SUMMARY
NF-κB (RelA) is constitutively active in many cancers where it up-regulates anti-apoptotic and other oncogenic genes. While proinflammatory stimulus-induced NF-κB activation involves IKK-dependent nuclear translocation, mechanisms for maintaining constitutive NF-κB activity in tumors have not been elucidated. We show here that maintenance of NF-κB activity in tumors requires Stat3 that is also frequently constitutively activated in cancer. Stat3 prolongs NF-κB nuclear retention through acetyltransferase p300-mediated RelA acetylation, thereby interfering with NF-κB nuclear export. Stat3-mediated maintenance of NF-κB activity occurs both in cancer cells and in tumor-associated hematopoietic cells. Both murine and human cancers display highly acetylated RelA, which is associated with Stat3 activity. This Stat3/NF-κB interaction is thus central to both the transformed and nontransformed elements in tumors.
INTRODUCTION
NF-κB is a central transcription factor in both innate and adaptive immunity. There has been increasing interest in NF-κB’s role in both cancer initiation (in particular, inflammation-induced carcinogenesis) as well as maintenance of established cancer, where it is frequently constitutively activated and plays a major role in the transcriptional activation of multiple anti-apoptotic and other oncogenic genes. NF-κB consists of five Rel-related proteins and the prototypical NF-κB complex is a RelA/p50 heterodimer, which is important for NF-κB-mediated anti-apoptotic effects (Karin et al., 2002). In the absence of appropriate stimuli, NF-κB is sequestered in the cytoplasm by IκBα protein. IκB kinases (IKKs) are activated upon stimulation of Toll-like receptors and intracellular sensors such as RIG-I, MDA-5 and NOD1&2 by various pathogen-associated molecular patterns (PAMPs) or proinflammatory cytokines such as TNF, leading to serine phosphorylation of IκBα and its subsequent proteasome-mediated degradation, which is critical for NF-κB nuclear translocation (Ghosh et al., 1998). An important role of IKKβ-dependent NF-κB activation involving RelA/p50 has been documented both during pathogen infection and in cancers caused by chronic inflammation and other stimuli (Naugler et al., 2007; Stancovski and Baltimore, 1997). However, with the exception of lymphoid tumors, where activating mutations of upstream IKK activators such as CARD11 have been identified (Lenz et al., 2008; Pomerantz et al., 2002), IKK is not often continuously activated in cultured cancer cells, but rather is inducible by proinflammatory stimuli. Several studies have provided evidence that secretion of cytokines and growth factors, many of which are encoded by NF-κB target genes, is critical for constitutive activation of NF-κB in cancer cells (Lu et al., 2004; Lu and Stark, 2004). Since IKK is not often constitutively activated in tumors, the question remains whether additional mechanism(s) involving signaling pathways or molecules downstream of some of these cytokines and growth factors might directly contribute to constitutive activation of NF-κB.
It has been suggested that NF-κB:IκBα can shuttle in and out of the nucleus in the absence of stimuli, although the rate of nuclear export is greater than nuclear import (Huang et al., 2000). Recent studies demonstrated that the amplitude and half-life of nuclear NF-κB can be influenced by acetylation of RelA (Chen and Greene, 2004), which requires prior RelA phosphorylation (Chen et al., 2005). In particular, endogenous RelA is acetylated in a signal-coupled manner following stimulation (Chen et al., 2001; Chen and Greene, 2004; Chen et al., 2002). The p300/CBP co-factors are acetyltransferases that mediate RelA acetylation, which is subject to deacetylation by HDACs (Chen et al., 2001). Reversible acetylation of RelA is essential for the duration of NF-κB activity, due to its role in regulating the assembly of RelA-IκBα complexes necessary for RelA nuclear export and its presence in the cytoplasm. Acetylated RelA only weakly interacts with IκBα, while deacetylation of RelA by HDAC markedly increases the binding of RelA to IκBα (Chen et al., 2001; Chen and Greene, 2004). Although RelA acetylation has been studied in the context of inflammatory stimuli, whether it has a role in constitutive NF-κB activation in cancer is unknown.
Recent research has documented the importance of cytokines and growth factors secreted by tumor cells, which often depend on IKK-mediated NF-κB activation for their production, as a causative factor in constitutive NF-κB activation in cancer cells and tumors (Greten et al., 2004; Lu et al., 2004; Lu and Stark, 2004). Some of these cytokines and growth factors, such as IL-6 and FGF, are activators of signal transducer and activator of transcription 3 (Stat3) (Deo et al., 2002; Zhong et al., 1994). Stat3 is a transcription factor that can promote oncogenesis (Bromberg et al., 1999), and it is commonly activated in cancer (Darnell, 2002; Yu and Jove, 2004), as well as tumor-associated myeloid cells (Kortylewski et al., 2005; Kujawski et al., 2008). Stat3 and NF-κB stimulate a highly overlapping repertoire of pro-survival, proliferative and pro-angiogenic genes (Catlett-Falcone et al., 1999; Darnell, 2002; Lo et al., 2005; Yu and Jove, 2004). A recent study further demonstrated that Stat3 interaction with RelA led to upregulation of the immunosuppressive IL-23/p19 gene (Kortylewski et al., 2009). Although Stat3 has been implicated in inhibiting IKK activity in normal immune cells (Welte et al., 2003), whether constitutively-activated Stat3 and RelA directly interact in both cancer cells and immune cells within the tumor microenvironment remains unknown. In the current study, we explored the possibility that constitutively-activated Stat3 maintains NF-κB activity in tumor.
RESULTS
Stat3 Is Required to Maintain Tumor NF-κB Activity
It has been shown that phospho-IκBα (p-IκBα) levels are increased in Stat3−/− DCs, suggesting that Stat3 signaling inhibits IKK activity in the context of normal immune responses (Welte et al., 2003). We confirmed that Stat3 negatively regulates IKK activity in normal immune cells by determining the ratio of p-IκBα/IκBα in splenic cells with or without Stat3. The generation of mice containing a functional deletion of Stat3 alleles in the myeloid compartment has been described previously (Lee et al., 2002). Data from these experiments showed that the p-IκBα/IκBα ratio was higher in Stat3-deficient splenocytes relative to their wild-type (WT) counterparts (Supplementary Figure 1A). Using in vitro IKK kinase assays we further showed that in tumor cells with both constitutive Stat3 and NF-κB activity, such as human A2058 melanoma and DU145 prostate cancer cell lines, blocking Stat3 by a small-molecule Stat3 inhibitor (Turkson et al., 2004) or siRNA also increased IκBα phosphorylation by IKK, which was further upregulated by TNFα (Supplementary Figure 1B).
