Abstract
Malignant melanoma has a high propensity for metastatic spread, making it the most deadly form of skin cancer. B-RAF has been identified as the most mutated gene in these invasive cells and therefore an attractive therapeutic target. However, for uncertain reasons, chemotherapy inhibiting B-Raf has not been clinically effective. This has raised questions whether this pathway is important in melanoma metastasis or whether targeting a protein other than B-Raf in the signaling cascade could more effectively inhibit this pathway to reduce lung metastases. Here, we investigated the role played by V600EB-Raf in melanoma metastasis and showed that targeting this signaling cascade significantly reduces lung metastases. Small interfering RNA (siRNA)–mediated inhibition was used in mice to reduce expression (activity) of each member of the signaling cascade and effects on metastasis development were measured. Targeting any member of the signaling cascade reduced metastasis but inhibition of mitogen-activated protein kinase/extracellular signal–regulated kinase kinase (Mek) 1 and Mek 2 almost completely prevented lung tumor development. Mechanistically, metastatic inhibition was mediated through reduction of melanoma cell extravasation through the endothelium and decreased proliferative capacity. Targeting B-Raf with the pharmacologic inhibitor BAY 43-9006, which was found ineffective in clinical trials and seems to act primarily as an angiogenesis inhibitor, did not decrease metastasis, whereas inhibition of Mek using U0126 decreased cellular proliferative capacity, thereby effectively reducing number and size of lung metastases. In summary, this study provides a mechanistic basis for targeting Mek and not B-Raf in the mutant V600EB-Raf signaling cascade to inhibit melanoma metastases.
Introduction
Malignant melanoma is the most deadly form of skin cancer due to its highly metastatic nature (1–3). The disease develops in a stepwise manner as evolving cancer cells undergo cellular changes, resulting from genetic alteration, which promote development of more aggressive metastatic tumors (1, 4). These changes include accelerated cell growth, enhanced survival under conditions that would kill a normal cell, development of blood vessels as tumors increase in size, and, finally, invasion of cancer cells into surrounding tissues culminating in metastasis to distant organs (1, 4). Although clinical trials have tested a wide range of approaches ranging from immunotherapy, radiotherapy, and chemotherapy, no effective therapy exists for patients to inhibit the metastatic spread of melanoma (5–8).
Several studies have implicated the mitogen-activated protein (MAP) kinase [Ras/Raf/MAP kinase-extracellular signal–regulated kinase (Erk) kinase (Mek)/Erk] pathway in melanoma metastasis by promoting processes such as cell proliferation, survival, invasion, and tumor angiogenesis (9–12). The signaling cascade is activated in ~60% of melanomas by a single-base mutation in B-RAF converting T to A at nucleotide 1,799, substituting a valine for a glutamic acid at codon 600 (V600E) in exon 15 (13). The mutation was originally misidentified as occurring at codon 599 instead of codon 600; therefore, it is referred to as either V600E or V599E in the literature (13–15). B-RAF mutation is acquired during development of sporadic melanomas and therefore not inherited in familial melanomas (16–18).
The role of B-Raf in the signaling cascade is as an intermediate kinase relaying extracellular signals via an ordered series of consecutive phosphorylation events from cell surface through Ras to nucleus (9, 11). The V600EB-Raf mutation leads to constitutive activation of B-Raf and increased activity or expression of downstream members of the signaling cascade (13–15). For example, increased phosphorylation of Mek and Erk, as well as increased expression of cyclin D1, has been reported in cells expressing V600EB-Raf protein (14, 15). Metastasis requires that melanoma cells pass into the vascular system (intravasation), survive the stresses encountered during blood flow, pass through the endothelial lining of vessels into the tissue (extravasation), and finally proliferate in the new tissue environment (19). The role played by mutant V600EB-Raf protein to facilitate these processes remains uncertain.
Because B-RAF is the most mutated gene in melanomas, it is an attractive therapeutic target to inhibit metastatic spread (14). Unfortunately, targeting B-Raf in melanomas using a Raf kinase inhibitor, called BAY 43-9006 (Sorafenib), has not been clinically effective (14, 20). This has raised concerns about the importance of this pathway in promoting melanoma metastasis (15, 21, 22). Furthermore, it remains uncertain whether lack of therapeutic success was due to the mechanism of action of the Raf kinase inhibitor BAY 43-9006 or whether targeting mutant V600EB-Raf is not the most efficient strategy to inhibit this signaling cascade (14, 20, 23–25). Targeting another member of this signaling cascade might more effectively inhibit this pathway, thereby more efficiently reducing melanoma metastasis (26–28). Finally, the mechanism by which V600EB-Raf promotes metastasis remains uncertain (14).
This study investigates these questions by identifying the role played by V600EB-Raf in melanoma metastasis and determining whether targeting this signaling cascade reduces metastatic tumor development. Results show that targeting any member of the signaling cascade (V600EB-Raf, Mek, Erk, or cyclin D1) reduced metastasis; however, simultaneous inhibition of Mek 1 and Mek 2 almost completely prevented tumor development in the lungs. Mechanistically, inhibition of metastasis occurred through a reduction of melanoma cell extravasation into lung tissue and by slowed cell proliferation in the lung microenvironment. Thus, these studies provide a rationale and a mechanistic basis for therapeutically targeting mutant V600EB-Raf signaling to inhibit melanoma metastasis.
Materials and Methods
Human melanoma cell lines, culture conditions, and B-RAF mutational status
The human melanoma cell lines 1205 Lu, A375M, C8161.Cl9, UACC 903, and UACC 903M were grown in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Hyclone, Logan, UT). Green fluorescent protein (GFP)–tagged versions of 1205 Lu, UACC 903M, and C8161.Cl9 were used for metastasis assays. UACC 903M, a highly metastatic variant of the UACC 903 cell line, was derived by i.v. injecting GFP-tagged UACC 903 cells into nude mice and, 30 days later, establishing cell lines from tumors, which had formed in the lungs. The resultant cell line called UACC 903M was highly metastatic to the lungs following i.v. injection of the cells. The presence or absence of the T1799A B-RAF mutation in cell lines was undertaken as previously described (29).
In vitro siRNA studies
SiRNA (100 pmol) was introduced into 1 × 106 1205 Lu, A375M, C8161.Cl9, or UACC 903 cells via nucleofection with an Amaxa Nucleofector (Koeln, Germany), as described in refs. 12, 30. Solution R/program K-17 was used for 1205 Lu, C8161.Cl9, and UACC 903 cell lines whereas Solution R/program A-23 was used for A375M cells. The resultant transfection efficiency following nucleofection was >90%. Duplexed Stealth siRNA (Invitrogen) was used for these studies. The sequence for each respective siRNA is as follows: scrambled siRNA, AAUUCUCCGAACGUGU-CACGUGAGA; C-RAF, GGUCAAUGUGCGAAAUGGAAUGAGC; WT B-RAF (Com4 or 4), GGACAAAGAAUUGGAUCUGGAUCAU; WT/MUT B-RAF (MuA or A), GGUCUAGCUACAGAGAAAUCUCGAU; MEK 1, CCCGCAAUCCGGAAC-CAGAUCAUAA; MEK 2, GAACUCAAAGACGAUGACUUCGAAA; ERK 1, CCCUGGAAGCCAUGAGAGAUGUCUA; ERK 2, CCGAAGCACCAUUCAA-GUUCGACAU; and CYCLIN D1, CCACAGAUGUGAAGUUCAUUUCCAA. Following nucleofection, cells were replated and, 72 hours later, protein lysates were harvested for Western blot analysis. Duration of siRNA knockdown was measured in replated cells harvested 0, 2, 4, 6, and 8 days following nucleofection with siRNA to B-RAF, C-RAF, MEK 1, MEK 2, ERK 1, ERK 2, and CYCLIN D1 and cell lysates were subjected to Western blot analysis.
