Abstract
Background
Optimal vitamin D status for the prevention of osteoporosis has been inferred from examinations of the serum 25-hydroxyvitamin D [25(OH)D] concentration below which there is an increase in serum parathyroid hormone (PTH).
Objective
The objectives of the study were to ascertain whether a threshold for serum 25(OH)D exists below which serum PTH increases and whether persons with 25(OH)D above this threshold have lower rates of bone loss than do persons with 25(OH)D below the threshold.
Design
The relation of serum 25(OH)D to serum PTH was analyzed in 208 African American women studied longitudinally for 3 y. These healthy women in midlife were randomly assigned to receive placebo or 800 IU vitamin D3/d; after 2 y, the vitamin D3 supplementation was increased to 2000 IU/d. Both groups received calcium supplements to ensure an adequate calcium intake. A systematic literature review found a wide range of threshold values in part due to varied calcium intake.
Results
A Loess plot suggested a breakpoint between 40 and 50 nmol/L for serum 25(OH)D. A line-line model was fitted to the data, and it showed a spline knot at 44 nmol/L. A heuristic approach verified that PTH does not decline as rapidly when the serum concentration of 25(OH)D is >40 nmol/L as when it is <40 nmol/L. We found no significant difference in rates of bone loss between persons with 25(OH)D concentrations above and below 40 nmol/L.
Conclusion
Although a threshold for 25(OH)D can be identified, we suggest that it should not be used to recommend optimal vitamin D status.
Keywords: African Americans, vitamin D, parathyroid hormone, PTH, osteoporosis, calcium intake, secondary hyperparathyroidism
INTRODUCTION
The purpose of calcium intake in midlife is to replace the calcium lost through excretion so that the loss does not have to be offset from the skeleton (1). There is general agreement that an intake of 1000–1500 mg Ca/d should be recommended for white postmenopausal women (2, 3). There is insufficient information to make a definitive recommendation for other ethnic groups.
Vitamin D is necessary for active intestinal absorption of calcium. Vitamin D is an atypical nutrient in that it is primarily obtained from sunlight via its interaction with the skin rather than from food. The current recommendation for vitamin D intake is 10 μg/d for women aged 50–70 y (4). This quantity is sufficient to prevent vitamin D deficiency (rickets and osteomalacia), but the optimal vitamin D status for bone health would maximize bone mass and reduce the occurrence of osteoporosis.
Many investigators have estimated optimal vitamin D status by examining the relation between serum 25-hydroxyvitamin D [25(OH)D], which is the best estimate of vitamin D status, and serum parathyroid hormone [(PTH) 5–49]. The concept behind these estimates is that there is a threshold for serum 25(OH)D below which secondary hyperparathyroidism (and bone loss) occurs. The serum concentration of 25(OH)D below which PTH begins to rise has been estimated to be between 25 and 122 nmol/L (5–49). The wide range of these estimates may be related to the varied ethnicity and ages of the populations studied, varied calcium intake, the presence of illness that may affect PTH concentrations in the elderly, renal insufficiency, and lack of standardization of assays for 25(OH)D. Moreover, whereas the overall shape of scatter plots of PTH and 25(OH)D reported in the literature are remarkably similar, no consensus exists as to the ideal form of the mathematical relation between PTH and 25(OH)D.
Most of the reported studies are also limited because they are cross-sectional in design. We recently completed a prospective study of the effect of vitamin D supplementation on bone loss in healthy, postmenopausal, African American women (5). African Americans were selected for study because they have low serum 25(OH)D concentrations because of the low cutaneous synthesis of vitamin D that is due to their greater skin pigmentation (23, 50). A calcium intake of 1200–1500 mg/d was ensured in these women by the provision of calcium supplements. In this report, we examine the relation between serum 25(OH)D and PTH to ascertain whether evidence exists for a threshold of serum 25(OH)D below which PTH begins to rise in calcium-sufficient African American women in midlife. We explored whether such a “threshold” was useful in predicting bone loss in this population.
SUBJECTS AND METHODS
Subjects
Healthy, ambulatory, postmenopausal African American women who were not taking hormone replacement therapy were recruited. All subjects were assessed with a medical history and a physical exam that was performed on site by a physician. A cohort of 208 healthy, postmenopausal, African American women aged 50–75 y met the entry criteria and were enrolled in the study.
All participants provided written informed consent. The trial was approved by the Institutional Review Board at Winthrop University Hospital.
Study protocol
All subjects received calcium supplements after assessment of their dietary calcium to ensure a total calcium intake of ≈ 1200–1500 mg/d. The participants were randomly assigned to receive daily either 20 μg (800 IU) of oral vitamin D3 or a matched placebo. At the end of the 24-mo period, the dose of vitamin D3 was raised to 50 μg/d (2000 IU/d). Subjects were seen on-site every 3 mo. Study drug and calcium supplements were dispensed every 3 mo. A food-frequency questionnaire for calcium and vitamin D intakes was completed by participants with the assistance of a nurse at each visit to assess the supplemental calcium requirement (total daily intake of 1200–1500 mg). In addition, a 3-d dietary log was filled out by subjects at baseline and 24 mo, and it was analyzed by using NUTRITIONIST PRO software (version 1.2.207; First Data Bank Inc, San Bruno, CA). A fasting blood sample was collected for analysis of serum 25(OH)D and PTH at baseline and at 3, 6, 12, 18, 24, 27, 30, and 36 mo. The participants were advised to take the study drug every day at bedtime. The calcium supplements were provided as calcium carbonate (Major Pharmaceutical, Livonia, MI); each tablet contained the equivalent of 600 mg elemental calcium.
Vitamin D3 (20- and 50-μg capsules) and matched placebo capsules were custom-manufactured for the study (Tishcon Corp, Westbury, NY). Three batches of the study drug were prepared; one was supplied at the beginning of the study, and the others were supplied annually thereafter.
Laboratory tests
Serum PTH was measured by using the Allegro intact-PTH immunoassay [Nichols Institute Diagnostics, San Juan Capistrano, CA (5, 51)]. The intraassay CV was 5.2% and the inter-assay CV was 9.0%. Serum 25(OH)D was measured by using a radioimmunoassay (RIA) kit (DiaSorin Inc, Stillwater, MN; 5, 52). The intraassay CV was 4.1%, and the interassay CV was 7.0%.