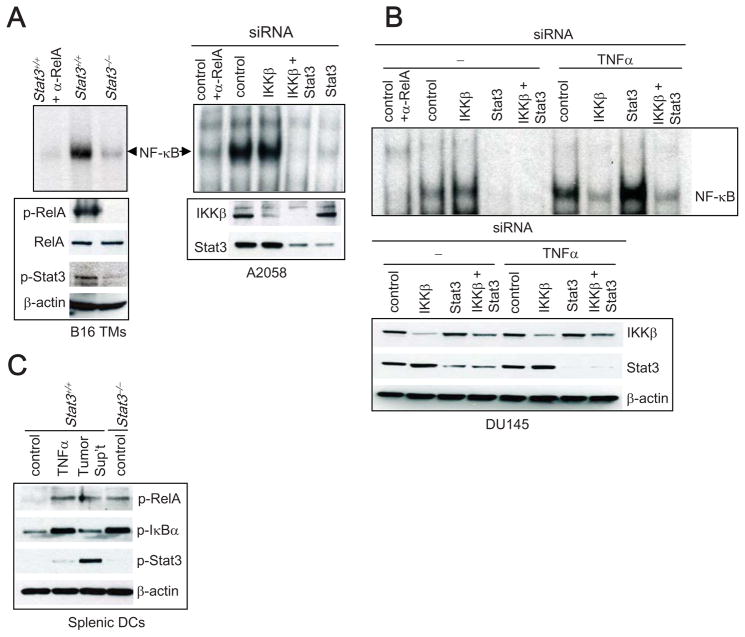
Our data, and that of others (Welte et al., 2003), suggest that Stat3 signaling has inhibitory effects on stimulus-induced IKK in both immune and tumor cells. However, these findings raise the question of how constitutively-activated Stat3 and NF-κB can co-exist in cancer cells. When we examined growing B16 melanoma tumors for NF-κB activity, we found that tumor RelA was constitutively bound to its consensus DNA sequence, as determined by EMSA (Figure 1A, left); we also observed increased RelA (Ser536) and Stat3 phosphorylation (Tyr705). B16 tumors growing in mice with Stat3−/− myeloid cells displayed reduced Stat3 activity (Figure 1A, left), likely due to the interruption of Stat3-mediated crosstalk between tumor cells and tumor stromal myeloid cells (Kortylewski et al., 2005; Kujawski et al., 2008). To our surprise, there was little constitutive RelA activity in B16 tumors when Stat3 activity was abrogated (Figure 1A, left). These observations suggested a possible requirement of Stat3 for constitutive RelA activity in tumors. The human A2058 melanoma cell line represents a typical example of a tumor with both constitutive Stat3 (Niu et al., 2002) and NF-κB activity (Figure 1A, right). Similar to what occurs in B16 tumors, when Stat3 is silenced, NF-κB (RelA) activity is greatly diminished in the tumor cells. These results obtained from both murine tumors and human tumor cells are the opposite of what would be predicted if the primary effect of Stat3 on the NF-κB pathway in tumors were inhibition of IKK, and if IKK were mainly responsible for maintaining constitutive NF-κB activity in tumor cells. In contrast to Stat3 siRNA treatment, silencing IKKβ alone did not affect nuclear NF-κB activity over a 48 h period (Figure 1A). Similarly, knocking down IKKα alone had no effect on NF-κB in tumor cells (data not shown). However, silencing both Stat3 and IKKβ with siRNA greatly diminished NF-κB activity in cancer cells (Figure 1A). While these results indicated that maintenance of existing constitutive NF-κB activity in tumors depends more on Stat3 than IKK activity, they did not rule out the need for IKK to initiate NF-κB activation by facilitating its nuclear translocation. They none-the-less reveal an important IKK-independent downstream mechanism for enhanced NF-κB activity in tumors.
Figure 1. Stat3 is Required for Maintaining Constitutive NF-κB Activity in Tumors.
(A) Stat3, but not IKKβ, is required to maintain constitutive NF-κB (RelA) activity in tumors. Left panels, DNA-binding of NF-κB was determined by EMSA using nuclear extracts prepared from B16 tumors grown in mice with Stat3+/+ and Stat3−/− myeloid cells. Phospho-RelA(S536) (p-RelA) and phospho-Stat3 (Y705) (p-Stat3) protein levels in tumors are shown by Western blot (lower panel). Right, DNA binding activity of endogenous NF-κB in A2058 tumor cells transfected with the indicated siRNAs (upper right). Supershifting with an anti-RelA antibody (αRelA) was included in the first lane to verify that the band corresponded to RelA complex. The effects of siRNA treatments are shown in the lower panel. (B) Stat3 is required for maintaining endogenous RelA activity while inhibiting IKK-induced RelA activation in DU145 cancer cells. Cells were transfected with indicated siRNAs and treated (15 min) with TNFα. NF-κB activity was determined by EMSA. (C) TNFα induces RelA phosphorylation through activating IKK/pIκBα, whereas RelA phosphorylation induced by tumor-factors is associated with Stat3 activation. Freshly isolated splenic DCs (from mice with Stat3+/+ and Stat3−/− hematopoietic system) were treated with either TNFα or medium conditioned with tumor supernatant then subjected to Western blotting with indicated antibodies.
Given our data suggesting Stat3 also inhibited IKK (Supplementary Figure 1A and B), we investigated whether the effect of Stat3 on NF-κB activity depended on NF-κB activity being driven by a proinflammatory stimulus (and is thus dependent on IKK activity) or whether it was constitutively activated, independent of immune stimuli. A2058 tumor cells display relatively high RelA activity, which is difficult to further increase by additional proinflammatory stimuli. However, DU145 prostate cancer cells have lower RelA activity (Figure 1B) and were thus used to confirm our findings that Stat3 facilitated maintenance of constitutive NF-κB activity in tumor cells, and to further test the role of Stat3 in inhibiting inflammatory signal-induced NF-κB activity. As in A2058 tumor cells, siRNA knockdown of Stat3, but not siRNA knockdown of IKKβ, resulted in the reduction of endogenous RelA activity in DU145 tumor cells (Figure 1B). The immunostimulatory cytokine TNFα can further stimulate RelA activity in DU145 cells. In contrast to the constitutive, proinflammatory signal-independent RelA activity, TNFα– induced RelA activity was diminished by IKKβ silencing while Stat3 silencing resulted in upregulation of TNFα-induced RelA activity due to abrogation of IKKβ inhibition (Figure 1B). The different effects of Stat3 on RelA activity through two distinct mechanisms were not restricted to tumor cells, as they were also observed in myeloid cells exposed to a proinflammatory stimulus vs. tumor-derived factors that activated Stat3 (Figure 1C). Both TNFα and conditioned medium with 10% tumor supernatant prepared from cultured C-4 mouse melanoma tumor cells, which have high Stat3 activity and whose secreted factors present in the supernatant have been shown to activate Stat3 effectively (Kujawski et al., 2008), activated RelA in splenic DCs, as indicated by an increase in RelA phosphorylation. However, while TNFα-induced phosphorylation of RelA was associated with an increase in P-IκBα, tumor factor-induced RelA phosphorylation was associated with Stat3 activation but not with an increase in P-IκBα (Figure 1C). Additionally, Stat3 gene functional deletion resulted in an increase in P-IκBα, consistent with published data (Welte et al., 2003) and results shown in Supplementary Figure 1A. These data suggested that Stat3 activity, which is elevated in diverse immune cells in the tumor microenvironment, might also contribute to constitutive NF-κB activation in tumor-associated immune cells.
