.108.114389. [DOI] [PubMed] [Google Scholar]
- 10.Langford HG, Cutter G, Oberman A, Kansal P, Russell G. The effect of thiazide therapy on glucose, insulin and cholesterol metabolism and of glucose on potassium: results of a cross-sectional study in patients from the hypertension detection and followup program. J Hum Hypertens. 1990;4:491–500. [PubMed] [Google Scholar]
- 11.Shafi T, Appel LJ, Miller ER, Klag MJ, Parekh RS. Changes in serum potassium mediate thiazide-induced diabetes. Hypertension. 2008;52:1022–9. doi: 10.1161/HYPERTENSIONAHA.108.119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–24. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 13.Plavinik FL, Rodrigues CI, Zanella MT, Ribeiro AB. Hypokalemia, glucose intolerance, and hyperinsulinemia during diuretic therapy. Hypertension. 1992;19(suppl II):II26–9. doi: 10.1161/01.hyp.19.2_suppl.ii26. [DOI] [PubMed] [Google Scholar]
- 14.McFarland KF, Carr AA. Changes in the fasting blood sugar after hydrochlorothiazide and potassium supplementation. J Clin Pharmacol. 1977;17:13–17. doi: 10.1002/j.1552-4604.1977.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 15.Veterans Administration Cooperative Study Group Effects of treatment on morbidity in hypertension. III. Influence of age, diastolic pressure, and prior cardiovascular disease: further analysis of side effects. Circulation. 1972;45:991–1004. doi: 10.1161/01.cir.45.5.991. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson JW, Jansson P, Carlberg B, et al. Hydrochlorothiazide, but not candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of candesartan (MEDICA) study. Hypertension. 2008;52:1030–7. doi: 10.1161/HYPERTENSIONAHA.108.119404. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins RW. New drugs for the treatment of hypertension. Ann Intern Med. 1959;50:1–10. doi: 10.7326/0003-4819-50-1-1. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport MI. Thiazide-induced glucose intolerance treated with potassium. Arch Intern Med. 1964;113:405–8. doi: 10.1001/archinte.1964.00280090091014. [DOI] [PubMed] [Google Scholar]
- 19.Goldner MG, Zarowitz H, Akgun S. Hyperglycemia and glycosuria due to thiazide derivatives administered in diabetes mellitus. N Engl J Med. 1960;262:403–5. doi: 10.1056/NEJM196002252620807. [DOI] [PubMed] [Google Scholar]
- 20.Sagild U, Andersen V, Andreasen PB. Glucose tolerance and insulin responsiveness in experimental potassium depletion. Acta Medica Scandinavica. 1961;169:243–51. doi: 10.1111/j.0954-6820.1961.tb07829.x. [DOI] [PubMed] [Google Scholar]
- 21.Carter BL. Preventing thiazide-induced hyperglycemia: opportunities for clinical pharmacists. Pharmacotherapy. 2008;28:1425–8. doi: 10.1592/phco.28.12.1425. [DOI] [PubMed] [Google Scholar]
- 22.Mariosa LS, Ribeiro-Filho FF, Batista MC, et al. Abdominal obesity is associated with potassium depletion and changes in glucose homeostasis during diuretic therapy. J Clin Hypertens. 2008;10:443–9. doi: 10.1111/j.1751-7176.2008.07817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JA, Boerwinkle E, Zineh I, et al. Pharmacogenomics of antihypertensive drugs: rationale and design of the pharmacogenomic evaluation of antihypertensive responses (PEAR) study. Am Heart J. 2009;157:442–9. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III): final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 26.Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4–9. doi: 10.1161/01.HYP.0000103632.19915.0E. [DOI] [PubMed] [Google Scholar]
- 27.Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–8. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 28.Menon DV, Arbique D, Wang Z, et al. Differential effects of chlorthalidone vs spironolactone on muscle sympathetic nerve activity in hypertensive patients. J Clin Endocrin Metab. 2009;94:1361–6. doi: 10.1210/jc.2008-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angarwal R. Hypertension, hypokalemia, and thiazide-induced diabetes: a 3-way connection. Hypertension. 2008;52:1012–13. doi: 10.1161/HYPERTENSIONAHA.108.121970. [DOI] [PubMed] [Google Scholar]