Abstract
Interactions between nicotine and learning could contribute to nicotine addiction. Although previous research indicates that nicotine withdrawal disrupts contextual learning, the effects of nicotine withdrawal on contextual memories acquired before withdrawal are unknown. The present study investigated whether nicotine withdrawal disrupted recall of prior contextual memories by examining the effects of nicotine withdrawal on recall of nicotine conditioned place preference (CPP) and contextual fear conditioning. C57BL/6J mice trained in CPP exhibited a significant preference for an initially non-preferred chamber that was paired with 0.35 mg/kg nicotine. Following CPP, mice were implanted with mini-osmotic pumps containing 6.3 mg/kg/d nicotine or saline. Pumps were removed twelve days later and nicotine CPP was retested 24 hours later. Mice withdrawn from chronic nicotine exhibited CPP, suggesting that older drug-context associations are not disrupted by nicotine withdrawal. One hour later, the same mice were trained in contextual and cued fear conditioning; nicotine withdrawal disrupted contextual but not cued fear conditioning. A subsequent experiment demonstrated that nicotine withdrawal did not disrupt recall of contextual or cued fear conditioning when acquisition occurred before nicotine withdrawal. These data suggest that nicotine withdrawal disrupts new contextual learning, but does not alter contextual learning that occurred before withdrawal.
Keywords: Nicotine, Withdrawal, Learning, Reward, Addiction, Hippocampus
Introduction
Investigations into the neurobiology of addiction have revealed that drugs of abuse can alter learning and memory and that many of the neural substrates disrupted by drug addiction also play an important role in learning and memory (Davis and Gould, 2008; Gould, 2006; Hyman, 2005; Kelley, 2004). However, the overlap between the neurobiology of nicotine addiction and learning and memory is only beginning to be understood. Several studies have reported that the effects of nicotine can be associated with contextual or discrete cues. Lazev and colleagues (1999) found that neutral environmental cues can be associated with cigarette smoking and will elicit cravings when presented alone. Furthermore, smoking-related stimuli can produce increases in cravings in both withdrawn smokers and non-withdrawn smokers and are associated with changes in the function of brain regions previously implicated in both addiction and learning, such as the hippocampus, amygdala, and the ventral tegmental area (Due et al., 2002; Franklin et al., 2007; Geier et al., 2000). In addition, contextual cues that indicate smoking availability increase cravings for smoking (Dols et al., 2002; Dols et al., 2000; Thewissen et al., 2005). Together, these studies suggest that maladaptive associations between the effects of nicotine and contextual stimuli may contribute to cravings that facilitate the development and maintenance of nicotine addiction.
Further support for the link between nicotine-induced changes to learning and memory and nicotine addiction comes from studies investigating nicotine conditioned place preference (CPP) in rodents, and the effects of nicotine on contextual fear conditioning in mice. In the nicotine CPP procedure, rodents develop a preference for a chamber that is consistently paired with nicotine (Calcagnetti and Schechter, 1994; Fudala et al., 1985; Risinger and Oakes, 1995), which suggests that an association is made between the rewarding properties of nicotine and contextual stimuli. Thus, environmental/contextual stimuli associated with nicotine may facilitate drug seeking/taking behavior. In support, nicotine self-administration can be enhanced or reinstated by the presentation of environmental stimuli that were previously associated with nicotine self-administration (Caggiula et al., 2001, 2002; LeSage et al., 2004). In addition to nicotine being able to enter into associations with contextual features, nicotine may also enhance contextual learning (Davis et al., 2005; Gould, 2003; Gould and Higgins, 2003; Gould and Wehner, 1999; Kenney and Gould, 2008). Thus, nicotine can facilitate contextual learning which may lead to the formation of strong but maladaptive drug-context associations that could evoke cravings.
The ability of nicotine to facilitate contextual learning may not be the only way in which the effects of nicotine on learning contribute to nicotine addiction; nicotine withdrawal could disrupt learning-related processes and smokers may relapse in order to ameliorate the deficits (Gould, 2006). Nicotine withdrawal produces numerous symptoms in humans that include disruption of cognitive processes such as attention (Jacobsen et al., 2005; Rukstalis et al., 2005), concentration (Hughes et al., 1991; Hughes et al., 1994), and learning and memory (Jacobsen et al., 2005; Jacobsen et al., 2007; Mendrek et al., 2006). In fact, changes in cognition during abstinence from tobacco predict relapse (Rukstalis et al., 2005). Consistent with these data, withdrawal from chronic nicotine produces deficits in contextual fear conditioning in mice (André et al., 2008; Davis et al., 2005; Portugal and Gould, 2007; Portugal et al., 2008), and these deficits are ameliorated by drugs that are efficacious in treating nicotine addiction (Portugal and Gould, 2007; Raybuck et al., 2008). Thus, the aversive effects of nicotine withdrawal on cognitive processes may contribute to maintenance of nicotine addiction. However, these studies withdrew nicotine prior to training; therefore, it is unknown if withdrawal from chronic nicotine treatment disrupts acquisition and/or recall of contextual memories.
