Abstract
Objective
This study examined MRI hippocampal volume and cavum septi pellucidi (CSP) in female subjects with schizotypal personality disorder (SPD) and comparison subjects.
Method
MRI was performed on 20 SPD and 29 comparison subjects with delineation of left and right hippocampi. Number of slices containing the CSP was counted. Subjects were given a working memory task, the Delayed Alternation task and other measures of working memory including the Wechsler Memory Test-Revised and the California Verbal Learning Test. Clinical measures were derived from the SCID-II.
Results
SPD females evinced bilaterally smaller hippocampal volumes compared with non-psychiatric female subjects (15.1% on left, 15.7% on right). Additionally, SPD subjects showed statistically significantly more slices containing CSP, and a trend level difference when large CSP was defined as four or more slices (20% vs. 6.9%). SPD subjects demonstrated more errors, more perseverations, and a trend toward more failure to maintain set on the Delayed Alternating task, which were associated with smaller left hippocampal volumes. There was no difference between groups in logical memory, verbal learning or semantic clustering nor a significant correlation between these measures and hippocampal volumes. Clinically, in SPD subjects, right hippocampal volumes correlated negatively with odd appearance/behavior and positively with suspiciousness/paranoia, and odd speech was positively correlated with the number of slices containing a CSP in exploratory analyses.
Conclusions
Female SPD subjects showed bilaterally smaller hippocampal volumes and larger CSP than comparison subjects, similar to what has been shown in schizophrenia. Moreover, these abnormalities have clinically significant associations which may help to explain some of the manifestations of the disorder.
Keywords: Schizophrenia, Schizotypal personality disorder, Hippocampus, Cavum septum pellucidum, Delayed alternation task, Spatial working memory
1. Introduction
The hippocampus is critical for episodic memory consolidation (Squire and Zola-Morgan, 1991) and aspects of working memory (Lipska et al., 2002). It has been shown to be structurally abnormal in 74% of 49 studies of schizophrenics (Shenton et al., 2001), including both abnormalities in volume (Shenton et al., 1992; Gur et al., 2000; Schulze et al., 2003; Bryant et al., 1999), and in shape (Tepest et al., 2003; Csernansky et al., 2002; Shenton et al., 2002), with some negative studies (e.g. DeLisi et al., 1991). Unaffected relatives of schizophrenics have similarly demonstrated reduced volume of hippocampus (Tepest et al., 2003; Seidman et al., 2002; van Erp et al., 2004). Post-mortem data of the hippocampus in schizophrenics have demonstrated alterations of synaptic circuitry involving primarily glutamatergic neurons (for review see Harrison, 2004).
Abnormalities of hippocampal development may also have the secondary effect of lessening the degree of fusion of the two leaflets of the septum pellucidum, which separates the two lateral ventricles (Galarza et al., 2004). During normal development the growth of hippocampus and corpus callosum exert pressure on the two leaflets, facilitating fusion. Incomplete fusion is termed cavum septi pellucidi (CSP). CSP can be visualized by ultrasound at 16 weeks gestation and continues to expand throughout pregnancy to approximately 34 weeks, at which point the CSP begins to decline in size (Serhatlioglu et al., 2003), with 2/3 of CSP closed at birth, all part of normal development (Mott et al., 1992). Although a persistently large CSP can be normal (10.3%in (Kwon et al., 1998)), it is generally associated with a range of neuropsychiatric, anatomic, and genetic abnormalities (Serhatlioglu et al., 2003), including schizophrenia (0.15–58.8%) (Kwon et al., 1998; Nopoulos et al., 1997). Even within schizophrenics, however, the increased presence of CSP could be secondary to other developmental defects in other regions, including corpus callosum (Galarza et al., 2004).
One major complication in interpreting structural MRI data from patients diagnosed with schizophrenia, however, is the potential effects of neuroleptics (Chakos et al., 1994), other medications (Sassi et al., 2002), and the extreme stress (Sapolsky et al., 1986) of having a serious and chronic illness on various brain regions of interest (ROI), including the hippocampus (Sapolsky et al., 1986; Wood et al., 2004). To avoid these potential confounds, the study of subjects with schizotypal personality disorder (SPD), who share the same genetic diathesis as schizophrenia (Kendler et al., 1993), offers an opportunity to examine subjects in the schizophrenia spectrum who are relatively free of such confounds.
Previously we examined the hippocampus and CSP in males recruited from the community who were neuroleptic-naive and who met DSM-IV criteria for SPD. Male SPD subjects demonstrated an elevated rate of large CSP (18.8%) (Kwon et al., 1998). This finding suggested that the brains of SPD subjects, similar to the brains of patients diagnosed with schizophrenia, might have neurodevelopmental abnormalities. The hippocampus in male SPD subjects, however, was shown to have volumes comparable to age-matched male comparison subjects (Dickey et al., 1999). The brain structure of female SPD subjects, including the hippocampus and CSP, has, unfortunately, been a neglected area of research. As the hippocampus is rich in estrogen and androgen receptors during development (Goldstein et al., 2001), this might be expected to affect its development (Romeo et al., 2005), although two studies failed to find an effect of gender on hippocampal volume in schizophrenia (Bryant et al., 1999; Goldstein et al., 2002). Gender may, however, play a role in terms of the effect of co-morbid disorders on hippocampal volumes. Specifically, hippocampal volumes have been shown to be abnormal in major depression (e.g. Neumeister et al., 2005), including in a meta-analysis of 12 studies (Videbech and Ravnkilde, 2004b), with the duration of the untreated depression correlating with smaller volumes (Sheline et al., 2003).
Moreover, cognitive deficits in the schizophrenia spectrum, including SPD, attributable to impaired hippocampal function have been demonstrated. These include poor encoding (Voglmaier et al., 2000a; Jessen et al., 2003), poor working memory (Mitropoulou et al., 2005; Farmer et al., 2000; Barch et al., 2004; Trestman et al., 1995; Buchsbaum et al., 2002; Siegel et al., 1996), and inefficient visuospatial working memory (Levitt et al., 2004) demonstrated by fMRI (Koenigsberg et al., 2005). Finally, animal models have demonstrated that stereotactic infusions of NMDA will lesion hippocampi (Bardgett et al., 2006). These rats have deficits on delayed spatial alternation tasks. Deficits, which can be reversed by chronic clozapine, suggesting that spatial alternation tasks may be an effective probe of hippocampal function in schizophrenia spectrum subjects (Bardgett et al., 2006).
