Abstract
Aims/hypothesis
The pyruvate-malate shuttle is a metabolic cycle in pancreatic beta-cells and important for beta-cell function. Cytosolic malic enzyme (ME) carries out an essential step in the shuttle by converting malate to pyruvate and generating NADPH. In rat islets the pyruvate-malate shuttle may regulate insulin secretion and it has been shown to play a critical role in adaptation to obesity and insulin resistance. However, ME has not been demonstrated in mouse islets and three reports indicates that mouse islets contain no ME activity. If mouse islets lack ME then rat and mouse islets must regulate insulin secretion by different mechanisms.
Methods
We measured ME activity by a flourometric enzymatic assay and ME mRNA by real-time-PCR. ME activity was also measured in STZ-treated mouse islets, FACS purified beta-cells that were from MIP-GFP mouse islets, Agouti-L obese mouse islets and mouse beta-cell line MIN-6. Insulin secretion and NADPH/NADP+ ratios were measured in ME siRNA treated beta-cells.
Results
ME activity and mRNA were present in C57BL/6 mouse islets. ME activity was reduced in STZ-treated mouse islets. ME activity in FACS purified mouse beta-cells was also measurable. In addition, ME activity was significantly increased in obese Agouti-L mouse islets and mouse MIN-6 cell line. ME siRNA inhibited ME activity and reduced glucose stimulated insulin secretion and also inhibited NADPH products.
Conclusions/interpretation
Our results demonstrate that mouse islets contain ME and that ME plays a significant role in regulating insulin secretion.
Keywords: Malic enzyme, insulin secretion, pyruvate-malate shuttle, islet of Langerhans, MIN-6 cell
Introduction
Cytosolic malic enzyme (ME or ME1, EC1.1.1.40) is a key component in the pyruvate-malate shuttle (1;2) which is a major source of cytoplasmic NADPH. In this shuttle, pyruvate is converted to oxaloacetate by pyruvate carboxylase within the mitochondria. Malate dehydrogenase converts the oxaloacetate to malate, which is released into the cytosol and converted back to pyruvate by ME. The conversion of malate to pyruvate by ME produces one molecule of NADPH. In pancreatic beta-cells the pyruvate-malate shuttle produces significantly more NADPH than the pentose phosphate shuttle (1). NADPH-producing enzymes are essential to all living organisms because they play a key role in biosynthetic processes and in regulating cellular redox status (3). Specifically, NADPH is pivotal in lipid and fatty acids synthesis (4;5), cellular replication and inhibiting apoptosis (6–8). In addition, oxaloacetate, an intermediate in the pyruvate-malate shuttle, can be converted to aspartate, a precursor for protein synthesis (9). In the pancreatic beta-cell, an additional important role of NADPH may be regulation of glucose stimulated insulin secretion (GSIS) (1;10;11). Therefore, in the beta-cell the pyruvate-malate shuttle plays a role in metabolism, insulin secretion and cell proliferation. This has been demonstrated in rat islets (1;12–17).
ME is also part of an active pyruvate-citrate shuttle, and this cycle may also regulate GSIS (18;19). In this cycle, pyruvate enters into the Krebs cycle via pyruvate carboxylase and then oxaloacetate (20). The latter is converted to citrate that accumulates within the mitochondria. Mitochondrial citrate is then exported into the cytoplasm by the tricarboxylate carrier in exchange with malate. In the cytosol, ATP-citrate lyase cleaves citrate into acetyl-CoA and oxaloacetate. The latter is converted to malate by malate dehydrogenase. Malate is then converted back into pyruvate by ME, and produces NADPH.
Previous studies (21;22) reported that mouse islets, unlike rat islets do not possess ME activity. This observation was supported by another report that showed ME activity in mouse islets was below the level of detection (23). This would be critical because it would imply that mouse and rat beta-cells use different mechanisms to regulate insulin secretion. Since we have been studying pyruvate carboxylase and the pyruvate-malate shuttle in mouse beta-cells for some time, it was of interest to re-evaluate whether ME is present in mouse islets. Thus, in this study, we measured ME activity and ME mRNA in C57BL/6 mouse islets. We also measured ME activity in islets with or without in vitro streptozotocin (STZ) treatment, in islets of obese Agouti-L mice (AyL, C57BL/6 background) and in purified beta-cells from C57BL/6 mouse islets. All of these results indicated that ME is present in mouse beta-cells, albeit it at lower levels than in rat islets. Secondly, using small interfering RNA (siRNA) to inhibit ME activity we showed that ME modulates NADPH production and GSIS in dispersed beta-cells from C57BL/6 mouse islets and in the mouse beta-cell line, MIN6.
Research design and methods
Animals
The principles of animal laboratory care under the guidelines of NIH, the University of Louisville and The Research Institute in New Orleans Children’s Hospital’s Animal Care Committee were strictly followed. Male C57BL/6 mice, AyL mice (all from Jackson Laboratory, Bar Harbor, ME) and Sprague-Dawley (SD) rats (Taconic, Germantown, NY) from 9 to 15 weeks of age were used for this research. The animals received normal chow, and were housed in the Animal Care Facility at the University of Louisville and The Research Institute in New Orleans Children’s Hospital and maintained at 25 °C with a 12-h light/dark cycle. MIP-GFP C57BL/6 mice, carrying a transgene for green fluorescent protein regulated by the mouse insulin promoter, were provided by Dr. Manami Hara (24) at the University of Chicago and were housed in the Joslin Diabetes Center.
Islet isolation and separation of islets into single cells
Islets were isolated from rats and mice by an adaptation of the Gotoh method (25): pancreas duct infiltration with collagenase, Histopaque gradient separation and hand selection. For separation of islets into single cells, mouse islets were digested with 0.25% trypsin for 5 min; incompletely digested islets were separated into single cells by passing through a 200 µl pipette tip. Islet cells were cultured at 37°C in humidified air and 5% CO2 in RPMI 1640 media containing 5.5 mmol/l glucose, 10% newborn calf serum, 2 mmol/l glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin (all from Gibco, Grand Island, NY).
