Abstract
Recovery from aphasia can be achieved through recruitment of either peri-lesional brain regions in the affected hemisphere or homologous language regions in the non-lesional hemisphere. For patients with large left-hemisphere lesions, recovery through the right hemisphere may be the only possible path. The right hemisphere regions most likely to play a role in this recovery process are the superior temporal lobe (important for auditory feedback control), premotor regions/posterior inferior frontal gyrus (important for planning and sequencing of motor actions and for auditory-motor mapping) and the primary motor cortex (important for execution of vocal motor actions). These regions are connected reciprocally via a major fiber tract called the arcuate fasciculus (AF), but this tract is usually not as well developed in the non-dominant right hemisphere. We tested whether an intonation-based speech therapy (i.e., Melodic Intonation Therapy) which is typically administered in an intense fashion with 75–80 daily therapy sessions, would lead to changes in white matter tracts, particularly the AF. Using diffusion tensor imaging (DTI), we found a significant increase in the number of AF fibers and AF volume comparing post with pre-treatment assessments in 6 patients that could not be attributed to scan-to-scan variability. This suggests that intense, long-term Melodic Intonation Therapy leads to remodeling of the right AF and may provide an explanation for the sustained therapy effects that were seen in these 6 patients.
Keywords: Melodic Intonation Therapy, Speech Therapy, Plasticity, Diffusion Tensor Imaging, Aphasia
Introduction
Aphasia is a condition characterized by either partial or total loss of the ability to communicate verbally. Aphasic disorders are classified according to fluency of verbal output, and subdivided into one of two categories: either fluent or nonfluent aphasia. Nonfluent aphasia most commonly results from a lesion in the left frontal lobe involving the left posterior inferior frontal region known as Broca’s area1.Patients who are nonfluent usually have the ability to comprehend the speech of others, but are unable to produce words themselves. Fluent aphasia generally results from a lesion involving the posterior superior temporal lobe known as Wernicke’s area. While these patients’ verbal output can be relatively fluent, they may have a prominent comprehension deficit and their jargon-like and/or nonsensical speech is often incomprehensible. The posterior superior temporal region (Wernicke’s area) and the posterior inferior frontal region (Broca’s area) as well as the adjacent premotor cortex are connected via a prominent fiber bundle called the arcuate fasciculus2.A disruption of just this fiber bundle results in a characteristic aphasic disorder known as conduction aphasia. Patients with conduction aphasia are fluent and have relatively good comprehension, however, their ability to repeat words and phrases is significantly impaired1. If a stroke affects the AF fiber bundle and its anterior target regions, then the clinical presentation is usually that of a nonfluent or dysfluent Broca’s aphasia with a greater or lesser degree of impairment to repetition, but relatively intact comprehension. Most patients with aphasia undergo speech therapy in the subacute to chronic phase, however, the outcome in moderate to severely nonfluent patients with large left-hemisphere lesions is often dismal, even with intensive speech therapy. Although the efficacy of speech therapy in general has been shown in several meta-analyses, these analyses have also revealed that therapies might only lead to a measurable effect if the intervention is both intense and long-term3.
Functional imaging methods such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have been used to reveal the functional neural correlates of language recovery, mostly using one assessment either at the presumed end of natural recovery or at the end of an experimental study. The general consensus across all of these studies is that there are two routes to recovery. In patients with small lesions, there tends to be more activation of left-hemisphere peri-lesional cortex and variable right-hemisphere activation either during the recovery process or after recovery. In patients with large left-hemisphere lesions involving most, if not all of the language-capable regions in the left frontotemporal lobes, there tends to be more activation of homologous right-hemisphere language regions4–11. Interestingly, relatively few studies have examined the neural correlates of an aphasia treatment by contrasting pre- and post-therapy assessments10–15.
Structural correlates of language recovery have not yet been examined. Voxel-based morphometry methods may be sensitive enough to detect changes in gray matter densities or gray matter volume, but more meaningful methods might be those capable of detecting changes in connectivity between regions that must function in concert in order to restore any degree of language function after a stroke. In the present study, we aimed to use diffusion tensor imaging to examine possible connectivity changes in chronic aphasic patients undergoing intensive therapy. Diffusion Tensor Imaging (DTI) is a Magnetic Resonance Imaging (MRI) technique that provides information about the diffusion of water molecules (Brownian motion) in the brain. The diffusivity of water molecules provides information about the brain’s microstructure: in regions with high diffusion that has directionality, nerve fibers are likely to go in a similar direction. There has been increasing interest in the DTI technique not only because it allows us to study normal white matter anatomy and structural connectivity16,17, but also because it allows us to study potential remodeling of white matter tracts in stroke patients who are undergoing intense rehabilitation. The method allows filtering of fibers that pass through desired regions of interest (ROI). The resulting isolated tracts are depicted in a probabilistic map reflecting the likelihood of a structural connection between selected regions of the brain18.