Research regarding possible anatomic contributions to clinical and social impairments in SPD is limited. In schizophrenia, however, more work has been done. For example, smaller right hippocampus has been correlated with negative symptoms (Szendi et al., 2006). Moreover, it has been hypothesized that the delusions experienced by paranoid schizophrenics may be linked to hyperactivity of the dopamine-sensitive CA1 region neurons (Krieckhaus et al., 1992). With respect to the CSP, larger CSP have been associated with abnormalities in the verbal domain, specifically, with lower verbal IQ scores (Nopoulos et al., 2000); with the thinking disturbance factor from the BPRS (Kasai et al., 2004); and with poorer prognosis as measured by years of hospitalization (Fukuzako and Kodama, 1998).
Using high-resolution MRI, this study examined the CSP and hippocampal volume in a neuroleptic-naive group of SPD females. The diagnostic criteria of SPD and potential associations with left and right hippocampus and CSP were explored. Further statistical relationships were evaluated between hippocampal volumes and clinical/cognitive measures also thought to be associated with hippocampal function. Specifically, these were the Beck Depression Inventory, important as 56% of female SPD subjects in a larger sample met criteria for life-time major depression (Dickey et al., 2005); the Delayed Alternation task (Oscar-Berman, 1991), a task of spatial working memory previously shown to be impaired in schizophrenia (Seidman et al., 1995); the Wechsler Memory Test-Revised Logical Memory Score (WMS-R); and California Verbal Learning Test (CVLT) (Delis et al., 1987), words learned trials 1–5 and semantic clustering, a verbal learning task previously shown by our laboratory to be abnormal in SPD subjects (Voglmaier et al., 2000b).
2. Methods
2.1. Subject recruitment
Twenty female SPD and 29 female comparison subjects were recruited from the community via newspaper advertisements, and all met the following inclusion criteria: right-handed; age 18–55 years; English as first language; no history of neurological disorder; no history of serious medical disorder or treatment which could affect cognition as assessed by questionnaire, ECT, drug or alcohol dependence ever or abuse in the last year; a normal brain MRI as evaluated by a clinical neuroradiologist; and no current use of psychoactive medications. Female comparison subjects also had no history of mental illness or personality disorder in themselves or major mental illness in their first-degree relatives as assessed by the Family Interview for Genetic Studies (FIGS) (Maxwell, 1992). After a complete description of the study, written informed consent was obtained. All subjects underwent a SCID and SCID-II interview. Groups did not differ on age or parental socio-economic status (SES), although SPD subjects had lower personal SES and completed fewer years of education despite similar IQ scores, findings likely attributable to the disorder (Dickey et al., 2005) (see Table 1). Note our lab initially focused on males with SPD, but, in recent years, has begun recruitment of females and examination of gender differences.
Table 1.
Subject demographics, cognitive measures, and ROI volumes
| SPD mean (S.D.) |
Comparison mean (S.D.) |
F, p or Mann– Whitney U ◊ |
||||
|---|---|---|---|---|---|---|
| Age | 28.8 | (8.1) | 30.8 | (10.1) | .566 | 0.45 |
| PSES | 4.2 | (.9) | 4.4 | (.7) | .789 | 0.38 |
| SES | 3.0 | (1.0) | 4.0 | (1.0) | 12.258 | 0.001 |
| Years of education | 15.0 | (1.9) | 16.4 | (1.5) | 6.618 | 0.005 |
| WAIS-R vocabulary |
13.1 | (1.7) | 13.9 | (1.8) | 2.142 | 0.15 |
| WAIS-R block design |
12.5 | (2.7) | 12.1 | (2.1) | .272 | 0.6 |
| DA total errors | 12.8 | (15.0) | 5.6 | (7.5) | 4.313 | 0.04 |
| DA perseverations | 2.7 | (3.9) | .56 | (1.0) | 7.142 | 0.01 |
| DA failure to maintain set |
.85 | (1.3) | .28 | (.6) | 3.561 | <0.07 |
| WMS-R Logical Memory 1 (%) |
32.2 | (5.6) | 34.2 | (4.9) | 1.364 | <0.3 |
| CVLT total words | 60.5 | (4.6) | 63.3 | (6.5) | 2.608 | 0.113 |
| CVLT Semantic Clustering |
2.6 | (.8) | 2.7 | (.7) | .296 | <0.6 |
| Left hippocampus ◊ |
2.75 | (.2) | 3.20 | (.2) | 54.0 | <0.0005 |
| Right hippocampus ◊ |
2.88 | (.3) | 3.37 | (.3) | 54.0 | <0.0005 |
| CSP (# slices) ◊ | 2.8 | (1.7) | 1.69 | (1.2) | 177.0 | <0.02 |
Subject demographics, cognitive measures, and hippocampal volumes. Hippocampus given as absolute volumes, statistics performed on ICC corrected volumes. Hippocampus volume measures were not normally distributed on the left for SPD subjects (Shapiro–Wilk=0.870, p=0.012), nor for the number of slices containing a CSP for both SPD and comparison subjects (SPD: Shapiro–Wilk=0.861, p=0.008; comparison subjects Shapiro–Wilk=0.894, p=0.007).
2.2. Clinical measures
IQ was measured with the Vocabulary and Block Design sub-scales from WAIS-R for 18 SPD and 21 comparison subjects (2 SPD and 7 comparison subjects recruited later were given WAIS-III; data from one comparison subject were missing). All SPD subjects met full DSM-IV diagnostic criteria for SPD based on the SCID II, with the 9 items in the SPD diagnostic criteria used as correlative measures, for example, degree and nature of clinical impairment due to odd speech or behavior. The Beck Depression Inventory (Bean et al., 1991) was given to quantify current mood. Subjects were also given the Delayed Alternation test (Oscar- Berman, 1991), a classic delayed response test of working memory. In this task a penny is placed in a cup, the screen is lowered, and the subject has to indicate the cup containing the penny. If correct, the penny is next placed in the alternate cup and the task is repeated. If incorrect, the penny remains in the first cup until the subject indicates the correct cup. This task tests spatial working memory (Lipska et al., 2002; Maruki et al., 2001), and executive functioning (perseveration), processes shown to be abnormal in SPD (Koenigsberg et al., 2005). It was selected also because aberrant scores on the task have correlated with morphometric anomalies in males with SPD (Levitt et al., 2002), and may also serve as a probe of hippocampal function in the schizophrenia spectrum (Bardgett et al., 2006). Subjects were given additional probes of memory function to assess potential correlations with hippocampal volumes including the WMS-R Logical Memory Score thought to measure context-related verbal memory and the CVLT, words learned trials 1–5 thought to measure verbal encoding and semantic clustering score thought to effect more frontally-based semantic organization (Delis et al., 1987) (N, SPD=15, NC=20). Not all subjects were available to participate in all three tests of memory function: for Delayed Alternation (SPD=20, NC=25), for WMS-R (SPD=18, NC=19), and for CVLT (SPD=15, NC=20).