Models used in the statistical analysis
To ensure that the data were examined with the fewest a priori assumptions about the shape of the curve that described the relation between serum 25(OH)D and PTH, the Loess method was used. The Loess method is a technique for determining the shape of the function that best summarizes the scatter plot between 2 continuous variables (53). The method does require the input of a “smoothing parameter,” which is the fraction of the data that is used around each point. An algorithm for choosing an optimal value for the smoothing parameter according to objective criteria, described by Hurvich and Simonoff (54), was used in this analysis. As described below, the Loess analysis suggested either a line-line model or an exponential decay model. So that the putative threshold would not depend on the model chosen, various models and techniques were used to produce converging evidence for a threshold.
The second approach used, the line-line or spline model, of 2 straight lines joined at a “knot,” was previously used in the analysis of the data on the relation between PTH and 25(OH)D [PTH–25(OH)D] (22, 37). A repeated-measures regression model implemented in SAS PROC MIXED software (version 9.1; SAS Institute, Cary, NC) was used to test whether the line-line model provides a statistically better fit than does the simple linear regression model in ascertaining whether a specific 25(OH)D concentration exists.
Finally, the exponential decay model was also used in the analysis below because it was visually suggested by the results of the Loess analysis. This model is included here also because of precedent: it has been used by several researchers to model the PTH–25(OH)D relation (7). Another, more heuristic technique identifies a threshold by comparing changes in PTH from before treatment to after treatment with 25(OH)D in cohorts determined by baseline 25(OH)D (55). There should not be any significant change in PTH in those patients who began the study with 25(OH)D above the threshold. Conversely, the existence of a threshold implies significant PTH change in patients with 25(OH)D below this point. Paired t tests were used to quantify the significance of PTH change.
The longitudinal design of the current study has several advantages. First, our longitudinal data set allows us to examine the influence of serum 25(OH)D on PTH at various time points and doses and also to study the influence of the change in PTH on the change in PTH at those points. Second, because we are measuring each patient as many as 9 times over 36 mo, we are able to generate a correlation between PTH and 25(OH)D and then to use that information in the modeling process. Having multiple observations per patient also allows us to model changes in bone mineral density over time and at the same time to automatically control for extraneous factors (eg, age or weight differences between participants) that may influence the rate of bone loss measured in the group as a whole. Third, we can merge the observations at each time point into a much larger set (n = 1240) of measurements of PTH–25(OH)D pairs and examine the effect of variation in 25(OH)D on PTH. This study takes particular advantage of this third point. We did not use data collected at baseline (a time when patients were not yet reliably calcium replete) in the mathematical analysis of the Loess, line-line, or exponential decay models. The repeated-measures or longitudinal structure of our data set required that we incorporate the correlation between observations in individual patients into the analyses. This was done by implementing the line-line models by using SAS PROC MIXED. To establish the linear component of the association between PTH and vitamin D, Pearson correlation coefficients were computed for various time points and changes between time points. Independent t tests were used to analyze the group differences in continuous baseline values. Paired t tests were used to analyze differences over time. Nonparametric methods such as Spearman’s correlation coefficient were used to confirm parametric results. Fisher’s exact test and the chi-square statistic were used for establishing the significance of the relation between categorical variables. Model parameter estimates and their SEs were generated by the use of maximum likelihood functions. The significance threshold for all hypothesis tests was set at α = 0.05. Alternative hypotheses were 2-sided.
Systematic review of literature reporting a parathyroid hormone–25-hydroxyvitamin D threshold
We report below a summary of the PTH–25(OH)D relation literature. Included studies satisfied the following criteria: inclusion in Ovid Medline between 1 January 1995 and 15 December 2005; English language; human-subject studies that had the key words parathyroid hormone, PTH, calcidiol, or hydroxyvitamin D. This search yielded almost 500 abstracts. We then scanned these abstracts to determine whether they specifically discussed a relation between PTH and 25(OH)D, eg, correlation, association, and regression. This scanning identified 44 such publications (5–49), which dealt mainly with normal subjects or subjects seeking care at an osteoporosis center and specifically discussed the bivariate relation between various vitamin D concentrations by examining the relation between PTH and 25(OH)D. Of the 44 publications, 29 provided an estimate of “optimal” 25(OH)D or of a 25(OH)D threshold by examining the relation between 25(OH)D and PTH. One of these 29 publications found no evidence for a threshold after log transformation of the data (48). Another of the 29 publications (31) provided a separate estimate for each sex and is considered as having 2 separate thresholds. Our own data (5), subjected to the analyses reported below, produced a 31st threshold. Fifteen of the reported thresholds used the DiaSorin RIA for the 25(OH)D assay, and 15 used a more heterogeneous set of assays. These included mainly in-house competitive protein–binding (CPB) assays or Nichols Automated Chemiluminescence Assays (Nichols Advantage, San Clemente, CA). We wished to evaluate the reported thresholds as a linear function of other population parameters such as mean serum 25(OH)D and calcium intake. Because of the variability introduced by the inconsistent assay methods, this analysis was done in several ways. We first confined the data set to those DiaSorin RIA–based studies with reported dietary calcium intake. Because only 12 such studies had been identified, we then relaxed the inclusion criteria to allow into the regression analyses the 17 studies that used both DiaSorin and non-DiaSorin assays and that reported dietary calcium intake. A sensitivity analysis incorporating a series of hypothetical CPB-to-RIA conversion factors allowed us to examine the potential effect of including non-DiaSorin assays in the regressions. The results did not differ qualitatively when the different sets of studies were used, and thus the results presented below use the maximal set of those 17 studies that report all necessary data. In all analyses, each data point was weighted proportionally to the sample size of that study. A second weighting scheme using weights proportional to the inverse of the SEM of reported 25(OH)D was also tried. This produced very similar results, and therefore only the weighting by sample size is reported.
The 30 publications summarized in Table 1 and analyzed below represent a collective sample of 14 577 patients. The sub-sample of 16 DiaSorin RIA–based studies represent a collective sample of 8093 patients. The sample of 18 publications with dietary calcium information represents a collective sample of 7176 patients.
TABLE 1.