The one intervention that specifically seeks to engage homologous right-hemisphere language regions and appears effective at doing so when it is done intensively over a long period of time, is Melodic Intonation Therapy (MIT)19,20. This method was developed in response to the observation that severely aphasic patients can often produce well articulated, linguistically accurate words while singing, but not during speech21–25. MIT is a hierarchically structured treatment that uses intoned (sung) patterns to exaggerate the normal melodic content of speech by translating prosodic speech patterns (spoken phrases) into melodically-intoned patterns using just two pitches11. MIT contains two unique elements that set it apart from other, non-intonation-based therapies: 1) the melodic intonation (singing) with its inherent continuous voicing, and 2) the rhythmic tapping of each syllable (using the patient’s left hand) while phrases are intoned and repeated. Another important characteristic of MIT is that, unlike many therapies administered in the chronic phase that involve one to two short sessions per week, MIT engages patients in intensive treatment totaling 1.5 hrs/day, five days/week until the patient has mastered all three levels of MIT, usually 75–80 or more sessions.
Considering that MIT is applied in a very intense fashion, we aimed to examine whether the intensity of this intonation-based speech therapy in chronic nonfluent aphasic patients with relatively large left-hemisphere lesions would not only lead to functional changes in the brain as reported previously11, but would also change brain structure. It is possible that the sustained therapy effects may actually be due to structural changes in a language network, whether it be one that already exists or one that must be established during the course of treatment in order for the therapy to successfully restore some degree of fluency. The critical structure that facilitates both speech production and its feedforward and feedback control system is the arcuate fasciculus. This structure connects the posterior part of the temporal lobe and inferior parietal region with the inferior frontal region of the brain and is considered to be part of a larger structure, the superior longitudinal fasciculus. In the dominant hemisphere, this pathway is thought to connect Wernicke’s area with Broca’s area. We sought to determine whether or not the arcuate fasciculus in the undamaged right hemisphere would show structural changes as a result of intensive, long-term treatment with MIT.
Material and Methods
Patients
We selected 6 patients from a larger group of patients who had participated either in our MIT-pilot studies or in our ongoing randomized clinical trial examining the behavioral and neural correlates of two speech therapies in nonfluent aphasic patients. These 6 patients were selected because they had moderate to severe nonfluent aphasia with relatively preserved comprehension and were at least 1 year since their first (and only) left-hemisphere stroke. In addition, they had undergone high-resolution MRI studies that had included DTI acquisitions both before and after therapy. In addition, several of these patients had two separate DTI studies done before therapy which allowed us to examine possible scan-to-scan variability in DTI-derived measures and relate that variability to therapy-induced changes in DTI-derived measures. Behavioral assessments which were done several times before therapy, after 75 therapy sessions and again 1 month later included the number of Correct Information Units (CIU)/min produced during spontaneous speech, picture descriptions, and descriptions of common procedures. Secondary outcome measures included correctly named items on standard picture naming tests, as well as syllables per phrase11
Imaging assessments
Structural MRI with DTI was performed using a 3-Tesla General Electric scanner. Anatomical images were acquired using a T1-weighted, three-dimensional, magnetization-prepared, rapid-acquisition, gradient-echo (MPRAGE) volume acquisition with a voxel resolution of 0.93x0.93x1.5mm. DTI was performed using a diffusion-weighted, single-shot, spin-echo, echo-planar, imaging sequence (TE1 = 86.9ms, TR = 10000 milliseconds, FOV = 240 mm, matrix size 128x128 voxels, slice thickness = 5.0 mm, no skip, NEX = 1). Twenty-five noncollinear directions with a b-value of 1000 s/mm2 and one direction with a b-value of 0 s/mm2 were acquired. Fractional anisotropy (FA) values, a measure of the degree of directional preference of water diffusion, were calculated within each brain voxel.