Requirement of Stat3 for Phospho-RelA Nuclear Retention in Cancer Cells
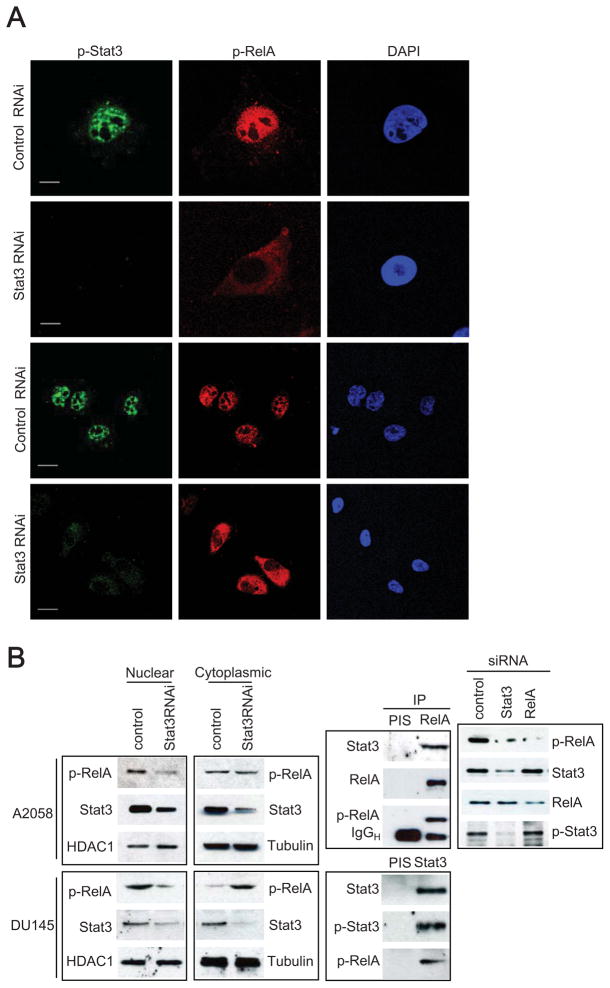
To assess how Stat3 might enhance RelA activity in cancer cells, we utilized immunofluorescent staining and confocal microscopy to detect phospho-Stat3 (p-Stat3) and phospho-RelA (p-RelA) in A2058 cancer cells transfected with control and Stat3 siRNAs. Our results showed that knocking down Stat3 expression by siRNA decreased p-RelA levels in the nucleus (Figure 2A). To confirm this, nuclear and cytoplasmic extracts were prepared from tumor cells and levels of phospho- and total Stat3 and RelA proteins were determined by Western blot. Consistent with data in Figure 2A, both A2058 and DU145 cells contained decreased nuclear p-RelA levels after Stat3 knockdown (Figure 2B, left), whereas nuclear protein HDAC1 was not affected. P-Stat3 and p-RelA also physically interacted with each other in A2058 cells, as demonstrated by immunoprecipitation with either anti-RelA (Figure 2B, middle, top) or anti-Stat3 antibody (middle, bottom). Stat3 affected nuclear p-RelA levels, but not total RelA or RelA stability (Figure 2B, right).
Figure 2. Stat3 Modulates Nuclear NF-κB Retention in Tumor Cells.
(A) Cellular localization of p-Stat3 (green) and p-RelA (red) was observed by confocal microscopy after immunofluorescent staining of A2058 cancer cells treated with Stat3 or control siRNAs. Photos with higher magnification were shown in the upper panels. Scale = 20μm. (B) Stat3-dependent p-RelA accumulation in tumor cells. Left: Western blot analyses of nuclear and cytoplasmic p-RelA in indicated cancer cells transfected with either control or Stat3 siRNAs. HDAC1 and tubulin proteins were used as nuclear and cytoplasmic controls, respectively. Middle panel: Physical interaction of p-RelA with p-Stat3 in tumors. Nuclear extracts from A2058 tumor cells were immunoprecipitated using anti-RelA antibody or anti-Stat3 or pre-immune serum (PIS) control, followed by immunoblotting analysis with specified antibodies. Right panel: Effects of knocking down Stat3 and RelA on p-RelA vs. total RelA in the nucleus.
Stat3 Facilitates Constitutive Activation of NF-κB via RelA Acetylation
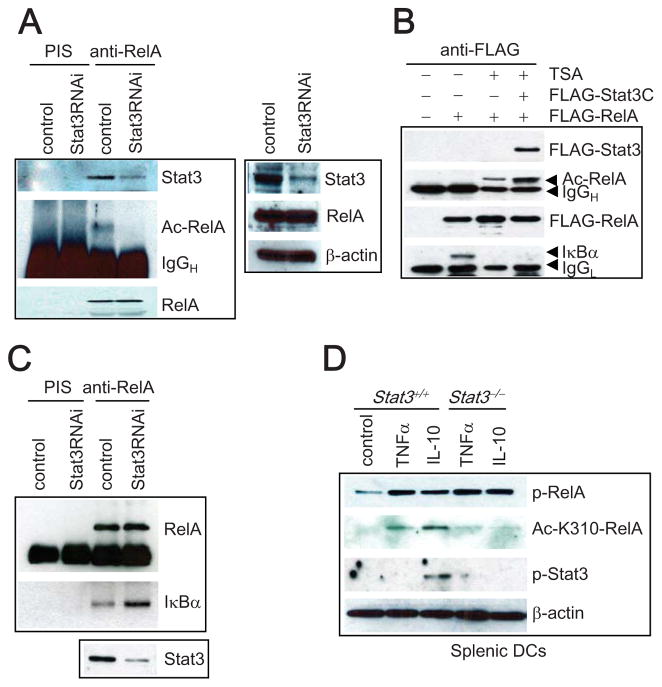
Our data led us to further explore the potential mechanism(s) that could facilitate Stat3-mediated NF-κB (p-RelA) nuclear accumulation in cancer cells. It has been shown that RelA acetylation can prolong its nuclear retention (Chen et al., 2001). Importantly, acetylation of RelA requires prior phosphorylation of serines 276 and 536 (Chen et al., 2005). We therefore tested the possibility that constitutively-activated Stat3 might mediate enhanced RelA acetylation, thereby prolonging retention of p-RelA in cancer cell nuclei. While Stat3 knockdown in A2058 cancer cells by siRNA did not affect total levels of endogenous RelA protein (Figure 3A), it reduced the relative amount of acetylated RelA (Ac-RelA) (Figure 3A). Given recent findings suggesting NF-κB p50 is an acetylated protein (Chen and Greene, 2003; Deng and Wu, 2003), we tested whether p50 acetylation was also regulated by Stat3. Compared to RelA, the p50 acetylation level was relatively low in A2058 tumor cells and was not affected by Stat3 knockdown (Supplementary Figure 2).
Figure 3. RelA Acetylation Is Mediated by Stat3.
(A) Inhibition of Stat3 decreases RelA acetylation in A2058 cells. siRNA transfected cells were immunoprecipitated with anti-RelA antibody; endogenously acetylated RelA protein (Ac-RelA) was then detected by anti-Ac lysine antibody (left panel). Stat3 inhibition by siRNA is shown in the right panel. (B) Constitutively active Stat3C enhances TSA-mediated RelA acetylation while inhibiting interaction between RelA and IκBα. BALB/c 3T3 cells were transfected with indicated expression vectors and treated (18 h) with TSA. Western blot analyses with specified antibodies are shown. (C) Stat3 activity inhibits tumor nuclear RelA’s affinity to IκBα. Nuclear RelA from A2058 tumor cells treated with control or Stat3 siRNA was allowed to interact with recombinant IκBα, followed by Western blot analysis. The effects of Stat3 siRNA are shown at the bottom. (D) Stat3 activators frequently elevated in tumor, such as IL-10, induce acetylation of RelA in primary immune cells in a Stat3-dependent manner. Splenic DCs isolated from Stat3+/+ or Stat3−/−were treated with either TNFα or IL-10, followed by Western blot analyses.
These findings prompted us to further investigate a potential role of Stat3 in regulating RelA acetylation. Trichostatin A, a selective inhibitor of multiple histone deacetylases (HDACs), has been shown to increase acetylation of RelA and inhibit its interaction with IκBα (Chen et al., 2001). Treatment of A2058 melanoma cells with TSA increased the proportion of acetylated RelA (Figure 3B). Moreover, the presence of Stat3C, a constitutively-activated Stat3 mutant (Bromberg et al., 1999), led to further enhancement of RelA acetylation accompanied by the loss of RelA-IκBα complexes, as shown by the absence of IκBα protein in RelA complexes assessed by immunoprecipitation (Figure 3B). This finding suggested that Stat3 activity inhibited RelA affinity for IκBα. To test this, nuclear RelA complexes were isolated from A2058 tumor cells transfected with either a control siRNA or Stat3 siRNA, followed by incubation with recombinant IκBα. We found that nuclear RelA from Stat3 knockdown tumor cells showed increased affinity for IκBα (Figure 3C).