2.3. MRI procedures and ROI definition
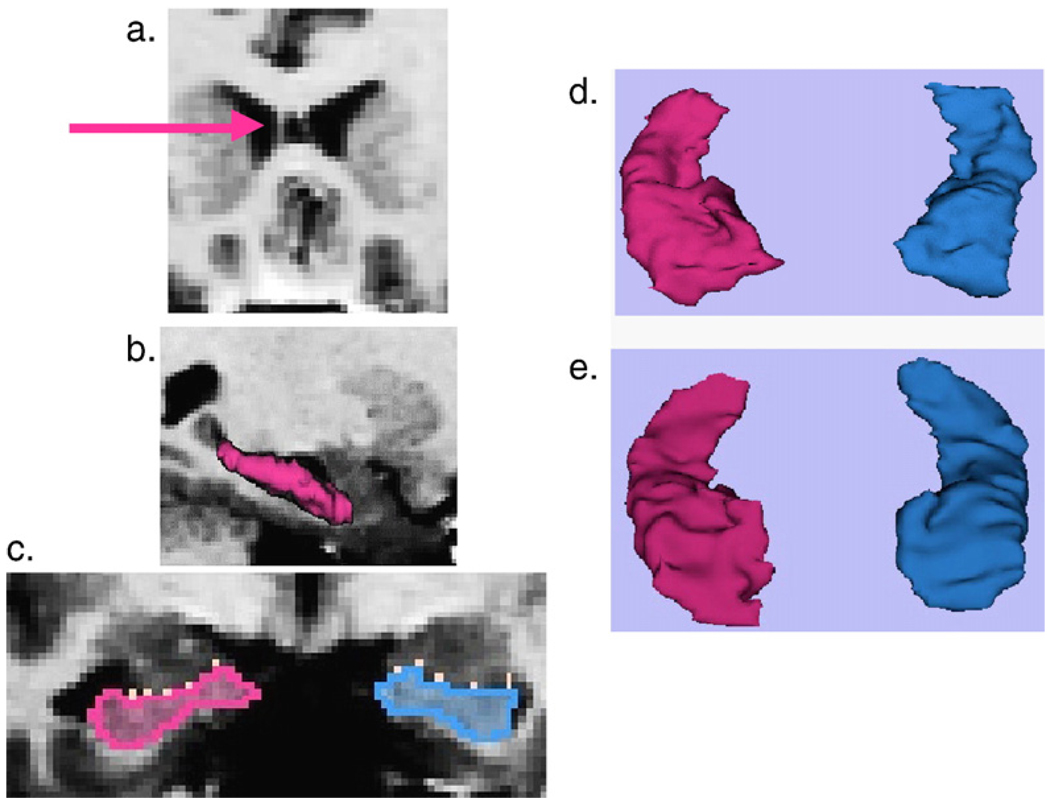
MR images 1.5 mm thick were acquired in the coronal plane on a 1.5T GE system similar to our male sample protocol (Dickey et al., 1999). The measurement of number of slices of CSP followed our previous protocol (Kwon et al., 1998). The extent of the cavum was calculated by multiplying the number of slices containing CSP (Fig. 1a) by 1.5 mm slice thickness. For compatibility with the previous literature, a threshold definition of abnormality was employed, with CSP visible on 1–3 slices (1.5–4.5 mm) categorized as normal, and on four or more slices (>6 mm) categorized as abnormal (Kwon et al., 1998; Nopoulos et al., 1997).
Fig. 1.
Female subjects, a–d images are from the same SPD subject, e is from a comparison subject. (a) Pink arrow points to leaflet of large CSP. (b) 3D model of left hippocampus distinguishing it from the more anterior (toward right and dark gray) amygdala. Note that the guide line used to separate the hippocampus from the amygdala would have been drawn in the CSF space between the amygdala and hippocampus. That guide line would be visible in the coronal plane as seen in the white dots in C. (c) White dots demarcate the outline from the sagittal image of separation between the amygdala (superior and dark gray) and hippocampus (pink is left). (d) 3D model of left and right hippocampus. (e) 3D model of left and right hippocampus similar to d, except that the image for e taken from comparison subject. Anterior aspect, or head, of hippocampus is in lower part of image d and e. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the hippocampal ROI, the posterior extent of the hippocampus was defined as the slice that contained the complete crux of the fornix. A manually delineated line separating the hippocampus from the amygdala was drawn on the sagittal slice which most clearly demonstrated the separation of the two structures by CSF (Fig. 1b,c), a process not technically possible on the previously reported male data (Dickey et al., 1999). This guide between hippocampus and amygdala is the only step that differed from our previous report on male SPD subjects, which separated the amygdala/anterior hippocampus using the mamillary body. The current method was considered to follow the anatomy more closely than our previous method, and was made possible by new developments derived from the 3D-Slicer program (www.slicer.org) with drawing capabilities in all three planes. The use of an anatomic boundary rather than the arbitrary yet reliable mamillary body boundary, with the use of 3D-Slicer, represents a technological advancement for our laboratory.
2.4. Statistical methods
Hippocampal volumes were regressed with intracranial contents (ICC) and residuals were used in all subsequent analyses. Non-parametric Mann–Whitney U tests were employed. ICC were normally distributed and compared using one-way ANOVA. All tests were two-tailed. The total % difference for hippocampal volumes was defined as |volc−volspd| / [.5*(volc+volspd)] where vol=mean volume and c=comparison subjects. Spearman's Rho correlations were performed between the nine SPD diagnostic criteria, right and left hippocampal volumes and number of CSP slices. In addition, right and left hippocampal volumes were correlated with Beck Depression Inventory (total score), Delayed Alternation test (number of errors, number of errors, number of perseverations and failures to maintain set), WMS-R Logical Memory 1, and California Verbal Learning Test (total words learned trials 1–5 and semantic clustering). These correlations were performed without Bonferonni correction given the exploratory nature of the work.