Data Analysis
Tractography was applied to the DTI data to reconstruct white matter tracts by successively following the path of preferred direction of water diffusion when FA is higher than a selected threshold26–28. Using MedINRIA software version 1.5.3 (http://www-sop.inria.fr/asclepios/software/MedINRIA)29 diffusion tensors were calculated from all voxels within the brain and fiber tracts were calculated by connecting adjacent voxels with similar principal eigenvectors, using a threshold FA value of 0.2 and a smoothness factor (a parameter ranging from 0 to 1 corresponding to the straightness of each fiber) of 0.2 for continuous fiber reconstruction30. Only fibers with lengths of >10 mm were included. These parameters are similar to those used by others who applied a fiber assignment by continuous tracking algorithm31,32. Regions of interest were drawn in each brain on sagittal slices (with visual control of the region in the other two orthogonal planes) by a single coder, who was blind to the status of the participants. We identified the arcuate fasciculus according to published DTI atlases33,34. We drew ROIs in the white matter underlying the posterior middle temporal gyrus (pMTG) which contains the largest posterior branch of the AF, and the posterior inferior frontal gyrus (pIFG) to constrain fiber tracts. Fibers were reconstructed using voxels in the pMTG as seed regions and voxels in the pIFG ROIs as the target region.
In addition to the AF fiber tract, we also identified and traced the corticospinal tract (CST) in order to relate AF fibers at each timepoint to an internal control fiber bundle to minimize possible whole brain differences in diffusivity from timepoint to timepoint. There was no reason to expect that the CST in the non-affected hemisphere would change in this group of chronic stroke patients who did not undergo occupational therapy during the time that they were enrolled in our study. The CST was determined by drawing ROIs in the posterior limb of the internal capsule (PLIC), the brainstem at a pontine level, and the white matter underlying the precentral gyrus in each hemisphere on the color-coded FA images. The analysis was started by drawing an ROI in the PLIC which is known to include the CST from anatomical35 and MRI studies36–38. The next ROI was drawn at a pontine level using a slice on which the superior cerebellar peduncle was visible, corresponding to z = −26 of a spatially normalized brain in Talairach and Tournoux space39. We added a logical AND-function so that only fibers passing through both ROIs were considered for further analysis. The third ROI was drawn in the precentral gyrus including its underlying white matter at a level that corresponded to z = 64 mm of a spatially-normalized brain. A logical AND-function was also added for this ROI so that only fibers that started in the precentral gyrus, passed through the PLIC and the pons were designated as the CST. After applying tractography, the identified fiber bundles were compared for tract fiber number and volume.
Results
The arcuate fasciculus (AF) was successfully identified in the right hemisphere of all six aphasic patients, but could not be identified to its full extent in the left hemisphere because each of the 6 patients’ strokes had destroyed the majority of the tract. Therefore, our analysis is restricted to post- vs pre-treatment comparisons of right-hemisphere tracts.
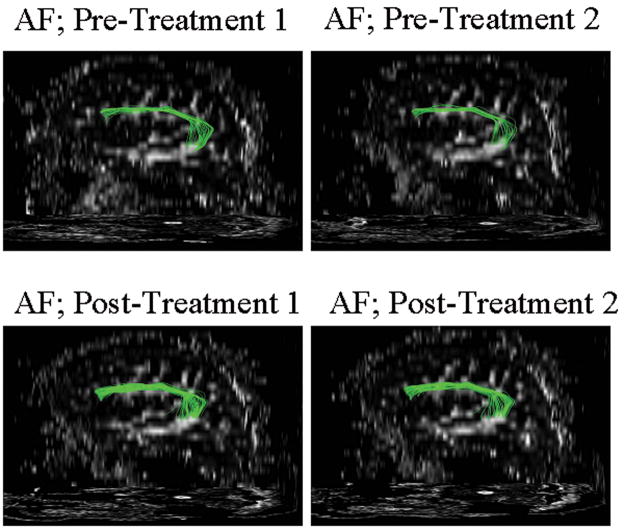
All six patients showed a significant increase in the absolute number of fibers in the right AF comparing post- versus pre-treatment DTI studies (paired t-test, p=0.04). An example of one patient is shown in figure 1. This patient showed not only an increase in the absolute fibers of the AF, but also an increase in the fiber length after therapy. The two pre-treatment DTI studies did not show any significant difference in the overall AF tract or in the total number of fibers. In order to normalize the pre- and the post-treatment measurement, we calculated a ratio between the fibers in the AF and the CST. Similar to the absolute differences seen in the post- vs pre-assessments, we also found a significant difference (paired t-test, p=0.02) in the relative fiber numbers when the AF fibers were normalized with the fibers in the pyramidal tract (Fig. 2a+b).
