Abstract
Positive selection is a critical T cell developmental checkpoint that is driven by TCR signals. Enhanced positive selection toward the CD4 lineage occurs in the absence of Ikaros. One explanation for this phenotype is that Ikaros establishes the TCR signaling threshold that must be overcome for positive selection to occur. In the current study, this possibility is explored through the use of CD3ζ ITAM transgenic mice that express a CD3 ζ-chain with zero, one, or three ITAMs and an MHC class II (DO11.10)- or MHC class I (H-Y)-restricted TCR transgene. Using this system, we demonstrate that in the absence of Ikaros, thymocytes are able to mature into the CD4 lineage with reduced TCR signaling potential compared with that required to drive the maturation of wild-type thymocytes. We also demonstrate that maturation into the CD8 lineage is enhanced under conditions of reduced TCR signaling potential in the absence of Ikaros.
Positive selection is a TCR-driven event during T cell development. It ensures that only T cells that can recognize a peptide in the context of self-MHC (pMHC) are permitted to develop. Double positive (DP)5 thymocytes expressing TCRs that are unable to interact with self-pMHC undergo apoptosis. However, if TCR recognizes self-pMHC, survival and differentiation signals are delivered (1). Based on whether the DP thymocyte interacts with MHC class I or II, it differentiates into a CD8 or CD4 single positive (SP) T cell, respectively (2).
Signaling capacity of the TCR is provided by the four CD3 proteins, largely through their ITAMs. Upon TCR engagement, ITAMs become phosphorylated, which permits their association with other signaling components such as ZAP-70. CD3δ, γ, and ε each have one ITAM, whereas CD3ζ contains three ITAMs. The TCR is comprised of two ζ, two ε, one δ, and one γ CD3 protein, resulting in a total of 10 ITAMs.
A role for CD3ζ in T cell development was revealed through analyses of CD3ζ knockout (CD3ζ–/–) mice (3). In the absence of CD3ζ, very few mature SP thymocytes or peripheral T cells are observed, indicating that CD3ζ is required for efficient positive selection. To examine the role of the ITAMs, deletions were engineered in the CD3ζ-chain to create proteins containing zero (ζ0), one (ζ1), or three (ζ3) ITAMs, which were then used to reconstitute ζ expression in CD3ζ–/– mice (4). Mice expressing ζ0 had a block at the DP stage of development on a TCR transgenic (Tg) background (4, 5). As the number of ITAMs increased, the amount of positive selection also increased.
Analyses of Ikaros (IK) null (IK–/–) mice support a role for IK in the establishment of thresholds of activation that must be overcome for positive selection to occur. Peripheral T cells in IK–/– mice proliferate in response to levels of TCR signal that are insufficient to drive proliferation of wild-type T cells, suggesting that the lack of IK lowers T cell activation thresholds in mature T cells (6, 7). Thymocytes also have established TCR signaling thresholds. However, when they are overcome, survival and differentiation are the results. IK–/– thymocytes display altered differentiation profiles, which suggests that they also have lowered TCR signaling thresholds. IK–/– thymocytes differentiate preferentially toward the CD4 lineage, a lineage that has been postulated to be the consequence of stronger TCR signals delivered during positive selection (7–9).
To determine whether these phenotypes are the result of a role for IK in establishing TCR signaling thresholds during positive selection, we used the CD3ζ ITAM Tg mouse model system (3, 4). Using this system, the efficiency of positive selection in the context of progressively decreasing TCR signaling capacity can be compared in the thymuses of IK–/– and wild-type mice.
The CD3ζ ITAM Tg were studied in conjunction with the DO11.10 MHC class II-restricted or H-Y MHC class I-restricted TCR Tg, which exclusively drive development of CD4 or CD8 SP T cells, respectively (10, 11). Use of the TCR Tg models are required because, on a polyclonal background, expression of the ζ0Tg in CD3ζ–/– mice almost completely restores CD4/CD8 thymic profiles (4). This is likely the result of the polyclonal repertoire obscuring the effect of decreasing TCR signaling efficiency on positive selection. However, on the DO11.10 and H-Y TCR Tg backgrounds, there is a progressive increase in the percentage of mature SP T cells with increasing numbers of CD3ζ ITAMs (5, 12).