Western blot analysis
For Western blot analysis, cell lysates were harvested in Petri dishes by the addition of lysis buffer containing 50 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 10 mmol/L EDTA, 10% glycerol, 1% Triton X-100, 1 mmol/L sodium orthovandate, 0.1 mmol/L sodium molybdate, 1 mmol/L phenylmethylsulfonyl fluoride, 20 μg/mL aprotinin, and 5 μg/mL leupeptin. Whole-cell lysates were centrifuged (≥10,000 × g) for 10 minutes at 4°C to remove cell debris. Protein concentration was quantified using the BCA Assay from Pierce (Rockford, IL) and 30 μg of protein lysates per lane were loaded onto a NuPage Gel (Life Technologies, Inc., Carlsbad, CA). Following electrophoresis, samples were transferred to polyvinylidene difluoride membrane (Pall Corporation, Pensacola, FL). The blots were probed with antibodies according to the recommendations of suppliers: anti-pErk and anti-pMek from Cell Signaling Technologies (Beverly, MA); antibodies to B-Raf, C-Raf, Erk 2, cyclin D1, p27, and α-enolase from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were conjugated with horseradish peroxidase and obtained from Santa Cruz Biotechnology. The immunoblots were developed using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ).
In vitro extravasation assays
Three days after nucleofection, migration of melanoma cells across an endothelial-like cell layer under flow conditions was examined using a modified 48-well Boyden flow migration chamber (31, 32). The device contains 48 wells in the bottom plate with an inlet and outlet connected to the upper chamber to enable flow conditions. A monolayer of confluent EI cells was grown (typically 36–48 hours after cell seeding) on a sterile 8-μm pore size polyvinylpyrrolidone-free polycarbonate filter (NeuroProbe, Gaithersburg, MD) precoated for 3 hours with 30 μg/mL fibronectin (Sigma, St. Louis, MO). EI cells, fibroblast L-cells transfected to express human E-selectin and intercellular adhesion molecule 1, were generously provided for these studies by Dr. Scott Simon (University of California at Davis, Davis, CA) and maintained as previously described (33). The 12 center wells in the bottom plate were filled with soluble type IV collagen chemoattractant (100 μg/mL in RPMI 1640/0.1% BSA; BD Biosciences, Lexington, KY). Surrounding control wells were filled with RPMI 1640 supplemented with 0.1% BSA. Apparatus was assembled by placing filter on plate followed by addition of a sealing gasket and top plate. Flow was initiated using 37°C medium to purge bubbles from the system. For migration assays, 5 × 105 melanomas cells only or melanoma cells together with neutrophils (5 × 105 of each) were pipetted into the chamber subjected to static conditions or 4 dyn/cm2 shear flow for 4 hours in a 37°C, 5% CO2 incubator. Human neutrophils were isolated according to a protocol approved by The Pennsylvania State University Institutional Review Board that involved isolating cells from whole blood using a Ficoll-Hypqaue (Histopaque, Sigma, Milwaukee, WI) density gradient according to the protocol of the manufacturer. Following isolation, neutrophils were stored in PBS supplemented with 0.1% human serum albumin at 4°C until use. Migration was quantified by staining of the filter using Protocol Brand Hema3 solution (Fisher Scientific, Pittsburgh, PA). Melanoma cells migrating to the underside of the filter were photographed using an inverted microscope (Diaphot 330, Nikon, Japan) with NIH Image software (v.β4.0.2) and counted.
In vivo siRNA and pharmacologic studies
Animal experimentation was undertaken according to protocols approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University College of Medicine. SiRNA (100 pmol) was nucleofected into GFP-tagged 1205 Lu, UACC 903M, or C8161.Cl9 cells, which were then replated in culture dishes. Thirty-six hours later, 1 × 106 1205 Lu or C8161.Cl9 cells or 0.5 × 106 UACC 903M cells in 0.2 mL of HBSS were injected i.v. into the lateral tail vein of nude mice. Mice were sacrificed 17 days later, necropsied, and lungs were analyzed for the presence of fluorescent tumors using a Nikon SMZ 1500 dissecting microscope with a Plan Apo 1.6× objective and fluorescence detection capabilities. Images of five random fields were photographed at a magnification of 4.8× from the ventral surface of each lung and the number of fluorescent tumors as well as area scored in pixels occupied by each tumor was quantified using IP lab imaging software (Scanalytics, Fairfax, VA). Duplicate experiments consisting of eight animals were used per group. For tumor proliferation studies, 5 × 106 1205 Lu cells nucleofected with siRNA were s.c. injected into mice and tumors harvested 4 days later to measure changes in cell proliferation or apoptosis, as previously described (12, 30, 34).
Effect of the Mek inhibitor U0126 (35) or Raf kinase inhibitor BAY 43-9006 (12, 24, 36) on metastasis development was measured by pretreatment of mice with compound (U0126, 10 mg/kg body weight; BAY 43-9006, 75 mg/kg body weight) by i.p. injection twice (−4 and −2 days) before i.v. injection of 1205 Lu, UACC 903M, or C8161.Cl9 cells and every 2 days thereafter. Tumor proliferation rates following U0126 or BAY 43-9006 treatment were determined by comparing tumors of the same size exposed to DMSO vehicle 4 days after s.c. injection of melanoma cells into nude mice. U0126 was obtained from Cell Signaling whereas the BAY 43-9006 compound used for these studies was synthesized as described in refs. 12, 37.
Cell proliferation rates in formalin-fixed tumor sections were measured using the RPN 20 cell proliferation kit (Amersham Biosciences) that uses bromodeoxyuridine (BrdUrd) incorporation and immunocytochemistry. Two hours before sacrificing, 1 mg/100 gm body weight of BrdUrd was injected i.p. into mice and tumors were processed according to the proliferation kit instructions. The number of BrdUrd stained cells was scored as the percentage of total cells of tumors treated with siRNA, U0126, BAY 43-9006, or vehicle (DMSO). For all tumor analyses, a minimum of four different tumors with four to six fields per tumor were analyzed and results represented as the average ± SE.