Summary of systematic review of literature reporting a parathyroid hormone (PTH)– 25-hydroxyvitamin D [25(OH)D] threshold1
| Reference | n | Health of population | Sex | Age | Optimal 25 (OH)D | 25(OH)D | PTH | Correlation2 | PTH plateau | Dietary vitamin D | Dietary calcium | 25(OH)D assay method | Assay manufacturer3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| y | nmol/L | nmol/L | pg/mL | pg/mL | μg/d | mg/d | |||||||
| Aloia et al (5) | 208 | Healthy | F | 60.5 ± 6.254,5 | 44 | 57.0 ± 27.35 | 37.8 ±16.65 | −0.23 | 33 | 4.6 ± 4.55 | 1330 ± 1815 | RIA | DiaSorin |
| Chapuy et al (7) | 1569 | Healthy | M/F | 50 ± 6 | 78 | 61.0 ± 30.0 | 40.0 ± 16.0 | −0.076 | 36 | 3.4 ± 7.6 | 849 ± 481 | RIA | DiaSorin |
| Cheng et al (8) | 193 | Healthy | F | 117 | 408 | 32.6 ± 10.85 | 39.0 ± 13.05 | −0.31 | 33 | 2.7 | 733 | RIA | DiaSorin |
| Dawson-Hughes et al (9) | 391 | Osteoporosis | M/F | 70.8 ± 4.55 | 110 | 75.2 ± 33.95 | 37.3 ± 16.25 | NA | NA | 4.75 ± 2.675 | 734 ± 3555 | CPB | —9 |
| Fuleihan et al (11) | 346 | Healthy | M/F | 13.3 ± 1.655 | 75 | 49.0 ± 18.85 | 42.510 | −0.37 | NA | 3.75 ± 3.985 | 661 ± 4585 | RIA | DiaSorin |
| Gallagher et al (12) | 735 | Osteoporosis | M/F | 70.9 ± 45 | 100 | 76.3 ± 24.35 | 36.8 ± 13.44 | −0.31 | 28 | 3.95 ± 2.525 | 640 ± 3765 | CPB | — |
| Gannage-Yared et al (13) | 316 | Healthy | M/F | 40 ± 5.6 | 25 | 24.2 ± 17.7 | 43.3 ± 21.4 | −0.35 | NA | 2.51 ± 1.70 | 683 ± 276 | RIA | DiaSorin |
| Gloth et al (16) | 208 | Other11 | M/F | 77.9 ± 7.25 | 37.5 | 42.2 ± 19.35 | 43.7 ± 22.45 | −0.42 | NA | NA | NA | RIA | — |
| Gomez-Alonso et al (17) | 268 | Osteoporosis | M/F | 68 ± 8.55 | 75 | 41.0 ± 21.8 | 51.5 ± 22.55 | −0.30 | NA | NA | NA | RIA | IDS |
| Guillemant et al (20) | 394 | Healthy | M | 157 | 82.8 | 39.6 ± 8.25 | 33.1 ± 10.45 | −0.50 | 23 | NA | 809 ± 409 | CPB | — |
| Haden et al (21) | 237 | Osteoporosis | F | 56 ± 14 | 62.5 | 57.5 ± 27.512,13 | 37.012 | −0.30 | 49 | NA | 80812 | RIA | DiaSorin |
| Harkness and Cromer (22) | 393 | Healthy | F | 15.5 ± 1.6 | 90 | 55.0 ± 30.4 | 39.4 ± 20.6 | −0.34 | 30 | NA | NA | CPB | Nichols |
| Harris and Cromer (23) | 246 | Osteoporosis | M/F | 75.6 ± 7.65 | 50 | 54.1 ± 26.8 | 58.1 ± 38.3 | −0.30 | NA | NA | NA | CPB | — |
| Holick et al (24) | 1536 | Osteoporosis | F | 71.1 ± 9.0 | 74.5 | 76.0 ± 33.0 | 31.010 | −0.29 | 27.2 | NA | NA | CPB | Nichols |
| Isaia et al (25) | 700 | Osteoporosis | F | 67.8 ± 5.7 | 82.5 | 27.3 ± 25.0 | 36.6 ± 28.3 | −0.38 | NA | NA | 784 ± 395 | RIA | DiaSorin |
| Jesudason et al (26) | 486 | Osteoporosis | F | 63 ± 9.5 | 60 | 62.8 ± 25.0 | 50.4 ± 27.6 | −0.24 | 47.5 | NA | NA | RIA | IDS |
| Kauppinen-Makelin et al (27) | 205 | Other14 | M/F | 54.3 | 50 | 35.3 ± 18.013 | 39.0 | −0.236 | NA | NA | NA | RIA | DiaSorin |
| Kinyamu et al (30) | 376 | Osteoporosis | F | 71 ± 35 | 122 | 78.6 ± 24.95 | 35.6 ± 13.15 | −0.33 | 28 | 6.95 ± 2.15 | 744 ± 3075 | CPB | — |
| Lamberg-Allardt et al (31) | 202 | Healthy | F | 38 ± 3 | 80 | 47.0 ± 34.0 | 30.0 ± 13.0 | −0.235 | 26 | 4.7 ± 2.5 | 962 ± 423 | RIA | DiaSorin |
| Lamberg-Allardt et al (31) | 126 | Healthy | M | 37 ± 4 | 40 | 45.0 ± 35.0 | 24.0 ± 12.0 | −0.206 | 23 | 5.6 ± 3.2 | 1037 ± 489 | RIA | DiaSorin |
| Malabanan et al (33) | 35 | Healthy | 67 ± 8 | 50 | 87.5 ± 14.8 | 45.0 ± 17.8 | NA | NA | NA | 1250 | CPB | Nichols | |
| Melin et al (34) | 104 | Osteoporosis | M/F | 85 ± 45 | 75 | 66.1 ± 28.55 | 51.1 ± 21.75 | −0.30 | 43 | NA | 525 ± 2395 | RIA | DiaSorin |
| Need et al (35) | 496 | Osteoporosis | F | 62 ± 8.7 | 40 | 61.0 ± 24.5 | 41.6 ± 19.5 | −0.28 | NA | NA | NA | CPB | — |
| Need et al (36) | 918 | Osteoporosis | F | 64 ± 9.3 | 50 | 61.0 ± 25.0 | 47.0 ± 24.0 | −0.21 | NA | NA | NA | RIA | DiaSorin |
| Ooms et al (37) | 330 | Healthy | F | 80.3 ± 5.6 | 25 | 28.1 ± 13.0 | 37.1 ± 22.8 | NA | NA | NA | 1100 | CPB | — |
| Outila et al (38) | 178 | Healthy | F | 15.3 ± 0.6 | 408 | 39.0 ± 14.0 | 30.0 ± 14.0 | −0.186 | 30 | 4.3 ± 2.8 | 1216 ± 591 | RIA | DiaSorin |
| Soontrupa et al (44) | 106 | Healthy | F | 69.4 ± 6.8 | 87.5 | 83.3 ± 17.8 | 32.3 ± 18.0 | −0.423 | 25 | NA | NA | RIA | DiaSorin |
| Souberbielle et al (45) | 280 | Healthy | M/F | 69.57 | 30 | 28.8 ± 9.3 | 35.0 ± 20.0 | −0.31 | NA | NA | NA | CPB | — |
| Steingrimsdottir et al (46) | 944 | Healthy | M/F | 55.7 ± 15.35 | 45 | 46.2 ± 19.55 | 38.0 ± 16.15 | NA | NA | 13.0 ± 9.85 | 1233 ± 5405 | RIA | DiaSorin |
| Thomas et al (47) | 290 | Other15 | M/F | 62.0 ± 19.0 | 37.5 | 37.5 ± 22.5 | 46.8 ± 44.45 | NA | NA | 7.5 ± 7.3 | NA | CPB | Nichols |
| Vieth et al (48) | 1741 | Other16 | M/F | 56.9 ± 14.8 | 7317 | 52.5 ± 22.08,13 | 40.48 | NA | 29.517 | NA | NA | RIA | DiaSorin |
RIA, radioimmunoassay; CPB, competitive protein binding; IDS, Immuno Diagnostic Systems; NA, not available.