3. Results
3.1. ICC
There was no difference in ICC volume between groups (F(1,47)=1.370, p=0.2).
3.2. Hippocampus
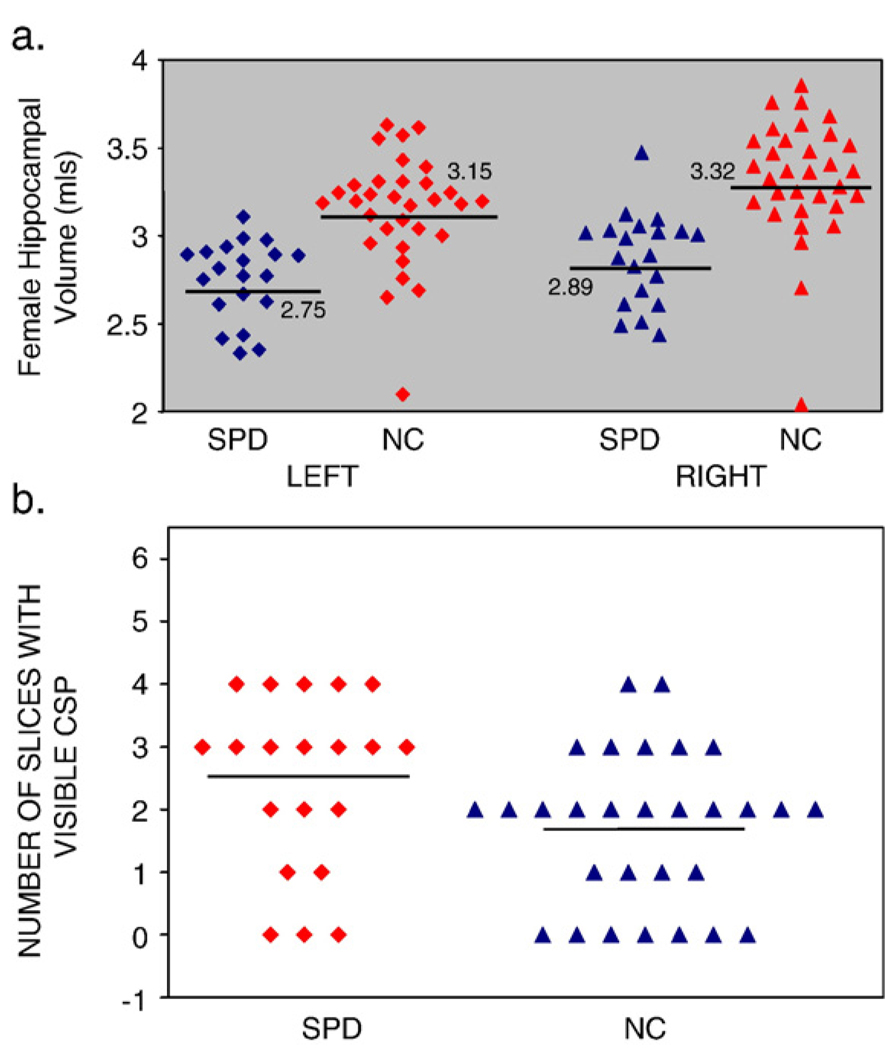
Both left hippocampus and right hippocampus showed significantly smaller volumes in subjects with SPD contrasted with comparison subjects (left: U=54.0, p<0.0005, 15.1% difference; right: U=54.0, p<0.0005, 15.7% difference) (Fig. 1d,e and Fig 2a).
Fig. 2.
(a) Scatterplot of absolute volumes (in ml) of left and right hippocampus of SPD and comparison subjects. Mean volumes indicated. (b) Scatterplot of number of slices with a visible CSP.
3.3. CSP
The mean number of slices for CSP for comparison subjects was 1.69 (S.D.=1.22) (2.53 mm extent) and for SPD subjects, was 2.45 (S.D.=1.4) (3.7 mm extent). This was a statistically significant group difference, with SPD subjects having more extensive CSP (U=190.5, p=0.038). When we used the threshold definition of abnormality (abnormal CSP defined as >4 slices or 6 mm), the frequency of large CSP was greater in SPD subjects (N=5/20, 20%) than in comparison subjects (N=2/29, 6.9%), but this threshold definition showed only a trend level significance (U=237.5, p=0.078; effect size=.24, 60 subjects per group required to show a difference) (Fig. 2b). Contrary to our prediction, there was no correlation between hippocampal volumes and number of CSP slices for either group.
3.4. Clinical–cognitive measures
Seventeen (85%) of the SPD subjects had a past history of Axis I disorder and received psychotherapy, but only 3 subjects were treated pharmacologically prior to enrolling in the study. Within the SPD sample, note there was no significant correlation between the score on Beck Depression Inventory (BDI) and hippocampal volumes. There was, however, a group difference on the BDI, likely due to exclusion criteria for major depression for comparison subjects (F(1,47)=24.415, p<0.0005).
Female SPD subjects had memory deficits compared with controls (Table 1). Specifically, SPD subjects demonstrated difficulties with spatial working memory and executive functioning as measured by the Delayed Alternation task. SPD subjects had more errors, more perseverations, and a trend toward more failure to maintain set. There was no difference between groups on logical memory, encoding on the CVLT or semantic clustering on CVLT.
3.5. Clinical–neuroanatomic correlations
Female SPD subjects did demonstrate significant correlations between right hippocampal volumes and two of the nine SPD criteria: smaller right hippocampus volumes and impairment due to odd appearance and behavior (rho=−.548, N=20, p=0.012) and larger right hippocampus volumes and impairment due to suspiciousness/paranoia (rho=.467, N=20, p=0.038). Female SPD subjects also exhibited a significant correlation between extent of CSP and impairment due to odd speech (rho=.450 N=20, p= 0.046).
In SPD subjects, the smaller the left hippocampal volume, the larger were: the number of errors (rho=−.528, N=20, p=0.017); the total number of perseverations (rho=−.435, N=20, p=0.056, trend level) and the failures to maintain set (rho=−.468, N=20, p=0.037). The finding of a significant correlation with smaller hippocampal volume and poor memory function in SPD subjects appears to be unique to the Delayed Alternation Task as there were no statistically significant correlations with WMS-R nor the CVLT.
