Abstract
Activation of V1 vasopressin (VP) receptors prevents serum deprivation-induced apoptosis in neuronal H32 cells, partially through mitogen activated protein (MAP) kinase-mediated Bad phosphorylation. Here we investigated the role of protein kinases C (PKC) and B (PKB) mediating VP induced antiapoptosis in H32 cells. Serum deprivation increased PKCδ but not PKCα or PKCβ activity, while VP increased PKCα and PKCβ without affecting PKCδ activity. Inhibition of PKCδ prevented caspase-3 activation, indicating that PKCδ mediates the proapoptotic actions of serum deprivation. Simultaneous inhibition of PKC α&β and MAP kinase abolished VP-induced Bad phosphorylation, but it only partially prevented caspase-3 inhibition. Complete abolition of the protective effect of VP on serum deprivation-induced caspase 3 activity required additional blockade of PI3K/protein kinase B (Akt). The data demonstrate that VP exerts antiapoptosis through multiple pathways; while PKCα&β together with ERK/MAP kinase activation mediates Bad phosphorylation (inactivation), the full protective action of VP requires additional activation of PKB (PI3K/Akt) pathway.
Keywords: Vasopressin, V1a receptor, Apoptosis, PKC, ERK, Bad
Introduction
The neuropeptide vasopressin (VP) is well recognized for its peripheral actions on water conservation and cardiovascular homeostasis (McCann et al., 1989; Knepper et al., 1994). In addition the peptide is secreted within the brain, where it facilitates memory and modulates social behavior (Caffe et al., 1987; Landgraf et al., 1998; Alescio-Lautier et al., 2000; Landgraf and Neumann, 2004; Donaldson and Young, 2008). As in smooth muscle and liver, the predominant VP receptors found in the brain are the V1 subtype (Alescio-Lautier et al., 2000). We have recently shown that VP has protective effects against serum deprivation-induced apoptosis in the hypothalamic neuroendocrine cell line, H32 (Chen et al., 2008), and in hippocampal neuronal primary cultures (Chen and Aguilera, 2009). This suggests that VP acts as a neuroprotective agent in the brain. Since VP is released within the brain during stress and non-peptide V1 antagonists are sought as antidepressant agents (Griebel et al., 2002; Overstreet and Griebel, 2005), it is critical to fully understand the mechanisms of the antiapoptotic actions of VP in neuronal cells. The antiapoptotic effect of VP is mediated by V1 receptors and involves phosphorylation (leading to inactivation) of the pro-apoptotic protein Bad. This effect is largely mediated by transactivation of the epidermal growth factor receptors (EGFR) with consecutive activation of extracellular signal-regulated kinases (ERK) (Volpi et al., 2006) and the 90 kDa ribosomal S6 kinase (RSK) (Chen et al., 2008). Bad phosphorylation prevents the release of mitochondrial cytochrome c and the consequent activation of the caspase cascade, promoting neuronal survival (Chen et al., 2008). However, the inhibitory effect of VP on serum deprivation induced caspase 3 activation is only partially blocked by inhibitors the EGFR or ERK kinase, or by dominant negative forms of RSK (Chen et al., 2008), suggesting that activation of additional signaling pathways by VP are necessary for the full protective action of VP. Moreover, the fact that blockade of the EGFR/ERK/RSK pathway does not fully repress the ability of VP to phosphorylate Bad is also indicative multiple pathways are required for the antiapoptotic actions of VP in neurons.
The major pathway activated by binding of VP to V1 receptors is activation of phospholipase C (PLC), leading to diacylglycerol (DAG) formation and stimulation of protein kinase C (PKC) (Thibonnier, 1992; Lolait et al., 1995). PKC activation has an important role in mediating the actions of VP on neuronal function, including transactivation of the EGF receptor and activation on the MAP kinase cascade (Volpi et al., 2004). There is extensive evidence supporting a role of PKC in cell survival (Wakade et al., 1988; Bhave et al., 1990; Zirrgiebel et al., 1995; Maher, 2001) and it is likely that activation of PKC is part of the mechanism of the neuroprotective actions of VP. Since the hypothalamic neuronal cell line, H32, expresses functional V1 receptors (mostly V1a and a small proportion of V1b receptors) (Volpi et al., 2006), these cells provide an ideal experimental model to study the effects of VP in neurons. In addition, H32 cells are extremely sensitive to apoptotic cell death when exposed to serum deprivation. For these reasons, the neuronal cell line H32 is a good model for studying the mechanisms of action of VP in neurons. The aim of the present study is to test the hypothesis that, in conjuction with the previously demonstrated participation of the ERK/RSK pathway, activation of the PLC/PKC pathway is part of the mechanism mediating the antiapoptotic actions of VP in neurons.