In this report, we demonstrate that in IK–/– thymocytes positive selection into the CD4 lineage in mice expressing the DO11.10 TCR Tg occurs with fewer ζ ITAMs than those required for positive selection of IK+/+ thymocytes. However, in mice expressing the H-Y TCR Tg, lack of IK cannot drive selection into the CD8 lineage with fewer ζ ITAMs. Nevertheless, the efficiency of positive selection into the CD8 lineage is dramatically increased in the absence of IK. Also, we provide evidence that one mechanism behind the role of IK in positive selection may be as a regulator downstream of a signaling pathway that functions as a modulator of TCR signaling strength.
Materials and Methods
Mouse strains
IK–/– mice (C57BL/6 × 129) were crossed to CD3ζ–/– mice (The Jackson Laboratory) and ζ0, ζ1, and ζ3 ITAM Tg mice (gift of Dr. W. Shores, National Institutes of Health, Bethesda, MD). IK–/–CD3ζ–/– mice and IK+/+ CD3ζ–/– mice expressing each of the ITAM Tg were then crossed to DO11.10 TCR Tg mice (gift of Dr. S. Miller, Northwestern University Feinberg School of Medicine, Chicago, IL) or the H-Y TCR Tg mice (The Jackson Laboratory). All mice were crossed to a Rag1–/– background.
Mice were analyzed between 3 and 4 wk of age using age-matched controls. They were bred and maintained in the Northwestern University Center for Comparative Medicine (Chicago, IL). All animal studies were approved by Northwestern University's Animal Care and Use Committee.
Lymphocyte preparation and analyses
Lymphocyte suspensions were made from thymus and spleen as previously described (8). The following Abs (Abs) were used for flow cytometric analyses: anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-TCRβ (H57-597), and anti-CD40-L (MR1). All Abs were purchased from eBioscience and were allophycocyanin, FITC, or PE conjugates. Cells were analyzed on a FACSCalibur (BD Biosciences) flow cytometer and analyses performed using FloJo software.
CD40 ligand (CD40L) expression assays
Flat – bottom, 96-well plates were coated with anti-CD3ε (145-2C11) and anti-CD28 (37.51) at 10 μg/ml each. Thymocytes were cultured at 5 × 106 cells/well in RPMI 1640, 10% FCS, 500 U/ml each streptomycin and penicillin, and 50 μM 2-ME (RPMI complete medium) for4hat 37°C. Alternatively, cells were cultured in RPMI complete medium with PMA (20 ng/ml) and ionomycin (500 ng/ml) for 5 h. Cells were then stained with fluorochrome-conjugated Abs against CD4, CD8, and CD40L, followed by analyses on a FACSCalibur (BD Biosciences) flow cytometer.
Quantitative RT-PCR
CD4+CD8+ DP thymocytes were sorted using a DakoCytomation MoFlo. Purity was consistently ≥98%. mRNA was prepared using an SV total RNA isolation kit (Promega), followed by the generation of cDNA using a Super-Script II kit (Invitrogen). Quantitative RT-PCR (qRT-PCR) was performed using a Bio-Rad iQ5 Real Time PCR machine and iQ SYBR Green Supermix. Results were analyzed with the Pfaffl method. Primers used for qRT-PCR were generated using the Beacon Design program and synthesized by IDT DNA Technologies. Sequences are available upon request.