Cytokines and growth factors elevated in the tumor environment are known to activate Stat3 in normal cells, including DCs, macrophages and myeloid derived suppressor cells in the tumor stroma, promoting tumor immunosuppression and angiogenesis (Kortylewski et al., 2005; Kujawski et al., 2008; Yu et al., 2007). NF-κB is also constitutively activated in immune cells in the tumor stroma (Greten et al., 2004). To determine whether Stat3-mediated RelA acetylation and nuclear retention observed in cancer cells was also operative in immune cells exposed to the tumor milieu, we tested whether Stat3 activity promoted RelA acetylation in DCs stimulated by the Stat3 activator IL-10, which is frequently elevated in cancer. Normal splenic DCs were isolated from mice with Stat3+/+ and Stat3−/− myeloid compartment. We found that IL-10 activation of Stat3 in DCs from mouse spleens was coupled with an increase in RelA acetylation that was greater than what was observed with TNFα stimulation (Figure 3D). Lack of Stat3 in Stat3−/− splenic DCs effectively blocked IL-10-induced RelA acetylation (Figure 3D), indicating a critical requirement of Stat3 for RelA acetylation in immune cells exposed to the tumor milieu.
RelA Acetylation by p300 Acetyltransferase Requires Stat3 Phosphorylation and DNA-binding
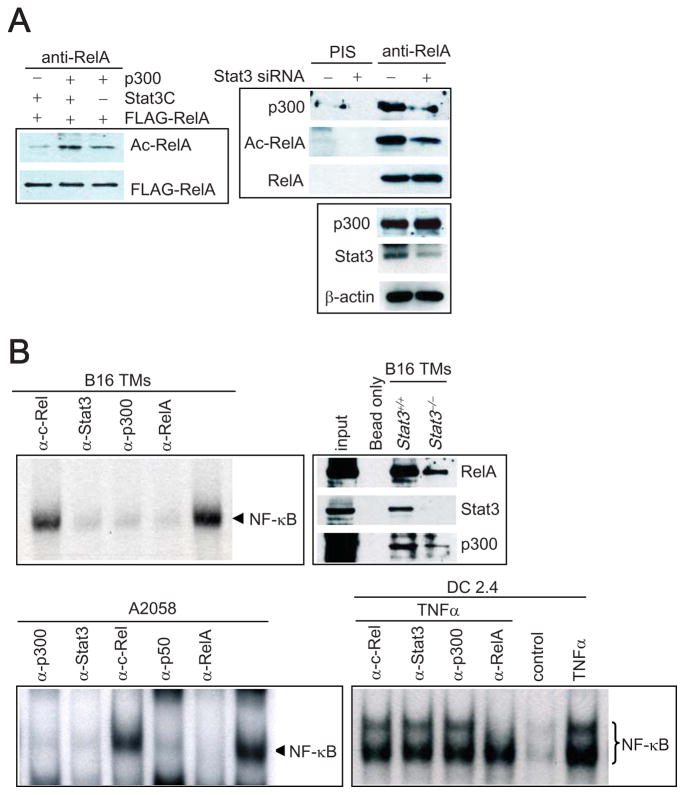
To further test Stat3’s role in mediating RelA acetylation, we investigated whether Stat3 facilitates RelA acetylation through p300, as previous studies have shown that RelA acetylation is mediated by p300 (Chen et al., 2001), which also can serve as a Stat3 co-activator (Darnell, 2002). Co-transfection of p300 with Stat3C, a constitutively-activated Stat3 mutant (Bromberg et al., 1999), led to greater acetylation of endogenous RelA than transfecting either one alone (Figure 4A, left). Co-expression of Stat3C and p300 in 3T3 fibroblasts also resulted in increased nuclear RelA levels (data not shown). Furthermore, levels of p300 and acetylated RelA in the complex were Stat3-dependent in both B16 melanoma (Figure 4A, right) and A2058 cancer cells (data not shown). To demonstrate that RelA activity in tumors was dependant upon the interaction with Stat3 and p300, we performed NF-κB DNA-binding assays using an NF-κB DNA-binding oligonucleotide and nuclear extracts prepared from growing B16 melanoma tumors. Constitutive NF-κB activity in tumors was blocked by pre-incubation with either Stat3 or p300 antibody (Figure 4B), but not by anti-c-Rel antibody, suggesting that constitutive activation of NF-κB complex in tumors involved both Stat3 and p300 (Figure 4B, top left). To further confirm that the NF-κB DNA binding complex contained Stat3 and p300, an oligo-binding assay using biotin-labeled NF-κB DNA-binding sequences was performed. After incubation with nuclear extract, NF-κB complexes were pulled down by streptavidin-conjugated magnetic beads, followed by Western blot analysis. Our results suggested Stat3, p300 and NF-κB were in the same DNA-binding complex (Fig. 4B, top right). Moreover, the interaction between DNA-bound RelA and p300 was reduced in growing tumors from Stat3−/− mice (Fig. 4B, top right). Similar to what was noted in murine melanoma tumors, p300, Stat3 and RelA were in the same DNA-binding complex in A2058 melanoma cells (Fig. 4, bottom left), and the RelA-p50 heterodimer was detected in the DNA-binding complex (Fig. 4, bottom, left). In contrast, NF-κB DNA complexes induced by TNFα in DC 2.4 cells, which have low Stat3 activation levels (data not shown), did not contain Stat3 and p300 (bottom, right).
Figure 4. NF-κB Interaction with p300 Acetyltransferase Requires Stat3 Activity.
(A). Stat3-associated RelA acetylation is mediated by p300. Left: Activated Stat3 increases p300-mediated RelA acetylation. 3T3 cells were transiently transfected with plasmid expressing p300 and/or Stat3C together with RelA, followed by Western blotting. Right: p300 protein interacted with acetylated RelA in a Stat3-dependent manner. B16 melanoma cells were transfected with either control or Stat3 siRNA. Nuclear RelA proteins were immunoprecipitated with anti-RelA antibody followed by Western blot analysis with indicated antibody. Total p300 levels were determined in whole cell extracts (bottom panel). (B). Tumor NF-κB-DNA complex contains both p300 and Stat3, whereas TNFα-induced NF-κB-DNA complex in DCs does not. Top left: Nuclear extracts prepared from B16 tumors were subjected to EMSA to detect NF-κB DNA-binding activity. Specific antibodies as indicated were used to identify Stat3, RelA and p300 in the binding complex. Top right: Nuclear extracts from B16 tumors were incubated with biotin-labeled oligonucleotides containing NF-κB sites and the DNA-bound RelA complex was pulled down by Streptavindin beads, followed by Western blotting. Bottom left: NF-κB RelA/p50 complexes with p300 and Stat3 in A2058 cancer cells. Bottom right: NF-κB-DNA complexes in DC2.4 cells after TNFα treatment was distinct from that found in tumor cells with constitutively-activated Stat3 (bottom, right).
