Abstract
African Americans have lower serum 25-hydroxyvitamin D concentrations and a lower risk of fragility fractures than do other populations. I review the evidence on factors other than vitamin D that might explain this paradox and the calcium economy in different life stages. Researchers are actively trying to explain this genetically programmed advantage. Factors that could protect African Americans against fracture include their higher peak bone mass, increased obesity rates, greater muscle mass, lower bone turnover rates, and advantageous femur geometry. In addition, bone histomorphometry in young adults shows longer periods of bone formation. Although African Americans fall as frequently as do whites, the direction of their falls and their manner of breaking falls could protect them from fractures. African American girls accrue more calcium than do white girls during adolescence as the result of increased calcium absorption and superior renal calcium conservation. In adulthood, higher parathyroid hormone concentrations do not result in increased bone loss in African Americans because of their skeletal resistance to parathyroid hormone, and their superior renal conservation of calcium persists. These advantages diminish in the elderly, in whom further increases in parathyroid hormone result in increased bone turnover and bone loss. Ultimately, I explain the paradox by multiple factors associated with fracture risk and calcium economy in African Americans. Despite African Americans’ reduced risk of osteoporotic fractures, such fractures remain an important public health problem for this population that vitamin D intervention studies have not addressed.
INTRODUCTION
Physiologic experiments on the role of vitamin D in calcium absorption and the prevention of secondary hyperparathyroidism and the relation of serum 25-hydroxyvitamin D [25(OH)D] to bone density have provided evidence suggesting that vitamin D plays a role in preventing fragility fractures (1, 2). Clinical studies, including randomized clinical trials, have confirmed that increasing vitamin D intake prevents falls and fractures (3–5). Thus, it seems paradoxical that African Americans have fewer fragility fractures than do other ethnic groups despite having lower concentrations of serum 25(OH)D. I conjecture that examining this apparent paradox could lead to insights concerning the role of vitamin D in preventing osteoporosis and explaining the lower risk of osteoporosis in African Americans. In this article, I explore the factors that could explain this paradox and review the calcium economy in each life segment in African Americans.
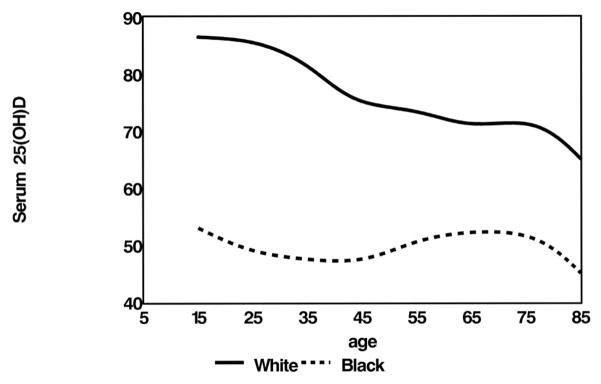
The distribution of serum 25(OH)D concentrations by age in African Americans and whites from the third National Health and Nutrition Examination Survey (NHANES III) is shown in Figure 1. At all ages, African Americans have lower serum 25(OH)D concentrations than do whites. Clearly, factors other than vitamin D must provide protection against osteoporotic fractures in African Americans.
FIGURE 1.
Serum 25-hydroxyvitamin D [25(OH)D] distribution in all African American and white participants in the third National Health and Nutrition Examination Survey (with sampling weights), by age.