Cell doubling time
The in vitro doubling time of 1205 Lu, A375M, and UACC 903 cells nucleofected with control or experimental siRNA was estimated by plating 3 × 103 per well for UACC 903, 2.5 × 103 per well for A375M, or 4 × 103 per well for 1205 Lu in 200 μL of DMEM supplemented with 10% FBS in multiple rows of wells in five 96-well plates. Growth was measured colorimetrically using the sulforhodamine B binding assay (Sigma Chemical Co., St. Louis, MO) every 24 hours over a period of 5 days on one plate each day. Absorbance was measured at 570 nm using a Perkin-Elmer HTS 700+ Bioassay Plate Reader (Foster City, CA). Doubling times were calculated using the exponential equation of a best-fit line (y = y0eax) for each condition (12). Values are averages ± SE obtained from three independent experiments by calculating the amount of time (x) it took the absorbance (y) to double.
Statistics
For statistical analysis, the Student’s t test was used for pairwise comparisons and the ANOVA or the Kruskal-Wallis one-way ANOVA test was used for groupwise comparisons, followed by the appropriate post hoc tests (Dunnett’s, Tukey’s, or Dunn’s test). Results were considered significant at P < 0.05.
Results
Targeting mutant V600EB-Raf signaling in melanoma cells inhibits development of lung metastases
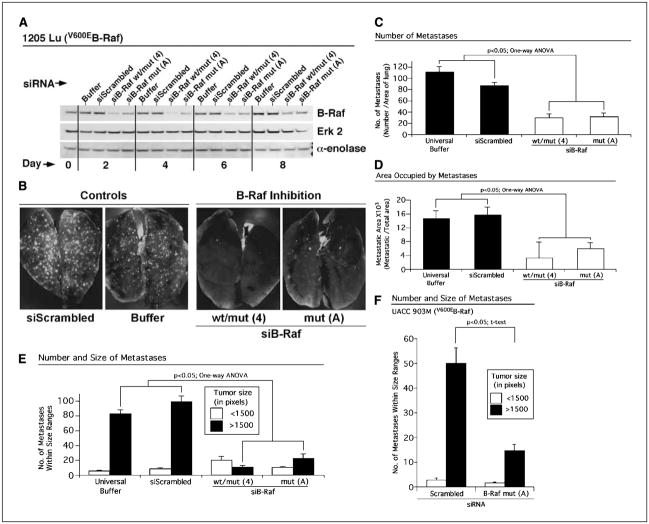
Although mutant V600EB-Raf protein has been shown to play an important role in melanoma tumor development (12, 38, 39), its role in metastasis remains uncertain (14). To develop a more thorough understanding of its function in metastasis, expression (activity) of B-Raf was decreased in metastatic human melanoma cells containing V600EB-Raf (1205 Lu, A375M, and UACC 903) and compared with the C8161.Cl9 cell line lacking the T1799A mutation. SiRNA specifically designed to inhibit expression of mutant V600EB-Raf protein (MuA or A) or to simultaneously reduce expression of both wild-type and mutant protein (Com4 or 4) were used for this study (12, 38). Experimental metastasis development was studied by nucleofecting siRNA into melanoma cells, allowing recovery in culture for 1.5 days (36 hours) followed by i.v. injection of cells into the tail vein of nude mice. Seventeen days later, the lungs of mice were removed and number as well as area occupied by GFP-expressing tumors was quantified. Nucleofection resulted in siRNA transfection efficiencies of >90% (12, 30) and knockdown of B-Raf protein in 1205 Lu melanoma cells persisted beyond 8 days in culture compared with control cells nucleofected with scrambled siRNA (Fig. 1A; ref. 12). Similar levels and durations of siRNA-mediated B-Raf protein knockdown were observed for UACC 903M and C8161.Cl9 cell lines (not shown). Reducing expression (activity) of V600EB-Raf significantly retarded the metastatic potential of 1205 Lu (Fig. 1B–E;P < 0.05, one-way ANOVA) and UACC 903M (Fig. 1F; P < 0.05, t test) cells containing mutant V600EB-Raf but not of C8161.Cl9 cells lacking it (not shown). Fewer fluorescent tumors were observed in the lungs of animals injected with melanoma cells in which V600EB-Raf had been inhibited, suggesting that less cells extravasated through the endothelial lining or become entrapped in lung vessels and proliferated in the lung tissue (Fig. 1C). In contrast, control animals injected with cells nucleofected with scrambled siRNA or buffer had significantly more metastases, occupying a larger overall area of lung than cells in which B-Raf had been inhibited (Fig. 1D; P < 0.05, one-way ANOVA). Metastases were placed into two pixel-based categories based on size (i.e., those <1,500 and >1,500 pixels). A significant 5- to 7-fold decrease in tumors >1,500 pixels was observed following V600EB-Raf inhibition compared with control 1205 Lu cells nucleofected with buffer or scrambled siRNA (Fig. 1E). Similar results were observed in UACC 903M cells following V600EB-Raf inhibition in which there was a ~2- to 3-fold decrease in metastases >1,500 pixels compared with a scrambled siRNA control (Fig. 1F; P < 0.05, t test). In stark contrast, inhibition of B-RAF in the C8161.Cl9 cell line lacking the T1799A mutation had no effect on metastatic potential of these cells (not shown). Collectively, these data show that inhibition of B-Raf in melanoma cells containing, but not those lacking, the T1799A B-Raf mutation reduces development of metastatic lung tumors.
Figure 1.
Inhibition of mutant V600EB-Raf reduces the metastatic potential of melanoma cells. A, siRNA-mediated reduction of mutant V600EB-Raf protein expression persists for 8 to 10 days in melanoma cells. SiRNA-mediated knockdown of B-Raf reduces protein expression in cells for 8 to 10 days following nucleofection compared with controls nucleofected with buffer or scrambled siRNA. Erk 2 and α-enolase were used as controls for protein loading. B, siRNA-mediated reduction of mutant V600EB-Raf protein decreased formation of melanoma metastases in the lungs of nude mice. SiRNA against B-RAF or scrambled siRNA was introduced into GFP-tagged 1205 Lu cells; 36 hours later, cells were i.v. injected into nude mice. Photographs show presence of GFP-tagged tumors in the lungs of mice 17 days later. Control cells were nucleofected with buffer only or scrambled siRNA. SiRNA mediated down-regulation of B-Raf protein expression reduced tumor number (C), area (D), and large-sized metastases (E) in the lungs of nude mice. Number of tumors and area occupied by GFP-tumors were quantified in a minimum of six fields per lung from 5 to 10 animals. Tumors were grouped into two pixel-based size ranges (<1,500 or >1,500 pixels). F, siRNA-mediated reduction of mutant V600EB-Raf expression (activity) in UACC 903M cells decreased formation of lung metastases. SiRNA against V600EB-RAF or a scrambled siRNA control were introduced into GFP-tagged UACC 903M cells; 36 hours later, cells were i.v. injected into nude mice, and 17 days later, lung metastases were quantified. Numbers of tumors within particular size ranges (<1,500 or >1,500 pixels) were quantified in a minimum of six fields per lung from 5 to 10 animals.