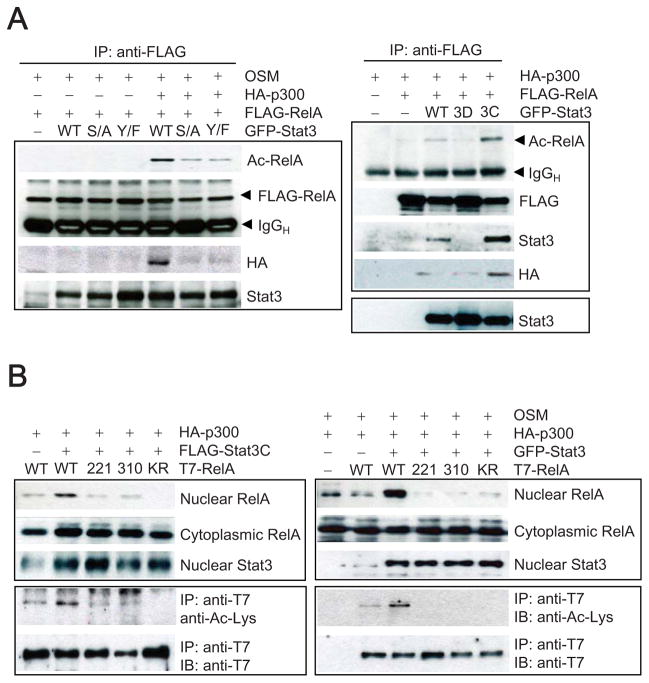
To explore this interaction in more detail, we tested which part of the Stat3 protein was critical for p300-mediated RelA acetylation. Co-transfection of RelA, Stat3 and p300 expression vectors into Stat3-deficient MEFs, followed by OSM treatment to stimulate Stat3, led to an increase in RelA acetylation-mediated by p300 and Stat3 (Figure 5A, left). Because Stat3 phosphorylation at Ser727 is known to be important for interaction with p300 (Schuringa et al., 2001), we tested whether a Ser to Ala mutation in Stat3 at the 727 residue (S/A) would interfere with p300-mediated RelA acetylation. Co-transfecting the T7-tagged RelA with a GFP-Stat3(S/A) reduced p300 incorporation into the RelA protein complex as well as levels of acetylated RelA (Figure 5A). Similarly, when a Stat3 tyrosine 705 residue mutant (Y/F) was overexpressed, which does not efficiently translocate into the nucleus but is found in both the cytoplasm and nucleus (Pranada et al., 2004), RelA acetylation was inhibited upon OSM stimulation, relative to the WT Stat3. Furthermore, when a Stat3 DNA-binding mutant (3D) was expressed in Stat3-deficient MEF cells, RelA acetylation was compromised relative to the constitutively-activated Stat3 mutant, Stat3C (Figure 5A, right). A critical role of the DNA-binding domain in mediating Stat3 interaction with NF-κB has been described (Yu and Kone, 2004).
Figure 5. Stat3-Mediated p300 Interaction Regulates RelA Acetylation that Modulates Its Nuclear Retention.
(A) Stat3 Ser727, Y705 and Stat3 DNA binding are all important for RelA acetylation. Stat3-deficient MEFs were transfected with Stat3 WT, or Stat3 S727A or Y705, Stat3D (dominant-negative DNA-binding mutant) or Stat3C (constitutively activated mutant). Samples shown in the left panels were treated with OSM to stimulate Stat3 activation. (B). RelA acetylation is critical for Stat3-mediated RelA nuclear retention. Left panel: Stat3C and p300 were co-transfected with WT RelA, or RelAK221R, K310R or K218/221/310R. RelA levels in nuclear and cytoplasmic extracts were determined by Western blot. Co-immunoprecipitation to determine RelA acetylation was performed using the nuclear extract. Right panel: OSM was used to stimulate Stat3 in Stat3−/− MEFs transfected with indicated vectors. Nuclear and acetylation levels of RelA were detected as described above.
These results led us to test whether RelA acetylation was required for Stat3-mediated RelA nuclear retention. We co-transfected Stat3C into Stat3−/− MEFs with vectors encoding either WT RelA, RelA-K221R (221), K310R (310) or RelA-K218/221/310R (K/R) mutants, all of which have been reported to have markedly reduced acetylation (Chen et al., 2002). While Stat3C induced nuclear accumulation of WT RelA, it failed to mediate stable nuclear retention of RelA K/R mutants (Figure 5B, left). Additional experiments using OSM to stimulate Stat3 further demonstrated that Stat3–mediated RelA nuclear retention required RelA acetylation (Figure 5B, right).
The results shown in Figure 5 could have resulted from a Stat3-mediated increase in p300 HAT activity. We assessed this possibility by transfecting either WT Stat3 or Stat3 S727A expression vectors into B16 tumor cells with relatively low endogenous Stat3 activity, followed by in vitro acetylation assays. Results of these experiments suggested that Stat3 facilitated p300 recruitment to RelA, but did not directly increase p300 HAT enzymatic activity (Supplementary Figure 3).
RelA Is Highly Acetylated in Mouse and Human Tumors: a Critical Role for Stat3
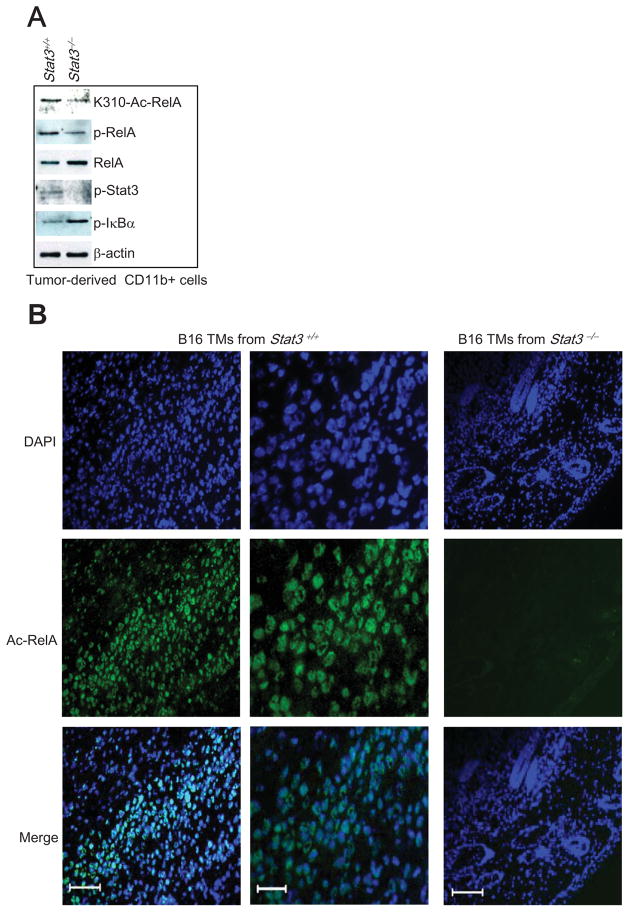
A correlation between Stat3 and NF-κB activity and cancer progression has been well documented. To validate the importance of Stat3-mediated RelA acetylation in cancer, we utilized tumor-associated myeloid cells to examine whether Stat3 was required for RelA acetylation in vivo. While CD11b+ myeloid cells isolated from B16 tumors contained both phosphorylated and acetylated RelA, in vivo targeted functional deletion of Stat3 in the myeloid compartment diminished the level of both phospho- and acetylated RelA (Figure 6A). Furthermore, immunohistochemical analyses confirmed the heavy presence of acetylated RelA in B16 tumors, mainly in the nuclear compartment (Figure 6B, left panels). In contrast, the level of acetylated RelA was greatly diminished (Figure 6B, right) in whole B16 tumors with minimal Stat3 activity, due to Stat3 ablation in the tumor-infiltrating myeloid cells (Figure 1A, left). Of the eight slides examined, only one section of the B16 tumor tissue from a mouse with a Stat3−/− myeloid compartment exhibited detectable, but low levels of acetylated RelA (data not shown). Other sections did not have detectable acetylated RelA (Figure 6B, right). These results emphasized the point that the Stat3-dependent NF-κB acetylation/nuclear retention described here was not totally cell-autonomous, but rather that crosstalk between tumor cells and non-transformed hematopoietic cells in the tumor microenvironment was very important to amplify this interaction in multiple cellular components within the tumor in vivo.
Figure 6. Acetylation of RelA in Growing Tumors Requires Stat3.
(A) CD11b+ myeloid cells were purified from B16 tumors harvested from mice with Stat3+/+ and Stat3−/− myeloid compartment, followed by Western blot analysis utilizing an antibody specific for Ac-K310 of RelA. (B) High levels of acetylated RelA in B16 tumor cell nuclei from mice with Stat3+/+ myeloid cells but not in tumors grown in mice with Stat3−/− myeloid cells. Frozen sections from OCT embedded tumor tissues were stained with an antibody against acetylated RelA (green) and mounted in medium containing DAPI to show nucleus (blue). Middle panels are enlarged images of the left panels. Left and right panels scale bar = 200μm; middle panels scale bar = 50μm.