Ex vivo TCR cross-linking and Western blot analyses
IK–/– and IK+/+ thymocytes (1 × 107) were activated by incubation with anti-CD3ε (hamster IgG isotype; 20 μg/ml), followed by cross-linking with goat anti-hamster IgG (20 μg/ml) (Jackson ImmunoResearch) for 1, 3, or 5 min at 37° C. Cells were lysed in 420 mM NaCl lysis buffer (20 mM Tris (pH 7.5), 0.1% BSA, 1 mM EDTA, and 1% Nonidet P-40) supplemented with protease and phosphatase inhibitors. Fifty μg of protein was electrophoresed on 8% denaturing SDS gels followed by transfer to polyvinylidene difluoride membranes. Membranes were incubated with primary Ab (anti-phosphotyrosine from Upstate Biotechnology and anti-Erk, anti-Akt, anti-phospho-Erk, and anti-phospho-Akt Abs from Cell Signaling Technology) followed by HRP-conjugated anti-rabbit or anti-mouse IgG secondary Ab (Jackson ImmunoResearch Laboratories). Proteins were visualized by incubation with ECL reagent and exposure to film.
Results and Discussion
Appearance of DO11.10 Tg CD4 SP cells occurs with decreased TCR signaling potential in IK–/– thymuses
The IK null (IK–/–) mutation was backcrossed onto a CD3ζ–/– background expressing the ζ0, ζ1, or ζ3 Tg. These mice were then crossed to mice expressing the DO11.10 TCR on a Rag1–/–, positively selecting (H-2d) background to determine whether, in the absence of IK as compared with the presence of IK, decreased TCR signaling potential would be sufficient for the maturation of CD4 T cells. Levels of DO11.10 TCR were not statistically different on double negative and CD4 populations in all strains of IK–/– mice as compared with their IK+/+ counterparts. However, a statistically greater level of TCR staining was observed in IK–/– DP populations in the ζ1Tg mice (p = 0.0007). This was due to a greater percentage of DP thymocytes that had up-regulated TCR as compared with their IK+/+ counterparts, as occurs during positive selection (supplemental Fig. S1).6
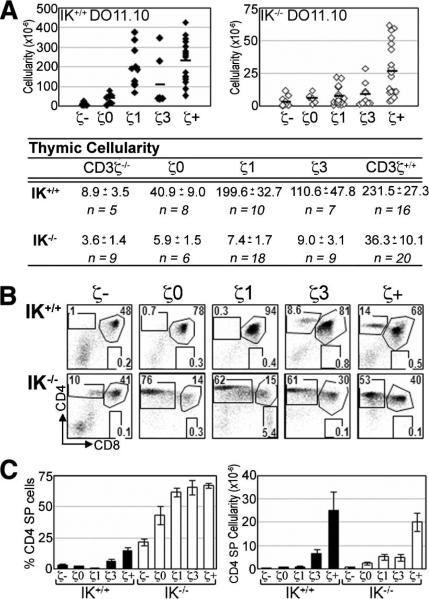
In the absence of the CD3 ζ-chain, TCR is not stably expressed on the cell surface (5). DP thymocytes develop in CD3ζ–/– mice, albeit with a reduced thymic cellularity due to the absence of the proliferative burst associated with β selection (Fig. 1A). IK wild-type (IK+/+) DO11.10 CD3ζ–/– mice had an average 26-fold reduction in thymic cellularity compared with their CD3ζ+/+ counterparts (Fig. 1A), similar to what has been reported previously on a polyclonal TCR background (3). IK–/– DO11.10 CD3ζ–/– mice had an average 10-fold reduction in thymic cellularity compared with their CD3ζ+/+ counterparts (Fig. 1A), showing that lack of IK does not decrease dependence on TCR signaling for thymocyte expansion.
FIGURE 1.
IK–/– thymocytes expressing a DO11.10 TCR develop into CD4 SP cells with reduced TCR signaling potential. A, Absolute numbers of thymocytes in IK+/+ (filled symbols) and IK–/– (open symbols) DO11.10 ζTg mice. Averages, SEM, and sample size (n) are listed. B, Representative flow cytometry plots showing CD4/CD8 profiles of IK+/+ (upper panels) and IK–/– (lower panel) DO11.10 ζTg thymuses. Numbers denote percentage of cells in each region. C, Bar graphs denoting average percentage (left panel) and absolute number (right panel) of CD4 SP cells in IK+/+ and IK–/– DO11.10 ζTg thymuses. Error bars indicate SEM. ζ–, CD3ζ–/–; ζ+, CD3ζ+/+.