Inhibition of proteins downstream of mutant V600EB-Raf retarded metastasis development and identified Mek 1/2 proteins as target to most effectively inhibit metastasis
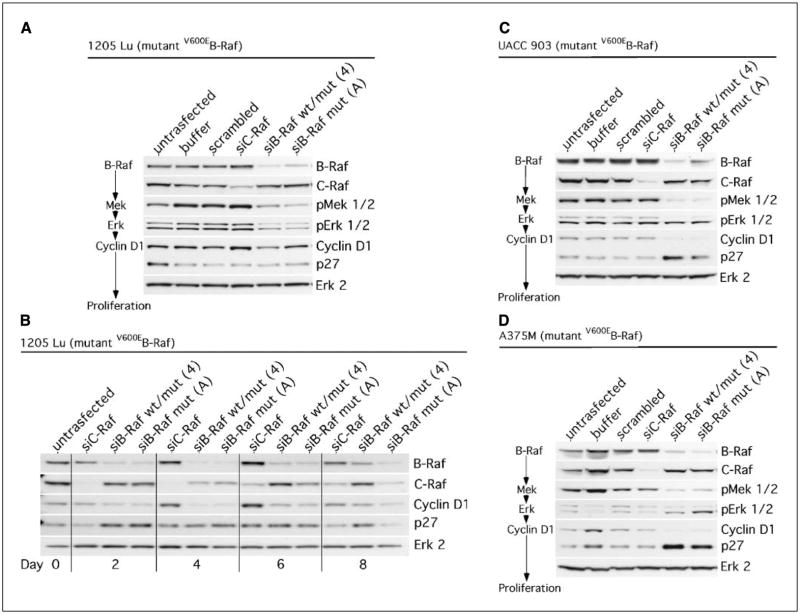
Whereas inhibiting V600EB-Raf can reduce melanoma cell lung metastasis (Fig. 1), it is unknown whether targeting another protein in the signaling cascade would more efficiently inhibit this process. To investigate this unknown possibility, signaling through the mutant V600EB-Raf pathway was initially confirmed by measuring changes in phosphorylation or expression levels of downstream proteins in the MAP kinase pathway following siRNA-mediated knockdown of V600EB-Raf protein (Fig. 2A; refs. 12, 38, 39). Western blot analysis showed reduced V600EB-Raf expression in 1205 Lu cells 72 hours after siRNA introduction with a corresponding decrease in phosphorylation (activity) levels of downstream targets Mek and Erk as well as reduced amounts of cyclin D1. In contrast, no changes were observed in phosphorylation levels of Mek, Erk, or cyclin D1 in control untransfected or cells transfected with buffer, scrambled siRNA, or siRNA against C-RAF (Fig. 2A). Because changes in cyclin D1 levels were subtle following V600EB-Raf knockdown 72 hours (3 days) after introduction of siRNA into cells, a time course was undertaken examining levels 2, 4, 6, and 8 days after siRNA-mediated knockdown of V600EB-Raf protein expression (Fig. 2B). Cyclin D1 levels decreased significantly between days 4 and 6 in 1205 Lu cells, indicating that targeting V600EB-Raf reduced downstream levels of this protein whereas inhibition of C-Raf did not. Signaling through the mutant V600EB-Raf signaling cascade was also verified in UACC 903 (Fig. 2C) and A375M (Fig. 2D) melanoma cell lines yielding similar results. A corresponding increase in p27 levels accompanied declining cyclin D1 levels in all cell lines (Fig. 2B–D). Low levels of pErk in A375M cells made altered activity levels difficult to measure; however, downstream cyclin D1 levels were decreased. These results confirmed that the mutant V600EB-Raf signaling cascade relayed signals from V600EB-Raf to Mek 1/2 to Erk 1/2 to cyclin D1 in melanoma cells, thereby regulating cellular proliferation. Thus, all these proteins were potential targets in the signaling cascade that could inhibit metastasis development.
Figure 2.
SiRNA-mediated reduction of mutant V600EB-Raf expression reduces the downstream activity of Mek and Erk as well as levels of cyclin D1 protein in melanoma cells. A, siRNA-mediated knockdown of V600EB-Raf, but not of C-Raf, reduces phosphorylation (activity) levels of downstream proteins Mek and Erk in 1205 Lu cells 72 hours after nucleofection. Western blots of cells probed for the various proteins in the V600EB-Raf signaling cascade or control proteins following introduction of siRNA into cells. Erk 2 served as a control for protein loading. B, decreased cyclin D1 levels were detected in 1205 Lu cells following knockdown of V600EB-Raf expression. A 2-, 4-, 6-, and 8-day time course is shown for 1205 Lu cells following mutant V600EB-Raf inhibition. A decrease in cyclin D1 levels was observed starting at day 2 and ending at day 6. A corresponding increase in p27 levels was detected at day 2. Erk 2 was used as a protein loading control. SiRNA-mediated reduction of V600EB-Raf expression (activity) in melanoma cell lines UACC 903 (C) and A375M (D) led to significant decreases in activity and expression of downstream proteins 72 hours after nucleofection into cells. Increased p27 levels accompanied decreased cyclin D1 levels. Scrambled siRNA or buffer alone were used as controls. Erk 2 served as a control for protein loading.
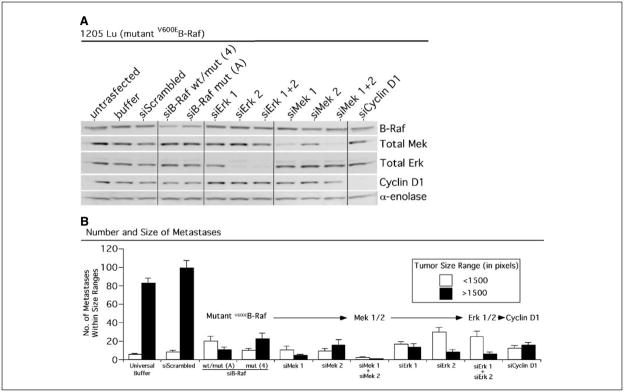
Having verified the signal relay through members of the V600EB-Raf pathway in melanoma cells, we next examined whether any particular protein of the signaling cascade would more efficiently inhibit metastatic tumor development in the lungs of animals compared with targeting mutant V600EB-Raf. Members of the signaling cascade, including V600EB-Raf, Mek 1/2, Erk 1/2, and cyclin D1, were inhibited using siRNA specific against each gene and consequences on lung metastasis development were compared. Each siRNA knocked down expression of its target protein to approximately equivalent levels, which persisted beyond 8 days in culture (not shown). Reduced protein expression 72 hours (3 days) after nucleofection of each respective siRNA into 1205 Lu cells is shown in Fig. 3A. Following knockdown of protein expression, number and size of fluorescent tumors present in the lungs of mice were measured 17 days after i.v. injecting cells nucleofected with either control or siRNA to each respective member of the V600EB-Raf signaling cascade (Fig. 3B). Targeted inhibition of each member of the V600EB-Raf signaling cascade significantly retarded development of lung metastases compared with buffer or scrambled siRNA controls (Fig. 3B; P < 0.05, Kruskal-Wallis ANOVA). However, the most dramatic reduction in metastasis development occurred following simultaneous inhibition of both Mek 1 and Mek 2, which nearly eliminated metastasis formation. In contrast to combined Mek 1/2 inhibition, targeting each independently or Erk 1 and Erk 2 simultaneously were not as effective at reducing metastasis development. Collectively, these data showed that siRNA-mediated inhibition of downstream members of the mutant V600EB-Raf signaling pathway reduced metastasis development. However, simultaneous inhibition of Mek 1 and Mek 2 most dramatically inhibited formation of metastases, suggesting that these proteins in the V600EB-Raf pathway should be targeted to inhibit effectively melanoma lung metastases.