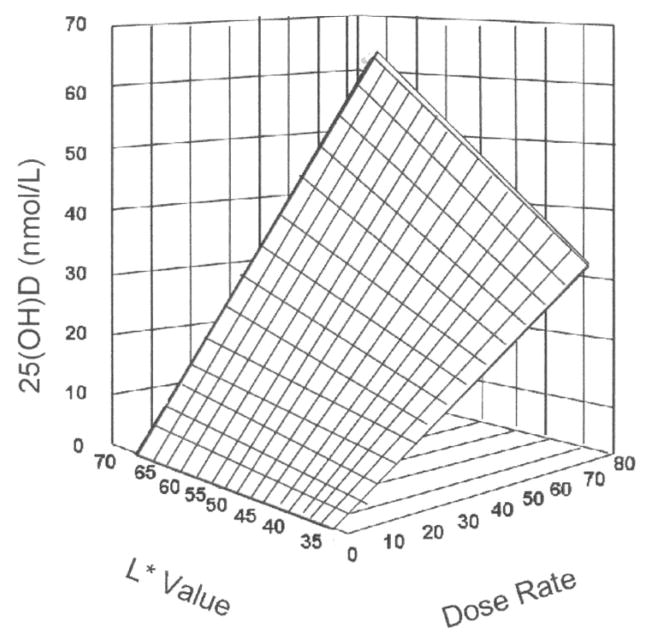
One explanation for the lower 25(OH)D concentration in African Americans is that the increased melanin pigmentation interferes with the absorption of ultraviolet B light and the formation of vitamin D in the skin. Armas et al (6) recently quantified skin pigmentation by using a reflective meter to measure its impact on ultraviolet B light absorption. In this study of 25(OH)D response to ultraviolet B radiation and skin color, the investigators used an L value, with 0 representing perfectly black and 100 representing perfectly white (Figure 2). They exposed 90% of the study participants’ skin to ultraviolet B radiation 3 times per week for 4 wk. As shown in Figure 2, the serum 25(OH)D response was proportional to the amount of ultraviolet exposure and skin pigmentation.
FIGURE 2.
Three-dimensional scatter plot of 4-wk serum 25-hydroxyvitamin D [25(OH)D] response change above baseline expressed as a function of both basic skin lightness (L*) and ultraviolet-B dose rate. The surface is a hyperboloid, plotting equation 1, and was fitted to the data by least-squares regression methods. Reproduced with permission from Elsevier (6).
African Americans have a lower risk of hip fracture, as shown in the National Osteoporosis Risk Assessment (NORA) study. In this prospective study of 197 848 postmenopausal women, the 7784 black participants had one-half the prevalence of osteoporosis and one-half the fracture risk of whites (7). Hence, the paradox exists with low serum 25(OH)D concentrations and lower fracture risk.
A definition of osteoporosis is reduced bone strength, which leads to an increased risk of fragility fractures, although many fragility fractures occur in persons who do not have osteoporosis. Even so, we examine the factors that influence the risk of osteoporotic fractures to identify the factors that might protect the African American population from such fractures. The factors discussed in this article are listed in Table 1.
TABLE 1.
Factors that influence osteoporotic fractures
| Bone mass |
| Muscle mass and obesity |
| Remodeling rate |
| Microarchitecture |
| Geometry |
| Neuromuscular instability and falls |
FACTORS THAT INFLUENCE OSTEOPOROTIC FRACTURE RISK
Bone mass
Eighty percent of a bone’s strength is related to its mass or density. However, factors other than bone mass can influence fracture risk. These factors include bone architecture and geometry, the bone remodeling rate, heritable factors, and propensity to fall.
Bone mass in African Americans is higher than in other populations. Studies at Brookhaven National Laboratory used radiographic absorptiometry and in vivo neutron activation analysis to show that bone mass in African American adults is ≈10–15% higher than in other populations throughout life (8, 9). More recent studies using dual-energy X-ray absorptiometry have found similar results, and a study of trabecular bone sites showed an even higher advantage in African Americans (10–18).
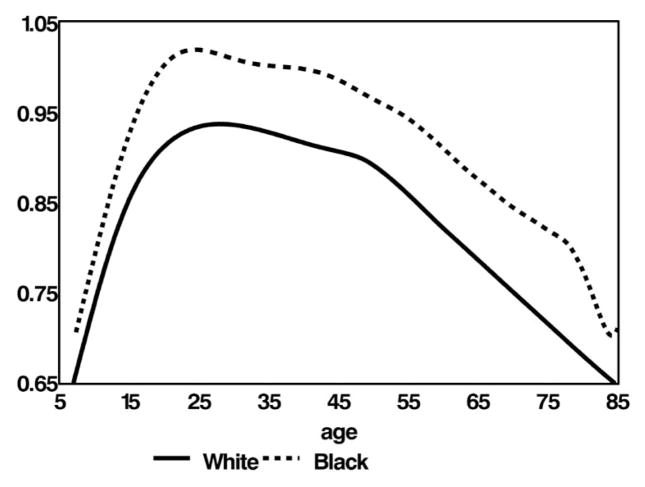
In Figure 3, we combined data from a longitudinal study of bone mineral density of the femur in 154 children aged 6–16 y with data on adults from the NHANES III study (19). This figure shows that the bone density advantage in African Americans begins in early childhood. This advantage was present at 6 y of age and increased during adolescence, a stage when the skeleton accrues 50% of its peak bone mass (19). The pattern was similar in the 2 races, with bone loss beginning at about the same age, accelerating during menopause in women, and continuing to decline with aging. The data suggest that bone loss after the age of 75 y is more rapid in African Americans than in whites.