Exploratory correlations were performed to investigate whether the reduced hippocampal volumes may have had implications for the manifestations of the clinical phenomena, specifically the nine clinical criteria for SPD. These correlations were not Bonferroni corrected and would not have withstood such a correction. The correlations are included as SPD is an understudied disorder and hypotheses regarding the clinical/pathophysiological findings are less well-developed (Table 2 and Table 3).
Table 2.
Spearman correlations between the nine diagnostic criteria for SPD, hippocampal volumes and number of slices of CSP
| SPD criteria | Right hippocampus |
Left hippocampus |
Number of slices of CSP |
|||
|---|---|---|---|---|---|---|
| Ideas of reference | .292 | .21 | .332 | .15 | −.395 | .085 |
| Social anxiety | .244 | .3 | .192 | .42 | −.223 | .35 |
| Odd beliefs/magical thinking |
−.022 | .93 | .000 | 1.00 | −.123 | .61 |
| Unusual perceptual experiences |
−.032 | .89 | −.079 | .74 | .263 | .26 |
| Odd behavior/ appearance |
−.548 | .012 | −.098 | .68 | −.004 | .99 |
| No close friends | −.176 | .46 | .062 | .79 | −.014 | .95 |
| Odd thinking/speech | −.16 | .50 | .230 | .33 | .450 | .046 |
| Inappropriate/ blunted affect |
−.349 | .131 | −.256 | .28 | .301 | .20 |
| Paranoid ideation | .467 | .038 | .171 | .472 | −.071 | .77 |
Values given are Sperman's rho followed by p values with significant correlations highlighted in bold.
Table 3.
Correlations between hippocampal volumes and measures of memory function
| Right hippocampus |
Left hippocampus |
|||
|---|---|---|---|---|
| Beck Depression Inventory | −.018 | .94 | −.133 | .57 |
| Delayed alternation: total number of errors |
.098 | .68 | −.528 | .017 |
| Delayed alternation: total number of perseverations |
.218 | .56 | −.435 |
.056 (trend) |
| Delayed alternation: failure to maintain set |
−.191 | .42 | −.468 | .037 |
| WMS-R Logical Memory 1 (age percentile) |
−.262 | .29 | −.104 | .68 |
| CVLT: total words learned trials 1–5 (age scaled score) |
−.205 | .46 | .112 | 69 |
Values given are Sperman's rho followed by p values with significant correlations highlighted in bold.
4. Discussion
Female SPD subjects compared with comparison subjects exhibited bilaterally smaller hippocampal volumes, and an increase in the extent of CSP. There also was a trend toward an increase in frequency of abnormally large CSP in SPD when measured by a threshold of >6 mm. Of note, the CSP finding for female SPD subjects is similar to what was previously reported in our laboratory for male SPD subjects (Kwon et al., 1998); with neither SPD group showing a correlation between hippocampus and CSP (Kwon et al., 1998).
In addition, smaller left hippocampal volumes in female SPD subjects correlated with deficits on the Delayed Alternation task, a measure of spatial working memory and executive functioning. Specifically, on the Delayed Alternation test, errors, perseverations, and a trend toward failure to maintain set correlated negatively with the left hippocampal volumes. Although the Delayed Alternation task has been studied little in schizophrenia (Seidman et al., 1995; Pantelis and Brewer, 1995), it nonetheless may serve as a useful tool in measuring perseveration (Koenen et al., 2001), a common deficit in the schizophrenia spectrum, including SPD (Levitt et al., 2002). Poor spatial working memory has been shown in subjects who are at “ultra high risk” for developing schizophrenia (Wood et al., 2003); and even normal subjects with features of SPD(Raine et al., 1992; Gooding and Tallent, 2003). Finally, data from animals with early hippocampal lesions demonstrate poor performance on Delayed Alternation tasks (Lipska et al., 2002) and aberrant adolescent behavior in animals, and thus is one model implicating the hippocampus in schizophrenia (Lipska et al., 2002; Weinberger, 1999).
In contrast, there was no difference between groups in WMS-R logical memory, verbal encoding as measured by CVLT words learned trials 1–5, nor semantic clustering. Moreover, there was no correlation between any of these verbally-mediated tasks and hippocampal volumes. This is consistent with previous reports from our laboratory of females SPD subjects performing better than male SPD subjects on verbal learning (Voglmaier et al., 2005) and relatively preserved logical memory (Voglmaier et al., 1997). The validation of the null-hypothesis on these measures in the setting of impaired spatial working memory suggests that this finding is specific to spatial working memory for female SPD subjects.
In female SPD subjects, impairment due to odd appearance/behavior correlated negatively with right hippocampal volumes, and impairment due to odd speech correlated with the extent of CSP. Thus, smaller hippocampi and larger CSP in female SPD subjects had notable associations with clinical manifestations of the disorder, which may have implications for social functioning in SPD subjects (Dickey et al., 2005). Less clear, however, is the positive association between suspiciousness/paranoia and larger right hippocampal volumes. As noted in the Results section, however, these exploratory correlations would not have withstood Bonferrroni correction. However, as this is the first study to examine the hippocampus in a female sample of neuroleptic-naïve SPD subjects, these correlations are included to guide future studies. In schizophrenia, many, but not all researchers (Flaum et al., 1995), have reported clinical/ size correlations. For example, in schizophrenia, shorter hippocampi bilaterally correlated with bizarre behavior (Fukuzako et al., 1996) and smaller hippocampal volumes with negative symptoms (Rajarethiam et al., 2001). Abnormal hippocampal volumes may be involved in the manifestation of such behavior across the spectrum.
Why SPD subjects, or female SPD subjects specifically, should have smaller hippocampus volumes is speculative. Evidence from animal studies that suggest that repeated stress can result in dendritic atrophy in the CA3 region of the hippocampus (Magarinos and McEwen, 1995), specifically, a reduction of apical length and branch points of pyramidal cells (Wood et al., 2004) (reviewed in (McEwen, 2004)). Although SPD subjects are not psychotic, SPD does result in significant impairment cognitively, occupationally, and socially (Dickey et al., 2005), the cumulative effect of which may be stressful for these individuals.