In vitro STZ treatment
STZ was prepared 1 minute before use by dissolving in 0.1 mol/l sodium citrate (pH 4.5) at 4°C at concentration of 200 mmol/l. Ten microliters of this solution were added to the islets to obtain 1 mmol/l STZ. Islets were then incubated overnight (18 h).
Islet DNA, protein and insulin content assay, and insulin protein determination
Islet DNA content was measured by the Labarca method (26) and protein content using a commercial kit (Bio-Rad, Hercules, CA) with BSA as standard. Insulin protein content in STZ-treated islets was determined by Western blot using insulin antibody.
MIN6 cell culture
Mouse insulinoma cell line, MIN6 cells, were provided by Dr. Sabire Ozcan (University of Kentucky) at passage 24–30. They were cultured as described previously (27) in DMEM containing 4.5 g/L glucose, 70 µmol/l beta mercaptoethanol, 1% penicillin/streptomycin and 15% FBS at 37°C in a humidified atmosphere with 5% CO2. The cells were subcultured by digestion with 0.25% trypsin at 37 °C for 5 min, followed by centrifugation. The medium was changed every 3 days.
Insulin secretion and insulin assay
Insulin secretion was performed by a previously described method (14). Insulin secreted into KRB buffer was measured using an ELISA insulin assay kit.
Purification of GFP expressing mouse beta-cells
Mouse islet isolation from 6–8 weeks old MIP-GFP transgenic mice and beta-cell purification were performed at the Joslin Diabetes Center. Islets were isolated using a modified method of Gotoh et al (25). The hand-picked islets were dispersed to single cells as previously described (28). In brief, the islets were washed with PBS and suspended in a trypsin (Sigma) and DNAse 1 (Roche) solution then incubated for 15 minutes at 37 °C. The islets were gently vortexed at 5-minute intervals, washed at the end of the digestion and then filtered through a 40-µm filter for sorting. Single cells were sorted for GFP expression on a Mo-Flo (Dako Cytomation) facs sorter. The green insulin producing cells were collected and washed with PBS then lysed in 30 µl homogenization buffer (see ME activity assay section) by sonication (Sonic Dismembrator, Fisher Sci) and frozen. The frozen extracts were shipped overnight to our laboratory in Louisville for enzyme activity assay. Intact islets were homogenized, frozen and shipped similarly to the purified beta-cells, for measurement of ME activity.
ME activity assay
Islets, beta-cells or liver tissues were washed 3 times with PBS then homogenized in buffer containing 10 mmol/l HEPES, pH 7.4, 250 mmol/l sucrose, 2.5 mmol/l EDTA, 2 mmol/l cysteine and 0.02% BSA (wt/vol). To compare the effects of different homogenization buffers on ME activity, rat or mouse islets were also homogenized in a buffer containing 220 mM mannitol, 70 mmol/l sucrose, 5 mmol/l potassium HEPES buffer, pH 7.5 and 1 mmol/l dithiothreitol (DTT), as previously described (21). The homogenate was centrifuged at 10,000×g for 15 minutes and the supernatant was collected for ME activity and protein assays. All buffers prior to enzyme assay were kept cold on ice. Cell extract (20–40 µg protein) or NADPH standard (1–100 nmol) were added to 1 ml of reaction buffer (50 mmol/l Tris/HCl, pH 7.8, 4 mmol/l MgCl2, 0.1 mmol/l NADP+ and 1 mmol/l malate). The change in fluorescence from 1 min to 10 min was measured at room temperature at excitation 340 nm and emission 420 nm using a Hitachi F-2500 Fluorescence Spectrophotometer. Actual sample values were obtained by subtracting blank values (without cell extracts) from the sample values (with cell extracts). ME activity in C57BL/6 mouse liver and SD rat islet extracts were measured for positive controls; partial extracts were heated at 100 °C for 10 min for negative control.
Total RNA extraction from liver tissues and cultured islets, qPCR and real-time-PCR
A small piece of tissue (about 10–30 mg) was cut from right leaf of the liver and sliced. These sliced liver tissues were put into a 1.5 ml RNase free pellet pestle/homogenizing tube (Kontes Glass Company), and 600 µl RLT lysis buffer was added. The liver tissues were homogenized by thoroughly grinding and centrifuged at 15,000 ×g for 3 min. Islets were washed with PBS (Mg++ and Ca++ free), digested with 0.25% trypsin (Invitrogen) for 5--15 min, then ice cold RPMI 1640 (containing 10% newborn calf serum) was added to stop the digestion. The cells and liver tissues were transferred to a 10 ml sterile RNase-free tube and were washed and centrifuged 3 times at 300×g at 4°C for 4 min. Total cellular RNA was isolated from islets and liver tissues using an RNeasy Mini kit (Qiagen) according to protocol and RNA concentration was determined using a RiboGreen RNA Quantization kit (Molecular Probes). cDNA synthesis was carried out using Brilliant qPCR kit (Stratagene) according to the manufacturers protocol. Universal PCR Master Mix (Applied Biosystems) was diluted according to the manufacturer’s procedure for qPCR (29). The final concentration of primer was 0.9 µmol/l and the probe was 0.25 µmol/l. Relative copy numbers were calculated using the Pfaffl method (30). The sequences designed for detecting ME gene expression by real-time-PCR in mouse beta-cells are shown in the electronic supplementary material (ESM) Table 1.
ME siRNA transfection. PC siRNA transfection
PC siRNA transfection was carried out according to Stealth RNA transfection protocol (Invitrogen), and the cells were cultured for 48 hours. The sequences designed for inhibiting ME gene expression in mouse beta-cells are shown in the electronic supplementary material (ESM) Table 1.
NADPH and NADP+ measurement
NADPH or NADP+ contents in the extract were measured by a modified method (Xu et al., Diabetologia, 2008, in press) based on a cycling method described by Lower and Passonneau (31).