FIGURE 3.
Total femur bone mineral density (BMD) in African American and white females, by age, in the BMD Child Study and the third National Health and Nutrition Examination Survey. Reproduced with permission from the Journal of Clinical Endocrinology and Metabolism (19). Copyright 2007, the Endocrine Society.
In the Study of Osteoporotic Fractures, a large longitudinal study of fracture risk in a diverse population (20), African Americans had a lower risk of fracture than did whites, even when they had the same bone density. Thus, factors in addition to bone density appear to protect African Americans from fractures.
Heredity
Epidemiologic studies have shown that a maternal history of fragility fractures increases the risk of fracture (21). Furthermore, genetic factors are apparently responsible for skeletal accretion during childhood. Therefore, several studies have attempted to identify the differences between black and white genotypes to illuminate the development of peak bone mass and bone loss and support the development of interventions to maximize bone mass (22–28).
Environmental factors influence osteoporosis, which is a polygenic disorder (25). The search for relevant polymorphic differences between African Americans and whites has yielded both positive and negative results (22, 24, 26, 27). Most genetic studies have concentrated on the vitamin D receptor gene. Although an early study of only 101 African American women showed no ethnic differences in the black-white genotype distribution for bone mineral density (28), a more recent study used admixture marking in African Americans to separate their African and European heritage in the search for population-specific allele frequencies (23). This appears to be a useful approach for identifying the genetic contribution to the skeletal variables of interest.
Because of the similarity between mouse and human genotypes, studies in mice might prove fruitful in understanding differences between African Americans and whites (25). For example, Edderkaoui et al (29) recently identified the mouse Duffy antigen receptor for chemokines (Darc, which increases osteoclast formation) as a bone mineral density quantitative trait locus site gene. Duffy-negative individuals are African American, so this gene could influence the rate of bone turnover in adulthood.
Body composition
Obesity protects people from bone density decreases and fractures. The many explanations given for this include that greater body weight might mechanically stimulate bone formation, resulting in a higher bone density. Another possible explanation is that high estrogen levels, which are typical in obese women, might protect obese women from bone loss. Finally, hip padding as a result of fat accumulation could protect people from fractures when they fall.
Like bone density, obesity has a paradoxical relation with vitamin D in that people who are more obese tend to have lower 25(OH)D concentrations. Obesity is more prevalent in African Americans than in whites, and this could explain their additional protection against fracture. In addition, like bone mass, muscle mass is higher in African Americans, and muscle mass in healthy populations has a strong relation to bone mass (8, 30–32). Cross-sectional data indicate that African Americans experience less sarcopenia than do whites with aging, although not all studies have shown this (33, 34).
The NORA study researchers were unable to explain the skeletal mass advantage of African Americans by increased weight (7). In a body-composition study that matched whites with African Americans by body size, the researchers concluded that body size did not explain the higher skeletal mass in African Americans (8). The Health, Aging and Body Composition study examined the relation of lean mass and fat mass to bone mineral content in 2619 persons (35) and could not explain the difference in bone densitometry by height, weight, lean, or fat mass.
Bone turnover
Many studies have identified high bone turnover as a fracture risk independent of bone density. Several of these studies have shown lower levels of bone turnover markers in adult African Americans than in whites (9, 11). An early study by Weinstein and Bell (36) suggested that bone turnover could be lower in African Americans. The bone turnover markers have been of bone resorption (C-terminal telopeptide of type I collagen, N-telopeptide of collagen type 1, hydroxyproline) and bone formation (osteocalcin, alkaline phosphatase). However, the Study of Women’s Health Across the Nation, which examined the health of perimenopausal women, found no difference in bone turnover markers in African Americans and whites (37). Similarly, studies have not shown any differences in bone turnover markers in African American and white children (38). Bone turnover increases in elderly compared with adult African Americans (39, 40). These differences in results at different life stages demonstrate the importance of considering life stage when one examines differences between ethnic groups. Bone turnover might be lower in African Americans than in whites in adulthood but not in other life segments. The sample size of studies in childhood is too small to be conclusive.