Correlation between PTH and 25(OH)D.
DiaSorin, Stillwater, MN; IDS, Boldon, United Kingdom; Nichols Advantage, San Clemente, CA.
x̄ ± SD (all such values).
For some studies with subgroups, the mean ± SD was reported only for each subgroup for some variables; in these cases, the weighted mean was computed and the overall SD was estimated from the pooled variance.
For a few studies, the correlation was computed but not reported; in these cases, the correlation was estimated from the reported sample size and P value.
For a few studies, the mean age was not reported; in these cases, it was estimated by taking the average of the minimum and maximum ages.
Outila et al (38) inferred the optimal vitamin D status from concentrations of PTH above the mean; Cheng et al (8) simply confirmed higher PTH values below the value reported by Outila et al.
Not specified in the report.
For a few studies, the mean was estimated from the provided graphic—eg, box plot or bar graph.
Elderly patients (homebound, nursing home residents, and ambulatory).
Median.
Estimated from the range.
Inpatients on general medical wards and healthy outpatients.
Inpatients on general medical wards.
Patients with thyroid disease or osteoporosis.
The threshold was not detected after log transformation, and Vieth et al concluded that there is no threshold.
RESULTS
Baseline characteristics of postmenopausal African American women
The baseline characteristics and demographic profile of the population were reported previously (5). The mean age of the participants was 60 y. Mean body mass index (BMI; in kg/m2) was 29.8 ± 4.6. The dietary intakes of vitamin D and calcium were generally low—184 ± 181 IU/d and 759 ± 582 mg/d, respectively, in the 2 groups. At baseline, ≈47% of the women were taking supplemental calcium or multivitamins. The baseline demographic profile did not differ significantly between the 2 groups. The baseline serum 25(OH)D ranged from 10.8 to 99.7 nmol/L, and the mean concentration was 45.1 ± 18.8 nmol/L. At enrollment, 67% of the women had serum concentrations of 25(OH)D < 50 nmol/L, and 95% had concentrations below 80 nmol/L. The highest serum PTH was 126.7 pg/mL, and the mean concentration was 41.5 ± 19.5 pg/mL. Eleven percent of the women had serum concentrations of PTH > 65 pg/mL.
Negative correlation between parathyroid hormone and 25-hydroxyvitamin D
Data from the studies that examine the relation between PTH and 25(OH)D almost always find a significant negative correlation in the range of −0.15 to −0.45. We, too, observed this result. In our sample of 208 women who entered the study with baseline measurements, we noted a statistically significant negative linear correlation between PTH and serum 25(OH)D. This relation expresses itself in various ways. For example, the correlation between the 3-mo changes from baseline in PTH and 25(OH)D is negative (r = −0.30, P < 0.0001; n = 163), as is the correlation between the 12-mo changes in PTH and 25(OH)D (r = −0.40, P < 0.0001; n = 164). The correlations between these 2 variables at baseline (r = −0.18, P <0.01; n =204), 3 mo (r = −0.23, P < 0.01; n = 163), 12 mo (r = −0.26, P < 0.001; n = 164), and 36 mo (r = −0.22, P < 0.005; n = 152) are also negative. Finally, incorporation of all 1240 available PTH–25(OH)D pairs yields a result of r = −0.23 (P < 0.01). The equivalence between the Pearson correlation and a simple linear model indicates that there is, at least as a first approximation, a significant inverse relation between serum PTH and 25(OH)D.
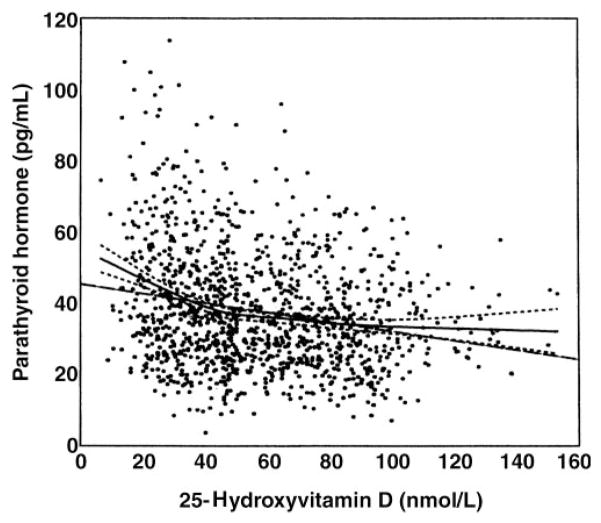
Loess model results
The Loess model results are depicted in Figure 1. The Hurvich and Simonoff (54) selection method for using the Akaike Information Criteria resulted in a smoothing parameter of 0.6. A heuristic inspection of the smoothed data indicated a “natural” break point between ≈40 and ≈50 nmol/L. Note that the linear fit (thin solid line) is outside the 95% CI boundaries (light dashed lines), which indicates a relative lack of fit for the linear model. This result is confirmed more formally in the mixed-model analysis of variance implementation of the line-line model below.