Data presentation and statistical methods
All data are expressed as mean ± S.E.M. The listed n values represent the number of a single experiment performed and each experiment was duplicated. Comparisons between two groups were performed by Student’s t test. Comparisons between multiple groups were performed by one- or two-way ANOVA (Tukey post hoc test). A value of p<0.05 was considered significant.
Results
ME activity and mRNA in mouse islets
Initially ME activity was measured in C57BL/6 mouse liver and SD rat islet to serve as positive controls (Figure 1). ME activity in C57BL/6 mouse liver was about 120 nmol/min/mg protein and activity in SD rat islets was just under 80 nmol/min/mg protein. In C57BL/6 mouse islets, ME activity was about 35 nmol/min/mg protein (29% of liver ME activity); demonstrating that ME activity was detectable in mouse islets. Mouse islet ME activity was significantly reduced by DTT, but rat islet ME was not. The previous report (21) that ME was not detectable in mouse islets may be in part due to the inclusion of DTT in the homogenization buffer used in that study.
Figure 1.
ME activity was measurable in C57BL/6 mouse islets. ME activity levels in C57BL/6 mouse liver and rat islet were used as positive controls. Data are mean ± S.E.M, n=4. −DTT indicates standard homogenization buffer which contained no DTT; +DTT indicates that the homogenization buffer contained 1 mmol/l DTT. NS means not significant.
To make sure measured ME activity was real enzyme activity, we performed several blank and negative control measurements. As shown in Table 1, we could not measure ME activity if islet extract, NADP+ or malate were not added to the reaction buffer. Rat islet and mouse liver extracts were used for positive controls. As a negative control, we denatured islet or liver extract by heating them at 100 °C for 10 minutes. No enzyme activity could be measured in these denatured extracts. These control assays indicates that ME activity can be measured in our assay system.
Table 1.
Various assay conditions for measuring ME activity. The components and concentrations of NADP+ and malate in the reaction solution are described in the Method section. Data are mean ± S.E.M. n=5
| Assay conditions | ME activity, nmol/min/mg protein | |||
|---|---|---|---|---|
| Tissue extract* | NADP+ | Malate | Heating | |
| No extract | + | + | − | 0 |
| Mouse islet | − | − | − | 0 |
| Mouse islet | + | − | − | 0 |
| Mouse islet | − | + | − | 0 |
| Mouse islet | + | + | − | 28.2±6.4 |
| Mouse islet | + | + | + | 0 |
| Rat islet | + | − | − | 0 |
| Rat islet | − | + | − | 0 |
| Rat islet | + | + | − | 82.4±21.6 |
| Rat islet | + | + | + | 0 |
| Mouse liver | + | + | − | 115.6±32.8 |
| Mouse liver | + | + | + | 0 |
40 µg of mouse islet protein and 20 µg of rat islet and mouse liver protein were used for an individual enzyme activity assay.
Heating means the tissue extracts were heated in 100 °C for 10 minutes before enzyme activity assay.
means with; − means without.
To further support our findings of ME activity in mouse islets, we measured ME mRNA in mouse islets by Taqman real-time PCR. As shown in Table 2, ME mRNA was expressed in C57BL/6 mouse islets, with a Ct about 5 cycles greater that the Ct for actin mRNA. As a positive control, C57BL/6 mouse liver was found to contain higher levels of ME mRNA, with a Ct 3 cycles after actin. Based on the calculation, ME mRNA expression level in the islets was 22% of liver ME mRNA. This percentage of ME mRNA level in the islets comparing to liver is similar to those in enzyme activity level (29%, Figure 1).
Table 2.
Relative quantitation of ME mRNA expression in mouse liver and islet using a comparative Ct method [Applied Biosystems, User Bulletin #2, December 11, 1997 (updated 10/2001) (http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_040980.pdf)]. ME mRNA expression levels in C56BL/6 mouse islet and liver were determined by real-time PCR. ME mRNA expression levels in liver were used as a positive control, ME mRNA expression levels in mouse islets were compared with those in liver and presented as a relative value. n=4.
| Tissues | ME average Ct | Actin average Ct | ΔCt ME-actin a | ΔΔCt ΔCt-ΔCt, liverb | ME Relative to liverc |
|---|---|---|---|---|---|
| Liver | 17.41±0.39 | 14.32±0.23 | 3.09±0.45 | 0.00±0.45 | 1.01 (0.73–1.37) |
| Islet | 20.75±0.28 | 15.50±0.04 | 5.25±0.28 | 2.16±0.28 | 0.22 (0.18–0.27) |
The ΔCt value is determined by subtracting the average actin Ct value from the average ME Ct value. The standard deviation of the difference is calculated from the standard deviations of the ME and actin values.
The calculation of ΔΔCt involves subtraction by the ΔCt calibrator value. This is subtraction of an arbitrary constant, so the standard deviation of ΔΔCt is the same as the standard deviation of the ΔCt value.
The range given for ME relative to brain is determined by evaluating the expression: 2−ΔCT with ΔCt + s and ΔΔCt − s, where s = the standard deviation of the ΔΔCt value.