Bone histomorphometry
The microarchitecture of bone can have a substantial influence on fracture risk, but only a few studies have compared bone histomorphometry between African Americans and whites. Two studies compared bone histomorphometry in South African black and white men and women aged 21–84 y (41, 42). These studies suggested that African Americans have more trabecular and cortical bone and thicker trabecular and cortical bone with less porous cortices than do whites. The studies also suggested that the age-related rate of decline in bone mass is the same in whites and African Americans and that bone turnover might actually increase rather than decrease with age.
A subsequent study in adult white and African American women in the United States provided a potential explanation for the lower rate of bone loss in adult African Americans (43). In this study, the bone formation period was longer in African American women, and white women had more inactive periods in the life span of bone formation units. The longer period of bone formation in African Americans results in greater overall deposition of bone mineral and could produce better bone quality than that in whites. A recent histomorphometric analysis suggested that the reason for the longer bone formation period in African Americans might be diminished osteoblast apoptosis (44, 45).
Bone geometry
The shape of bone could influence its propensity to fracture. Research has identified hip axis length as a characteristic of femur shape that could influence fracture risk (46). Shorter hip axis length protects against osteoporotic fractures. African American adult women have a shorter hip axis length than do whites (47, 48). Recent studies have used densitometry to structurally analyze the hip. A study comparing African American and other postmenopausal women suggested that the spatial distribution of bone in the femoral neck in African Americans is arranged in such a way as to resist greater loading (10).
Falls
Some data are available from the Study of Osteoporotic Fractures concerning falls in African Americans and whites (49). This was a longitudinal study of 6007 white and 482 African American women with a mean age >70 y. If African Americans had a lower fall rate, this would partially explain why they have fewer fractures. However, the Study of Osteoporotic Fractures found no significant difference in the rate of falling between African Americans and whites; indeed, the rate was higher (but not significantly) in whites. However, whites tended to fall laterally rather than forward, which resulted in a greater risk of hip fracture. In addition to tending to fall forward, African Americans tended to break their falls with their wrists, which would also protect them from hip fracture.
CALCIUM ECONOMY
Calcium economy in adolescents and adults
One can glean insight into the higher peak bone mass in African Americans from studies in adolescents because people accrue one-half their bone mass during adolescence. In a study on calcium balance and kinetics in African American and white adolescent girls (38), African Americans had greater calcium retention and a higher bone formation rate. This cohort was unusual in that the African American girls did not have lower serum 25(OH)D concentrations than did the white girls. The African American girls also had higher calcium absorption efficiency, probably because of their higher concentrations of serum 1,25-dihydroxyvitamin D [1,25(OH)2D]. These girls also had lower urinary calcium excretion, indicating superior renal conservation. Bone turnover markers were not significantly different, but the study was not sufficiently powered to detect a difference.
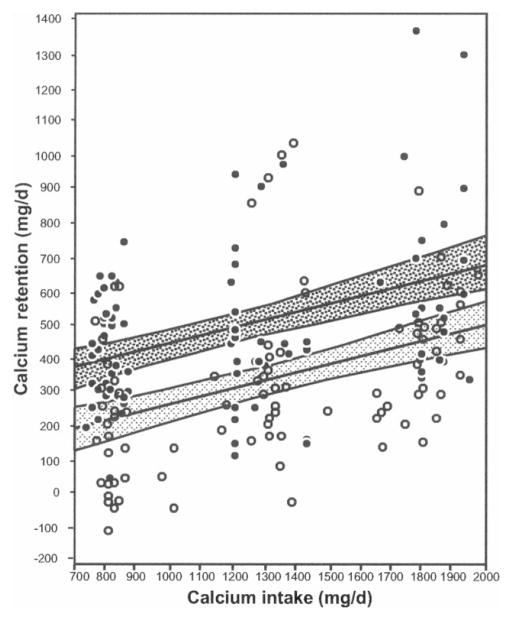
The results of a subsequent 3-wk balance study in 55 African American and 66 white girls by the same investigators are shown in Figure 4 (50). As Figure 4 shows, the African American girls retained more calcium at every calcium intake. The retention curves are parallel, with African American girls retaining 185 ± 32 mg/d more calcium than did the white girls. Calcium retention generally has a threshold above which calcium retention no longer increases with calcium intake. Clearly, intakes of up to 2000 mg/d did not reach that threshold in the African American girls. Thus, the authors concluded that calcium requirements are the same in adolescent girls of both races. We need further studies to determine whether even higher calcium intakes are beneficial to African American girls.