The effects of nicotine and nicotine withdrawal on learning may work together to promote nicotine addiction. As reviewed, nicotine could facilitate the formation of maladaptive drug-context associations that could lead to drug-seeking behavior when re-exposed to the associated contexts, and nicotine withdrawal-related disruption of cognitive processes could trigger relapse. If maladaptive drug-context associations acquired before nicotine withdrawal are intact during withdrawal, then cravings elicited by contextual stimuli during withdrawal may act in combination with withdrawal-related learning impairments to further facilitate relapse. Although contextual fear conditioning that occurs during nicotine withdrawal is disrupted, the effects of nicotine withdrawal on contextual memories acquired prior to withdrawal remain unknown. The present study examined the effects of nicotine withdrawal on contextual learning that occurred prior to withdrawal and learning that occurred during nicotine withdrawal.
Methods
Subjects
C57BL/6J mice were trained at 8–12 weeks of age, with 8 mice per condition in each experiment. Mice were housed in groups of four per cage and were maintained on a 12 hour light-dark cycle (lights on from 7:00 am – 7:00 pm) with unrestricted access to food and water. Experiments took place during the light phase of the cycle. All behavioral and surgical procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
The training and testing of nicotine CPP occurred in four identical boxes (41.4 cm × 21.6 cm × 21.6 cm); each box contained two distinct chambers that measure 19.7 cm × 20.3 cm × 18.3 cm and were modeled after those used by Walters and colleagues (2005). In one chamber, the walls and floor were composed of gray Plexiglas, whereas the other chamber had gray Plexiglas walls with 1.3 cm-wide white stripes and a stainless steel mesh floor. Both chambers had clear Plexiglas lids. The wall that separated the chambers had an opening (5.1 cm × 5.1 cm) so that mice could access both sides; this wall was replaced during conditioning trials with another wall that prevented mice from moving between chambers. Data were collected with a ceiling-mounted video camera that was connected to a television and VCR. All sessions were recorded onto VHS and the preference for each chamber was scored manually.
The training and testing of contextual fear conditioning occurred in four identical chambers (17.78 cm × 19.05 cm × 38.10 cm) that were housed in sound attenuating boxes (Med-Associates, St. Albans, VT). The front, back, and top chamber walls of the conditioning chambers were made of clear Plexiglas, and side walls were made of stainless steel. The floors of the chambers were composed of 18 stainless steel bars that were connected to a shock generator and scrambler. Ventilation fans mounted on the right wall of the sound attenuating boxes provided air exchange and background noise (69 dB), and speakers attached to the right wall of each chamber were used to administer the white noise conditioned stimulus (CS). The chambers were connected to a computer that used Med-PC software (Med-Associates, St. Albans, VT) to control stimulus administration. Mice were tested for cued fear conditioning in four identical altered chambers (20.32 × 22.86 × 17.78 cm) that were housed in sound attenuating boxes and located in a different room from the training chambers. The front, back, and top walls of these chambers were comprised of clear Plexiglas, and the side walls of the chambers were made of aluminum. The chamber floors were made of white plastic. Speakers were mounted on the left wall of each chamber and were used to administer the white noise CS. A vanilla extract olfactory cue was added to further distinguish these chambers from the training chambers.
Drugs and Administration
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in saline at 0.35 mg/kg (all doses reported in freebase nicotine weight), and was administered subcutaneously with an injection volume of 10 ml/kg. This dose of nicotine was based on prior research with C57BL/6J mice demonstrating that 0.3 and 0.5 mg/kg nicotine will produce nicotine CPP (Grabus et al., 2006; Walters et al., 2006). In order to investigate the effects of nicotine withdrawal on nicotine CPP and fear conditioning, nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in saline and was loaded into mini-osmotic pumps (model 1002; Alzet, Cupertino, CA). Mini-osmotic pumps that contained either 6.3 mg/kg/day nicotine or saline were implanted subcutaneously. This dose of chronic nicotine was chosen because previous research has demonstrated that withdrawal from 6.3 mg/kg/day nicotine produces deficits in contextual fear conditioning in C57BL/6J mice (André et al., 2008; Davis et al., 2005; Portugal and Gould, 2007; Raybuck et al., 2008). Furthermore, this dose of chronic nicotine produces plasma nicotine levels within the range observed in smokers (Benowitz et al., 1989; Davis et al., 2005; Henningfield and Keenan, 1993). Mini-osmotic pumps were removed from all mice after 12 days of chronic treatment. Mice were anesthetized with 5% isoflurane (Abbott Laboratories, North Chicago, IL) for pump insertion and removal surgeries.
Experiment 1: The effects of withdrawal on nicotine conditioned place preference and fear conditioning
Handling and Habituation
The first experiment investigated the effects of nicotine withdrawal on contextual associations that were acquired either before nicotine withdrawal or during nicotine withdrawal. In the first phase of the experiment, mice were handled and were habituated to the experimental room the week prior to the nicotine CPP experiment. This procedure was used because previous research found that handling and habituation facilitates nicotine CPP (Grabus et al., 2006). Mice were transported from the animal colony to the experimental room and were habituated to the experimental room for 6 hours (9:00 am – 3:00 pm) on the Wednesday – Friday of the week before the experiment commenced. After the first hour of habituation, each mouse was handled for ~1 min. During handling, mice were stroked on the neck and back and then held as if a subcutaneous injection was to be administered. After handling, mice remained undisturbed in the experimental room for approximately 3 hours until handled again in the same manner as described. Two hours later, mice were returned to the animal colony.