ME activity in STZ-treated C57BL/6 mouse islets
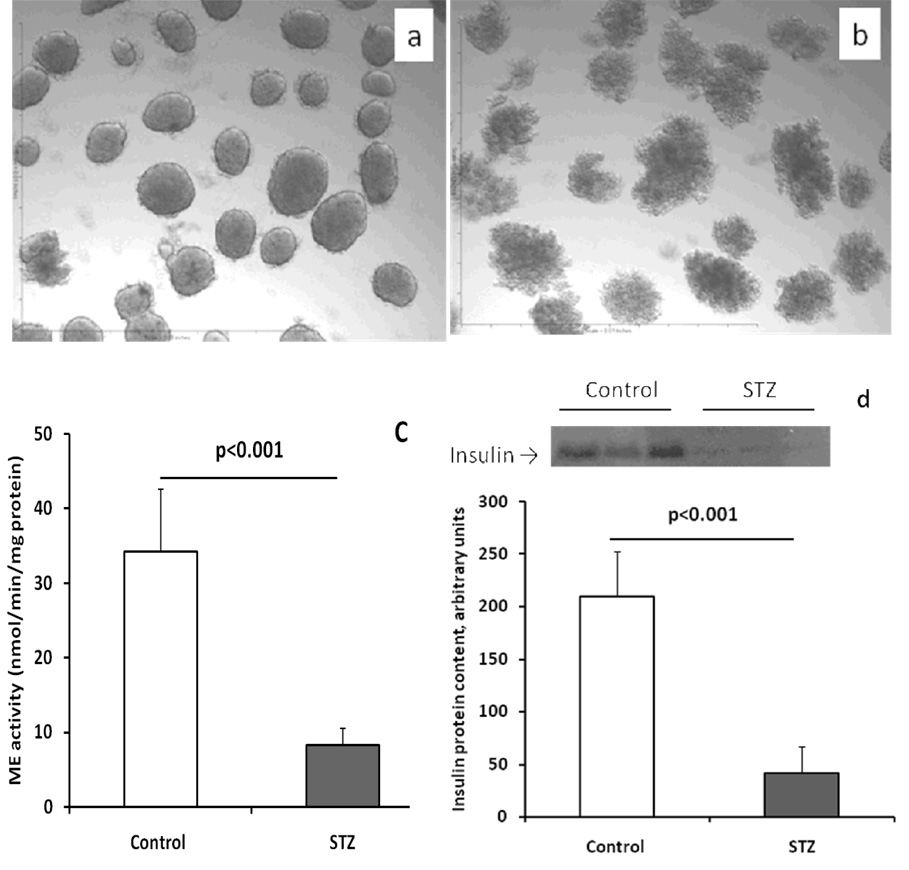
Since STZ specifically destroys beta-cells (32), it was used in the following experiment: Isolated C57BL/6 mouse islets were treated with 1 mmol/l STZ for 18 hours (Figures 2b), followed by measurement of islet protein, DNA and ME activity. Protein and DNA contents were reduced to about 22% and 39% of controls, respectively, in STZ-treated islets; suggesting that most beta-cells were destroyed. As shown in Figure 2c, the ME activity in STZ treated islets was reduced to 24% of control; mostly likely due to the destruction of many beta-cells in the islets. The loss of beta-cells is demonstrated in Figure 2d, insulin contents detected by Western blot in STZ-treated islets were significantly reduced (20% of control).
Figure 2.
STZ treatment (1 mmol/l for 18 hrs) destroyed most beta-cells and significantly reduced ME activity. a, a representative micrograph of control islets; b, a representative micrograph of STZ treated islets; c, islet ME activity; and d, insulin protein levels in the islets detected by Western blot: a picture on the top shows the result of Western blot [each lane in control or STZ treated group in this picture was loaded with islet (30 islets per lane) protein extract obtained from one mouse], graph shows the data of protein band quantification. The data in graphs show the mean ± S.E.M. In c, n=4; in d, n=3.
ME activity in purified mouse islet beta-cells
In order to exclude all non-beta-cells from the ME assays, we purified primary beta-cells from the islets of MIP-GFP transgenic mice, which express GFP only in beta-cells, by FACS sorting. As shown in Figure 3, ME activity was measurable (about 9 nmol/min/mg protein) in pure C57BL/6 mouse beta-cells, thus demonstrating mouse beta-cells possess ME activity. ME activity in pure beta-cell was about 30% of whole islet ME activity (about 28 nmol/min/mg protein); suggesting that ME activity is lost during the purification process.
Figure 3.
ME activity was detectable in primary beta-cells purified by FACS sorting from C57BL/6 beta-cell GFP transgenic islets. A portion of the whole islets was saved prior to beta-cell purification for use as positive control. Data are mean ± S.E.M. n=3.
ME activity in obese mouse islets and MIN-6 cells
We measured islet ME activity in 15-week old obese AyL mice. These mice have enlarged beta-cell mass in response to insulin resistance of obesity but are not diabetic (33). As shown in Figure 4a, islet ME activity in AyL was increased more than 2 fold. ME is known to express in mouse MIN-6 cells (21). To know whether ME activity is increased in MIN-6 cells comparing to primary mouse islets, we measured ME activity in MIN-6 cells and C57BL/6 mouse islets. As shown in Figure 4b, ME activity was 2.5-fold increased in MIN-6 cells comparing to C57BL/6 mouse islets; indicating ME was up-regulated by transformation.
Figure 4.
ME activity was increased in the islets isolated from 15 weeks old obese Agouti-L mice (a) and in MIN-6 cells (b). AyL indicates Agouti-L obese mouse. Data are mean ± S.E.M. n=6.