FIGURE 4.
Mean calcium retention and 95% CIs for regression lines across different calcium intakes, by race. The darker shading represents African American girls (●, 84 observations in 55 girls) and the lighter shading represents white girls (○, 98 observations for 66 girls). Reproduced with permission from the American Journal of Clinical Nutrition (50). Copyright 2007, the American Society for Nutrition.
African American adults have lower concentrations of bone turnover markers than do white adults, as we have indicated earlier. In addition, they have higher parathyroid hormone (PTH) concentrations as a result of their low 25(OH)D concentrations. Serum 25(OH)D is inversely related to serum PTH concentrations. As 25(OH)D availability declines, serum 1,25(OH)2D declines, and this results in reduced calcium absorption, a transient decline in serum calcium concentration, and stimulation of PTH secretion. Despite their higher PTH concentrations, African American adults have slower bone loss and reduced concentrations of bone turnover markers, and some authors have argued that this reflects skeletal resistance to PTH (9, 11).
The lower serum 25(OH)D concentration in African American adults than in white adults could result in higher PTH concentrations, which reduce the loss of calcium through the kidneys and thereby increase protection from fractures. However, because the skeleton resists PTH, bone loss does not accelerate in times of calcium insufficiency. Studies using PTH have supported the concept of skeletal resistance to PTH in the adult life stage in African Americans (51), which highlights the importance of considering life stage when assessing differences between ethnic groups.
Vitamin D supplements in midlife
We conducted a 3-y randomized, double-blind, placebo-controlled, parallel study of the efficacy of vitamin D3 in preventing bone loss in 208 postmenopausal African American women (mean age: 60 y; 52). One-half the women received a placebo and one-half received 800 IU vitamin D3 (cholecalciferol) for 2 y, followed by 2000 IU for 1 y. These women also took dietary calcium supplements, so their calcium intake was between 1200 and 1500 mg/d. The women’s baseline serum 25(OH)D concentration was 47 nmol/L.
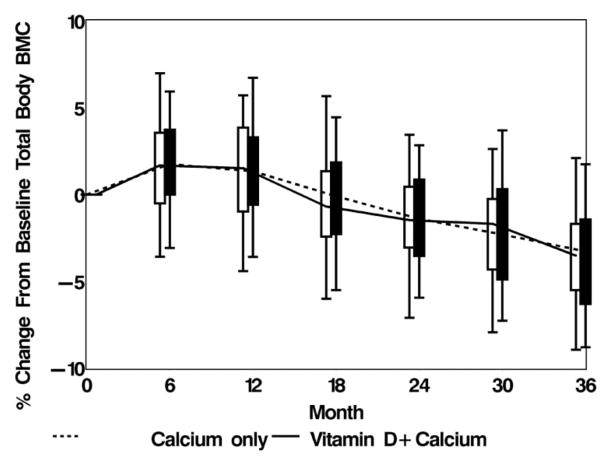
The change in total-body bone mineral content in both groups of study participants is shown in Figure 5 (7). We found no difference in bone loss rate between the women who received vitamin D3 and those in the placebo group and no relation between serum 25(OH)D concentration and bone loss rates in either group. The initial increase in bone density at 6 mo was probably due to a remodeling transient resulting from increased dietary calcium; we took this into account in the study design and analyses. In the remodeling transient phenomenon, when an intervention (such as increased dietary calcium) affects the PTH axis, bone density increases for one remodeling cycle and this change is subsequently lost. We concluded that vitamin D supplementation of up to 2000 IU/d did not prevent bone loss in these calcium-sufficient African American women in midlife. We noted no serious adverse events related to vitamin D, and the participants did not demonstrate hypercalcemia or hypercalciuria.
FIGURE 5.
Box and whisker plot of the percentage change in total-body bone mineral content (BMC) from baseline. Reproduced with permission from the Archives of Internal Medicine (52). Copyright 2005, American Medical Association. All rights reserved.