Conditioned Place Preference
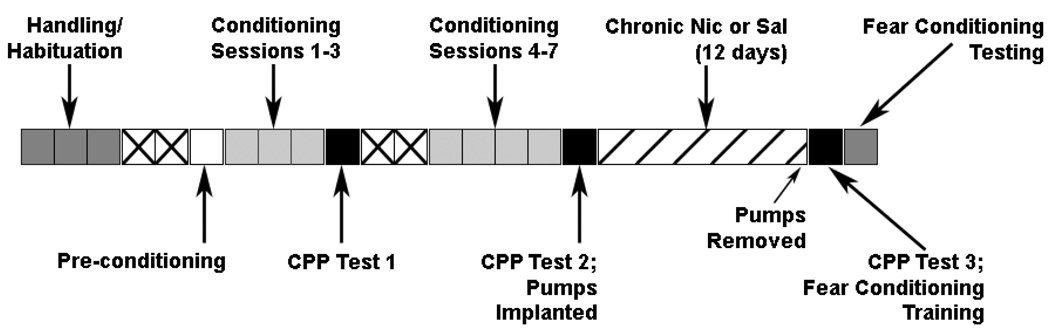
The nicotine CPP experiment began on the Monday after the last day of handling and habituation (see Figure 1 for a graphic depiction of the experimental design). The first day was a pre-conditioning day: mice were randomly placed into one of the two chambers and had unrestricted access to both chambers for 15 minutes. Entry into a chamber was defined as instances when the head and all four paws of the mouse had crossed over into the chamber (Vastola et al., 2002). The amount of time spent in both chambers was recorded, and these data were used to determine which chamber was paired with nicotine. Specifically, nicotine was paired with the chamber that was least preferred by mice on the pre-conditioning day. On pre-conditioning day, most subjects preferred the chamber with striped walls: 75% of mice assigned to nicotine CPP and 69% of mice assigned to saline CPP preferred the striped chamber. On average, mice in the nicotine CPP group spent 495.3 ± 14.6 seconds out of 900 seconds in the preferred striped chamber, and saline CPP mice spent 494.6 ± 18.1 seconds out of 900 seconds in the preferred striped chamber. This initial preference for the striped chamber is within the range reported in other studies of nicotine CPP that used a biased design (Korkosz et al., 2006; Rauhut et al., 2008; Tammimaki et al., 2008). Following pre-conditioning, mice were trained with conditioning sessions that occurred on days 2 – 4. During conditioning, mice in nicotine CPP groups received a subcutaneous injection of either 0.35 mg/kg nicotine or saline and were immediately confined to the nicotine or saline-paired chamber, respectively, for 15 minutes. Five hours later, mice were treated with the alternate drug condition and were immediately placed in the opposite chamber for 15 minutes. To rule out the possibility of order effects, the schedule of injections was counterbalanced within conditioning sessions so that half of the mice received saline and half of the mice received nicotine during the first conditioning trial. The order of injections was also counterbalanced between days, such that mice were administered alternating injections of nicotine or saline for the first conditioning trial across multiple days. Mice that were assigned to saline CPP groups received subcutaneous injections of saline before being placed in both chambers for 15 minutes. On day 5 (testing day), mice were placed in the saline-paired chamber and were allowed to move freely between the two chambers for 15 minutes.
Figure 1.
A schematic of the design for experiment 1. Each box represents a day and the color coding and text indicates the experimental manipulations that took place on that day. Boxes with an “X” indicate days in which no experimental manipulations occurred.
Additional Conditioning and Testing
In the next phase of the experiment, mice received four additional days of conditioning that began on the Monday following the first test of nicotine CPP. The use of additional conditioning trials was based on previous research which demonstrated that additional conditioning produces robust nicotine CPP in rats (Wilkinson and Bevins, 2008). These conditioning sessions were identical to those described in the previous section. Overall, mice assigned to nicotine CPP groups received 7 drug and 7 saline conditioning trials. Twenty-four hours after the last conditioning session, a second test of nicotine CPP was conducted in the same manner as described. Two hours after the second CPP test, mice were implanted with mini-osmotic pumps containing 6.3 mg/kg/d nicotine or saline. Thus, mice were assigned to four possible conditions: nicotine CPP/withdrawal from chronic nicotine (WCN), nicotine CPP/withdrawal from chronic saline (WCS), saline CPP/WCN, and saline CPP/WCS (n = 8 for all groups). Chronic nicotine or saline administration continued for 12 days following pump implantation, and all pumps were removed on the 12th day of chronic nicotine or saline treatment. A final test of nicotine CPP, which was identical to the previous tests, was conducted 24 hours after the removal of mini-osmotic pumps. One hour following the final CPP test, the training of contextual and cued fear conditioning commenced.