Figure 3.
Inhibition of each protein downstream of mutant V600EB-Raf reduces formation of metastatic tumors in the lungs of mice. A, siRNA-mediated reduction of mutant V599EB-Raf, Mek, Erk, and cyclin D1 reduced expression of each respective protein 72 hours after nucleofection into 1205 Lu melanoma cells. Western blots probed with antibodies against each respective protein indicates specificity of knockdown. α-Enolase served as a control for protein loading. B, siRNA-mediated reduction of mutant V600EB-Raf, Mek, Erk, and cyclin D1 protein decreased formation of melanoma metastases in the lungs of mice. SiRNA against each respective protein or a scrambled siRNA control was introduced into GFP-tagged 1205 Lu cells; 36 hours later, cells were i.v. injected into nude mice, and 17 days later, lung metastases were quantified. Numbers of tumors within particular size ranges (<1,500 or >1,500 pixels) were scored in a minimum of six fields per lung from 5 to 10 animals.
Reduced melanoma cell extravasation and proliferation following inhibition of V600EB-Raf signaling retard melanoma lung metastases
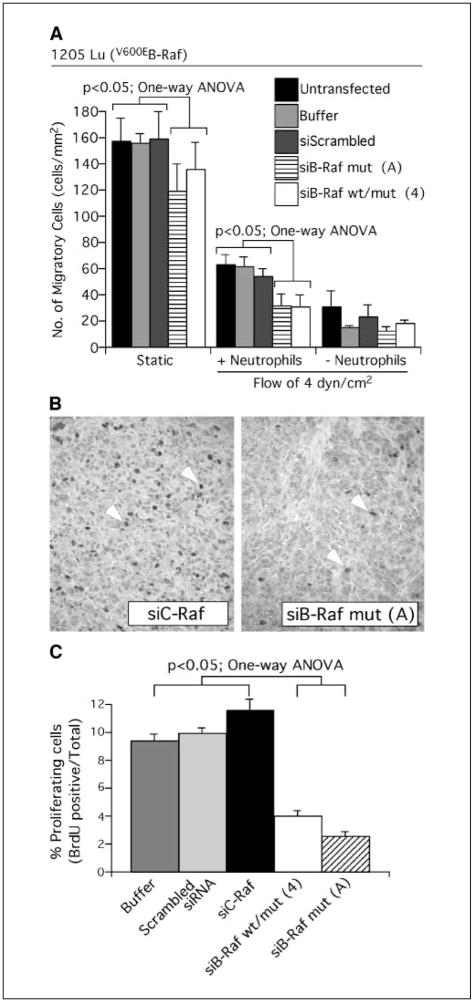
Melanoma cell lung metastasis involves extravasation through the endothelial lining of lung vessels or entrapment in the lung microvasculature followed by active proliferation in lung tissue (19). Disruption of any of these processes can reduce development of melanoma metastases (19). To determine which of these processes V600EB-Raf signaling regulated to promote metastasis, the pathway was inhibited and effect on extravasation and proliferation examined. Extravasation of melanoma cells through the endothelial lining of lung vessels was evaluated using an in vitro flow-migration assay (31, 32). Using this model, migration of melanoma cells alone or in combination with neutrophils across an endothelial-like layer was measured by placing cells into a chamber in which fluid movement could be controlled and quantifying migration across an endothelial cell like layer in response to collagen IV chemoattractant placed beneath the cell layer (Fig. 4A). Migration under flow conditions (4 dyn/cm2) recapitulates the physical dynamics of a blood capillary where cell adhesion to the endothelial-like layer corresponded to binding to the vessel wall and migration of tumor cells from the top to the bottom compartment represented extravasation into the tissue space (31, 32, 40). Under static conditions and in the absence of neutrophils, melanoma cell migration was significantly reduced following B-Raf inhibition (Fig. 4A, Static; P < 0.05, one-way ANOVA). In contrast, under flow conditions of 4 dyn/cm2, no migratory difference was observed between control cells or those in which B-Raf had been inhibited. However, combining neutrophils with 1205 Lu melanoma cells promoted significant migration under flow conditions of 4 dyn/cm2 (Fig. 4A, +Neutrophil; P < 0.05, one-way ANOVA) compared with melanoma cells alone (Fig. 4A, −Neutrophil). In the presence of neutrophils, inhibiting V600EB-Raf in melanoma cells significantly retarded migration (P < 0.05, one-way ANOVA). Thus, inhibition of mutant V600EB-Raf signaling significantly reduced extravasation of melanoma-neutrophil cell complexes or melanoma cells alone across an endothelial-like cell layer, suggesting one potential mechanism for preventing metastasis development.
Figure 4.
Inhibition of mutant V600EB-Raf in melanoma cells reduces neutrophil-mediated extravasation through the endothelium and lowers the proliferative capacity of melanoma cells. A, siRNA-mediated inhibition of V600EB-Raf decreased neutrophil-mediated extravasation across an endothelial-like cell layer. Following siRNA-mediated knockdown of V600EB-Raf, migration of 1205 Lu melanoma cell across an endothelial-like cell layer in response to collagen IV (100 μg/mL) over a 4-hour period was quantified by scoring number of migrated cells. Comparisons were made under static or flow conditions of 4 dyn/cm2 in the presence or absence of neutrophils. Untransfected cells or cells nucleofected with scrambled siRNA or buffer alone were used as controls for comparison. B, siRNA-mediated knockdown of B-Raf protein reduced the number of proliferating cells in tumors. Percentage proliferating cells was determined by scoring BrdUrd-positive cells in tumors established from 1205 Lu cells nucleofected with siRNA against V600EB-RAF and compared with control tumors formed from cells transfected with buffer, scrambled siRNA, or siRNA against C-RAF. C, siRNA-mediated inhibition of V600EB-Raf reduced melanoma tumor proliferative capacity by 2- to 3-fold. Columns, mean percentage of proliferating cells determined from four to six fields analyzed from each of six tumors; bars, SE. Untransfected cells or cells nucleofected with scrambled siRNA or buffer alone were used as controls for comparison.