FIGURE 1.
Loess plot (solid line) and 95% CI of the Loess plot (dotted lines) of parathyroid hormone as a function of 25-hydroxyvitamin D. n = 124. The linear regression line (- - -) is outside the 95% CI for 25-hydroxyvitamin D concentrations <30 or >110 nmol/L.
Line-line results
The Loess model results suggest that a 2-slope spline model will fit the data. The mixed model discussed above estimates the threshold to be 44 nmol/L. The slope regressing PTH on 25(OH)D of the first segment is different from zero (slope = −0.44, P = 0.0001). The slope of the second segment (−0.05) does not differ significantly from zero (P > 0 0.05), and the difference between the slopes of the first and second segments is highly significant (P = 0.0001), which indicates a change in the rate at which PTH increases as 25(OH)D drops below 44 nmol/L. Most important, the nonlinear threshold model is significantly better than the simple straight line (P < 0.0001).
Figure 1 indicates that our study data are consistent with a model with a threshold of ≈40–50 nmol/L. A series of statistical models gives converging evidence that a threshold value of ≈44 nmol/L fits the data. At this value, R2 was increased to 6.9%; this was higher than the R2 of any other threshold model and was a significantly better fit than the linear model (F1,184 = 24.3, P < 0.0001).
These results were obtained after adjustment for the correlation structure between the multiple measurements obtained from each subject. A mixed-model analysis of variance was used to compare the incremental contribution of using a knot and a simple linear model. A model with a knot of 44 nmol/L and an “unstructured” correlation structure were found to fit the data by using the minimum Akaike Information Criteria in conjunction with the criterion, imposed for model simplification, that the slope after the threshold not differ significantly from zero. (When this restriction is relaxed, the threshold with the best fit is 40 nmol/L. But the slope of the regression line after 40 nmol/L does not differ clinically or significantly from the slopes after 44 nmol/L.)
Several authors have used an exponential decay model to model the PTH–25(OH)D relation. Figure 1 also indicates that this curve should fit the data. Indeed, it is difficult to visually distinguish the 2 curves (not shown). In a graph of the exponential decay model, a plateau appears between 40 and 50 nmol/L. The actual fitted equation is
| (1) |
These parameters are almost identical to those reported by Chapuy et al (7) in a publication commonly cited by others who are using an exponential decay model. Although the fit of the line-line and the exponential models is almost identical from a statistical perspective, we suggest that the line-line model has several advantages over the exponential model. First, the shape of the Loess model result is most similar to that of the line-line model. Second, the line-line model allows for further decline in PTH beyond the threshold; it is expected that “mega-doses” of vitamin D will further suppress PTH. Finally, the line-line model allows for a clear-cut determination of the threshold point, whereas the exponential model does not.
The threshold estimate of 40–50 nmol 25(OH)D/L is confirmed by noting that the maximal ratio of PTH change in patients below the threshold to the change in patients above the threshold occurred—ie, the difference between the change in PTH (baseline to 1 y = −13.4) in patients with < 42 nmol 25(OH)D/L at baseline and the change in PTH (baseline to 1 y = −2.8) in patients with > 42 nmol 25(OH)D/L at baseline was greatest—when 42 nmol 25(OHD)/L was used as the threshold. Patients with 25(OH)D concentrations below this point did not show clinically and statistically significant changes as a result of their treatment with vitamin D3. Finally, there were highly significant differences via paired t tests in the change in PTH from baseline to 1 y in those active patients who began the study with a 25(OH)D value < 42 nmol/L. Conversely, the group with 25(OH)D concentrations > 42 nmol/L did not experience any significant change in PTH. Threshold values close to 42 nmol 25(OH)D/L had similar statistical characteristics.
Systematic review of the literature on optimal 25-hydroxyvitamin D concentrations
Data from the systematic literature review are summarized in Table 1. The reported studies included subjects with age ranges from 10 y old to the elderly; some studies include both men and women. When the earlier studies were performed, the CPB assay was still in use. Most recent studies used RIAs, in particular the DiaSorin assay. BMI was generally not reported, although it is a determinant of serum PTH (56). Dietary intakes of calcium and vitamin D were generally below those recommended by the Food and Nutrition Board. Surprisingly, the calcium or vitamin D intake was not even reported in some studies. In no study did estimated vitamin D intake approach the currently recommended intake. The estimated optimal serum concentration of 25(OH)D varied from 25 to 122 nmol/L. The highest estimates were reported for either CPB (57) or Nichols Advantage (58) assays, both of which give inappropriately high values for serum 25(OH)D. Indeed, the 4 highest thresholds were all from studies that used the CPB assay. Half of the studies provided estimates of ≤50 nmol/L for optimal 25(OH)D and a third (10 studies) provided estimates between 40 and 50 nmol 25(OH)D/L.
Although the variability in threshold estimates is multifactorial, varied calcium intake and vitamin D status appear to be 2 of the more significant factors. A cross-tabulation of dichotomous dietary calcium intakes (above and below 1000 mg/d) with dichotomous thresholds (above and below 50 nmol/L) indicates that a lower calcium intake is associated with a higher reported threshold (chi-square test: 7.6; P < 0.02). The Pearson correlation between these 2 variables of −0.62 (P = 0.01; n = 18) confirms this inverse relation. A univariate analysis of all 30 studies (31 reported thresholds) documents the effect that mean 25(OH)D has on the reported threshold. A highly significant (P = 0.001) Pearson correlation of 0.55 indicates the linear association between these 2 variables. The following linear equation implies that there is an almost one-to-one increase in the computed threshold for each 1-nmol/L increase in the mean 25(OH)D concentration.
| (2) |
Part of the variability in these estimates can also be explained by the fact that different models and methods were used to estimate the threshold. These varied from the sophisticated mixed-model approach to a naïve, intuitive, visual approach (5, 20, 25, 26).
Multiple regression analysis suggests that serum 25(OH)D and dietary calcium influence the reported threshold independently; together they account for ≈67% of the variance in reported thresholds among the 18 available studies. The overall model P value was 0.0003 (F2,15 = 15.1), and the contribution of dietary calcium to the prediction of the threshold remained significant even after control for serum 25(OH)D. Partial P values were both < 0.01. Because calcium intake was not reported in 13 studies, conclusions based on dietary calcium are only suggestive.