ME siRNA treatment reduced ME activity and GSIS but not KCl stimulated insulin secretion in beta-cells
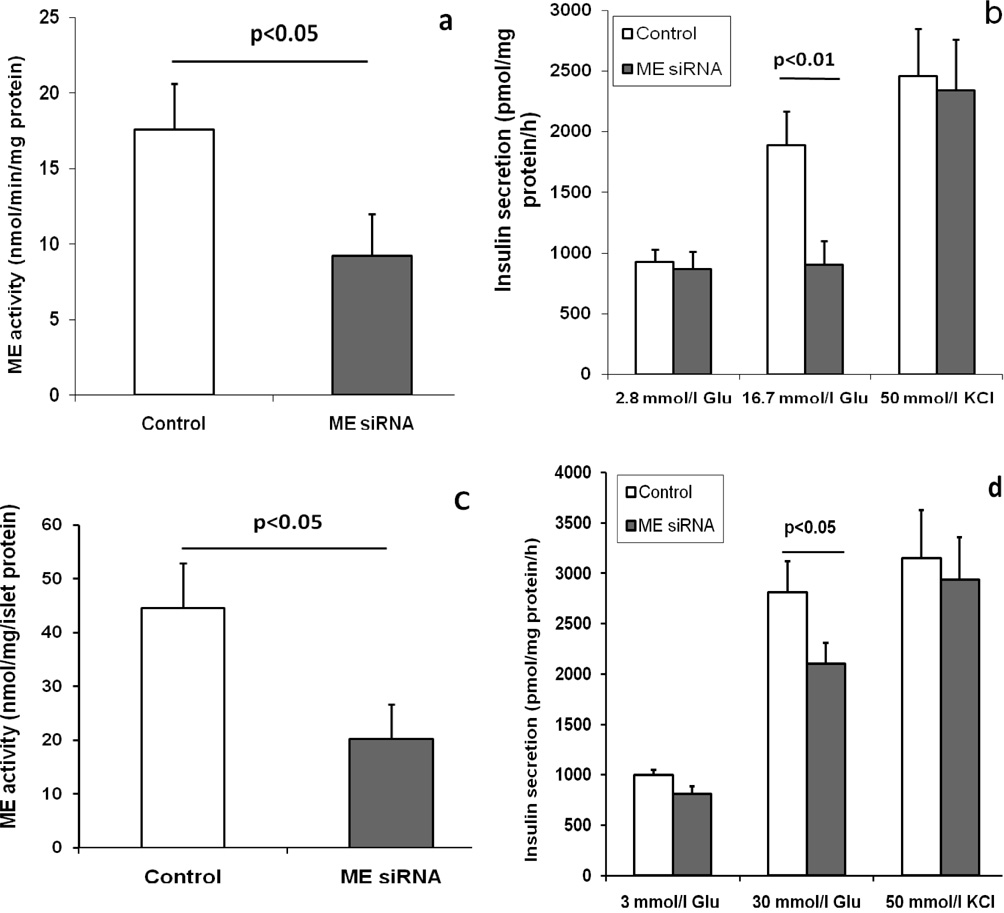
ME is a key component of the pyruvate-malate shuttle and its product NADPH may be involved in regulation of insulin secretion. To determine if ME activity is involved in regulation of GSIS, we treated dispersed single cells obtained from C57BL/6 mouse islets and the mouse beta-cell line, MIN6, with ME siRNA to reduce ME activity. Two days following siRNA treatment, ME activity was reduced by about 50% in both cell types (Figures 5a and c). Since there is no commercially available antibody for ME, we did not determine ME protein levels in the siRNA-treated cells. As shown in Figures 5b and d, GSIS was significantly reduced in both cell types; supporting a role for ME in determining the rate of insulin secretion. However, ME siRNA could not block KCl stimulated insulin secretion in the beta-cells (Figures 5b and d); suggesting KCl and ME regulated insulin secretion by different mechanisms.
Figure 5.
ME siRNA treatment significantly reduced ME activity (a and c) and GSIS (b and d) in both dispersed single cells of C57BL/6 mouse islets (a and b) and MIN6 cells (c and d), but could not reduce KCl stimulated insulin secretion (b and d) in these cells. Control cells were treated with scrambled RNA sequences (see ESM Table 1). Glu means glucose. Data are mean ± S.E.M. n=4.
NADPH/NADP+ ratios in beta-cells were elevated in response to high glucose and this elevation was suppressed by ME siRNA
In beta-cells, pyruvate-malate shuttle plays a predominant role to produce NADPH that may regulate insulin secretion (1); therefore, we measured NADPH/NADP+ ratios in dispersed C57BL/6 mouse islet single cells and MIN6 cells treated with or without ME siRNA. As shown in Figure 6, high glucose elevated NADPH/NADP+ ratios in the cells without siRNA treatment, and ME siRNA partially but significantly reduced this elevation; supporting ME produces NADPH and contributes to the regulation of GSIS.
Figure 6.
NADPH/NADP+ ratios were elevated in response to high glucose in both dispersed single cells of C57BL/6 mouse islets and MIN-6 cells, this elevation was significantly suppressed by ME siRNA treatment. After 2-day siRNA treatment, the cells were incubated in culture medium with different concentrations of glucose shown in the figure for 1h, and then the cells were used for NADPH and NADP+ assays. White and gray color columns mean the cells were treated with scrambled siRNA. Data are mean ± S.E.M. n=5.
Discussion
We have obtained several lines of evidence to support the presence and functional importance of ME in mouse islets: 1), measurable ME activity and ME mRNA were detected in C57BL/6 mouse islets; 2), ME activity was reduced in STZ treated C57BL/6 mouse islets; 3), ME activity can be measured in FACS purified C57BL/6 mouse beta-cells; 4), elevated ME activity was observed in the islets isolated from obese AyL mice and in MIN-6 cells; 5), ME siRNA significantly reduced ME activity, NADPH products and GSIS in dispersed single cells of C57BL/6 mouse islets and MIN6 cells. In addition, our results are supported by a very recent report from Matschinsky’s laboratory (34); the authors have demonstrated that 1) mouse islets express ME mRNA, and 2) cytosolic ME is involved in pyruvate-malate shuttle activity.
MacDonald previously reported (21) that ME is not present in mouse islets. In our assay we used a more sensitive flourometric assay of ME and we used a different homogenization buffer than MacDonald; his buffer included 1 mmol/l DTT and ours did not. Perhaps our homogenization buffer helps preserve ME activity in mouse islets. We tested and found that 1 mmol/l DTT reduced ME activity in mouse islets by about two fold, but this level of DTT did not affect activity in rat islets (Figure 1); suggesting that mouse islet ME might be particularly sensitive to DTT. Even in the absence of DTT, mouse islet ME activity was 60% lower than rat islet ME activity. It is possible that the previous study (21) did not demonstrate mouse islet ME activity because it is very low and it is inhibited by DTT. We have tested MacDonald’s assay condition, and found that much more mouse islets were needed for a single assay to measure ME activity using a spectrometer (data not shown). In another report (23), the authors used a sensitive fluorimeter and showed that ME activity in mouse islets was below the level of detection. We have also tested their assay conditions (23), our result showed that 20 µg of islet protein was not enough for a single assay to measure ME activity (data not shown).