CD4 SP cells are not observed in IK+/+ DO11.10 ζTg thymuses unless all three CD3ζ ITAMs (IK+/+ DO11.10 ζ3-Tg and IK+/+ DO11.10 CD3ζ+/+) are present (Fig. 1, B and C). In contrast, CD4 SP cells are observed in all of the IK–/– thymuses, including IK–/– DO11.10 CD3ζ–/– thymuses (Fig. 1, B and C).
CD8 SP cells are observed in IK–/– DO11 ζ1Tg thymuses
IK–/– mice display aberrant development of “CD4-like” SP thymocytes when expressing MHC class I-restricted TCR Tg (8). However, aberrant development toward the CD8 lineage was not observed in IK–/– mice expressing an MHC class II-restricted TCR Tg (8). Thus, it was surprising to observe CD8 SP cells in thymuses of IK–/– DO11.10 ζ1Tg mice (Fig. 1). These CD8 SP cells have up-regulated TCR surface expression compared with their DP counterparts, implying that they are not immature thymocytes that have aberrantly down-regulated CD4 (supplemental Fig. S2). However, they are not found in the periphery, suggesting that they are not fully mature T cells (supplemental Fig. S3).
CD4 SP thymocytes in IK–/– DO11.10 ζ1Tg mice up-regulate CD40L
In the absence of IK, the efficiency of positive selection toward the CD4 lineage reaches its maximum with the ζ1Tg, as determined by the percentage of CD4 SP thymocytes (Fig. 1C). To determine whether CD4 SP thymocytes in IK–/– DO11.10 ζ1Tg thymuses are mature, thereby providing evidence that they have undergone positive selection, their ability to up-regulate CD40L was tested (8). Only mature CD4 T cells can up-regulate expression of this cell surface marker in response to activation (13, 14).
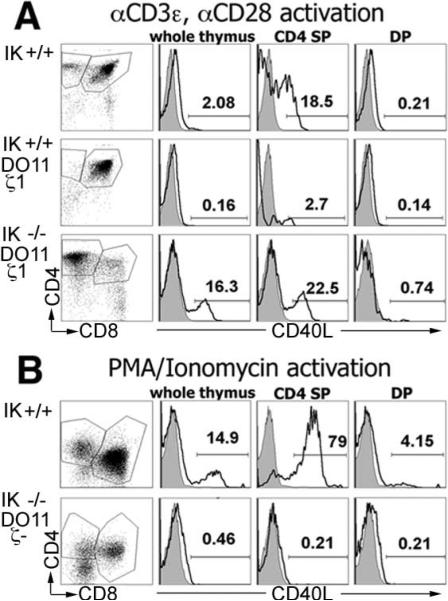
As expected due to the lack of CD4 SP cells, no CD40L up-regulation was observed with IK+/+ DO11.10 ζ1Tg thymocytes (Fig. 2A). In contrast, IK–/– DO11.10 ζ1Tg CD4 SP thymocytes up-regulated CD40L in a quantitatively equivalent fashion to that observed in CD4 SP thymocytes from wild-type mice (Fig. 2A). IK–/– DO11.10 ζ1Tg DP thymocytes were unable to up-regulate CD40L (Fig. 2A), obviating the concern that immature IK–/– thymocytes may have this ability. These data provide evidence that IK–/– DO11.10 ζ1Tg CD4 cells are mature T cells.
FIGURE 2.