Contextual and Cued Fear Conditioning
In the final phase of the experiment, mice withdrawn from chronic nicotine or saline were trained and tested in fear conditioning. Freezing, defined as the lack of all movement other than respiration (Blanchard and Blanchard, 1969) was used as the measure of conditioned fear. Freezing was scored using a time-sampling procedure, in which mice were observed for one second during 10 second bins and were scored as freezing or active (Gould and Wehner, 1999). Training began one hour after the final test of nicotine CPP. Mice were placed in the chamber and baseline activity was scored for 120 seconds. After baseline, mice were exposed to two CS (85 dB white noise) – unconditioned stimulus (US; 0.57 mA footshock) pairings separated by a 120 second inter-trial interval. The white noise CS presentation lasted for 30 seconds and co-terminated with a 2 second footshock US. Immediate freezing was scored during the 120 second inter-trial interval that occurred before the second CS – US pairing. Following the last CS – US pairing, the training session ended with a 30 second period during which freezing behavior was not recorded. The testing of contextual fear conditioning occurred 24 hours after training. Mice were returned to the training chambers and freezing was scored for 5 minutes. One hour later, mice were placed in an altered context to test for both generalized freezing and for cued fear conditioning. Specifically, mice were loaded into the altered context chambers and generalized freezing was scored for the first 3 minutes. After generalized freezing was scored, the CS was continuously activated and cued fear conditioning was scored for the next 3 minutes.
Experiment 2: Contextual fear conditioning is not disrupted if acquired before nicotine withdrawal
The second experiment investigated the effects of withdrawal from chronic nicotine on fear conditioning if withdrawal occurred 15 days after the training of contextual and cued fear conditioning. On the first day of the experiment, mice were trained in contextual and cued fear conditioning as described in the previous section. Forty-eight hours after training, mini-osmotic pumps containing either 6.3 mg/kg/d nicotine (n = 8) or saline (n = 8) were implanted. Chronic nicotine or saline pumps were removed 12 days later. The test of contextual and cued fear conditioning occurred 24 hours after pump removal (15 days after training); mice were tested in the same manner as described previously.
Statistical Analyses
For the nicotine CPP experiment, preference scores were calculated by subtracting the time spent in the nicotine-paired chamber on pre-conditioning day from the time spent in the same chamber on testing day (expressed in seconds). For each of the first two nicotine CPP tests, preference scores from nicotine CPP/WCN and nicotine CPP/WCS groups were pooled together and were compared to pooled saline CPP data using independent sample t-tests because these mice had not yet received chronic nicotine treatment and so there was no chronic treatment difference between pooled groups. The preference scores from the final nicotine CPP test that occurred after nicotine withdrawal was analyzed using 2 (withdrawal condition: WCN, WCS) × 2 (CPP condition: nicotine CPP, saline CPP) ANOVA. A linear contrast was used to make a planned comparison between nicotine CPP/WCN mice and saline CPP/WCN mice. A separate linear contrast compared nicotine CPP/WCS mice to saline CPP/WCS mice. Following nicotine withdrawal, mice were trained and tested in contextual and cued fear conditioning. These data were analyzed using a 2 (withdrawal condition: WCN, WCS) × 2 (CPP condition: nicotine CPP, saline CPP) ANOVA, and Tukey post-hoc tests were used to test pair-wise comparisons between specific experimental conditions. Fear conditioning data from the final experiment was analyzed using an independent samples t-test.