In detecting ME activity in mouse islets, a concern is contamination from exocrine cells that contain almost tenfold higher ME activity than mouse islets (data not shown). Thus it would require 10% contamination with acinar cells to obtain the level of ME activity we found in mouse islets. As described in the Methods section, islet went through 4 washes including one hand-picked wash. This produces over a billion-fold dilution of any extracellular enzyme. We also carefully counted cell contamination under the microscope in our final preparation and this was less than 50 contaminating cells (not stained for amylase) in each batch of 40 islets. This would produce at most a 0.125% level of contamination, which is far too low to account for the ME activity we found in mouse islets.
Detection of ME mRNA in islets provides further evidence consistent with the presence of ME enzyme in islets. In addition, ME activity was significantly reduced five-fold in STZ-treated mouse islets (Figure 2c); this was consistent with the remained beta-cells in STZ-treated mouse islets (Figure 2d). Since STZ primarily damages beta-cells this suggests that islet ME activity declined after STZ treatment because it was present in beta-cells. Further evidence was obtained by the detection of ME activity in FACS purified MIP-GFP mouse beta-cells (Figure 3) that excluded all non-beta-cells. However, the activity in the purified beta-cells was significantly lower than that in whole islets. Two possible explanations for this result are: 1) Most islet ME activity is present in non-beta-cells; 2) ME may be sensitive to the purification procedures and this could reduce ME activity.
To test if ME has a role in GSIS we used siRNA to inhibit ME activity in two beta-cell types, dispersed single cells of C57BL/6 mouse islets and mouse beta-cell line MIN-6 cells. We then measured GSIS in the presence of low and high glucose. Measurable ME activity in MIN-6 cells has previously been reported (21;35). We found that in MIN-6 cells and dispersed C57BL/6 beta-cells, ME siRNA significantly reduced both ME activity and GSIS (Figure 5). However, ME siRNA could not reduce KCl induced insulin secretion because KCl stimulates insulin secretion by regulating Ca2+ flux (36;37). The mechanism of reduced GSIS in these cells following siRNA inhibition of ME is uncertain but could be due to a decrease in flux through the pyruvate-malate shuttle with a resultant decline in NADPH production (2;38). Our data shown in Figure 6 supports this hypothesis; ME siRNA significantly reduced NADPH/NADP+ ratios in beta-cells. Most recently, Pongratz et al (39) showed that ME is important to insulin secretion in rat INS-1 cells. However this finding would be less important if ME was neither present nor important to insulin secretion in mouse beta-cells. Our results not only show that ME is present in mouse islets but they also demonstrate that ME participates in the regulation of GSIS.
The pyruvate–malate and pyruvate–citrate shuttles are part of anaplerosis and cataplerosis (40;41). Anaplerosis is initiated by PC, which produces oxaloacetate. In the pyruvate-citrate shuttle, citrate is converted to oxaloacetate and accumulates within the mitochondria (42) or it can be exported into the cytoplasm. In the cytosol, citrate is converted to acetyl-CoA and oxaloacetate. The latter is converted to malate by malate dehydrogenase. Malate is then converted back to pyruvate by ME, and NADPH is produced in this reaction. Cytosolic citrate may be an essential component of a pyruvate-citrate shuttle, generating cytosolic acetyl-CoA, NAD+, and NADPH (41). Schuit et al. (42) have reported that citrate and malate are accumulated in beta-cells under stimulation of high glucose, and this accumulation is associated with insulin release. On the other hand, mitochondrial citrate may be the precursor of cataplerotic signaling molecules such as malonyl-CoA that are implicated in the K+ATP-channel-independent pathway of insulin secretion (43;44). In addition, the entry of glutamine into the cycle (one of anaplerotic pathways) is also known to regulate insulin secretion (45;46), and glutamine metabolism may be involved in the pyruvate–malate shuttle (34). Because ME is located in anaplerotic/cataplerotic pathways, it may play a role in balancing these pathways.
ME activity level in mouse primary islets is lower comparing to those in rat islets, and our results are consistent with previous observation (47); this might be the reason why ME activity could not be measured by a routine method that was used to measure rat islet ME activity (21–23), and why methyl succinate does increase insulin secretion in rat islets but not in mouse islets (21). In addition, it has been reported that blood glucose and insulin levels are normal in the Mod-1 mice which lack cytoplasmic ME in all tissues (48;49); and this phenomenon argues against a critical role for the pyruvate-malate shuttle in insulin secretion. This might suggest that the roles of ME are limited in mouse primary beta-cells in normal physiological condition. This phenomenon forced us to reconsider the roles of ME in mouse islets. Low level of islet ME might be allowable and not affect energy metabolism or not be important in normal mice. However, ME may play important roles in some pathophysiological conditions. For examples, ME might be important for beta-cell adaptation to insulin resistance in obese mice. As shown in Figure 4A, ME activity was more than 2 fold increased in AyL obese and insulin resistant mice. ME perhaps plays important roles in enhancing beta-cell adaptation to insulin resistance, secreting more insulin to form hyperinsulinemia during obesity. If there is no ME in beta-cells, obese mice might more easily develop type 2 diabetes. We have shown that the pyruvate-malate shuttle plays an important role in insulin secretion (2;38) and beta-cell adaptation (13;15), and mouse islet ME has recently been reported to be involved in pyruvate-malate shuttle activity (34).
In summary, we have demonstrated that mouse islets possess ME activity and that it helps regulate insulin secretion. Increased ME activity in the islets of obese mice might play important roles in beta-cell adaptation to insulin resistance.