Figure 1.
Absolute and relative fiber number of the right arcuate fasciculus (AF) before (gray bars) and after therapy (black bars) in all 6 participants.
Figure 2.
Right AF in one patient with two scans before therapy and two scans after 75 sessions of Melodic Intonation Therapy. The scan-to-scan variability is minimal before therapy and a clear difference in the number of fibers and fiber volume can be seen comparing the AF before and after therapy.
Because we had two pre-treatment DTI studies in several patients we were able to assess scan-to-scan variations in the number of fibers present prior to therapy and compare this to the post vs pre-therapy differences. The scan-to-scan variation pre-treatment were very small or the fiber numbers were even identical. Thus, the post- vs pre-treatment difference cannot be explained by scan-to-scan variability.
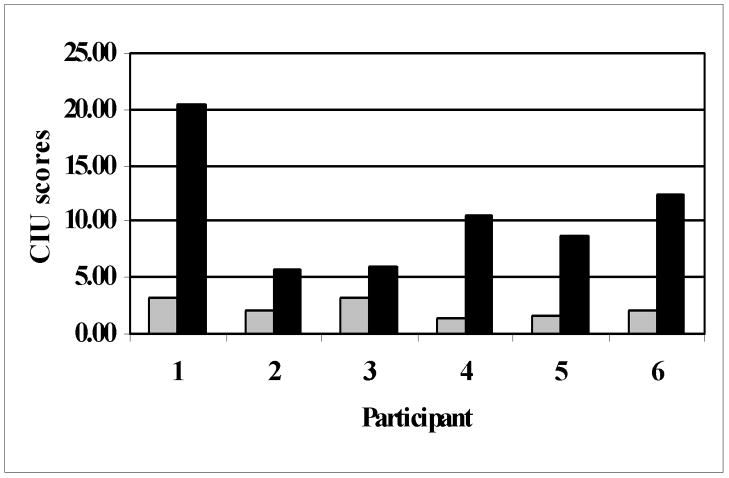
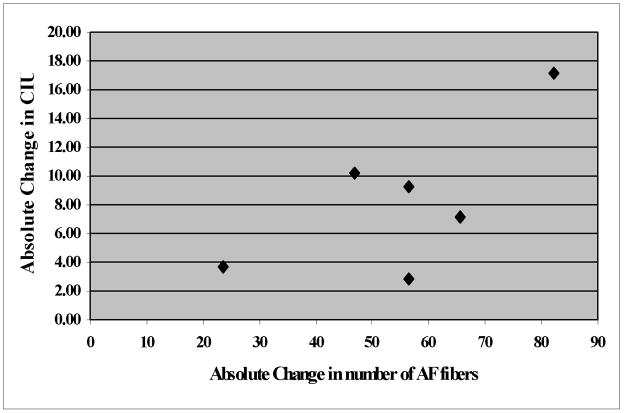
All six patients showed a significant improvement in speech outcome measures such as the Correct Information Units (CIU) while eliciting spontaneous speech through conversations with the patient and description of complex pictures as well as common procedures, the Picture Naming Test, and the number of syllables per phrase (see Fig. 3). By regressing change in CIU/min with change in AF fiber number, we found a strong trend for a correlation that may not have reached significance due to the relatively low number of patients. The more a patient improved after therapy compared to before therapy, the more AF fibers were detected (r=0.7; p=0.1) (see Fig. 4).
Figure 3.
CIU/min before (gray bars) and after therapy (black bars) for all 6 participants.
Figure 4.
Correlation between absolute change in CIU/min and absolute change in number of AF fibers.
Discussion
The small amount of empirical data available supports a bi-hemispheric role in the execution and sensorimotor control of vocal production for both speaking and singing40–44, with a tendency toward greater left-lateralization for speaking under normal physiological conditions (i.e., faster rates of production during speaking than singing). The asymmetry of the language fiber tracts in fiber number/volume and fiber extent, among them the arcuate fasciculus, might be a structural correlate of the left-hemisphere advantage for language functions, although the right hemisphere also plays a role in expressive language function.