Unlike the hippocampus, which recent work has shown can be affected by stress and depression (McEwen, 2005; Bianchi et al., 2005) (reviewed in Charney and Manji, 2004) large CSP reflects potential abnormalities in neonatal development. Therefore, the increased prevalence of large CSP in male schizophrenics (Nopoulos et al., 1997), male SPD subjects (Kwon et al., 1998) and now females with SPD, suggests that the pathogenesis of schizophrenia spectrum disorders begins early in neurodevelopment.
Speculatively, anatomic abnormalities in SPD may be driven in part by neurodevelopment, with additional effects of co-morbid diagnoses and stress, whereas in the much more clinically severe schizophrenia, the overwhelming effects of abnormal development and genetic factors may obscure additional effects of co-morbid disorders. Such developmental and genetic factors may help explain why there were negative correlations between large CSP and hippocampal volumes (Kwon et al., 1998) and parahippocampal volumes (Kasai et al., 2004) in schizophrenia, but not in SPD. Nonetheless, the neurodevelopmental abnormalities which are manifest by having a larger CSP may be clinically important for SPD subjects, as suggested by the correlation between the clinical criteria of impairment due to odd speech and number of MRI slices containing a CSP in SPD subjects. Indeed, in schizophrenia, a larger CSP was correlated with thinking disturbances (Kasai et al., 2004), similar to what was shown here.
There were several limitations to this study. One such limitation is that we can not rule out the effects of co-morbidity differences, which commonly fall along gender lines. Specifically, perhaps the higher incidence of histories of co-morbid Axis I disorders in this female sample (17/20 subjects or 85%) compared to our male sample (25%) (Dickey et al., 1999) may account for the current finding of reduced hippocampi in SPD females while not in male SPD subjects. Additionally, small hippocampal volumes have been noted in several psychiatric disorders (Heckers, 2001), including major depression,(Caetano et al., 2004), with the total number of episodes correlating with reduced right hippcampal volumes (Videbech and Ravnkilde, 2004a) and the longer duration of untreated depression the smaller hippocampal volumes (Sheline et al., 2003). Given the high co-morbidity of depression in this female SPD sample, the hypothesis of major depression contributing to the small hippocampal volumes must be considered, but the small number of female SPD subjects without co-morbid disorders (N=3) prohibited a direct test of this hypothesis. The lack of correlation between Beck Depression scores and hippocampal volumes is some-what reassuring, but the Beck Depression scores reflect a cross-sectional view of mood, not mood over time, which is more likely to affect hippocampal morphology. The degree of chronic stress was not evaluated in these subjects and may have contributed to the current findings. However, the lack of hippocampal volume findings in male SPD subjects, who generally have a more serious disorder (Dickey et al., 2005), argues against this possibility. Neuroanatomic boundaries for defining ROI are critical for evaluating possible measurement differences among MRI studies. The original male study (Kwon et al., 1998) had a different anterior boundary for the hippocampus, the mammillary body, which has been used in other reports (Shenton et al., 1992). Moreover, it was the anterior boundary which differed between the two protocols, and the head or anterior portion of the hippocampus is more likely affected in schizophrenics (Csernansky et al., 2002; Lee et al., 2004) and their unaffected relatives (Tepest et al., 2003) in terms of shape and volume (Heckers, 2001) (compare images 1d and 1e). Perhaps, differing methodology in delineating that region could affect MRI volume measurements, particularly in a small ROI. Indeed it may be that the anterior portion of the hippcampus, that region which interdigitates with the amygdala, is most affected by schizophrenia spectrum disorders (Szeszko et al., 2003). Future hippocampal shape analyses along with the inclusion of a second control group of subjects with major depression may help to differentiate these potential confounds. Finally, the correlations in the current report were exploratory, not Bonferroni corrected, necessitating further confirmation and limiting the interpretation of the findings. They are included in the current report to encourage future exploration of the clinical/anatomic relationships in this understudied disorder.
In conclusion, female SPD subjects display smaller hippocampi bilaterally compared with comparison subjects, a finding not previously demonstrated in male subjects (Dickey et al., 1999). Hippocampal volume reduction on the left also correlated with deficits in spatial working memory, whereas reduction on the right correlated with odd appearance and behavior. Moreover, female SPD subjects like their male counter-parts, showed an increased incidence of large CSP, likely due to abnormalities of neurodevelopment. Moreover, the larger the CSP, the more odd the speech in SPD females. The interrelationship between neuro-development and lifelong stressors and their effects on the brains of individuals suffering from schizophrenia spectrum disorders warrants further investigation.
Acknowledgments
This research was supported, in part, by the following grant support: VA Advanced Career Development Award (C.C.D.), VA REAP Award (R.W.M and M.E.S), VA Merit Awards (R.W.M. and M.E.S.), NIMH MH RO1 52807 (R.W.M.); NIMH MH RO1 40799 (R.W.M.); NIMH MH K05 01110 (M.E.S.); NIMH MH RO1 50740 (M.E.S.), Stanley Fellowship from the Stanley Medical Research Institute (M.X.). We wish to acknowledge Ms. Marie Fairbanks for her administrative support and Ms. Susan Demeo for her technical support on this project.