Materials and Methods
Materials
Gö6983, BIM, Gö6976, Rottlerin and LY294002 were purchased from BIOMOL Research Laboratory (Plymouth Meeting, PA); UO126 was from Calbiochem (San Diego, CA). Arginine-vasopressin, HPLC purified, was purchased from Bachem Americas, Inc. Torrance, CA. Antibodies against phospho-Bad (Ser112), Bad, phospho-Akt and Akt were purchased from Cell Signaling Technology™ (Beverly, MA); PKCα, PKCβI, PKCβII and PKCδ antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). β-tubulin antibody was purchased from Sigma (Saint Louis, MO).
Cell culture and treatments
The hypothalamic neuroendocrine cell line H32, provided by Dr Joachim Spiess, Goettingen, Germany, was cultured in Dulbecco's modified Eagle's medium (Life Technologies, Inc., Gaithersburg, MD) containing 10% fetal bovine serum (Life Technologies, Inc.), 10% horse serum, and 1% penicillin/streptomycin (Life Technologies, Inc.). After 24h culture in 100mm plates (1.5×106 cells per plate), at 37°C, under 5% CO2/95% air, the medium was changed to serum-free medium containing 0.1% BSA, with or without VP. To determine the signaling pathways mediating the effect of VP, cells were incubated in the presence and absence of inhibitors. After incubation for the time periods indicated in results and figure legends, cells were processed for caspase-3 activity or Western blot analysis.
Plasmids and transfection
PKCα, PKCβI, PKCβII and PKCδ dominant negative mutants, cloned into pcDNA3 vector, were provided by Dr. Jae-Won Soh (Inha University, Incheon, Korea). Transient transfection was performed in Opti-MEM I Reduced Serum Medium (Invitrogen) using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's recommendations. The transfection efficiency is about 70-80%. Cells were subjected to the experimental treatments 24h after transfection.
Western blot analysis
Cells were lysed with T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with proteinase and phosphatase inhibitor cocktail (Sigma). Protein concentrations were determined by BCA™ Protein Assay (Pierce) and 20 μg of protein were loaded and separated in a 4-20% SDS-PAGE (Invitrogen). Proteins were transferred from the gel to a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Piscataway, NJ), incubated with 5% nonfat dried milk in Tri-buffered saline (TBS plus 0.1% Tween-20 [TBST]) for 1h and incubated with the antibodies at a 1:1,000 dilution overnight. After washing in TBST, membranes were incubated for 2h with peroxidase-linked anti-Rabbit IgG at a 1:10,000 dilution or anti-mouse IgG at a 1:5,000. β-actin was used to correct for protein loading. Detection of immunoreactive bands was performed by using ECL Plus TM reagents (Amersham Pharmacia Biotech) and exposure to BioMax MR film (Kodak, Rochester, NY). Densitometric quantification of the immunoblots was performed using the public domain NIH Image program (ImageJ 1.36b) developed at the US National Institutes of Health and available on the Internet at: http://rsb.Info.nih.gov/nih-image).
Immunoprecipitation
For immunoprecipitation of endogenous PKC isoforms, cells were lysed using T-PER™ Tissue Protein Extraction Reagent (Pierce, Rockford, IL) and after protein concentrations were determined using the BCA protein assay (Pierce), 400 μg of protein in a final volume of 500 μl were pre-incubated for 2h at 4°C with 50 μl of protein G-agarose (Roche, Indianapolis, IN). After a quick spin to remove the beads, lysate was incubated overnight with 5 μl of PKCα, PKCβI, PKCβII or PKCδ (1:100) antibody at 4°C. Fifty μl of protein G-agarose was added and incubated overnight at 4°C. The beads were spun down at 12,000g for 1 min and the immunocomplexes were washed three times with lysis buffer supplemented with proteinase and phosphatase inhibitors (Sigma).