Results
The effects of withdrawal on nicotine conditioned place preference and fear conditioning
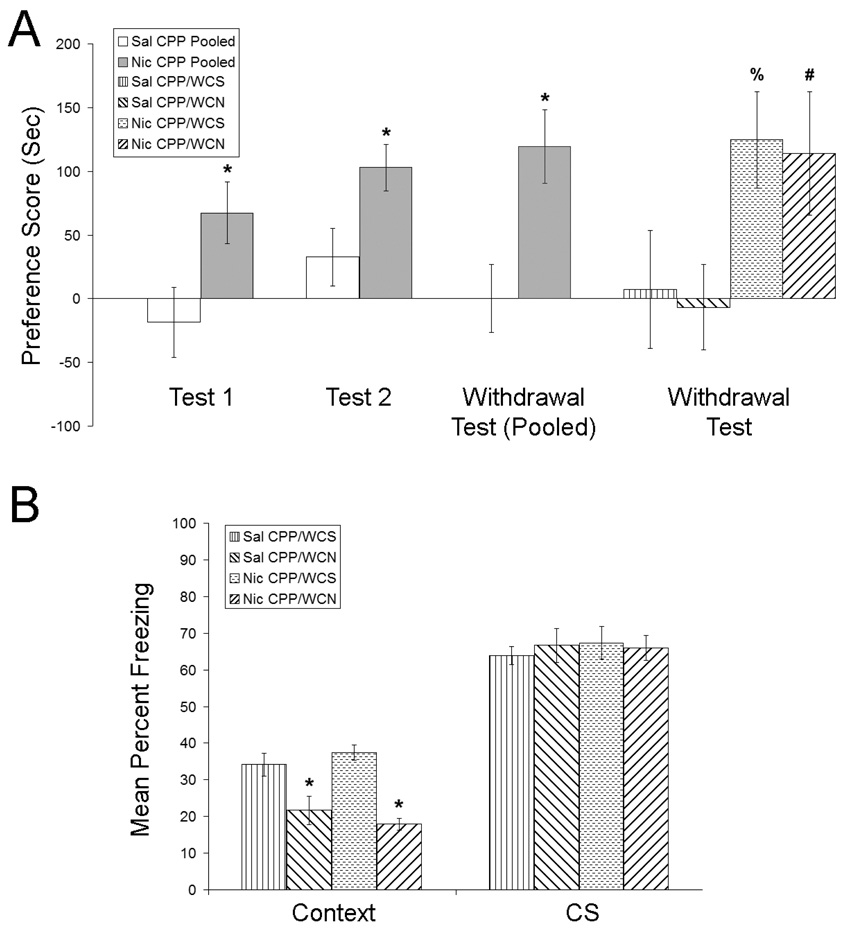
To investigate whether nicotine withdrawal alters contextual associations that were acquired before withdrawal, mice were trained in the nicotine CPP procedure (Figure 2a). An independent samples t-test revealed that mice treated with 0.35 mg/kg nicotine exhibited CPP for the nicotine-paired chamber relative to saline-treated controls following 3 conditioning sessions [t(30) = 2.41, p < 0.05]. Similarly, an independent samples t-test revealed a significant CPP for nicotine at the second CPP test [t(30) = 2.50, p < 0.05]. For the 3rd test of nicotine CPP that occurred during nicotine withdrawal, a 2 (withdrawal condition) × 2 (CPP condition) ANOVA revealed a significant main effect for the CPP condition [F(1, 28) = 9.20, p < 0.05]. In contrast, no significant main effect for withdrawal (nicotine vs. saline) was observed (p > 0.05), nor was there a significant CPP by withdrawal interaction (p > 0.05). Planned comparisons revealed a significant difference between nicotine CPP/WCN mice and saline CPP/WCN mice [t(28) = 2.17, p < 0.05], and a significant difference between nicotine CPP/WCS mice and saline CPP/WCS mice [t(28) = 2.12, p < 0.05]. Following the final test of nicotine CPP, mice were trained and tested in fear conditioning (Figure 2b). A 2 (withdrawal condition) × 2 (CPP condition) ANOVA revealed a significant main effect for the withdrawal condition [F(1, 28) = 37.36, p < 0.05] when mice were tested for contextual fear conditioning. However, there was no significant main effect of the CPP condition that mice were previously assigned to (nicotine vs. saline CPP; p > 0.05), nor was there a significant CPP by withdrawal interaction (p > 0.05). Subsequent Tukey post-hoc tests revealed that both groups of mice withdrawn from chronic nicotine exhibited deficits in contextual fear conditioning when compared to both groups that were withdrawn from chronic saline (p < 0.05). No significant differences were observed in baseline or immediate freezing measured on training day, nor were there any differences between groups in pre-CS freezing or in cued fear conditioning (p > 0.05). Taken together, these data suggest that nicotine withdrawal does not alter drug-context associations that were acquired before withdrawal. However, if contextual associations are acquired during nicotine withdrawal, then deficits are observed.
Figure 2.
The effects of nicotine withdrawal on nicotine CPP acquired before chronic nicotine administration (2a). Mice treated with 0.35 mg/kg nicotine exhibited a significant preference for the nicotine-paired chamber following 3 conditioning trials, and after 4 more conditioning trials. This preference persisted following withdrawal from chronic nicotine or saline, suggesting that nicotine CPP is long lasting and is not altered by nicotine withdrawal. Error bars indicate SEM, (*) indicates p < 0.05 compared to saline CPP data, (%) indicates p < 0.05 compared to saline CPP mice withdrawn from chronic saline, and (#) indicates p < 0.05 compared to saline CPP mice withdrawn from chronic nicotine. The effects of nicotine withdrawal on contextual and cued fear conditioning (2b). During withdrawal from chronic nicotine or saline, mice were trained in contextual and cued fear conditioning after the final test of nicotine CPP. Mice withdrawn from 6.3 mg/kg/d nicotine exhibited deficits in contextual but not cued fear conditioning, suggesting that nicotine withdrawal disrupts contextual learning that occurs during withdrawal. Error bars indicate SEM, and (*) indicates p < 0.05 compared to mice withdrawn from chronic saline.