Extravasated or entrapped cells within lung tissue need to proliferate to form secondary tumors (19). To determine whether reduced growth of melanoma cells following V600EB-Raf inhibition also contributed to decreased lung metastases, growth of tumor cells was measured following siRNA-mediated inhibition of V600EB-Raf, Mek 1/2, Erk 1/2, and cyclin D1 and compared with controls (Table 1). 1205 Lu, A375M, and UACC 903 cells nucleofected with buffer or scrambled siRNA doubled in number in vitro every ~41, ~33, and ~27 hours, respectively (Table 1). In contrast, cells nucleofected with siRNA against V600EB-Raf grew 29% to 41% slower than controls, similar to prior reports (12, 38, 39). To identify which proteins downstream of B-Raf contributed to decreased proliferation, each downstream protein (Mek 1, Mek 2, Erk 1, Erk 2, or cyclin D1) was initially inhibited individually. No consistent pattern was observed that was common to all three cell lines. This result suggested that knockdown of one isoform might be compensated for by another isoform. Therefore, we simultaneously targeted Mek 1 and Mek 2 or Erk 1 and Erk 2. Similar to results observed following knockdown of V600EB-Raf, targeting Mek 1 and Mek 2 or Erk 1 and Erk 2 together consistently reduced cellular proliferation by 18% to 63% for all cell lines.
Table 1.
Growth properties of 1205 Lu, A375M, and UACC 903 cells treated with scrambled siRNA or siRNA against B-RAF, MEK, ERK, or CYCLIN D1
| SiRNA treatment | Melanoma cell line doubling time in vitro (hours ± SE) |
|||||
|---|---|---|---|---|---|---|
| 1205 Lu | A375M | UACC 903 | ||||
| Controls | ||||||
| Scrambled | 38 ± 3 | 33 ± 2 | 26 ± 1 | |||
| Universal buffer | 44 ± 7 | 32 ± 3 | 28 ± 0 | |||
| Average | 41 | 33 | 27 | |||
| Experimental | ||||||
| B-Raf mut (A) | 53 ± 10 | ↑29% | 46 ± 6 | ↑39% | 38 ± 3 | ↑41% |
| Mek 1 | 41 ± 3 | NC | 36 ± 3 | NC | 32 ± 0 | ↑19% |
| Mek 2 | 39 ± 3 | NC | 29 ± 2 | NC | 26 ± 0 | NC |
| Mek 1 + Mek 2 | 60 ± 9 | ↑46% | 39 ± 2 | ↑18% | 44 ± 5 | ↑63% |
| Erk 1 | 53 ± 0 | ↑29% | 34 ± 4 | NC | 26 ± 0 | NC |
| Erk 2 | 43 ± 4 | NC | 34 ± 2 | NC | 31 ± 1 | ↑15% |
| Erk 1 + Erk 2 | 64 ± 3 | ↑56% | 40 ± 2 | ↑18% | 37 ± 1 | ↑37% |
| Cyclin D1 | 52 ± 0 | ↑27% | 36 ± 3 | NC | 29 ± 0 | NC |
| Average | 51 | ↑24% | 36 | NC | 33 | ↑22% |
NOTE: To the right of each experimental value is shown relative changes in proliferative capacity between the control average and experimental value; NC, no significant change between control average and experimental value (i.e., a difference of ≤3 hours).
Consistent with the reduced proliferative capacity observed in vitro, decreased proliferation was observed in 1205 Lu tumors following inhibition of V600EB-Raf (Fig. 4B). Rates of tumor cell proliferation and apoptosis were measured 4 days after s.c. injection in nude mice. Whereas no significant difference was detected in numbers of cells undergoing apoptosis (not shown), siRNA-mediated inhibition of B-Raf led to 2- to 3-fold fewer proliferating cells in tumors compared with control cells nucleofected with buffer, scrambled siRNA, or C-RAF siRNA (Fig. 4C). The ~75% reduction in proliferative capacity of tumor cells nucleofected with B-RAF siRNA showed that decreased proliferative capacity contributed to reduced metastasis development. Thus, inhibition of the mutant V600EB-Raf signaling pathway decreased tumor cell extravasation and proliferation to inhibit metastasis.
Pharmacologic inhibition of Mek, but not of B-Raf, retards development of melanoma lung metastases
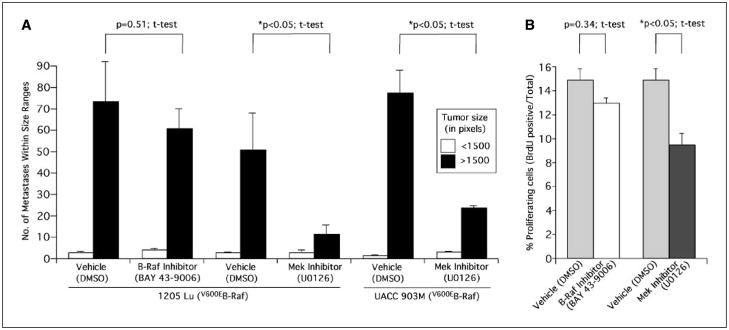
Because siRNA-mediated inhibition of mutant V600EB-Raf signaling reduced lung metastasis development, the effects of pharmacologic inhibition of B-Raf or Mek were examined next to determine whether a Mek inhibitor more effectively inhibited metastasis than a B-Raf inhibitor. BAY 43-9006, a nonspecific Raf kinase inhibitor, and U0126, a specific Mek inhibitor, were evaluated for effectiveness in preventing lung metastasis development. Mice were pretreated with each respective compound (75 mg/kg BAY 43-9006 or 10 mg/kg U0126) 4 and 2 days before i.v. injection of metastatic melanoma cells and every 2 days following i.v. injection of GFP-tagged 1205 Lu or UACC 903M tumor cells up to day 17 (12, 24, 35, 36). Both compounds inhibited the V600EB-Raf signaling pathway in culture (not shown), consistent with our prior reports and those of others (12, 23). However, in animals, BAY 43-9006 treatments had no effect on metastasis development of 1205 Lu (Fig. 5A) or C8161.Cl9 cells (not shown). Thus, irrespective of whether the metastatic cells contained or lacked V600EB-Raf, treatment with BAY 43-9006 had no effect on metastasis. In contrast, Mek inhibition using U0126 significantly reduced metastasis in both 1205 Lu and UACC 903M cell lines compared with mice exposed to DMSO vehicle (Fig. 5A; P < 0.05, t test). Both number and size of tumors developing in the lungs were reduced following Mek inhibition but not following B-Raf inhibition. Mechanistically, fewer proliferating cells were observed in mice exposed to the Mek inhibitor U0126 (Fig. 5B; P < 0.05, t test) compared with mice treated with the B-Raf inhibitor BAY 43-9006 (Fig. 5B; P = 0.34, t test). No significant difference was observed in apoptosis levels (not shown). Thus, pharmacologic inhibition of Mek in melanoma cells containing mutant V600EB-Raf reduced metastasis by decreasing the proliferative potential of the cells.
Figure 5.