A nonsignificant negative correlation of −0.27 was found between PTH and dietary calcium in the 18 studies with reported dietary calcium intakes. Recently, Steingrimsdottir et al (46) reported a calcium intake × optimal 25(OH)D interaction for an effect on PTH, which we are able to confirm through our literature review. We found that, in those studies with 25(OH)D of > 50 nmol/L, calcium intake did not affect PTH. But in those studies with a mean 25(OH)D of < 50 nmol/L, dietary calcium was inversely related to PTH: within this subset, PTH was 6 pg/mL lower in the set of studies with dietary calcium > 800 mg/d than in the studies with dietary calcium < 800 mg/d (interaction F1,14 = 3.5, P = 0.08).
DISCUSSION
Our data suggest that a serum concentration of 40–50 nmol 25(OH)D/L is needed to prevent a rise in PTH concentrations in calcium-sufficient African American women in midlife. We reviewed the English-language literature that reported a threshold estimate and found that most estimates clustered between 40 and 50 nmol/L or 70 and 80 nmol/L. Indeed, almost half of the studies in our literature review reported a threshold ≤50 nmol/L and one-third reported thresholds between 40 and 50 nmol/L, findings that are consistent with the value we observed. Thus, we take exception to the statement of Dawson-Hughes et al (59), “These estimates of the threshold serum 25(OH)D vary widely but there is a cluster in the 75–80 nmol range.” A equally evident cluster is found between 40 and 50 nmol/L.
The variability in the estimates for the 25(OH)D threshold may be explained by ethnic differences in calcium economy, the extent of vitamin D insufficiency, different calcium intakes, inaccuracy of 25(OH)D assays, the age and health of the populations studied, and the mathematical analyses used. We studied only African American women. Our findings may not be generalizable to other ethnic groups. It should be noted that osteoporotic fractures are less common and bone density is higher in African American women than in women of other races/ethnicities, despite the lower serum 25(OH)D of African Americans (60). Heaney (61) estimated that African American women require 300 mg/d less calcium intake than do white women.
Most of the studies examining optimal vitamin D status do not control for calcium intake. Consideration of optimal vitamin D intake without knowing calcium intake is problematic. In each study in which the calcium intake exceeded 1000 mg/d, the estimated optimal serum 25(OH)D was ≤50 nmol/L. It is of interest that the most recent Cochrane Database of Systematic Reviews concluded that, whereas vitamin D with calcium marginally reduced hip and other nonvertebral fractures, no effect was seen when vitamin D was given alone (62). Again, the interaction between vitamin D status and calcium intake should be considered in making nutritional recommendations.
Our population had a mean age of 60 y, whereas several of the studies from the literature were done in the elderly. Renal function declines with aging, and higher concentrations of 25(OH)D are needed to prevent a rise in serum PTH in the elderly (48). Indeed, a number of studies have documented secondary hyperparathyroidism in the elderly, and calcium with vitamin D supplementation has prevented fragility fractures in some (but not all) studies. Moreover, the effect of vitamin D effects on muscle may help prevent falls in the elderly, thereby reducing fracture risk (63).
Another cogent argument against recommending a vitamin D intake based mainly on a threshold derived from the scattergram of PTH versus 25(OH)D comes from our study (5). Using various models and techniques, we were able to consistently show a threshold value in our data. Despite our finding a threshold of 40–50 nmol 25(OH)D/L, those participants above and below the putative threshold did not differ significantly in loss of bone mineral density. Another analysis attempted to associate the rate of change in bone mineral density with 25(OH)D; no correlation was found between serum 25(OH)D and rates of bone loss (5).
The whole concept of a specific threshold is suspect because such a threshold may be partly an artifact of the reported serum 25(OH)D. In a global survey, Lips et al (57) found a wide range of mean serum concentrations of 25(OH)D across and within continents. Because the threshold is directly related to the observed serum 25(OH)D, it is not surprising that there is similar wide variability in reported thresholds across the 30 studies that we reviewed. Identifying a single optimal 25(OH)D value among this variability is problematic. Furthermore, the average reported correlation across the 25 studies that reported a correlation between PTH and vitamin D was −0.30. Thus, serum 25(OH)D “explains” ≈9% of the variance in PTH. A wide range in reported thresholds is found, because these thresholds are calculated from a wide range of populations, assays, and statistical techniques all applied to a weak biological phenomenon (ie, a linear r2 of 9%). The wide variability in threshold estimates is another reason for caution in using that concept in making dietary recommendations for heterogeneous populations.
There are 2 reasons for trying to identify a threshold. One reason has to do with the slope above the threshold. Several of the studies suggested that PTH concentrations above the threshold may continue to drift down with increased vitamin D (A Arabi et al, unpublished observations, 2004; 64, 65). A second reason for estimating a threshold has to do with the PTH concentrations below this point. Our theoretical concern is with the latter. As did Vieth and Fuleihan (66) and Heaney (67), we ultimately reject the clinical utility of the threshold as a way of identifying optimal vitamin D, but we first rigorously establish the statistical reality of such a point. Note that the slope of the line below the threshold is almost 10 times as big as the slope of the line above the threshold. The fact that it drifts down very slowly is not nearly as important as is the observation that, as vitamin D is reduced below the threshold, PTH increases much more rapidly. The potential conclusion that such a threshold may have implications for optimal vitamin D concentrations is one that we ultimately reject.
Finally, it must be stated that the establishment of an optimal vitamin D intake should also consider the noncalcemic effects of vitamin D that are believed to influence the prevention of some cancers, type 1 diabetes, heart disease, and falls in the elderly (17, 68). It is quite possible that African Americans (and others) may require less vitamin D for skeletal health but may require greater intake for prevention of these noncalcemic disorders. Vitamin D status and calcium intake recommendations should not be made independently but must be considered together.
Acknowledgments
We thank Sharon Sprintz for her expertise as a dual-energy X-ray absorptiometry technician and Jane Moore for their expertise as the Nurse Coordinator. We also thank Lynn Maier for preparation of the typescript.
Footnotes
Supported by grant no. RO1 AG15325 from the National Institute of Aging, National Institutes of Health.