Contextual fear conditioning is not disrupted if acquired before nicotine withdrawal
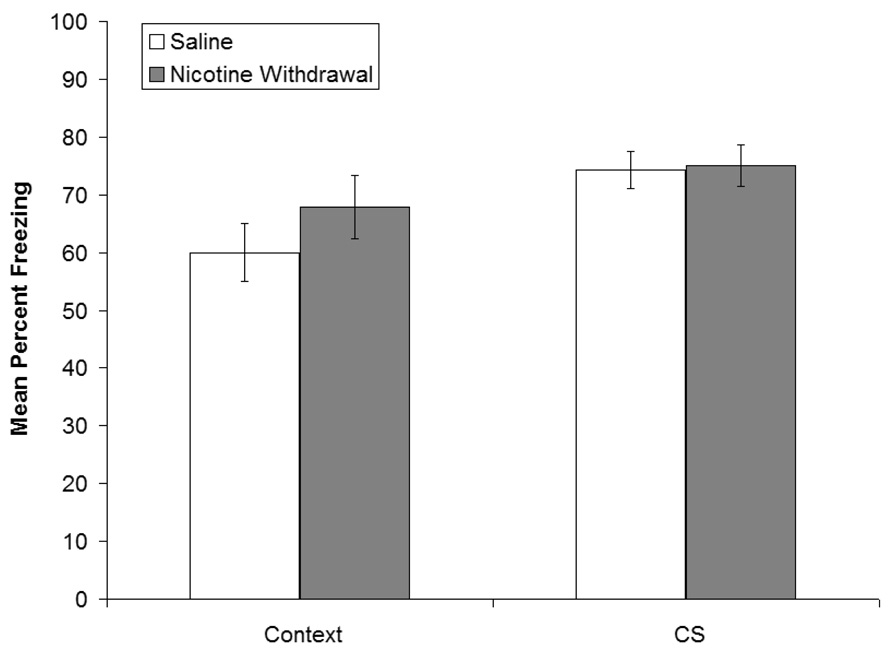
In order to determine whether nicotine withdrawal will disrupt aversive contextual associations that are learned before nicotine withdrawal, mice were trained in contextual and cued fear conditioning and were treated with chronic nicotine for 12 days starting 48 hours after training. Testing occurred during nicotine withdrawal, 15 days after training (Figure 3). No significant differences were observed in contextual fear conditioning between mice withdrawn from chronic nicotine or saline (p > 0.05). Furthermore, no significant differences were observed in baseline freezing, immediate freezing, pre-CS freezing or cued fear conditioning (WCN: mean = 75%, SEM = 3.55%; WCS: mean = 74.31%, SEM = 3.16%; p > 0.05). Thus, withdrawal from chronic nicotine did not disrupt recall of fear conditioning-associated memories.
Figure 3.
The effects of withdrawal from chronic nicotine on the recall of contextual and cued fear conditioning. Mice were trained in contextual and cued fear conditioning and osmotic mini-pumps that delivered chronic nicotine or saline were implanted 48 hours later. Chronic nicotine or saline was administered for 12 days and testing occurred during nicotine withdrawal, 15 days after training. No significant differences were observed in contextual or cued fear conditioning between mice withdrawn from chronic nicotine or saline. Error bars indicate SEM.
Discussion
The results of the present study are the first to reveal that withdrawal from chronic nicotine treatment differentially affects contextual learning depending upon whether acquisition takes place before or during nicotine withdrawal. Mice trained in nicotine CPP or contextual fear conditioning before chronic nicotine treatment and subsequent withdrawal showed unimpaired recall of contextual memories. In contrast, mice trained 24 hours after cessation of chronic nicotine treatment had deficits in contextual fear conditioning. Thus, nicotine withdrawal does not impair recall of contextual learning acquired before withdrawal but instead impairs acquisition of new contextual associations. Disrupted learning during withdrawal along with intact prior drug-context memories could be particularly problematic for smokers trying to quit. Cognitive deficits during withdrawal could interfere with learning adaptive behaviors while facilitating relapse as a means of ameliorating the deficits, and maladaptive drug-context memories could trigger cravings that also lead to relapse.
Another novel finding of the current study is that nicotine CPP-associated memories were long-lasting. The fact that mice exhibited nicotine CPP 13 days after the last conditioning session suggests that the association between the rewarding effects of nicotine and contextual stimuli enters into long-term memory storage. These data are consistent with research in humans demonstrating that smoking-related contextual cues can evoke cravings (Dols et al., 2002; Dols et al., 2000; Thewissen et al., 2005) and that evoked cravings last several weeks after nicotine withdrawal (Jarvis, 2004). The long-lasting ability of contextual stimuli to increase cravings may help explain why nicotine is one of the most addictive substances (Anthony et al., 1994). Although nicotine withdrawn mice did not exhibit deficits in nicotine CPP, it is possible that impairment may be observed if higher doses of chronic nicotine are administered. Therefore, future studies should examine whether withdrawal from higher doses of nicotine can disrupt the recall of nicotine CPP.
The finding that withdrawal from chronic nicotine treatment specifically disrupted acquisition of new contextual memories suggests that during chronic nicotine treatment, adaptations occur to neural processes involved in contextual learning. This is supported by a prior study which showed that the nAChR antagonist dihydro-beta-erythroidine (DHβE) had no effect in control mice but produced deficits in contextual fear conditioning in mice treated with chronic nicotine (Portugal et al., 2008), suggesting that changes occur with chronic nicotine treatment in nAChR function or in downstream processes. Furthermore, these data suggest that the acquisition of other types of contextual learning may similarly be disrupted during nicotine withdrawal. For instance, it has been demonstrated that the extinction of previously learned associations depends upon the context in which extinction is learned (Bouton et al., 2006; Frohardt et al., 2000). Thus, the acquisition of extinction may be disrupted if extinction training occurs during nicotine withdrawal. Also consistent with previous research (André et al., 2008; Davis et al., 2005; Portugal et al., 2008), the deficits observed were specific to contextual fear conditioning but not cued fear conditioning. This is an important observation because it demonstrates that the withdrawal deficits in contextual fear conditioning are due to changes in processes specific for acquisition of contextual fear conditioning, and are not due to non-specific changes that would affect both acquisition and recall of contextual and cued fear conditioning, such as changes in locomotor activity, changes in anxiety, or a generalized malaise. Thus, this specificity of the deficits in acquisition of contextual fear conditioning may help identify the underlying neural substrates.