References
- Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J. Abnorm. Psychology. 2004;113(4):556–568. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Griffith MS, Foltz RF, Hopkins JA, Massie CM, O'Connell SM. The effects of clozapine on delayed spatial alternation deficits in rats with hippocampal damage. Neurobiol. Learn. Mem. 2006;85(1):86–94. doi: 10.1016/j.nlm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Bean GJ, Rhodes AE, Martin BA. Electroconvulsive therapy: electric stimulus variables and the convulsive response. Can. J. Psychiatry. 1991;36(9):630–636. doi: 10.1177/070674379103600902. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Hagan JJ, Heidbreder CA. Neuronal plasticity, stress and depression: involvement of the cytoskeletal microtubular system? Curr. Drug Targets CNS Neurol. Disord. 2005;4(5):597–611. doi: 10.2174/156800705774322012. [DOI] [PubMed] [Google Scholar]
- Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am. J. Psychiatry. 1999;156(4):603–609. doi: 10.1176/ajp.156.4.603. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, Silverman JM, Siever LJ. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2002;54(1–2):141–150. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- Caetano S, Hatch J, Brambilla P, Sassi R, Nicoletti M, Mallinger A, Frank E, Kupfer D, Keshavan M, Soares J. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Research: Neuroimaging. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am. J. Psychiatry. 1994;151(10):1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE. 2004;2004(225):re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Csernansky J, Wang L, Jones D, Rastogi-Cruz D, Posener J, Heydebrand G, Miller J, Miller M. Hippocampal Deformities in Schizophrenia Characterized by High Dimensional Brain Mapping. 2002 doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test Manual-Research Edition. San Diego: The Psychological Corporation; 1987. [Google Scholar]
- DeLisi L, Hoff A, Schawrtz J, Shields G, Hathore S, Gupta S, Henn F, Anand A. Brain morphology in first-episode schizophrenia-like psychotic patients: a quantitative magnetic resonance imaging study. Biol. Psychiatry. 1991;29:159–175. doi: 10.1016/0006-3223(91)90044-m. [DOI] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Fischer I, Teh EK, Van Rhoads R, Jakab M, Kikinis R, Jolesz FA, Shenton ME. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol. Psychiatry. 1999;45(11):1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CC, McCarley RW, Niznikiewicz MA, Voglmaier MM, Seidman LJ, Kim S, Shenton ME. Clinical, cognitive, and social characteristics in a sample of neuroleptic-naive persons with schizotypal personality disorder. Schizophr. Res. 2005;78(2–3):297–308. doi: 10.1016/j.schres.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer CM, O'Donnell BF, Niznikiewicz MA, Voglmaier MM, McCarley RW, Shenton ME. Visual perception and working memory in schizotypal personality disorder. Am. J. Psychiatry. 2000;157(5):781–788. doi: 10.1176/appi.ajp.157.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaum M, O'Leary DS, Swayze VW, II, Miller DD, Arndt S, Andreasen NC. Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J. Psychiatr. Res. 1995;29(4):261–276. doi: 10.1016/0022-3956(94)00046-t. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Kodama S. Cavum septum pellucidum in schizophrenia [letter; comment] Biol. Psychiatry. 1998;43(6):467. doi: 10.1016/s0006-3223(97)00379-x. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Fukazako T, Hashiguchi T, Hokazono Y, Takeuchi K, Hirakawa K, Ueyama K, Takigawa M, Kajiya Y, Nakajo M, Fujimoto T. Reduction in hippocampal formation volume is caused mainly by its shortening in chronic schizophrenia: assessment by MRI. Biol. Psychiatry. 1996;39(11):938–945. doi: 10.1016/0006-3223(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Galarza M, Merlo A, Ingratta A, Albanese E, Albanese A. Cavum septum pellucidum and its increased prevalence in schizophrenia: a neuroembryological classification. J. Neuropsychiatry Clin. Neurosci. 2004;16:41–46. doi: 10.1176/jnp.16.1.41. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Seidman L, Horton N, Makris N, Kennedy D, Caviness V, Faraone S, Tsuang M. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O'Brien L, Horton N, Kennedy DN, Makris N, Caviness VS, Faraone SV, Tsuang MT. Impact of normal sexual dimorphisms on sex differences in structural brian abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch. Gen. Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA. Spatial, object, and affective working memory in social anhedonia: an exploratory study. Schizophr. Res. 2003;63(3):247–260. doi: 10.1016/s0920-9964(02)00326-2. [DOI] [PubMed] [Google Scholar]
- Gur R, Turetsky B, Cowell P, Finkelman C, Maany V, Grossman R, Arnold S, Bilker W, Gur R. Temporolimbic volume reductions in schizophrenia. Arch. Gen. Psychiatry. 2000;57(8):769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Harrison P. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–161. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn K-U, Maier W, Schild H, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am. J. Psychiatry. 2003;160(7):1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Kasai K, McCarley RW, Salisbury D, Onitsuka T, Demeo S, Yurgelun-Todd D, Kikinis R, Jolesz F, Shenton ME. Cavumsepti pellucidi in first-episode schizophrenia and first-episode affective psychosis: an MRI study. Schizophr. Res. 2004;71:65–76. doi: 10.1016/j.schres.2003.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, Spellman M, A OH, Walsh D. The Roscommon family study. II. The risk of nonschizophrenic nonaffective psychoses in relatives. Arch. Gen. Psychiatry. 1993;50(8):645–652. doi: 10.1001/archpsyc.1993.01820200059006. [DOI] [PubMed] [Google Scholar]
- Koenen K, Driver K, Oscar-Berman M, Wolfe J, Folsom S, Huang M, Schlesinger L. Measures of prefrontal system dysfunction in post-traumatic stress disorder. Brain Cogn. 2001;45:64–78. doi: 10.1006/brcg.2000.1256. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Buchsbaum MS, Buchsbaum BR, Schneiderman JS, Tang CY, New A, Goodman M, Siever LJ. Functional MRI of visuospatial working memory in schizotypal personality disorder: a region-of-interest analysis. Psychol. Med. 2005;35(7):1019–1030. doi: 10.1017/s0033291705004393. [DOI] [PubMed] [Google Scholar]
- Krieckhaus EE, Donahoe JW, Morgan MA. Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biol. Psychiatry. 1992;31(6):560–570. doi: 10.1016/0006-3223(92)90242-r. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Shenton ME, Hirayasu Y, Salisbury DF, Fischer IA, Dickey CC, Yurgelun-Todd D, Tohen M, Kikinis R, Jolesz FA, McCarley RW. MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am. J. Psychiatry. 1998;155(4):509–515. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Kim SH, Jang DP, Ha TH, Kim JJ, Kim IY, Kwon JS, Kim SI. Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. Neuroimage. 2004;22(2):831–840. doi: 10.1016/j.neuroimage.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Ciszewski AA, Kikinis R, Jolesz FA, Shenton ME. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am. J. Psychiatry. 2002;159(7):1190–1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Westin CF, Nestor PG, Estepar RS, Dickey CC, Voglmaier MM, Seidman LJ, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Shape of caudate nucleus and its cognitive correlates in neuroleptic-naive schizotypal personality disorder. Biol. Psychiatry. 2004;55(2):177–184. doi: 10.1016/j.biopsych.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska B, Aultman J, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- Magarinos A, McEwen B. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Hori K, Nomura M, Yamauchi T. Effects of rat ventral and dorsal hippocampus temporal inactivation on delayed alternation task. Brain Res. 2001;895:273–276. doi: 10.1016/s0006-8993(01)02084-4. [DOI] [PubMed] [Google Scholar]