CD4 SP cells that develop with reduced TCR signaling potential in the absence of IK are mature. Thymocytes were activated followed by staining with anti-CD4-allophycocyanin, anti-CD8α-FITC, and anti-CD40L-PE. Representative flow cytometry plots (far left column) of postactivation thymocytes with CD4 SP and DP gates that were used to analyze the up-regulation of CD40L are shown. Representative histograms (right columns) of anti-CD40L-PE staining (black line) compared with isotype control (shaded) in postactivation whole thymus, CD4 SP, and DP thymocytes are shown. A, Thymocytes from IK+/+,IK+/+ DO11.10 ζ1Tg, and IK–/– DO11.10 ζ1Tg mice activated with anti-CD3ε and anti-CD28. B, Thymocytes from IK+/+ and IK–/– DO11.10 ζ–/– mice activated with PMA/ionomycin.
Can IK null T cells differentiate toward the CD4 lineage in the absence of TCR signal, as suggested by the appearance of CD4 SP thymocytes in the IK–/– DO11.10 CD3ζ–/– thymuses? To answer this question, the maturity of CD4 SP thymocytes in these thymuses was assessed using PMA and ionomycin activation. Lack of TCR surface expression obviates the use of anti-CD3ε for stimulation. IK–/– DO11.10 CD3ζ–/– Tg CD4 SP thymocytes were unable to up-regulate CD40L, demonstrating that they are not functional T cells (Fig. 2B). This is not due to an inability of mature IK–/– T cells to up-regulate CD40L in response to PMA and ionomycin (supplemental Fig. S4). Therefore, lack of IK does not bypass the requirement for TCR surface expression in positive selection.
A second measure of positive selection is the export of T cells to the periphery. Therefore, spleens from IK–/– DO11.10 ζ0Tg and IK–/– DO11.10 ζ1Tg mice, as well as their IK+/+ counterparts, were analyzed for the presence of CD4 T cells. IK+/+ DO11.10 ζ0Tg and ζ1Tg spleens contained no CD4 T cells (supplemental Fig. S3). In contrast, spleens from IK–/– DO11.10 ζ0 and ζ1Tg mice contained CD4 T cells (supplemental Figure S3). CD4 T cells were not observed in the spleens of IK–/– DO11.10 CD3ζ–/– mice, another indication that CD4 T cells observed in the thymuses of these mice are not mature (supplemental Fig. S3).
CD8 SP cells do not develop with decreased signaling potential in IK–/– H-Y ζTg thymuses
If the lack of IK universally lowers signaling thresholds for positive selection, maturation of CD8 T cells should also require fewer ζITAM in IK–/– thymuses. Alternatively, IK may have differential roles in establishing signaling thresholds for the positive selection of CD4 vs CD8 T cells, in which case TCR signaling thresholds may not be altered for IK–/– CD8 T cells. To differentiate between these possibilities, IK–/– H-Y TCR Tg/Rag1–/– mice were crossed onto the CD3ζ–/–/ζTg backgrounds. Because the H-Y TCR interacts with a male-specific Ag with high affinity, only negative selection occurs in male mice (11). Therefore, female mice were analyzed. Levels of H-Y TCR were statistically equivalent on all thymic subsets in all strains of IK–/– mice as compared with their IK+/+ counterparts (supplemental Fig. S1).
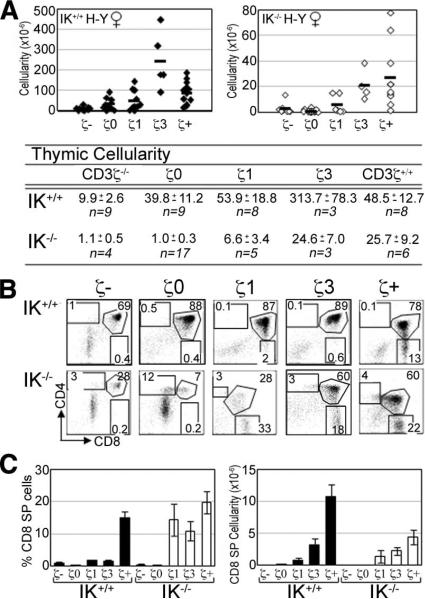
As in DO11.10 CD3ζ–/– mice, DP thymocytes are able to develop in H-Y CD3ζ–/– mice. Cellularity in IK+/+ H-Y CD3ζ–/– thymuses is reduced an average of 4.9-fold compared with their CD3ζ+/+ counterparts (Fig. 3A). IK–/– H-Y CD3ζ–/– thymuses also display a significantly reduced thymic cellularity compared with their CD3ζ+/+ counterparts (23-fold) (Fig. 3A).