Whereas both nicotine-context associations and nicotine withdrawal-related disruption of learning may contribute to nicotine addiction, it is not clear if these two processes share common neural, molecular, and genetic substrates. Previous studies demonstrated that contextual fear conditioning is hippocampus-dependent (Logue et al., 1997; Phillips and LeDoux, 1992), and infusions of nicotine into the dorsal hippocampus are sufficient to enhance contextual fear conditioning (Davis et al., 2007). The involvement of the hippocampus in nicotine CPP has not been directly tested. Only one study to date has investigated possible brain regions required for nicotine CPP; Spina and colleagues (2006) demonstrated that the infusion of a selective D1 antagonist (SCH 39166) into the nucleus accumbens (NAc) shell disrupted acquisition of nicotine CPP. Studies of other substances of abuse, however, suggest that the hippocampus plays an important role in CPP. Disruption of hippocampal function blocked CPP for morphine (Rezayof et al., 2003; Rezayof et al., 2006; Zarrindast et al., 2007), cocaine (Meyers et al., 2006), and amphetamine (Sakurai et al., 2007). The involvement of the hippocampus in multiple forms of drug CPP, along with evidence that the effects of nicotine on contextual fear conditioning are hippocampus-dependent, suggests that the hippocampus may be required for nicotine CPP; future research should investigate this issue.
Besides potentially sharing common hippocampal substrates, nicotine CPP and nicotine-induced changes in contextual fear conditioning may be mediated by similar nAChRs. β2-containing nAChRs are required for nicotine CPP, as well as for the effects of acute nicotine and nicotine withdrawal on contextual fear conditioning. Walters and colleagues (2006) demonstrated that β2 nAChR subunit knockout (KO) mice do not acquire nicotine CPP whereas wild-type (WT) mice do, and that DHβE, an antagonist that binds to high affinity nAChRs such as the α4β2 receptor (Harvey et al., 1996; Williams and Robinson, 1984), blocks nicotine CPP in C57BL/6J mice. Similarly, acute nicotine enhanced contextual fear conditioning in WT mice but not in β2 KO mice (Davis and Gould, 2007; Wehner et al., 2004), and DHβE blocked the enhancement of contextual fear conditioning by acute nicotine in C57BL/6J mice (Davis and Gould, 2006). Furthermore, nicotine withdrawal-related deficits in contextual fear conditioning were present in WT mice but not β2 KO mice, and DHβE-precipitated withdrawal disrupted contextual fear conditioning in C57BL/6J mice treated with chronic nicotine (Portugal et al., 2008). Together, these data suggest that the effects of nicotine on different forms of contextual learning (e.g. CPP vs. contextual fear conditioning) are regulated by common nAChRs.
If nicotine-context associations and nicotine withdrawal-associated disruption of cognitive processes involve the same neural substrates, then similar drugs should be able to treat both evoked cravings and withdrawal deficits in cognitive processes. Nicotine withdrawal-induced deficits in contextual fear conditioning were ameliorated in mice by pharmacotherapeutics for smoking cessation such as acute nicotine (nicotine replacement therapy), bupropion, and varenicline (Davis et al., 2005; Portugal and Gould, 2007; Raybuck et al., 2008). In addition, Brody and colleagues (2004) reported that bupropion reduced nicotine cravings elicited by exposure to smoking-related stimuli. Together, these results suggest that bupropion may disrupt recall of associations between the rewarding effects of nicotine and contextual stimuli and ameliorate nicotine withdrawal-associated cognitive deficits. However, it remains unknown whether other treatments would be similarly effective. Finding drugs that are efficacious for multiple symptoms of nicotine addiction should improve smoking cessation rates.
Similar to other research (Brielmaier et al., 2008; Korkosz et al., 2006; Le Foll and Goldberg, 2005; Rauhut et al., 2008; Tammimaki et al., 2008), the present study used a biased design for nicotine CPP. A recent study by Le Foll and Goldberg (2005) suggests that it is preferable to use a biased design to examine nicotine CPP; however, there are some limitations to using this approach. For instance it has been argued that the increased preference for the initially non-preferred chamber may be due to the anxiolytic effects of nicotine that are associated with the chamber, rather than an association between the rewarding properties of nicotine and the chamber (Bardo and Bevins, 2000; Tzschentke, 1998). It is also possible that a change in preference occurs because mice learn to avoid the chamber associated with saline administration. Although the use of a biased design does not rule out these possible explanations, the increase in preference still results from an association between the context and the effects of nicotine. Thus, the use of a biased design does not limit the finding that contextual associations are not disrupted when they are acquired prior to nicotine withdrawal.