One explanation for the lack of effect of vitamin D in this study is that calcium-sufficient people need less vitamin D. Indeed, based on their superior renal conservation of calcium, adult African Americans require 300 mg less calcium per day than do whites to replace their calcium losses from the body (53). In our study, we provided calcium supplementation to ensure that both active and placebo groups were calcium replete. Unfortunately, clinical studies often neglect the calcium–vitamin D interaction. Indeed, when we reviewed the literature on the interaction between serum 25(OH)D and PTH concentrations, we found that many studies did not even report calcium intake (34). Because vitamin D enables calcium absorption, researchers should consider these 2 nutrients together. Vitamin D appears to promote bone health in midlife, and 50 nmol/L of serum 25(OH)D is sufficient in African American women, provided that their calcium intake is adequate. This could also be the case in whites, but because the study did not include whites, its findings are only applicable to African Americans.
Secondary hyperparathyroidism
Although skeletal resistance to PTH characterizes the bone loss associated with aging in adult African Americans, this might not be true in elderly African Americans. The Study of Osteoporotic Fractures found accelerated bone loss in African Americans over 75 y of age (54), and the only longitudinal study of osteoporosis in African American men, the Baltimore Men’s Osteoporosis Study, found no difference in bone remodeling or adjusted rates of loss between races in the elderly (40). However, this study did not have sufficient power to detect a difference. We have conducted studies that showed an increase in bone turnover and bone loss with aging in African Americans. These studies suggest that African Americans might become more susceptible to the adverse effects of PTH in old age and could become more susceptible to fractures than at earlier ages.
CONCLUSIONS
I have reviewed several factors that could explain why, despite their lower 25(OH)D concentrations, African Americans have a lower risk of osteoporotic fractures than do whites. This paradox might be explained, at least in part, by the higher bone mass, obesity rates, more efficient calcium economy, and lower bone turnover in African Americans than in whites. In addition, African Americans have certain bone histomorphometric and geometric features and fall patterns that could protect them from fractures.
Despite the higher bone density of African Americans, osteoporosis is a significant health problem for this population (9). Although the risk of fracture is 50% less in African Americans than in whites, fractures in African Americans are a significant problem from a public health perspective. Moreover, the elderly African American population is rapidly increasing, and as this population ages, more and more women will experience declines in bone density and their risk of fracture will increase (55, 56).
Most intervention studies on calcium and vitamin D have not included African Americans. In fact, the only intervention study that included this population was the Women’s Health Initiative, which used only 400 IU vitamin D3 (57). Intervention studies that suggested that vitamin D could improve physical performance also failed to include African Americans.
Finally, one should not misinterpret the paradox of 25(OH)D and risk of osteoporosis in African Americans to mean that this population has a lower requirement for vitamin D than do whites. African Americans might have the same or higher extraskeletal requirements for vitamin D compared with whites. We need additional research to determine whether the high incidence of hypertension, diabetes, and cancer in African Americans is associated with increased susceptibility to the effect of vitamin D insufficiency on extraskeletal disorders.
Acknowledgments
I thank Lynn Maier for preparing this typescript.
Footnotes
Presented at the National Institutes of Health conference “Vitamin D and Health in the 21st Century: an Update,” held in Bethesda, MD, September 5–6, 2007.
Supported by grant number RO1 AG15325 from the National Institute of Aging, National Institutes of Health.
The author had no personal or financial conflicts of interest.