Supplementary Material
Acknowledgement
This work was supported by grants to YQL from the National Institutes of Health (P20 RR/DE17702 from the COBRE Program of the National Center for Research Resources, and 1R01 DK077624-01), the American Diabetes Association (Junior Faculty Award). This project was also supported by a grant (6931) from The Research Institute for Children, Children’s Hospital at New Orleans. Authors thank Dr. Sabire Ozcan in the University of Kentucky for providing MIN6 cells, and Dr. Manami Hara in the University of Chicago for the beta-cell GFP transgenic mice. Authors also thank Dr. Michael MacDonald in the University of Wisconsin Medical School for the communication on the ME activity assay.
Abbreviations
- ME
Cytosolic malic enzyme
- NADPH
Nicotinamide adenine dinucleotide phosphate
- siRNA
small interfering RNA
- AyL
Agouti-L obese mice (C57BL/6 background)
- STZ
streptozotocin
Footnotes
The preliminary data were presented at the 67th ADA Scientific Sessions Annual Meeting, 2007, Chicago, IL.
Duality of interest:
The authors declare that there is no duality of interest associated with this manuscript.
Reference List
- 1.Macdonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 2.Lu D, Mulder H, Zhao P, et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc.Natl.Acad.Sci.U.S.A. 2002;99:2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa Rosa LF, Curi R, Murphy C, Newsholme P. Effect of adrenaline and phorbol myristate acetate or bacterial lipopolysaccharide on stimulation of pathways of macrophage glucose, glutamine and O2 metabolism. Evidence for cyclic AMP-dependent protein kinase mediated inhibition of glucose-6-phosphate dehydrogenase and activation of NADP+-dependent 'malic' enzyme. Biochem.J. 1995;310(Pt 2):709–714. doi: 10.1042/bj3100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infante JP, Huszagh VA. Analysis of the putative role of 24-carbon polyunsaturated fatty acids in the biosynthesis of docosapentaenoic (22:5n-6) and docosahexaenoic (22:6n-3) acids. FEBS Lett. 1998;431:1–6. doi: 10.1016/s0014-5793(98)00720-0. [DOI] [PubMed] [Google Scholar]
- 5.Dmitriev LF. Activity of key enzymes in microsomal and mitochondrial membranes depends on the redox reactions involving lipid radicals. Membr.Cell Biol. 2001;14:649–662. [PubMed] [Google Scholar]
- 6.Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic.Biol.Med. 2002;32:1185–1196. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 7.Sheline CT, Choi DW. Neuronal death in cultured murine cortical cells is induced by inhibition of GAPDH and triosephosphate isomerase. Neurobiol.Dis. 1998;5:47–54. doi: 10.1006/nbdi.1998.0177. [DOI] [PubMed] [Google Scholar]
- 8.Brune B, Dimmeler S, Lapetina EG. NADPH: a stimulatory cofactor for nitric oxide-induced ADP-ribosylation reaction. Biochem.Biophys.Res.Commun. 1992;182:1166–1171. doi: 10.1016/0006-291x(92)91854-j. [DOI] [PubMed] [Google Scholar]
- 9.Menendez J, Delgado J, Gancedo C. Isolation of the Pichia pastoris PYC1 gene encoding pyruvate carboxylase and identification of a suppressor of the pyc phenotype. Yeast. 1998;14:647–654. doi: 10.1002/(SICI)1097-0061(199805)14:7<647::AID-YEA269>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald MJ. Export of metabolites from pancreatic islet mitochondria as a means to study anaplerosis in insulin secretion. Metabolism. 2003;52:993–998. doi: 10.1016/s0026-0495(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 11.Ivarsson R, Quintens R, Dejonghe S, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald MJ, Kaysen JH, Moran SM, Pomije CE. Pyruvate dehydrogenase and pyruvate carboxylase. Sites of pretranslational regulation by glucose of glucose-induced insulin release in pancreatic islets. J Biol Chem. 1991;266:22392–22397. [PubMed] [Google Scholar]
- 13.Liu YQ, Jetton TL, Leahy JL. beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J.Biol.Chem. 2002;277:39163–39168. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- 14.Liu YQ, Moibi JA, Leahy JL. Chronic high glucose lowers pyruvate dehydrogenase activity in islets through enhanced production of long chain acyl-CoA: prevention of impaired glucose oxidation by enhanced pyruvate recycling through the malate-pyruvate shuttle. J.Biol.Chem. 2004;279:7470–7475. doi: 10.1074/jbc.M307921200. [DOI] [PubMed] [Google Scholar]
- 15.Liu YQ, Han J, Epstein PN, Long YS. Enhanced rat beta-cell proliferation in 60% pancreatectomized islets by increased glucose metabolic flux through pyruvate carboxylase pathway. Am.J.Physiol Endocrinol.Metab. 2005;288:E471–E478. doi: 10.1152/ajpendo.00427.2004. [DOI] [PubMed] [Google Scholar]
- 16.Ronnebaum SM, Ilkayeva O, Burgess SC, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J.Biol.Chem. 2006;281:30593–30602. doi: 10.1074/jbc.M511908200. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MV, Joseph JW, Ilkayeva O, et al. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J.Biol.Chem. 2006;281:22342–22351. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am.J Physiol Endocrinol.Metab. 2005;288:E1–E15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 19.Guay C, Madiraju SR, Aumais A, Joly E, Prentki M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol.Chem. 2007;282:35657–35665. doi: 10.1074/jbc.M707294200. [DOI] [PubMed] [Google Scholar]
- 20.Khan A, Ling ZC, Landau BR. Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem. 1996;271:2539–2542. doi: 10.1074/jbc.271.5.2539. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald MJ. Differences between mouse and rat pancreatic islets: succinate responsiveness, malic enzyme, and anaplerosis. Am.J.Physiol Endocrinol.Metab. 2002;283:E302–E310. doi: 10.1152/ajpendo.00041.2002. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald MJ, Marshall LK. Survey of normal appearing mouse strain which lacks malic enzyme and Nad+-linked glycerol phosphate dehydrogenase: normal pancreatic beta cell function, but abnormal metabolite pattern in skeletal muscle. Mol Cell Biochem. 2001;220:117–125. doi: 10.1023/a:1010821821921. [DOI] [PubMed] [Google Scholar]