The two unique elements of MIT that, most likely, make the strongest contribution to the therapy’s beneficial effects are the melodic intonation (singing) with its inherent sustained vocalization, and tapping with the left hand. How might melodic intonation influence recovery? Functional imaging tasks targeting the perception of musical components that require a more global than local processing strategy (e.g., melodic contour, musical phrasing, and/or meter) tend to elicit greater activity in right-hemispheric brain regions than in left-hemispheric regions. It has been shown that tasks that emphasize spectral information over temporal information have shown more right-than left-hemispheric activation45. Similarly, patients with right-hemisphere lesions have greater difficulty with global processing (e.g., melody and contour processing) than those with left-hemisphere lesions46,47. Thus, it is possible that the melodic element of MIT engages the right hemisphere, particularly the right temporal lobe, more than therapies that do not make use of pitch or melody. Furthermore, using melody and emphasizing prosodic features will lead to a general reduction in the vocalization rate as syllables are lengthened and “chunked” into larger structures.
The effects of tapping the left hand should be considered in the same context. Once the right temporal lobe is specifically engaged by the melodic intonation and melodic contour, it is conceivable that the role of the left hand-tapping could be the activation and priming of a right-hemispheric sensorimotor network for articulation. Since concurrent speech and hand use occurs in daily life, and gestures are frequently used during speech, hand movements, possibly in synchrony with articulatory movements, may have a facilitating effect on speech production, but the precise role of this facilitation is unknown. We hypothesize that tapping the left hand may engage a right-hemispheric sensorimotor network that coordinates not only hand movements but orofacial and articulatory movements as well. There is some evidence in the literature that such superordinate centers exist in the premotor cortex and share neural substrates for hand and orofacial movements48–50. Furthermore, behavioral51, neurophysiological48,49 and fMRI studies52–54 have shown that motor and linguistic cortical representations of objects are closely linked, and that the premotor cortex may belong to an integrative network coordinating motor and linguistic expression. An additional or alternative explanation is that the left hand tapping may serve the same function as a pacemaker or metronome has in rehabilitation of other motor activities, and in so doing, may facilitate speech production through rhythmic anticipation, rhythmic entrainment, or auditory-motor coupling54–56.
The structural changes that we have detected in the arcuate fasciculus must be seen in the context of what MIT actually does and the potential benefit that a patient may derive from the therapy. It is very clear that for the therapy to work well, the temporal lobe must strengthen its connections with the frontal lobe in order to provide fast feedback mechanisms for vocal articulation and for auditory-motor coupling54–56 to take place in the right hemisphere, since mapping sounds to vocal motor actions may actually be much more of a left-hemisphere function and that function is typically destroyed as part of the stroke that causes Broca’s aphasia. Furthermore, the inferior frontal lobe needs to connect quickly with the premotor and motor regions in order to plan, prepare and execute vocal actions. This feedforward system may be under corrective and/or adaptive control of the sensory feedback system in order to improve the auditory-motor mapping54–56
In this context, it would make sense that the tract that provides the connections between these major regions undergoes remodeling as part of the long-term therapy, in particular, if the therapy specifically engages the brain components that are part of this tract. Moreover, since the right AF is known to have slightly less volume and appears to be slightly shorter than it is on the left, it is also not surprising that the major change that seems to be occurring is in fiber number/volume, and possibly fiber length as we have seen in some of our patients. The components that underlie this structural change are not clear. Because the software program suggests that there are more fibers, and we know that there is also more volume in this tract, it is possible that the myelinization of axons increases and there is either additional axon growth or axon collaterals are being formed57. Furthermore, it is also possible that there are other physical changes to membranes that make fibers more traceable. Experimental studies in monkeys have shown that remodeling of fiber tracts such as the formation of axon collateral can happen after a focal stroke57. The establishment of axon collaterals in the affected hemisphere may actually change the cytoarchitecture in these regions which, in turn, has direct effects on the diffusion of water molecules: the lower the alignment in the architecture, the lower the directionality in diffusion (and vice versa), consequently affecting fiber reconstruction.
It is clear that this change is not due to normal fluctuations in the measured parameters, since the variability between two DTI scans and their derived measures prior to therapy was very small, and the magnitude of change seen in the post- versus pre-therapy comparisons was way beyond the level of scan-to-scan variability. It is possible that the remodeling of the AF might be triggered by the need for stronger, more effective connections between speech-relevant brain regions in the right hemisphere. This remodeling could not only involve changes in myelination, but also in the axons themselves, possibly through the formation of axon collaterals which could account for the increased number of fibers detected after therapy.