References
- 1.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 2.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 4.Latham NK, Anderson CS, Lee A, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 6.Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588–93. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–94. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 8.Aloia JF, Vaswani A, Ma R, Flaster E. Comparison of body composition in black and white premenopausal women. J Lab Clin Med. 1997;129:294–9. doi: 10.1016/s0022-2143(97)90177-3. [DOI] [PubMed] [Google Scholar]
- 9.Aloia J, Vaswani J, Yeh J, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415–23. doi: 10.1007/BF00369203. [DOI] [PubMed] [Google Scholar]
- 10.Nelson DA, Jacobsen G, Barondess DA, Parfitt AM. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–7. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 11.Kleerekoper M, Nelson DA, Peterson EL, et al. Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55–75) postmenopausal white and black women. J Bone Miner Res. 1994;9:1267–76. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 12.Gilsanz V, Roe TF, Mora S, et al. Changes in vertebral bone density in black and white girls during childhood and puberty. N Engl J Med. 1991;325:1597–600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 13.Prentice A, Shaw J, Laskey MA, et al. Bone mineral content of British and rural Gambian women aged 18–80+ years. Bone Miner. 1991;12:210–4. doi: 10.1016/0169-6009(91)90033-v. [DOI] [PubMed] [Google Scholar]
- 14.Li JY, Specker BL, Ho ML, Tsang RC. Bone mineral content in black and white children 1 to 6 years of age. Early appearance of race and sex differences. Am J Dis Child. 1989;143:1346–9. doi: 10.1001/archpedi.1989.02150230104034. [DOI] [PubMed] [Google Scholar]
- 15.Luckey M, Meier D, Mandeli J, et al. Axial and appendicular bone density in white and black women: evidence of racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab. 1989;69:762–70. doi: 10.1210/jcem-69-4-762. [DOI] [PubMed] [Google Scholar]
- 16.Liel Y, Edwards J, Shary J, et al. The effect of race and body habitus on bone mineral density of the radius, hip, and spine in premenopuasal women. J Clin Endocrinol Metab. 1988;66:1247–50. doi: 10.1210/jcem-66-6-1247. [DOI] [PubMed] [Google Scholar]
- 17.Cohn SH, Abesamis C, Yasumura S, et al. Comparative skeletal mass and radial bone mineral content in black and white women. Metabolism. 1977;26:171–8. doi: 10.1016/0026-0495(77)90052-x. [DOI] [PubMed] [Google Scholar]
- 18.Garn S. Bone loss and aging. In: Goldman R, Rockstein ME, editors. Physiology and pathology of human aging. New York, NY: Academic Press; 1975. pp. 39–57. [Google Scholar]
- 19.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 20.Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–8. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 22.Fleet JC, Harris SS, Wood RJ, Dawson-Hughes B. The BsmI vitamin D receptor restriction fragment length polymorphism (BB) predicts low bone density in premenopausal black and white women. J Bone Miner Res. 1995;10:985–90. doi: 10.1002/jbmr.5650100621. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer JR, Kammerer CM, Reich D, et al. Genetic markers for ancestry are correlated with body composition traits in older African Americans. Osteoporos Int. 2007;18:733–41. doi: 10.1007/s00198-006-0316-6. [DOI] [PubMed] [Google Scholar]
- 24.Harris SS, Eccleshall TR, Gross C, et al. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997;12:1043–8. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- 25.Peacock M, Turner CH, Econs MJ, Foroud T. Genetics of osteoporosis. Endocr Rev. 2002;23:303–26. doi: 10.1210/edrv.23.3.0464. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DA, Vande Vord PJ, Wooley PH. Polymorphism in the vitamin D receptor gene and bone mass in African-American and white mothers and children: a preliminary report. Ann Rheum Dis. 2000;59:626–30. doi: 10.1136/ard.59.8.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zmuda JM, Cauley JA, Danielson ME, et al. Vitamin D receptor gene polymorphisms, bone turnover, and rates of bone loss in older African-American women. J Bone Miner Res. 1997;12:1446–52. doi: 10.1359/jbmr.1997.12.9.1446. [DOI] [PubMed] [Google Scholar]
- 28.Zmuda JM, Cauley JA, Danielson ME, et al. Vitamin D receptor translation initiation codon polymorphism and markers of osteoporotic risk in older African-American women. Osteoporos Int. 1999;9:214–9. doi: 10.1007/s001980050139. [DOI] [PubMed] [Google Scholar]