- 23.Heart E, Yaney GC, Corkey RF, et al. Ca2+, NAD(P)H and membrane potential changes in pancreatic beta-cells by methyl succinate: comparison with glucose. Biochem J. 2007;403:197–205. doi: 10.1042/BJ20061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara M, Wang X, Kawamura T, et al. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am.J.Physiol Endocrinol.Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 25.Gotoh M, Maki T, Satomi S, et al. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43:725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal.Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 27.Mosley AL, Ozcan S. The pancreatic duodenal homeobox-1 protein (Pdx-1) interacts with histone deacetylases Hdac-1 and Hdac-2 on low levels of glucose. J.Biol.Chem. 2004;279:54241–54247. doi: 10.1074/jbc.M410379200. [DOI] [PubMed] [Google Scholar]
- 28.Schuppin GT, Bonner-Weir S, Montana E, Kaiser N, Weir GC. Replication of adult pancreatic-beta cells cultured on bovine corneal endothelial cell extracellular matrix. Vitro Cell Dev.Biol.Anim. 1993;29A:339–344. doi: 10.1007/BF02633963. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Han J, Epstein PN, Liu YQ. Regulation of PDK mRNA by high fatty acid and glucose in pancreatic islets. Biochem.Biophys.Res.Commun. 2006;344:827–833. doi: 10.1016/j.bbrc.2006.03.211. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowry OH, Passonneau JV. Enzymatic Cycling. In: Lowry OH, Passonneau JV, editors. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. pp. 129–145. [Google Scholar]
- 32.Patel YC, Weir GC. Increased somatostatin content of islets from streptozotocin-diabetic rats. Clin.Endocrinol.(Oxf) 1976;5:191–194. doi: 10.1111/j.1365-2265.1976.tb02832.x. [DOI] [PubMed] [Google Scholar]
- 33.Soeller WC, Janson J, Hart SE, et al. Islet amyloid-associated diabetes in obese A(vy)/a mice expressing human islet amyloid polypeptide. Diabetes. 1998;47:743–750. doi: 10.2337/diabetes.47.5.743. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Nissim I, Chen P, et al. Elimination of KATP Channels in Mouse Islets Results in Elevated [U-13C]Glucose Metabolism, Glutaminolysis, and Pyruvate Cycling but a Decreased {gamma}-Aminobutyric Acid Shunt. J Biol.Chem. 2008;283:17238–17249. doi: 10.1074/jbc.M709235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iizuka K, Nakajima H, Namba M, et al. Metabolic consequence of long-term exposure of pancreatic beta cells to free fatty acid with special reference to glucose insensitivity. Biochim.Biophys.Acta. 2002;1586:23–31. doi: 10.1016/s0925-4439(01)00082-5. [DOI] [PubMed] [Google Scholar]
- 36.Eto K, Suga S, Wakui M, et al. NADH shuttle system regulates K(ATP) channel-dependent pathway and steps distal to cytosolic Ca(2+) concentration elevation in glucose-induced insulin secretion. J Biol.Chem. 1999;274:25386–25392. doi: 10.1074/jbc.274.36.25386. [DOI] [PubMed] [Google Scholar]
- 37.Min L, Leung YM, Tomas A, et al. Dynamin is functionally coupled to insulin granule exocytosis. J Biol.Chem. 2007;282:33530–33536. doi: 10.1074/jbc.M703402200. [DOI] [PubMed] [Google Scholar]
- 38.Cline GW, LePine RL, Papas KK, Kibbey RG, Shulman GI. 13 C-NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J.Biol.Chem. 2004;279:44370–44375. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- 39.Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J.Biol.Chem. 2007;282:200–207. doi: 10.1074/jbc.M602954200. [DOI] [PubMed] [Google Scholar]
- 40.Flamez D, Berger V, Kruhoffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2024. doi: 10.2337/diabetes.51.7.2018. [DOI] [PubMed] [Google Scholar]
- 41.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta- cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 42.Schuit F, De Vos A, Farfari S, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J.Biol.Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 43.Maechler P, Kennedy ED, Pozzan T, Wollheim CB. Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic beta-cells. EMBO J. 1997;16:3833–3841. doi: 10.1093/emboj/16.13.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wollheim CB, Maechler P. Beta-cell mitochondria and insulin secretion: messenger role of nucleotides and metabolites. Diabetes. 2002;51 Suppl 1:S37–S42. doi: 10.2337/diabetes.51.2007.s37. [DOI] [PubMed] [Google Scholar]
- 45.Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc.Trans. 2007;35:1180–1186. doi: 10.1042/BST0351180. [DOI] [PubMed] [Google Scholar]
- 46.Corless M, Kiely A, McClenaghan NH, Flatt PR, Newsholme P. Glutamine regulates expression of key transcription factor, signal transduction, metabolic gene, and protein expression in a clonal pancreatic beta-cell line. J Endocrinol. 2006;190:719–727. doi: 10.1677/joe.1.06892. [DOI] [PubMed] [Google Scholar]
- 47.Ashcroft SJ, Randle PJ. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem.J. 1970;119:5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CY, Lee SM, Lewis S, Johnson FM. Identification and biochemical analysis of mouse mutants deficient in cytoplasmic malic enzyme. Biochemistry. 1980;19:5098–5103. doi: 10.1021/bi00563a025. [DOI] [PubMed] [Google Scholar]
- 49.Macdonald MJ, Marshall LK. Survey of normal appearing mouse strain which lacks malic enzyme and Nad+-linked glycerol phosphate dehydrogenase: normal pancreatic beta cell function, but abnormal metabolite pattern in skeletal muscle. Mol.Cell Biochem. 2001;220:117–125. doi: 10.1023/a:1010821821921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.