FIGURE 3.
IK–/– thymocytes expressing an H-Y TCR do not develop into CD8 SP cells with reduced TCR signaling potential. A, Absolute numbers of thymocytes in IK+/+ (filled symbols) and IK–/– (open symbols) H-Y ζTg female mice. Averages, SEM, and sample size (n) are listed. B, Representative flow cytometry plots showing CD4/CD8 profiles of IK+/+ (upper panels) and IK–/– (lower panels) H-Y ζTg female thymuses. Numbers denote percentage of cells in each region. C, Bar graphs denoting average percentage (left panel) and absolute number (right panel) of CD8 SP cells in female IK+/+ and IK–/– H-Y ζTg female thymuses. Error bars represent the SEM. ζ–, CD3ζ–/–; ζ+, CD3ζ+/+.
CD8 SP cells are observed in IK+/+ H-Y ζ1Tg thymuses, although at a reduced percentage compared with their CD3ζ+/+ counterparts (Fig. 3B). In IK–/– H-Y ζTg mice, CD8 SP thymocytes also are first observed with the ζ1Tg, demonstrating that a lack of IK does not lower signaling requirements for maturation toward the CD8 lineage. However, there is enhanced selection toward the CD8 lineage in IK–/– thymuses, with efficient selection observed with ζ1Tg as determined by the percentage of CD8 SP thymocytes (Fig. 3C), in comparison with IK+/+ thymuses in which maximum selection was not observed unless all three ζITAMS were present.
Decreased dependency on TCR signal for the maturation of IK–/– CD4 T cells is not the result of altered expression of survival genes
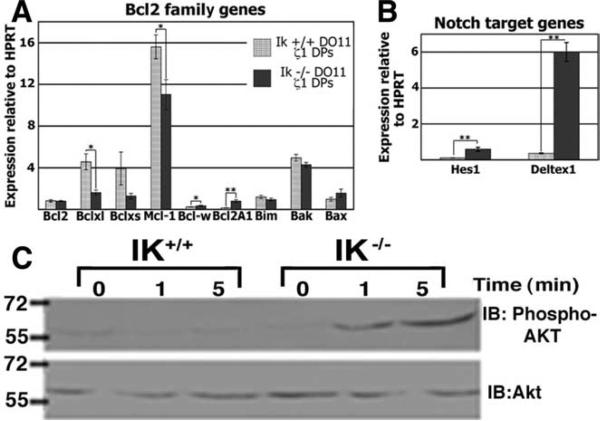
TCR signals that initiate positive selection induce the expression of Bcl2 family member genes, whose products play a critical role in the regulation of thymocyte survival vs apoptosis (15). Lack of IK may lead to altered expression of proapoptotic or antiapoptotic genes in thymocytes, which in turn could lead to the observed lower TCR signaling thresholds for the maturation of CD4 cells. To examine this possibility, expression of Bcl2 family member genes was compared between IK–/– DO11.10 ζ1Tg DP thymocytes that appear to undergo positive selection and their IK+/+ counterparts that do not undergo positive selection. DP thymocytes were chosen as the target population because these are the cells that would be initiating the changes in gene expression required for positive selection.