- 29.Edderkaoui B, Baylink DJ, Beamer WG, et al. Identification of mouse Duffy antigen receptor for chemokines (Darc) as a BMD QTL gene. Genome Res. 2007;17:577–85. doi: 10.1101/gr.6009507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohn SH, Abesamis C, Zanzi I, et al. Body elemental composition: comparison between black and white adults. Am J Physiol. 1977;232:E419–22. doi: 10.1152/ajpendo.1977.232.4.E419. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Bachrach L, Van Loan M, et al. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474–81. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 32.Aloia JF, Vaswani A, Ma R, Flaster E. Body composition in normal black women: the four-compartment model. J Clin Endocrinol Metab. 1996;81:2363–9. doi: 10.1210/jcem.81.6.8964878. [DOI] [PubMed] [Google Scholar]
- 33.Aloia JF, Vaswani A, Feuerman M, et al. Differences in skeletal and muscle mass with aging in black and white women. Am J Physiol Endocrinol Metab. 2000;278:E1153–7. doi: 10.1152/ajpendo.2000.278.6.E1153. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 35.Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein R, Bell N. Diminished rates of bone formation in normal black adults. N Engl J Med. 1988;319:1698–701. doi: 10.1056/NEJM198812293192603. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein JS, Sowers M, Greendale GA, et al. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3051–6. doi: 10.1210/jcem.87.7.8480. [DOI] [PubMed] [Google Scholar]
- 38.Bryant RJ, Wastney ME, Martin BR, et al. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88:1043–7. doi: 10.1210/jc.2002-021367. [DOI] [PubMed] [Google Scholar]
- 39.Harris SS, Soteriades E, Dawson-Hughes B. Secondary hyperparathyroidism and bone turnover in elderly blacks and whites. J Clin Endocrinol Metab. 2001;86:3801–4. doi: 10.1210/jcem.86.8.7783. [DOI] [PubMed] [Google Scholar]
- 40.Tracy JK, Meyer WA, Flores RH, et al. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–34. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 41.Schnitzler CM, Pettifor JM, Mesquita JM, et al. Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner. 1990;10:183–99. doi: 10.1016/0169-6009(90)90261-d. [DOI] [PubMed] [Google Scholar]
- 42.Schnitzler CM, Mesquita JM. Cortical bone histomorphometry of the iliac crest in normal black and white South African adults. Calcif Tissue Int. 2006;79:373–82. doi: 10.1007/s00223-006-0053-z. [DOI] [PubMed] [Google Scholar]
- 43.Parisien M, Cosman F, Morgan D, et al. Histomorphometric assessment of bone mass, structure, and remodeling: a comparison between healthy black and white premenopausal women. J Bone Miner Res. 1997;12:948–57. doi: 10.1359/jbmr.1997.12.6.948. [DOI] [PubMed] [Google Scholar]
- 44.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Differences in osteocyte and lacunar density between Black and White American women. Bone. 2006;38:130–5. doi: 10.1016/j.bone.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effect of ethnicity and age or menopause on the structure and geometry of iliac bone. J Bone Miner Res. 1996;11:1967–75. doi: 10.1002/jbmr.5650111219. [DOI] [PubMed] [Google Scholar]
- 46.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–7. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 47.Cummings SR, Cauley JA, Palermo L, et al. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4:226–9. doi: 10.1007/BF01623243. [DOI] [PubMed] [Google Scholar]
- 48.Mikhail MB, Vaswani AN, Aloia JF. Racial differences in femoral dimensions and their relation to hip fracture. Osteoporos Int. 1996;6:22–4. doi: 10.1007/BF01626533. [DOI] [PubMed] [Google Scholar]
- 49.Faulkner KA, Cauley JA, Zmuda JM, et al. Ethnic differences in the frequency and circumstances of falling in older community-dwelling women. J Am Geriatr Soc. 2005;53:1774–9. doi: 10.1111/j.1532-5415.2005.53514.x. [DOI] [PubMed] [Google Scholar]
- 50.Braun M, Palacios C, Wigertz K, et al. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr. 2007;85:1657–63. doi: 10.1093/ajcn/85.6.1657. [DOI] [PubMed] [Google Scholar]
- 51.Cosman F, Morgan DC, Nieves JW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12:958–66. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 52.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–23. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heaney RP. Ethnicity, bone status, and the calcium requirement. Nutr Res. 2002;22:153–78. [Google Scholar]
- 54.Cauley JA, Lui LY, Stone KL, et al. Longitudinal study of changes in hip bone mineral density in Caucasian and African–American women. J Am Geriatr Soc. 2005;53:183–9. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 55.Alam NM, Archer JA, Lee E. Osteoporotic fragility fractures in African Americans: under-recognized and undertreated. J Natl Med Assoc. 2004;96:1640–5. [PMC free article] [PubMed] [Google Scholar]
- 56.Wei GS, Jackson JL, Herbers JE., Jr Ethnic disparity in the treatment of women with established low bone mass. J Am Med Womens Assoc. 2003;58:173–7. [PubMed] [Google Scholar]
- 57.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]