This study is the first to use spontaneous withdrawal from chronic nicotine treatment to examine whether nicotine withdrawal disrupts acquisition or recall of learned processes. The advantage in using this method of withdrawal for these studies is twofold. First, spontaneous withdrawal closely resembles what occurs in smokers trying to abstain from tobacco. Second, while nAChR antagonist-precipitated withdrawal is a powerful tool for examining behavioral and neural changes that occur with chronic nicotine treatment, precipitated withdrawal does not always produce the same symptoms as spontaneous withdrawal. For example, Bruijnzeel and colleagues (2007) reported that changes to intracranial self stimulation (ICSS) during precipitated nicotine withdrawal were ameliorated by a CRF receptor antagonist, whereas these effects were not observed during spontaneous withdrawal. Furthermore, nicotine withdrawal increased light-enhanced startle (LES), but no changes to LES were observed during precipitated nicotine withdrawal (Jonkman et al., 2008). These results suggest that precipitated and spontaneous withdrawal may have different neural effects.
In summary, the results of the present study indicate that contextual learning is not altered by nicotine withdrawal if acquisition occurs prior to withdrawal. In contrast, contextual fear conditioning that occurs during nicotine withdrawal is impaired. Thus, during nicotine withdrawal, persistent drug-context associations along with symptoms of nicotine withdrawal that include deficits in learning could facilitate relapse and maintain nicotine addiction.
Acknowledgements
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG), and the National Cancer Institute/National Institute on Drug Transdisciplinary Tobacco Research Center Grant (P5084718 PI: Caryn Lerman PhD), and thank Virgil Portugal for his assistance with building the nicotine CPP apparatus.
Footnotes
Financial Disclosures: Each author of this manuscript reports no conflicts of interest, financial or otherwise.
References
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behav Brain Res. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., 3rd Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–287. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav. 2008;89:94–100. doi: 10.1016/j.pbb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, Jarvik ME, London ED. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:925–933. doi: 10.1016/0278-5846(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Associative Learning, the Hippocampus, and Nicotine Addiction. Current Drug Abuse Reviews. 2008;1:9–19. doi: 10.2174/1874473710801010009. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dols M, van den Hout M, Kindt M, Willems B. The urge to smoke depends on the expectation of smoking. Addiction. 2002;97:87–93. doi: 10.1046/j.1360-0443.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- Dols M, Willems B, van den Hout M, Bittoun R. Smokers can learn to influence their urge to smoke. Addict Behav. 2000;25:103–108. doi: 10.1016/s0306-4603(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Guarraci FA, Bouton ME. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav Neurosci. 2000;114:227–240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci. 2003;38:124–132. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ. Why people smoke. Bmj. 2004;328:277–279. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33:2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances contextual learning but not context-shock associative learning. Behav Neurosci. 2008 doi: 10.1037/a0012807. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkosz A, Zatorski P, Taracha E, Plaznik A, Kostowski W, Bienkowski P. Effects of ethanol on nicotine-induced conditioned place preference in C57BL/6J mice. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1283–1290. doi: 10.1016/j.pnpbp.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditions of environmental cues to cigarette smoking. Exp Clin Psychopharmacol. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol Biochem Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Hawrylak M, Mardekian SK. Bupropion differentially alters the aversive, locomotor and rewarding properties of nicotine in CD-1 mice. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline Ameliorates Nicotine Withdrawal-Induced Learning Deficits in C57BL/6 Mice. Behav Neurosci. 2008 doi: 10.1037/a0012601. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Zarrindast MR, Sahraei H, Haeri-Rohani A. Involvement of dopamine receptors of the dorsal hippocampus on the acquisition and expression of morphine-induced place preference in rats. J Psychopharmacol. 2003;17:415–423. doi: 10.1177/0269881103174005. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Zatali H, Haeri-Rohani A, Zarrindast MR. Dorsal hippocampal muscarinic and nicotinic receptors are involved in mediating morphine reward. Behav Brain Res. 2006;166:281–290. doi: 10.1016/j.bbr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Oakes RA. Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav. 1995;51:457–461. doi: 10.1016/0091-3057(95)00007-j. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Yu L, Tan SE. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav Pharmacol. 2007;18:497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G. Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacology (Berl) 2006;184:447–455. doi: 10.1007/s00213-005-0211-4. [DOI] [PubMed] [Google Scholar]
- Tammimaki A, Chistyakov V, Patkina N, Skippari J, Ahtee L, Zvartau E, Mannisto PT. Effect of forced chronic oral nicotine exposure on intravenous self-administration and rewarding properties of acute nicotine. Eur J Pharmacol. 2008 doi: 10.1016/j.ejphar.2008.06.081. [DOI] [PubMed] [Google Scholar]
- Thewissen R, van den Hout M, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100:387–396. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacol Biochem Behav. 2008;88:256–264. doi: 10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. J Neurosci. 1984;4:2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Lashgari R, Rezayof A, Motamedi F, Nazari-Serenjeh F. NMDA receptors of dorsal hippocampus are involved in the acquisition, but not in the expression of morphine-induced place preference. Eur J Pharmacol. 2007;568:192–198. doi: 10.1016/j.ejphar.2007.04.015. [DOI] [PubMed] [Google Scholar]