Pharmacologic inhibition of Mek, but not of V600EB-Raf, retards the metastatic potential of melanoma cells by altering proliferative capacity. A, pharmacologic inhibition of Mek, but not of V600EB-Raf, reduced the metastatic potential of melanoma cells. Four and two days before i.v. injection of metastatic melanoma cell lines 1205 Lu or UACC 903M into mice, animals were pretreated i.p. with a Raf inhibitor (BAY 43-9006; 75 mg/kg) or a Mek inhibitor (U0126; 10 mg/kg) dissolved in DMSO vehicle. Treatment was continued every 2 days up to day 17 when number and size of GFP-tagged tumors (<1,500 or >1,500 pixels) were quantified. Columns, mean of a minimum of six fields per lung from 5 to 10 animals; bars, SE. Controls were animals treated with DMSO vehicle only. B, pharmacologic inhibition of Mek, but not of V600EB-Raf, reduced the number of proliferating cells in tumors. Percentage proliferating cells was determined by scoring BrdUrd-positive cells in tumors 4 days after s.c. injection into mice. Animals treated with the DMSO vehicle served as controls. Columns, mean of four to six fields analyzed from six different tumors; bars, SE.
Discussion
Although mutant V600EB-Raf protein has been shown to play an important role in development of advanced stage melanomas by regulating proliferation, vascular development, and metastasis (12, 38, 39, 41), the mechanistic basis by which it regulates metastasis remained uncertain (14). Through use of a unique strategy involving transient siRNA-mediated knockdown of V600EB-Raf protein expression (activity), experimental lung metastases could be inhibited 4- to 5-fold in metastatic melanoma cells containing mutant B-Raf but not those lacking the mutant protein. The model focused on lung metastasis because lungs are a major organ to which melanoma cells metastasize; however, melanoma are highly aggressive and capable of invading any organ. It is important to note that inhibiting B-Raf in metastatic cells lacking the mutation did not reduce lung metastasis. Thus, targeting the MAP kinase signaling cascade may only be effective for ~60% of the melanoma patients whose tumors contain mutant V600EB-Raf protein (13, 42–44). This observation is supported by a recent study showing that melanomas containing mutated B-Raf are more responsive to agents targeting the MAP kinase pathway than those wild type for the protein or harboring a Ras mutation (45). Therefore, ascertaining the mutational status of B-RAF before use of therapeutics targeting the MAP kinase signaling cascade would be a potentially important indicator of clinical efficacy. This is also the first study to identify that targeting Mek 1/2 rather than V600EB-Raf in the signaling cascade can more efficiently inhibit lung metastasis development. SiRNA-mediated inhibition of V600EB-Raf and proteins downstream in the signaling cascade (Mek 1/2, Erk 1/2, and cyclin D1) all decreased metastasis development with simultaneous inhibition of Mek 1/2 having the most dramatic effect. This is not the first study to identify an important role for Mek in metastasis; pharmacologic inhibition of Mek has been shown previously to reduce melanoma metastasis (46). It is also intriguing that simultaneously targeting Erk 1/2 did not inhibit metastasis development to the same extent as Mek 1/2 inhibition, which further shows the importance of targeting Mek 1/2 to inhibit the V600EB-Raf pathway in melanoma cells.
A lack of clinical responsiveness in targeting B-Raf using the pharmacologic agent BAY 43-9006 has raised serious doubt about the usefulness of therapeutically targeting this signaling cascade to inhibit melanoma development (14, 20). We have shown that siRNA-mediated inhibition of mutant V600EB-Raf signaling reduced lung metastases, indicating that this pathway is important for melanoma metastasis. Furthermore, because siRNA-mediated inhibition of V600EB-Raf reduced lung metastasis development, it is reasonable to assume that a pharmacologic inhibitor of B-Raf would do the same (24, 47). However, our results show that Mek inhibition using U0126 could inhibit melanoma metastasis more efficiently than occurs when inhibiting B-Raf using BAY 43-9006. It is possible that the in vivo mechanism of action of BAY 43-9006 is not conducive for metastasis inhibition. In support of this possibility, BAY 43-9006 has been shown to inhibit melanoma tumor development in preclinical models by reducing vascular development (angiogenesis) mediated through decreased vascular endothelial growth factor (VEGF) secretion by the melanoma cells and through inhibition of VEGF receptors (12, 48, 49). Furthermore, others have reported that BAY 43-9006 did not block lung adenoma formation whereas Mek inhibitor CI-1040 did (23). It is confounding that both compounds inhibit V600EB-Raf signaling in melanoma cells in culture but are not equally effective at inhibiting metastasis development. Thus, in vitro efficacy does not necessarily translate into in vivo effectiveness. A possible explanation is that the mechanism of action of BAY 43-9006 involves regulating angiogenesis, which is not required for initial lung metastasis, making the compound ineffective (50, 51). In contrast, Mek inhibition reduced cell proliferation, which effectively decreased the number and size of metastasis forming in the lung stroma (23, 46). It remains to be determined whether other B-Raf inhibitors more specific than BAY 43-9006 would inhibit proliferation and thereby inhibit metastasis formation. However, even if a specific B-Raf inhibitor were developed, siRNA-mediated inhibition data presented in this manuscript show that targeting Mek and not mutant B-Raf in melanomas might be the most efficient approach to inhibit melanoma metastasis.
Metastasis can be inhibited by disrupting extravasation through the endothelial lining of lung vessels or by reducing proliferation in the lung microenvironment (19). We have shown that V600EB-Raf plays a critical role regulating both of these processes thereby regulating metastasis. Interaction of melanoma cells with neutrophils facilitates attachment to the endothelium, thereby promoting extravasation into lung stroma (52, 53). We have provided novel insight into these processes showing that, in melanoma, V600EB-Raf promotes metastasis by facilitating extravasation across the endothelial layer under flow conditions and by conferring increased proliferative potential in the lung microenvironment. V600EB-Raf enhanced extravasation by facilitating interaction with neutrophils, which aided in tethering the melanoma cell to the endothelial lining under flow conditions, thereby promoting movement of the melanoma cell through the endothelial lining (52, 53). Once extravasation has occurred into lung stroma, cells need to proliferate, also facilitated by V600EB-Raf. SiRNA-mediated inhibition of V600EB-Raf reduced the proliferative capacity of melanoma cells by 29% to 75%, the most dramatic inhibition occurring following Mek 1/2 inhibition.
In conclusion, we identified the role played by V600EB-Raf in melanoma metastasis and showed that targeting this signaling cascade would reduce metastatic tumor development. Targeting any member of the signaling cascade reduced metastasis, but inhibition of Mek 1 and Mek 2 almost prevented it. Inhibition is mediated through reduction of melanoma cell extravasation into lung tissue and decreased proliferative potential within the lung microenvironment. This study provides a mechanistic basis for therapeutically targeting mutant V600EB-Raf signaling to inhibit melanoma metastasis.
Acknowledgments
Grant support: American Cancer Society grant RSG-04-053-01-GMC (G.P. Robertson), The Melanoma Research Foundation (G.P. Robertson), The Foreman Foundation for Melanoma Research (G.P. Robertson), Johnson & Johnson Innovative Technology Research Seed Grant Program, and PA Health Research Grant (C. Dong and G.P. Robertson), Grace Woodward Grants in Engineering and Medicine (C. Dong and G.P. Robertson), NIH grant CA-97306 (C. Dong), and National Science Foundation grant BES-0138474 (C. Dong).