JFA, the principal investigator, designed and supervised the study and wrote the manuscript; SAT, the co-investigator, was responsible for medical supervision of the study participants; SP, the study statistician, was responsible for the data and statistical analyses and contributed to the writing of the manuscript; MF contributed to the data and statistical analyses, to the literature review, and to the writing of the manuscript; JKY, the laboratory director, was responsible for the biochemical assays. None of the authors had a personal or financial conflict of interest.
References
- 1.Heaney RP, Weaver CM. Calcium and vitamin D. Endocrinol Metab Clin North Am. 2003;32:181–94. doi: 10.1016/s0889-8529(02)00063-4. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Optimal calcium intake. NIH Consensus Development Panel on Optimal Calcium Intake. JAMA. 1994;272:1942–8. [PubMed] [Google Scholar]
- 3.Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17:1–45. [PubMed] [Google Scholar]
- 4.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine. Washington, DC: National Academy Press; 1997. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. [PubMed] [Google Scholar]
- 5.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–23. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapuy M, Schott A, Garnero P, Hans D, Delmas P, Meunier P. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab. 1996;81:1129–33. doi: 10.1210/jcem.81.3.8772587. [DOI] [PubMed] [Google Scholar]
- 7.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, Tylavsky FA, Kroger H, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and pre-pubertal Finnish girls. Am J Clin Nutr. 2003;78:485–92. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 9.Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65:67–71. doi: 10.1093/ajcn/65.1.67. [DOI] [PubMed] [Google Scholar]
- 10.Fradinger EE, Zanchetta JR. Vitamin D and bone mineral density in ambulatory women living in Buenos Aires, Argentina. Osteoporos Int. 2001;12:24–7. doi: 10.1007/s001980170153. [DOI] [PubMed] [Google Scholar]
- 11.El-Hajj Fuleihan G, Nabulsi M, Choucair M, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001;107:E53. doi: 10.1542/peds.107.4.e53. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher JC, Kinyamu HK, Fowler SE, Dawson-Hughes B, Dalsky GP, Sherman SS. Calciotropic hormones and bone markers in the elderly. J Bone Miner Res. 1998;13:475–82. doi: 10.1359/jbmr.1998.13.3.475. [DOI] [PubMed] [Google Scholar]
- 13.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000;15:1856–61. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- 14.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas P. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;13:337–49. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 15.Ghannam N, Hammami M, Bakheet S, Khan B. Bone mineral density of the spine and femur in healthy Saudi females: relation to vitamin D status, pregnancy, and lactation. Calcif Tissue Int. 1999;65:23–8. doi: 10.1007/s002239900652. [DOI] [PubMed] [Google Scholar]
- 16.Gloth FM, Gundberg CM, Hollis BW, Haddad JG, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–6. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Alonso C, Naves-Diaz ML, Fernandez-Martin JL, Diaz-Lopez JB, Fernandez-Coto MT, Cannata-Andia JB. Vitamin D status and secondary hyperparathyroidism: the importance of 25-hydroxyvitamin D cut-off levels. Kidney Int Suppl. 2003;85:S44–8. doi: 10.1046/j.1523-1755.63.s85.11.x. [DOI] [PubMed] [Google Scholar]
- 18.Goussous R, Song L, Dallal G, Dawson-Hughes B. Lack of effect of calcium intake on the 25-hydroxyvitamin D response to oral vitamin D3. J Clin Endocrinol Metab. 2005;90:707–11. doi: 10.1210/jc.2004-1380. [DOI] [PubMed] [Google Scholar]
- 19.Guillemant J, Guillemant S. Acute PTH response to oral calcium load and seasonal variation of vitamin D status in healthy young adult subjects. Eur J Clin Nutr. 1996;50:469–72. [PubMed] [Google Scholar]
- 20.Guillemant J, Taupin P, Le HT, et al. Vitamin D status during puberty in french healthy male adolescents. Osteoporos Int. 1999;10:222–5. doi: 10.1007/s001980050219. [DOI] [PubMed] [Google Scholar]
- 21.Haden ST, Fuleihan GE, Angell JE, Cotran NM, LeBoff MS. Calcidiol and PTH levels in women attending an osteoporosis program. Calcif Tissue Int. 1999;64:275–9. doi: 10.1007/s002239900618. [DOI] [PubMed] [Google Scholar]
- 22.Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16:109–13. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- 23.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85:4125–30. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Siris E, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 25.Isaia G, Giorgino R, Rini G, Bevilacqua M, Maugeri D, Adami S. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporos Int. 2003;14:577–82. doi: 10.1007/s00198-003-1390-7. [DOI] [PubMed] [Google Scholar]
- 26.Jesudason D, Need AG, Horowitz M, O’Loughlin P, Morris HA, Nordin B. Relationship between serum 25-hydroxyvitamin D and bone resorption markers in vitamin D insufficiency. Bone. 2002;31:626–30. doi: 10.1016/s8756-3282(02)00866-9. [DOI] [PubMed] [Google Scholar]
- 27.Kauppinen-Makelin R, Tahtela R, Loyttyniemi E, Karkkainen J, Valimaki M. A high prevalence of hypovitaminosis D in Finnish medical in- and outpatients. J Intern Med. 2001;249:559–63. doi: 10.1046/j.1365-2796.2001.00847.x. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Atkinson E, Melton LJ, Riggs BL. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab. 1997;82:1522–7. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- 29.Kinyamu HK, Gallagher JC, Balhorn KE, Petranick KM, Rafferty KA. Serum vitamin D metabolites and calcium absorption in normal young and elderly free-living women and in women living in nursing homes. Am J Clin Nutr. 1997;65:790–7. doi: 10.1093/ajcn/65.3.790. [DOI] [PubMed] [Google Scholar]
- 30.Kinyamu HK, Gallagher JC, Rafferty KA, Balhorn KE. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites. Am J Clin Nutr. 1998;67:342–8. doi: 10.1093/ajcn/67.2.342. [DOI] [PubMed] [Google Scholar]
- 31.Lamberg-Allardt CJ, Outila TA, Karkkaine MU, Rita HJ, Valsta LM. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res. 2001;16:2066–73. doi: 10.1359/jbmr.2001.16.11.2066. [DOI] [PubMed] [Google Scholar]