Acknowledgments
This work was supported in part by grants from the National Institute of Neurological Disease and Stroke (NS045049, DC008796), the Doris Duke Charitable Foundation, the Grammy Foundation, and The Mattina R. Proctor Foundation
References
- 1.Kertesz A, Lesk D, McCabe P. Isotope localization of infarcts in aphasia. Arch Neurol. 1977;100:1–18. doi: 10.1001/archneur.1977.00500220024004. [DOI] [PubMed] [Google Scholar]
- 2.Glasser MF, Rilling JK. DTI tractography of the human brain’s language pathway. Cereb Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- 3.Robey RR. The efficacy of treatment for aphasic persons: a metanalysis. Brain Lang. 1994;47:582–608. doi: 10.1006/brln.1994.1060. [DOI] [PubMed] [Google Scholar]
- 4.Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, Dutschka K, Woods RP, Noth J, Diener HC. Recovery from Wernicke’s aphasia: A positron emission tomographic study. Ann Neurol. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 5.Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Cappa S, Vallar G. The role of left and right hemispheres in recovery from aphasia. Aphasiology. 1992;6:356–372. [Google Scholar]
- 7.Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 8.Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–1763. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- 9.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 11.Schlaug G, Marchina S, Norton A. From singing to speaking: why singing may lead to recovery of expressive language function in patients with Broca’s aphasia. Music Perception. 2008;25:315–323. doi: 10.1525/MP.2008.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelissen K, Laine M, Tarkiainen A, Jarvensivu T, Martin N, Salmelin R. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15:444–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- 13.Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122:1781–1790. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- 14.Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang. 1998;62:298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- 15.Thompson CK, Shapiro LP. Treating agrammatic aphasia within a linguistic framework: Treatment of Underlying Forms. Aphasiology. 2005;19:10–11. doi: 10.1080/02687030544000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mori S, Zhang J. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch Neurol. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- 20.Sparks R, Helm N, Albert M. Aphasia rehabilitation resulting from melodic intonation therapy. Cortex. 1974;10:303–316. doi: 10.1016/s0010-9452(74)80024-9. [DOI] [PubMed] [Google Scholar]
- 21.Gerstman HL. A case of aphasia. J Speech Hear Dis. 1964;29:89–91. doi: 10.1044/jshd.2901.89. [DOI] [PubMed] [Google Scholar]
- 22.Geschwind N. Current concepts: Aphasia. New Engl J Med. 1971;284:654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- 23.Hebert S, Racette A, Gagnon L, Peretz I. Revisiting the dissociation between singing and speaking in expressive aphasia. Brain. 2003;126:1–13. doi: 10.1093/brain/awg186. [DOI] [PubMed] [Google Scholar]
- 24.Keith RL, Aronson AE. Singing as therapy for apraxia of speech and aphasia: Report of a case. Brain Lang. 1975;2:483–488. doi: 10.1016/s0093-934x(75)80085-x. [DOI] [PubMed] [Google Scholar]
- 25.Kinsella G, Prior MR, Murray G. Singing ability after right and left sided brain damage. A research note Cortex. 1988;24:165–169. doi: 10.1016/s0010-9452(88)80026-1. [DOI] [PubMed] [Google Scholar]
- 26.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractogrphy using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- 29.Fillard P, Toussaint N, Pennec X. MedINRIA: DT-MRI Processing and Visualization Software. Similar NoE Tensor Workshop; Las Palmas: 2006. [Google Scholar]
- 30.Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain. 2005;128:2562–2577. doi: 10.1093/brain/awh600. [DOI] [PubMed] [Google Scholar]
- 31.Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstein DM, Kindlmann GL, Lundberg EC. Tensorlines: Advection-diffusion based propagation through diffusion tensor fields. IEEE Visualization. 1999:249–254. [Google Scholar]
- 33.Lawes IN, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, Clarks DA. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. NeuroImage. 2008;39:62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 34.Wakana S, Jiang H, Nage-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 35.Kretschmann HJ. Localisation of the corticospinal fibres in the internal capsule in man. J Anat. 1988;160:219–225. [PMC free article] [PubMed] [Google Scholar]