Many Bcl2 family member genes were expressed at statistically equivalent levels in IK–/– DO11.10 ζ1Tg and IK+/+ DO11.10 ζ1Tg DP thymocytes (Fig. 4A). Exceptions were Bcl-xL and Mcl-1, which were expressed at lower levels, and Bcl-w and Bcl2A1, which were expressed at higher levels in IK–/– cells. Mcl-1 and Bcl-xL expression levels parallel each other and both are down-regulated by positive selection signals (16, 17), offering an explanation for their lower expression levels in IK–/– DO11.10 ζ1Tg DPs, which, by the evidence we have provided, are receiving these signals. Although expression levels of the antiapoptotic genes Bcl2A1 and Bcl-w were up-regulated in the absence of IK, it is unlikely that this is sufficient to lower the thresholds of positive selection. Bcl2A1 overexpression has been reported to prolong the survival of immature DP thymocytes, but it cannot drive positive selection in the absence of a sufficient TCR signal (18). Also, no role for Bcl-w has been reported in thymocytes and its expression levels, in both the presence and absence of IK, are extremely low (Fig. 4A).
FIGURE 4.
Comparison of expression levels of Bcl2 family and Notch target genes and levels of Akt phosphorylation in the presence and absence of IK. A, Expression levels of Bcl2 family genes were analyzed by qRT-PCR in IK+/+ and IK–/– DO11.10 ζ1Tg DP thymocytes. Bar graph shows levels relative to those of HPRT. Bcl2, Bclxl, Mcl-1, Bcl-w, and Bcl2A1 are antiapoptotic, whereas Bim, Bclxs, Bak, and Bax are proapoptotic. B, Expression levels of Notch target genes Hes1 and Deltex1 were analyzed by qRT-PCR in IK+/+ and IK–/– DO11.10 ζ1Tg DP thymocytes. Bar graph shows levels relative to those of HPRT. All experiments were performed at least three times in duplicate. Error bars represent the SEM. p values were calculated using paired Student t tests. *, p < 0.1; **, p < 0.05. C, Akt phosphorylation levels were analyzed by Western blot analyses using protein extracts prepared from IK–/– and IK+/+ thymocytes activated by TCR cross-linking for the indicated amounts of time. Levels of Akt were analyzed as a loading control. IB, Immunoblotting.
Deregulation of Notch target gene expression in IK –/– DO11.10 ζ 1Tg DP thymocytes
The complete process of positive selection cannot be induced by TCR cross-linking ex vivo unless thymocytes are cocultured with thymic epithelium. The thymic cortical epithelium expresses Notch ligands, and Notch signals can act to enhance TCR signal transduction in DP thymocytes (19, 20). Thus, deregulation of Notch target gene expression may also be hypothesized to reduce TCR signaling thresholds for positive selection. Lack of IK leads to deregulated Notch target gene expression in thymocytes (21–23), suggesting that deregulation of the Notch-induced genes in IK–/– DP thymocytes could facilitate positive selection with suboptimal signals. Therefore, expression levels for two canonical Notch target genes, Hes1 and Deltex1, were compared in IK+/+ DO11.10 ζ1Tg and IK–/– DO11.10 ζ1Tg DP thymocytes. Both genes were expressed at higher levels in IK–/– DO11.10 ζ1Tg DP thymocytes (Fig. 4B). These data suggest that the mechanism by which IK influences signaling thresholds for positive selection could be through its role in Notch target gene regulation. How this results in lowered thresholds specifically for the CD4 lineage is under investigation. Finally, we examined the impact of lack of IK on cytoplasmic phosphorylation events that occur in response to TCR signaling in thymocytes. Interestingly, although no observable differences in the gross levels of tyrosine phosphorylation were observed in IK–/– thymocytes as compared with their wild-type counterparts, levels of Erk phosphorylation appeared to be slightly sustained and levels of Akt phosphorylation were increased (Fig. 4C and supplemental Fig. S5). It is interesting to speculate that the enhanced Akt activation observed in IK–/– thymocytes could result in a survival advantage in the presence of less TCR signal and therefore could contribute to the phenotype reported in this study.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Elizabeth Shores for providing the ζ-chain transgenic mice, Jenny Lehman for excellent animal care and genotyping, and Dr. Richard Longnecker for providing Abs for the Western blot analyses.