We thank Xioali Lu, SubbaRao Madhunapantula, and Sung Jin Huh for technical assistance and Dr. Elliot Vessel for proofreading the manuscript.
References
- 1.Schalick WO, Albino AP, Reed JA, et al. Cutaneous oncology. Malden (MA): Blackwell Science, Inc; 1998. pp. 180–348. [Google Scholar]
- 2.Jemal A, Devesa SS, Fears TR, Hartge P. Cancer surveillance series: changing patterns of cutaneous malignant melanoma mortality rates among whites in the United States. J Natl Cancer Inst. 2000;92:811–8. doi: 10.1093/jnci/92.10.811. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–83. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 4.Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813–24. doi: 10.1172/JCI24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serrone L, Hersey P. The chemoresistance of human malignant melanoma: an update. Melanoma Res. 1999;9:51–8. doi: 10.1097/00008390-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19:21–34. [PubMed] [Google Scholar]
- 7.Atkins JH, Gershell LJ. Selective anticancer drugs. Nat Rev Drug Discov. 2002;1:491–2. doi: 10.1038/nrd842. [DOI] [PubMed] [Google Scholar]
- 8.Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138–51. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 9.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 10.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–9. [PubMed] [Google Scholar]
- 11.Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–32. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 13.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 14.Tuveson DA, Weber BL, Herlyn M. BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell. 2003;4:95–8. doi: 10.1016/s1535-6108(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 15.Reifenberger J, Knobbe CB, Sterzinger AA, et al. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int J Cancer. 2004;109:377–84. doi: 10.1002/ijc.11722. [DOI] [PubMed] [Google Scholar]
- 16.Lang J, Boxer M, MacKie R. Absence of exon 15 BRAF germline mutations in familial melanoma. Hum Mutat. 2003;21:327–30. doi: 10.1002/humu.10188. [DOI] [PubMed] [Google Scholar]
- 17.Laud K, Kannengiesser C, Avril MF, et al. BRAF as a melanoma susceptibility candidate gene? Cancer Res. 2003;63:3061–5. [PubMed] [Google Scholar]
- 18.Meyer P, Klaes R, Schmitt C, Boettger MB, Garbe C. Exclusion of BRAFV599E as a melanoma susceptibility mutation. Int J Cancer. 2003;106:78–80. doi: 10.1002/ijc.11199. [DOI] [PubMed] [Google Scholar]
- 19.Shevde LA, Welch DR. Metastasis suppressor pathways-an evolving paradigm. Cancer Lett. 2003;198:1–20. doi: 10.1016/s0304-3835(03)00304-5. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad T, Marais R, Pyle L, et al. BAY 43-9006 in patients with advanced melanoma: The Royal Marsden experience. Proc Am Soc Clin Oncol. 2004;23:708. [Google Scholar]
- 21.Haqq C, Nosrati M, Sudilovsky D, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci U S A. 2005;102:6092–7. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Angelini S, Czene K, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003;9:3362–8. [PubMed] [Google Scholar]
- 23.Kramer BW, Gotz R, Rapp UR. Use of mitogenic cascade blockers for treatment of C-Raf induced lung adenoma in vivo: CI-1040 strongly reduces growth and improves lung structure. BMC Cancer. 2004;4:24. doi: 10.1186/1471-2407-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8:219–25. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 25.Bollag G, Freeman S, Lyons JF, Post LE. Raf pathway inhibitors in oncology. Curr Opin Investig Drugs. 2003;4:1436–41. [PubMed] [Google Scholar]
- 26.Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002;25:511–8. doi: 10.1159/000068621. [DOI] [PubMed] [Google Scholar]
- 27.Webb CP, Van Aelst L, Wigler MH, Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci U S A. 1998;95:8773–8. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giehl K. Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386:193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- 29.Miller CJ, Cheung M, Sharma A, et al. Method of mutation analysis may contribute to discrepancies in reports of BRAF mutation frequencies in melanocytic neoplasms. J Invest Dermatol. 2004;123:990–2. doi: 10.1111/j.0022-202X.2004.23468.x. [DOI] [PubMed] [Google Scholar]
- 30.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 31.Slattery MJ, Dong C. Neutrophils influence melanoma adhesion and migration under flow conditions. Int J Cancer. 2003;106:713–22. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2004;288:831–9. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–58. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 34.Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–90. [PubMed] [Google Scholar]
- 35.Horiuchi H, Kawamata H, Fujimori T, Kuroda Y. A MEK inhibitor (U0126) prolongs survival in nude mice bearing human gallbladder cancer cells with K-ras mutation: analysis in a novel orthotopic inoculation model. Int J Oncol. 2003;23:957–63. [PubMed] [Google Scholar]
- 36.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 37.Bankston D, Dumas J, Natero R, Riedl B, Monahan M-K, Sibley R. A scaleable synthesis of BAY 43-9006: a potent Raf kinase inhibitor for the treatment of cancer. Organic Process Res Dev. 2002;6:777–81. [Google Scholar]
- 38.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–202. [PubMed] [Google Scholar]
- 39.Wellbrock C, Ogilvie L, Hedley D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–42. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 40.Dong C, Slattery MJ, Rank BM, You J. In vitro characterization and micromechanics of tumor cell chemotactic protrusion, locomotion, and extravasation. Ann Biomed Eng. 2002;30:344–55. doi: 10.1114/1.1468889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoeflich KP, Gray DC, Eby MT, et al. Oncogenic BRAF is required for tumor growth and maintenance in melanoma models. Cancer Res. 2006;66:999–1006. doi: 10.1158/0008-5472.CAN-05-2720. [DOI] [PubMed] [Google Scholar]
- 42.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 43.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 44.Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol. 2003;121:1160–2. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- 45.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collisson EA, De A, Suzuki H, Gambhir SS, Kolodney MS. Treatment of metastatic melanoma with an orally available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res. 2003;63:5669–73. [PubMed] [Google Scholar]
- 47.Lowinger TB, Riedl B, Dumas J, Smith RA. Design and discovery of small molecules targeting raf-1 kinase. Curr Pharm Des. 2002;8:2269–78. doi: 10.2174/1381612023393125. [DOI] [PubMed] [Google Scholar]
- 48.Schoffski P, Dumez H, Clement P, et al. Emerging role of tyrosine kinase inhibitors in the treatment of advanced renal cell cancer: a review. Ann Oncol. doi: 10.1093/annonc/mdj133. Epub 2006 Jan 17. [DOI] [PubMed] [Google Scholar]
- 49.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc) 2005;41:773–84. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 50.Kranenburg O, Gebbink MF, Voest EE. Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta. 2004;1654:23–37. doi: 10.1016/j.bbcan.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29:3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- 52.Dong C, Slattery M, Liang S. Micromechanics of tumor cell adhesion and migration under dynamic flow conditions. Front Biosci. 2005;10:379–84. doi: 10.2741/1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang S, Slattery MJ, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp Cell Res. 2005;310:282–92. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]