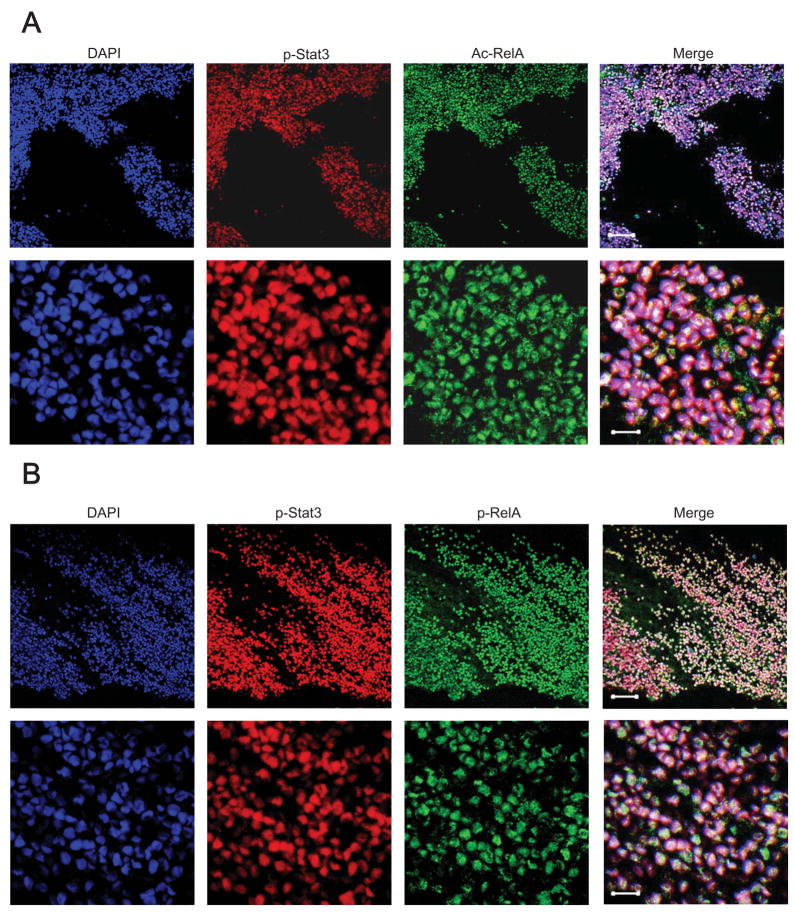
In order to determine whether our findings regarding Stat3-mediated RelA acetylation are important for human cancers, we next analyzed human tumors and normal tissues for phospho-RelA, acetylated RelA and phospho-Stat3. Human malignant tissues from different origins were subject to immunohistochemical staining and confocal microscopic analyses. We used serial sections of tumor tissues to co-stain with antibodies against phospho-Stat3 and acetylated RelA, or with anti-phospho-Stat3 and anti-phospho-RelA. Results from these analyses indicated that phospho-Stat3, acetylated RelA and phospho-RelA were highly elevated in human tumor cells and that they colocalized well in the tumor cell nuclei (Figure 7, Supplementary Figure 4A). Nuclear colocalization of constitutively-activated Stat3 and RelA is also observed in many other types of human tumors (Supplementary Figure 4B). Further, there was little detectable phospho-Stat3, phospho-RelA or acetylated RelA in nontransformed human tissues (shown are spleen and lung tissue slides as examples, Supplementary Figure 4C).
Figure 7. Acetylated RelA Is Prominently Present in Human Tumors and Colocalizes with Activated Stat3 in the Nuclei.
RelA is constitutively acetylated (A) and phosphorylated (B) in human tumors and colocalizes with p-Stat3. Sections of human lung cancer specimens were stained with the indicated antibodies, followed by confocal microscopic analyses. Lower panels are enlarged images of the top panels. Top panels scale bar = 40μm; bottom panels scale bar = 10μm.
DISCUSSION
Crosstalk between Stat3 and NF-κB has been demonstrated at multiple levels, including activation of Stat3 by NF-κB regulated factors such as IL-6 (Naugler et al., 2007; Zhong et al., 1994) and Cox-2 (Dalwadi et al., 2005), possible inhibition of IKK activity in normal immune cells by Stat3 (Welte et al., 2003), and nuclear translocation of unphosphorylated NF-κB by unphosphorylated Stat3 (Yang et al., 2007). However, how the two transcriptional factors, in phosphorylated forms, interact in the nucleus in cancer has not been elucidated. A number of recent studies have focused on the importance of NF-κB in both epithelial and myeloid cells in epithelial carcinogenesis (Karin and Greten, 2005). TNFα has been found to be frequently elevated in cancer and is critical for inflammation-induced cancer through activating NF-κB (Greten et al., 2004; Pikarsky et al., 2004). Our results do not contradict these findings but, rather, indicate that NF-κB activity regulating multiple critical oncogenic processes is determined in part by its interaction with activated Stat3. Our results therefore functionally link these two transcription factors that are frequently activated in cancer. Our data further suggest that the shift in equilibrium between acetylation and deacetylation of RelA towards hyperacetylation, which is driven by constitutively-activated Stat3, contributes to NF-κB activation in both tumor cells and the tumor microenvironment. Our studies also reconcile the roles of NF-κB and Stat3 in mediating the complex interactions between the tumor and its immune microenvironment.
The data generated by expressing various Stat3 mutants in MEF cells lacking intact Stat3 alleles suggest that Stat3-mediated RelA acetylation requires serine (727) and tyrosine (705) phosphorylation, as well as the DNA-binding domain of Stat3 protein. The reason serine phosphorylation is important for RelA acetylation is likely due to the fact it is the critical site for Stat3 interaction with p300 (Schuringa et al., 2001). It has been documented that both interaction of RelA with p300 and acetylation of RelA by p300 require phosphorylation of RelA (Chen et al., 2005). Because unphosphorylated Stat3 (Y705F) preferentially interacts with unphosphorylated RelA (Yang et al., 2007), it is plausible that only phosphorylated Stat3 is able to interact with p300/phosphorylated RelA efficiently, leading to RelA acetylation. As for why mutation of the DNA-binding domain of Stat3 inhibits its ability to activate NF-κB, it has been reported that the Stat3 DNA-binding domain is critical for mediating interaction with RelA (Yu and Kone, 2004). Our data are consistent with these reports in that S/A and Y/F Stat3 proteins were able to interact with RelA, but Stat3D was not (Fig. 5A). At the same time, these Stat3 mutants, which do not efficiently interact with either p300, RelA or phosphorylated RelA, were not able to facilitate RelA acetylation. Although our results suggest an important role of p300 in facilitating acetylation of phosphorylated RelA, it is possible that other acetyl transferases, such as Tip60 or NcoA/SRC1, which interacts with Stat3 (Giraud et al., 2002; Xiao et al., 2003), could also contribute to RelA acetylation.