Footnotes
This work was supported by American Heart Association Grant 0650111Z and National Institutes of Health Grant R01 CA104962-01A1 (to S.W.). J.A.U. was supported by Public Health Service Grant 5 T32 AI07476-08.
Abbreviations used in this paper: DP, double positive, CD40L, CD40 ligand; IK, Ikaros; qRT-PCR, quantitative RT-PCR; SP, single positive; Tg, transgene/transgenic.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr. Opin. Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 3.Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the ζ chain of the T cell antigen receptor complex. Science. 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- 4.Shores EW, Huang K, Tran T, Lee E, Grinberg A, Love PE. Role of TCR ζ chain in T cell development and selection. Science. 1994;266:1047–1050. doi: 10.1126/science.7526464. [DOI] [PubMed] [Google Scholar]
- 5.Shores EW, Tran T, Grinberg A, Sommers CL, Shen H, Love PE. Role of the multiple T cell receptor (TCR)-ζ chain signaling motifs in selection of the T cell repertoire. J. Exp. Med. 1997;185:893–900. doi: 10.1084/jem.185.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10:333–343. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 8.Urban JA, Winandy S. Ikaros null mice display defects in T cell selection and CD4 versus CD8 lineage decisions. J. Immunol. 2004;173:4470–4478. doi: 10.4049/jimmunol.173.7.4470. [DOI] [PubMed] [Google Scholar]
- 9.Itano A, Salmon P, Kioussis D, Tolaini M, Corbella P, Robey E. The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J. Exp. Med. 1996;183:731–741. doi: 10.1084/jem.183.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 11.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 12.Love PE, Lee J, Shores EW. Critical relationship between TCR signaling potential and TCR affinity during thymocyte selection. J. Immunol. 2000;165:3080–3087. doi: 10.4049/jimmunol.165.6.3080. [DOI] [PubMed] [Google Scholar]
- 13.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 14.Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J. Exp. Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 16.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J. Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao DT, Korsmeyer SJ. BCL-XL-regulated apoptosis in T cell development. Int. Immunol. 1997;9:1375–1384. doi: 10.1093/intimm/9.9.1375. [DOI] [PubMed] [Google Scholar]
- 18.Verschelde C, Michonneau D, Trescol-Biemont MC, Berberich I, Schimpl A, Bonnefoy-Berard N. Overexpression of the antiapoptotic protein A1 promotes the survival of double positive thymocytes awaiting positive selection. Cell Death Differ. 2006;13:1213–1221. doi: 10.1038/sj.cdd.4401814. [DOI] [PubMed] [Google Scholar]
- 19.Laky K, Fleischacker C, Fowlkes BJ. TCR and Notch signaling in CD4 and CD8 T-cell development. Immunol. Rev. 2006;209:274–283. doi: 10.1111/j.0105-2896.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang YH, Li D, Winoto A, Robey EA. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc. Natl. Acad. Sci. USA. 2004;101:4936–4941. doi: 10.1073/pnas.0401133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kathrein KL, Chari S, Winandy S. Ikaros directly represses the Notch target gene Hes1 in a leukemia T cell line: implications for CD4 regulation. J. Biol. Chem. 2008;283:10476–10484. doi: 10.1074/jbc.M709643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumortier A, Jeannet R, Kirstetter P, Kleinmann E, Sellars M, dos Santos NR, Thibault C, Barths J, Ghysdael J, Punt JA, et al. Notch activation is an early and critical event during T-cell leukemogenesis in Ikaros-deficient mice. Mol. Cell. Biol. 2006;26:209–220. doi: 10.1128/MCB.26.1.209-220.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chari S, Winandy S. Ikaros regulates Notch target gene expression in developing thymocytes. J. Immunol. 2008;181:6265–6274. doi: 10.4049/jimmunol.181.9.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.