- 36.Holodny AI, Gor DM, Watts R, Gutin PH, Ulug AM. Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: initial anatomic results in contradistinction to prior reports. Radiology. 2005;234:649–653. doi: 10.1148/radiol.2343032087. [DOI] [PubMed] [Google Scholar]
- 37.Kim YH, Kim DS, Hong JH, Park CH, Hua N, Bickart KC, Byun WM, Jang SH. Corticospinal tract location in internal capsule of human brain: diffusion tensor tractography and functional MRI study. Neuroreport. 2008;19:817–820. doi: 10.1097/WNR.0b013e328300a086. [DOI] [PubMed] [Google Scholar]
- 38.Zarei M, Johansen-Berg H, Jenkinson M, Ciccarelli O, Thompson AJ, Matthews PM. Two-dimensional population map of cortical connections in the human internal capsule. J Magn Reson Imaging. 2007;25:48–54. doi: 10.1002/jmri.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme, New York: Stuttgart; 1988. [Google Scholar]
- 40.Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- 41.Brown S, Martinez MJ, Hodges DA, Fox PT, Parsons LM. The song system of the human brain. Brain Res Cogn Brain Res. 2004;20:363–75. doi: 10.1016/j.cogbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Guenther FH, Hampson M, Johnson D. A theoretical investigation of reference frames for the planning of speech movements. Psychol Rev. 1998;105:611–633. doi: 10.1037/0033-295x.105.4.611-633. [DOI] [PubMed] [Google Scholar]
- 43.Jeffries KJ, Fritz JB, Braun AR. Words in melody: An H215O PET study of brain activation during singing and speaking. Neuroreport. 2003;14:749–745. doi: 10.1097/00001756-200304150-00018. [DOI] [PubMed] [Google Scholar]
- 44.Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33:628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- 46.Peretz I. Processing of local and global musical information by unilateral brain-damaged patients. Brain. 1990;113:1185–205. doi: 10.1093/brain/113.4.1185. [DOI] [PubMed] [Google Scholar]
- 47.Schuppert M, Munte TF, Wieringa BM, Altenmuller E. Receptive amusia: Evidence for cross-hemispheric neural networks underlying music processing strategies. Brain. 2000;123:546–559. doi: 10.1093/brain/123.3.546. [DOI] [PubMed] [Google Scholar]
- 48.Meister IG, Boroojerdi B, Foltys H, Sparing R, Huber W, Topper R. Motor cortex hand area and speech: Implications for the development of language. Neuropsychologia. 2003;41:401–406. doi: 10.1016/s0028-3932(02)00179-3. [DOI] [PubMed] [Google Scholar]
- 49.Tokimura H, Tokimura Y, Oliviero A, Asakura T, Rothwell JC. Speech-induced changes in corticospinal excitability. Ann Neurol. 1996;40:628–634. doi: 10.1002/ana.410400413. [DOI] [PubMed] [Google Scholar]
- 50.Uozumi T, Tamagawa A, Hashimoto T, Tsuji S. Motor hand representation in cortical area 44. Neurology. 2004;62:757–761. doi: 10.1212/01.wnl.0000113731.75479.25. [DOI] [PubMed] [Google Scholar]
- 51.Gentilucci M, Benuzzi F, Bertolani L, Daprati E, Gangitano M. Language and motor control. Exp Brain Res. 2000;133:468–490. doi: 10.1007/s002210000431. [DOI] [PubMed] [Google Scholar]
- 52.Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr Biol. 2006;16:1818–1823. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 53.Koelsch S, Fritz T, Schulze K, Alsop D, Schlaug G. Adults and children processing music: an fMRI study. Neuroimage. 2005;25:1068–1076. doi: 10.1016/j.neuroimage.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 54.Lahav A, Saltzman E, Schlaug G. Action representation of sound: Audiomotor recognition network while listening to newly-acquired actions. J Neurosci. 2007;27:308–314. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bangert M, Altenmuller E. Mapping perception to activation in piano practice: a longitudinal DC-EEG study. BMC Neurosci. 2003;4:26. doi: 10.1186/1471-2202-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bangert M, Peschel T, Schlaug G, Rotte M, Dresher D, Hinrichs H, Heinze HJ, Altenmuller E. Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjuction. NeuroImage. 2006a;30:917–926. doi: 10.1016/j.neuroimage.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 57.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina E, Stowe A, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]