While IKK gene silencing did not abrogate NF-κB (RelA) activity already present in the tumor cells tested, our results do not challenge the importance of IKK activity in the carcinogenic process. In fact, Stat3-mediated NF-κB nuclear retention during cancer development likely depends on IKK activation. Constitutive activation of NF-κB has been shown to be induced by secreted cytokines and growth factors in cancer cells (Lu et al., 2004; Lu and Stark, 2004). Many of these cytokines and growth factors are encoded by NF-κB target genes that require IKK activation. In several mouse carcinogen or tissue-damage-induced inflammation cancer models, IKK is critical for tumor initiation (Karin and Greten, 2005), which upregulates IL-6 and that, in turn, activates Stat3, leading to cancer development (Naugler et al., 2007). Interestingly, in the carcinogen/inflammatory murine cancer model, in which IKK is required for IL-6 production and tumor development, IKK activation is not constitutive whereas Stat3 is, by carcinogen treatment (Naugler et al., 2007). Recent publications further demonstrate a critical requirement of Stat3 for tumor growth in IL-6-mediated, murine inflammatory cancer models (Bollrath et al., 2009; Grivennikov et al., 2009). Although IKK activity is not constitutive, it is likely that periodic activation of IKK is required for NF-κB activity by facilitating its nuclear entry, while IL-6 production, which activates Stat3, is necessary to maintain oncogenic progression in such models, and in certain human cancers. A2058 melanoma cells, like many other tumor cells, sustain mutations, including c-Src, that activate Stat3 (Niu et al., 2002). In these cultured tumor cells, IKK activation may not be as critical for maintaining NF-κB activity. Nevertheless, a critical role of IKK, and many of its activators, including TNFα, in tumor initiation by tissue damage/inflammation and in chronic inflammation-associated cancer has been well documented (Hu et al., 2004; Karin and Greten, 2005; Naugler et al., 2007). At the same time, IKK-mediated cancer-promoting effects can be due to its interactions with molecules and pathways other than NF-κB (Hu et al., 2004; Lee et al., 2007). The interaction between Stat3 and RelA in both cancer cells and immune cells within the tumor microenvironment shown by our results is distinct, having opposite consequences for overall NF-κB activity in tumor versus normal immune cells responding to immunostimulatory signals. These findings define a cooperativity between Stat3 and NF-κB in cancer, and help explain why both transcription factors appear to stimulate a highly overlapping repertoire of pro-survival, proliferative and pro-angiogenic genes (Catlett-Falcone et al., 1999; Darnell, 2002; Lo et al., 2005; Yu and Jove, 2004). Our finding that this unique Stat3/NF-κB interaction extends to tumor-associated hematopoietic cells emphasizes its importance in the tumor microenvironment. Our study also provides evidence that in both murine tumors and human cancers, RelA is acetylated, which correlates with and is in part regulated by Stat3 activity. These findings may have implications for developing cancer therapeutics.
EXPERIMENT PROCEDURES
Cell Culture and Reagents
A2058 human melanoma and DU145 human prostate cancer cells were obtained from ATCC. DC 2.4 mouse dendritic cell line was obtained from Dr. K. L. Rock (University of Massachusetts Medical School). C-4 mouse melanoma cell line was kindly provided by Dr. I. Fidler (M.D. Anderson Cancer Center). Tumor supernatant was prepared with C-4 mouse melanoma cells; confluent cells received reduced amount of fresh medium, which was then collected 24 h later, filtered to remove cell debris, and added to fresh culture medium at 10% final concentration to stimulate Stat3 in DCs overnight, prior to cell harvesting. Polyclonal antibodies recognizing Stat3 (C-20 and C-20x) and RelA (C-20), and siRNA targeting Stat3, RelA, p50 and control siRNA, were from Santa Cruz Biotechnology; siRNA against p300 from Dharmacon; acetyl-lysine, IκB-α, phospho-IκBα and phospho-RelA (S536) antibodies from Cell Signaling Technology and anti-p300 from Upstate Biotechnology. T7-tagged RelA WT, K221R, K310 and RelA-K218/221/310R mutants as well as anti-acetyl K310RelA antibody were kindly provided by Dr. Greene (University of California, San Francisco, CA) and/or Abcam (for the antibody).
Transfection
Cells were seeded (5×105) in 100 mm plates 24 h before transfection with siRNA using Lipofectamine 2000 (Invitrogen). For some experiments, cells were treated (15 min, 20 ng/ml, 48 h post transfection) with TNFα (Endogen). To activate Stat3, cells received 10 ng/ml oncostatin M (Sigma) or 20 ng/ml IL-10 (PeproTech) for 20 min.
Coimmunoprecipitation and Immunoblotting Analysis
Stat3:NF-κB interaction was tested by coimmunoprecipitation analysis. Nuclear extract (50 μg) from A2058 cells was diluted 10 times with modified RIPA buffer containing 50 mM Tris, PH 7.4, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4 and protease inhibitor cocktail (Roche) and was then incubated overnight with 2 μg of either anti-RelA or anti-Stat3 antibody. Pre-immune serum was used as the antibody control in each experiment. Immune complex was pulled down by addition of protein A agarose (30 μl) followed by further incubation (1 h). After the extensive wash with modified RIPA, immunoprecipitates were separated by SDS-PAGE and subjected to immunoblotting. To detect the level of acetylated RelA protein, cells were transfected with either T7- or FLAG-tagged RelA plasmid DNA for 24 h, and treated overnight with TSA (400 ng/ml) before harvesting. For cell fractionation analysis, cells were transfected with siRNA for 48 h. Nuclear and cytoplasmic extracts were prepared as described (Catlett-Falcone et al., 1999).
Electrophoretic Mobility Shift Assay (EMSA)
DNA binding assays were carried out as previously described (Catlett-Falcone et al., 1999). Briefly, nuclear extracts (4 μg) were incubated (20 min, room temperature) with 50,000 cpm of 32P-labeled probe. The oligonucleotide probe sequence to detect NF-κB binding was: 5′-GATCCATTAGGGGATGCCCCTCAT-3′. For antibody supershift, antibody (1 μg) was pre-incubated with protein for 15 min prior to the addition of radiolabeled probe.
Oligonucleotide Pull Down Assay
Two complementary oligonucleotides containing NF-κB site (sequence shown above) were labeled with biotin according to manufacture’s instructions (Pierce). After labeling, oligonucleotides were annealed and 1 μg of double stranded oligonucleotides was incubated with 300 μg of nuclear extract from A2058 cells in 500 μl of binding buffer containing 12% glycerol, 12 mM HEPES, pH 7.9, 4 mM Tris, pH 7.9, 150 mM KCl, 1 mM EDTA, 1 mM DTT and 10 μg poly(dI-dC). Protein complexes bound to oligonucleotides were then pulled down by incubation with 50 μl of Streptavidin beads (Promega) pre-adsorbed with 1 mg/ml BSA, 50 μg poly(dI-dC) and 50 μg sheared salmon sperm DNA. After extensive washing with binding buffer, protein complexes were separated by SDS-PAGE, blotted and probed with antibodies. For in vitro IκBα pull down assay, tumor RelA complexes were isolated from the nuclei of A2058 cells by immunoprecipitation with anti-RelA antibody, then incubated (2 h, room temperature) with recombinant IκBα proteins (Biosource) Complexes were then detected by immunoblotting.
Immunofluorescent Staining and Confocal Microscopy
A2058 cells were seeded on coverslips in 6-well culture plates and transiently transfected with either control or Stat3 siRNA. Cells were fixed (20 min) with 2% paraformaldehyde, permeabilized (5 min) with PBS containing 0.1% Triton X-100 (PBS-T), quenched with 50 mM was performed using NH4Cl in PBS-T and blocked with 1% BSA in PBS-T. Immunostaining antibodies indicated in Figure 1B and images were acquired using a Zeiss LSM 510 META NLO confocal microscope with a plan-apo 63x/1.4 NA lens.