- Maxwell M. Manual for the Family Interview for Genetic Studies. Bethesda: National Institute for Mental Health; 1992. [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. Am. J. Psychiatry. 2005;162(10):1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- Mott S, Bodensteiner J, Allen W. The cavum septi pellucidi in term and preterm newborn infants. J. Clin. Neurol. 1992;7(1):35–38. doi: 10.1177/088307389200700106. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol. Psychiatry. 2005;57(8):935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh WT, Andreasen NC. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging [see comments] Biol. Psychiatry. 1997;41(11):1102–1108. doi: 10.1016/S0006-3223(96)00209-0. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Krie A, Andreasen NC. Enlarged cavum septi pellucidi in patients with schizophrenia: clinical and cognitive correlates. J. Neuropsychiatry Clin. Neurosci. 2000;12(3):344–349. doi: 10.1176/jnp.12.3.344. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Clinical and experimental approaches to varieties of memory. Int. J. Neurosci. 1991;58(3–4):135–150. doi: 10.3109/00207459108985429. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Brewer W. Neuropsychological and olfactory dysfunction in schizophrenia: relationship of frontal systems to syndromes of schizophrenia. Schizophr. Res. 1995;17:35–45. doi: 10.1016/0920-9964(95)00029-l. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Reynolds GP, Harrison G, Sheard C, Medley I, Reynolds LM, Cooper JE. An evaluation of structural and functional prefrontal deficits in schizophrenia: MRI and neuropsychological measures. Psychiatry Res. 1992;45(2):123–137. doi: 10.1016/0925-4927(92)90006-p. [DOI] [PubMed] [Google Scholar]
- Rajarethiam R, DeQuardo J, Miedler J, Arndt S, Kirbat R, Brunberg J, Tandon R. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psych. Res.: Neuroimaging Sec. 2001;108:79–87. doi: 10.1016/s0925-4927(01)00120-2. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology. 2005;81(6):391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen B. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrinol. Rev. 1986;7:7929–7939. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sassi R, Nicoletti M, Brambilla P, Mallinger A, Frank E, Kupfer D, Keshavan M, Soares J. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci. Lett. 2002;329(2):243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- Schulze K, McDonald C, Frangou S, Sham P, Grech A, Toulopoulou T, Walsh M, Sharma T, Sigmundsson T, Taylor M, Murray R. Hippocampal volume in familial and nonfamilial schizophrenic probands and their unaffected relatives. Biol. Psychiatry. 2003;53:562–570. doi: 10.1016/s0006-3223(02)01910-8. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Oscar-Berman M, Kalinowski A, Ajilore O, Kremen W, Faraone S, Tsuang M. Experimental and clinical neuropsychological measures of prefrontal dysfunction in schizophrenia. Neuropsychology. 1995;9:481–490. [Google Scholar]
- Seidman L, Faraone SV, Goldstein JM, Kremen W, Horton N, Nikos M, Toomey R, Kennedy D, Caviness VS, Tsuang MT. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch. Gen. Psychiatry. 2002;59(9):839–949. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Serhatlioglu S, Kocakoc E, Kiris A, Sapmaz E, Boztosun Y, Bozgeyik Z. Sonographic measurements of the fetal cerebellum, cisterna magna, and cavum septum pellucidum in normal fetuses in the second and third trimesters of pregnancy. J. Clin. Ultrasound. 2003;31(4):194–200. doi: 10.1002/jcu.10163. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Gado M, Kraemer H. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollack SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N. Engl. J. Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shenton M, Dickey C, Frumin M, McCarley R. A review of MRI findings in schizophrenia. Schizophr. Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Gerig G, McCarley RW, Szekely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BV, Jr., Trestman RL, O'Flaithbheartaigh S, Mitropoulou V, Amin F, Kirrane R, Silverman J, Schmeidler J, Keefe RS, Siever LJ. d-Amphetamine challenge effects onWisconsin Card Sort Test. Performance in schizotypal personality disorder. Schizophr. Res. 1996;20(1–2):29–32. doi: 10.1016/0920-9964(95)00002-x. [DOI] [PubMed] [Google Scholar]
- Squire L, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Szendi I, Kiss M, Racsmany M, Boda K, Cimmer C, Voros E, Kovacs ZA, Szekeres G, Galsi G, Pleh C, Csernay L, Janka Z. Correlations between clinical symptoms, working memory functions and structural brain abnormalities in men with schizophrenia. Psychiatry Res. 2006;147(1):47–55. doi: 10.1016/j.pscychresns.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Szeszko P, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra A, Lencz T, Bates J, Crandall D, Kane J, Bilder R. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am. J. Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- Tepest R, Wang L, Miller M, Falkai P, Csernansky J. Hippocampal deformaties in the unaffected siblings of schizophrenic subjects. Biol. Psychiatry. 2003;54:1234–1240. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- Trestman RL, Keefe RS, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, Davidson M, Aronson A, Silverman J, Siever LJ. Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res. 1995;59(1–2):127–136. doi: 10.1016/0165-1781(95)02709-2. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Saleh P, Huttunen MO, Lonnqvist J, Jaakko K, Salonen O, Valanne L, Poutanen V-P, Standertskjold-Nordenstam C-G, Cannon TD. Hippocampal volumes in schizophrenic twins. Arch. Gen. Psychiatry. 2004;61(4):346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004a;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004b;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Voglmaier M, Seidman L, Salisbury D, McCarley R. Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol. Psychiatry. 1997;41:530–540. doi: 10.1016/s0006-3223(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Voglmaier M, Seidman L, Niznikiewicz M, Dickey C, Shenton M, McCarley R. Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. Am. J. Psychiatry. 2000a;157:787–793. doi: 10.1176/appi.ajp.157.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW. Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. Am. J. Psychiatry. 2000b;157(5):787–793. doi: 10.1176/appi.ajp.157.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW. A comparative profile analysis of neuropsychological function in men and women with schizotypal personality disorder. Schizophr. Res. 2005;74(1):43–49. doi: 10.1016/j.schres.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol. Psychiatry. 1999;45:395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Proffitt T, Phillips LJ, Stuart GW, Buchanan JA, Mahony K, Brewer W, Smith DJ, McGorry PD. Spatial working memory ability is a marker of risk-for psychosis. Psychol. Med. 2003;33(7):1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]
- Wood G, Young L, Reagan L, Chen B, McEwen B. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. PNAS. 2004;101(11):3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]