- 32.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in post-menopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 33.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 34.Melin AL, Wilske J, Ringertz H, Saaf M. Vitamin D status, parathyroid function and femoral bone density in an elderly Swedish population living at home. Aging (Milano) 1999;11:200–7. [PubMed] [Google Scholar]
- 35.Need AG, Horowitz M, Morris HA, Nordin BC. Vitamin D status: effects on parathyroid hormone and 1, 25-dihydroxyvitamin D in post-menopausal women. Am J Clin Nutr. 2000;71:1577–81. doi: 10.1093/ajcn/71.6.1577. [DOI] [PubMed] [Google Scholar]
- 36.Need A, O’Loughlin P, Morris H, Horowitz M, Nordin BC. The effects of age and other variables on serum parathyroid hormone in postmenopausal women attending an osteoporosis center. J Clin Endocrinol Metab. 2004;89:1646–9. doi: 10.1210/jc.2003-031539. [DOI] [PubMed] [Google Scholar]
- 37.Ooms ME, Lips P, Roos JC, et al. Vitamin D status and sex hormone binding globulin: determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res. 1995;10:1177–84. doi: 10.1002/jbmr.5650100806. [DOI] [PubMed] [Google Scholar]
- 38.Outila TA, Karkkainen MU, Lamberg-Allardt CJ. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr. 2001;74:206–10. doi: 10.1093/ajcn/74.2.206. [DOI] [PubMed] [Google Scholar]
- 39.Reginster JY, Frederick I, Deroisy R, et al. Parathyroid hormone plasma concentrations in response to low 25-OH vitamin D circulating levels increases with age in elderly women. Osteoporos Int. 1998;8:390–2. doi: 10.1007/s001980050080. [DOI] [PubMed] [Google Scholar]
- 40.Rejnmark L, Jorgensen ME, Pedersen MB, et al. Vitamin D insufficiency in Greenlanders on a westernized fare: ethnic differences in calcitropic hormones between Greenlanders and Danes. Calcif Tissue Int. 2004;74:255–63. doi: 10.1007/s00223-003-0110-9. [DOI] [PubMed] [Google Scholar]
- 41.Sahota O, Masud T, San P, Hosking D. Vitamin D insufficiency increases bone turnover markers and enhances bone loss at the hip in patients with established vertebral osteoporosis. Clin Endocrinol (Oxf) 1999;51:217–21. doi: 10.1046/j.1365-2265.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- 42.Sahota O, Mundey M, San P, Godber N, Lawson N, Hosking D. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004;35:312–9. doi: 10.1016/j.bone.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone. 1998;23:555–7. doi: 10.1016/s8756-3282(98)00134-3. [DOI] [PubMed] [Google Scholar]
- 44.Soontrapa S, Pongchaiyakul C, Somboonporn W, Soontrapa S, Somboonporn C, Chailurkit L. Prevalence of hypovitaminosis D in elderly women living in urban area of Khon Kaen Province, Thailand. J Med Assoc Thai. 2001;84(suppl):S534–41. [PubMed] [Google Scholar]
- 45.Souberbielle JC, Cormier C, Kindermans C, et al. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab. 2001;86:3086–90. doi: 10.1210/jcem.86.7.7689. [DOI] [PubMed] [Google Scholar]
- 46.Steingrimsdottir L, Gunnarsson O, Indridason O, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 47.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 48.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185–91. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 49.Yan L, Prentice A, Zhang H, Wang X, Stirling D, Golden M. Vitamin D status and parathyroid hormone concentrations in Chinese women and men from north-east of the People’s Republic of China. Eur J Clin Nutr. 2000;54:68–72. doi: 10.1038/sj.ejcn.1600895. [DOI] [PubMed] [Google Scholar]
- 50.Looker AC, Orwoll ES, Johnston CC, Jr, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–8. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 51.Kao PC, Jiang NS, Klee GG, Purnell DC. Development and validation of a new radioimmunoassay for parathyrin (PTH) Clin Chem. 1982;28:69–74. [PubMed] [Google Scholar]
- 52.Hollis BW, Pittard WB., III Relative concentrations of 25-hydroxyvitamin D2/D3 and 1,25-dihydroxyvitamin D2/D3 in maternal plasma at delivery. Nutr Res. 1984;4:27–32. [Google Scholar]
- 53.Cleveland W, Devlin S. Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 54.Hurvich C, Simonoff J. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J Roy Stat Soc. 1998;60:271–93. [Google Scholar]
- 55.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 56.Aloia JF, Feuerman M, Yeh J. The reference range for serum parathyroid hormone. Endocr Pract. 2006;12:137–44. doi: 10.4158/EP.12.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–7. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 58.Carter G, Cater R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the International Vitamin D External Quality Assessment Scheme. Clin Chem. 2004;50:2195–7. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 59.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 60.Cauley JA, Lui L, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–8. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 61.Heaney RP. The importance of calcium intake for lifelong skeletal health. Calcif Tissue Int. 2002;70:70–3. doi: 10.1007/s00223-001-0032-3. [DOI] [PubMed] [Google Scholar]
- 62.Avenell A, Gillespie WJ, Gillespie L, O’Connell DL. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2005;(3):CD000227. doi: 10.1002/14651858.CD000227.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Janssen J, Samson M, Verhaar H. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–5. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 64.Fuleihan GE, Doeb M. Hypovitaminosis D in a sunny country. N Engl J Med. 1999;340:1840–1. doi: 10.1056/NEJM199906103402316. [DOI] [PubMed] [Google Scholar]
- 65.Fuleihan GE, Baddoura R, Awada H, Salam N, Salamoun M, Rizk P. Low peak bone mineral density in healthy Lebanese subjects. Bone. 2002;31:520–8. doi: 10.1016/s8756-3282(02)00845-1. [DOI] [PubMed] [Google Scholar]
- 66.Vieth R, Fuleihan GE. There is no lower threshold level for parathyroid hormone as 25-hydroxyvitamin D concentrations increase. J Endo Invest. 2005;28:183–6. doi: 10.1007/BF03345365. [DOI] [PubMed] [Google Scholar]
- 67.Heaney RP. Serum 25-hydroxyvitamin D and parathyroid hormone exhibit threshold behavior. J Endocrinol Invest. 2005;28:180–2. doi: 10.1007/BF03345364. [DOI] [PubMed] [Google Scholar]
- 68.Bischoff-Ferrari H, Dietrich T, Orav E, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]