To prepare frozen sections, B16 tumors harvested from Stat3flox/flox or Cre/Stat3flox/flox mice were embedded in OCT (Tissue-Tek) and frozen in liquid nitrogen. Sections were air dried, fixed in 2 % formaldehyde, and permeabilized in cold methanol before immunofluorescent staining with antibodies. The expression level of each protein in tumor tissues was visualized by a Nikon ECLIPSE TE2000-U microscope and imaged using SPOT software. Human tissue array slides (including both normal and malignant tissues), obtained from the archives of the Pathology Laboratory at City of Hope Medical Center, were used to detect acetylated vs. phosphorylated RelA and phosphorylated Stat3 proteins. Preparation of the tissue arrays was approved by the Institutional Review Board at City of Hope and use of these tissue array slides was exempt as anonymous, archived specimens. For antibody staining, tissue slides were deparaffinized, rehydrated through an alcohol series, and then boiled in Antigen Unmasking Solution (Vector). After incubation with a blocking solution containing 10 % goat serum (Sigma) in PBS, the sections were stained (overnight, 4°C) with a 1:50 dilution of a primary antibody. After incubation with a secondary antibody (Alexa 488 for green signal and Alexa 546 for red, Invitrogen), sections were mounted in Vectashield mounting medium containing 4′6′-diamidino-2-phenylindole (DAPI)(Vector Laboratories). Images obtained from a confocal microscopy were prepared by Zeiss LSM Image Browser software.
In vivo Experiments
Mouse care and experimental procedures were performed under pathogen-free conditions in accordance with established institutional guidance and approved protocols from the Institutional Animal Care and Use Committee of Beckman Research Institute at City of Hope Medical Center. Mx1-Cre mice were obtained from the Jackson Laboratory and Stat3flox/flox mice were generously provided by Drs. Shizuo Akira and Kiyoshi Takeda of Osaka University, Osaka, Japan. Generation of mice with Stat3−/− hematopoietic cells by inducible Mx-Cre recombinase system has been reported elsewhere (Kortylewski et al., 2005; Lee et al., 2002; Wang et al., 2004). Tumor challenges were performed in Stat3flox/flox or Cre/Stat3flox/flox mice 5 d after poly(I:C) treatment, which induces Stat3 ablation mainly in the hematopoietic system. Mice were sacrificed 2–3 wk after tumor challenge and spleens and tumor specimens were harvested. Purification of specific immune subsets has been previously described (Kortylewski et al., 2005). Protein and RNA were prepared from isolated immune cells, whole spleens and whole tumors for various analyses as indicated.
In vitro IP-Kinase Assay
IKKβ kinase complexes were immunoprecipitated from cells transfected with siRNA in the presence or absence of TNFα. Kinase activity was measured in 20 μl reaction buffer containing 1 mM ATP, 10 μCi 32P-γ-ATP and 1 μg recombinant IκBα. ELISA-based kinase assay was performed according to supplier’s instruction (SuperArray).
HAT Assay
The enzymatic activity of p300 was assayed by incubating (30 min, 30°C) p300 immunoprecipitates with either 2 μg of Histone H4 peptides (Upstate) or 0.5 μg of recombinant RelA (BIOSOURCE) as substrate. Reaction mixtures were spotted on p81 filters and washed extensively. Incorporation rate of 3H-AcetylcoA was measured by liquid scintillation counting.
Supplementary Material
Acknowledgments
We thank Dr. P.C. Heinrich for critical reading, Dr. S. Costa for editing, staff members of Analytic Microscopy Core, the Pathology Research Core, the DNA Synthesis Core and the Animal Research Center at City of Hope Medical Center for their technical assistance. This study was supported by National Institutes of Health (R01CA122976, R01CA115815, R01CA115674, P50 CA107399), Harry Lloyd Charitable Trust; the Board of Governors at City of Hope, gifts from the Topercer family, Mrs. Dorothy Needle, Mr. John Goldsmith, the Seraph Foundation and the Janney Fund. The antibody against K310-Ac-RelA and RelA acetylation mutants were from Dr. W. Greene of University of California San Francisco. The Flag-RelA plasmid DNA was kindly provided by Dr. M. W. Mayo, University of Virginia. The Stat3-deficient MEFs were from Dr. V. Poli of University of Turin, Italy. H. Yu, R. Jove and D. Pardoll wish to dedicate this article in memory of Dr. Tsai-Fan Yu, a woman physician-scientist active in medical science since the 1930s. Dr. Tsai-Fan Yu’s pioneering and seminal contributions to elucidating the metabolic basis and defining treatments for gout are a paragon of translational biomedical research.
Footnotes
SIGNIFICANCE
Development of innate and adaptive immunity in response to proinflammatory stimuli requires induction of NF-κB, which involves its nuclear translocation followed by expression of proinflammation/immunity related genes. In contrast, NF-κB can be constitutively activated without continuous proinflammatory stimuli in cancer cells, where it serves a very different role: up-regulating genes necessary for tumor progression. How NF-κB remains constitutively active in cancer remains to be fully defined. The current work reveals a mechanism whereby constitutively-activated Stat3 maintains constitutive NF-κB activity in cancers by inhibiting its export from the nucleus. This Stat3/NF-κB interaction observed in cancer provides insights to carcinogenesis and strategies for developing cancer therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med. 2003;81:549–557. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. Embo J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwadi H, Krysan K, Heuze-Vourc’h N, Dohadwala M, Elashoff D, Sharma S, Cacalano N, Lichtenstein A, Dubinett S. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res. 2005;11:7674–7682. doi: 10.1158/1078-0432.CCR-05-1205. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- Deng WG, Wu KK. Regulation of inducible nitric oxide synthase expression by p300 and p50 acetylation. J Immunol. 2003;171:6581–6588. doi: 10.4049/jimmunol.171.12.6581. [DOI] [PubMed] [Google Scholar]
- Deo DD, Axelrad TW, Robert EG, Marcheselli V, Bazan NG, Hunt JD. Phosphorylation of STAT-3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet-activating factor, JAK-2, and Src in human umbilical vein endothelial cells. Evidence for a dual kinase mechanism. J Biol Chem. 2002;277:21237–21245. doi: 10.1074/jbc.M110955200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Giraud S, Bienvenu F, Avril S, Gascan H, Heery DM, Coqueret O. Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J Biol Chem. 2002;277:8004–8011. doi: 10.1074/jbc.M111486200. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci U S A. 2000;97:1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 Balance by Stat3 Signaling in the Tumor Microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, Dave SS, Zhao H, Xu W, Rosenwald A, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lu T, Sathe SS, Swiatkowski SM, Hampole CV, Stark GR. Secretion of cytokines and growth factors as a general cause of constitutive NFkappaB activation in cancer. Oncogene. 2004;23:2138–2145. doi: 10.1038/sj.onc.1207332. [DOI] [PubMed] [Google Scholar]
- Lu T, Stark GR. Cytokine overexpression and constitutive NFkappaB in cancer. Cell Cycle. 2004;3:1114–1117. [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-kappaB by the T cell receptor complex. Embo J. 2002;21:5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranada AL, Metz S, Herrmann A, Heinrich PC, Muller-Newen G. Real time analysis of STAT3 nucleocytoplasmic shuttling. J Biol Chem. 2004;279:15114–15123. doi: 10.1074/jbc.M312530200. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ, Schepers H, Vellenga E, Kruijer W. Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett. 2001;495:71–76. doi: 10.1016/s0014-5793(01)02354-7. [DOI] [PubMed] [Google Scholar]
- Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Turkson J, Zhang S, Palmer J, Kay H, Stanko J, Mora LB, Sebti S, Yu H, Jove R. Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent antitumor activity. Mol Cancer Ther. 2004;3:1533–1542. [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, et al. STAT3 deletion during hematopoiesis causes Crohn’s disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Chung J, Kao HY, Yang YC. Tip60 is a co-repressor for STAT3. J Biol Chem. 2003;278:11197–11204. doi: 10.1074/jbc.M210816200. [DOI] [PubMed] [Google Scholar]
- Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- Yu Z, Kone BC. The STAT3 DNA-binding domain mediates interaction with NF-kappaB p65 and iuducible nitric oxide synthase transrepression in mesangial cells. J Am Soc Nephrol. 2004;15:585–591. doi: 10.1097/01.asn.0000114556.19556.f9. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.