PKC Activity Assay
PKC activity was measured by using a PKC kinase activity assay Kit (Stressgen, Ann Arbor, MI). For detection of PKC activity in the immunoprecipitates, immunocomplexes were suspended in 50 μl of the kinase assay dilution buffer and loaded on 96-well plates coated with PKC substrate peptide. The PKC assay was initiated by the addition of 10 μl of ATP (diluted 1 mg/ml) to each well at 30°C and assayed as per the manufacturer's instructions, measuring incorporation of phosphate into the substrate peptide at 50 min. The wells were then washed twice with antibody dilution buffer, and 40 μl of phosphospecific substrate antibodies were added to each well and incubated for 1 h. Each well was subsequently washed three times for 10 min with wash buffer and a 1:1,000 dilution of anti-rabbit IgG HRP-conjugated antibody preparation in dilution buffer and incubated for 30 min. The wells were washed three times, and 60 μl of tetramethylbenzidine substrate (Stressgen) was added and incubated in the wells for 45 min. The HRP reaction was quenched by addition of 20 μl of acid stop solution, and absorbance at 450 nm was measured. The reaction was found to be linear with protein concentrations between the range of 0 and 100μg, and time periods between 15 and 90 min.
Cytochemical Detection of Apoptotic Cell Death
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining was performed using In Situ Cell Death Detection Kit, Fluorescein (Roche, Penzberg, Germany). Briefly, H32 cells grown on glass coverslips were fixed with 3% paraformaldehyde and permeabilized with 0.1 % Triton X-100, and TUNEL staining was performed according to the manufacturer's protocol. Photomicrographs from 5 different fields in each coverslip were captured by Nikon Eclipse E800 fluorescent microscope. Images were digitized using 20× objectives. The number of TUNEL-positive (apoptotic) cells was counted form the images. Typically, ∼300 cells were analyzed for the number of TUNEL-positive (apoptotic) staining. Total numbers of cells were counted by DAPI staining. Apoptotic cell numbers were presented as the numbers of TUNEL-positive cells in total of 300 cells.
Caspase-3 activity measurement
Caspase-3 activity was measured using a Caspase-3/CPP32 fluorometric protease assay kit (BioSource International, Inc., Camarillo, CA) according to the manufacturer's protocol. Briefly, cells were washed with PBS, centrifuged for 5 min at 800× g, the supernatant removed and the pellet resuspended in ice cold lysis buffer. After 20 min incubation at room temperature, samples were centrifuged at 16,000 × g for 10 min at 4 °C, and protein concentrations in the supernatants determined using BCA™ protein Assay (PIERCE, Rockford, IL). Aliquots containing 100μg of protein were incubated with substrate DEVD (Asp-Glue-Val-Asp)-AFC (7-amino-4-trifluoromethyl coumarin) for 90 min at 37 °C. Upon cleavage of the substrate by Caspase-3, free AFC, which emits a yellow-green fluorescence, was measured by using a FLUOStar OPTIMA microplate reader (BMG Labtechnologies Inc, Durham, NC), with a 405 nm excitation and 505 nm emission filter.
Data analysis
Statistical significance of the differences between groups was calculated by one or two-way analysis of variance (ANOVA), or by Student's t test for paired data, as indicated in the legends to the figures. P values< 0.05 were considered significant. Data are presented as means ± standard error of the mean (SEM) from the values in the number of observations indicated in results or legends to Figures.
Results
PKC involvement in the protective effect of VP
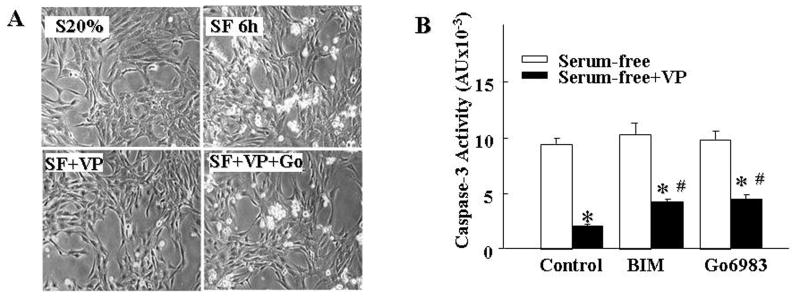
Incubation of H32 hypothalamic neuronal cells with serum-free medium for 6h resulted in morphological changes including retraction, rounding, shrinking and detachment from the culture plate (Fig 1A). This was accompanied by a marked increase in caspase 3 activity (9-fold, p<0.05) indicating apoptotic cell death (Fig 1B). In contrast, cells incubated in serum-free medium in the presence of 10nM VP did not display morphological changes or increases in caspase 3 activity (Fig 1A and 1B). Addition of the PKC inhibitor, Gö6983, 15min before VP attenuated the protective action of VP, as indicated by the presence of rounded-detached cells and a 3-fold increase in caspase 3 activity (Fig 1A and 1B). Similar inhibition of the protective effect of VP was observed after co-incubation with another generic PKC inhibitor, BIM. These results suggest that the antiapoptotic effect of VP is in part mediated through the PKC pathway.
Fig.1.
Effect of PKC on the inhibitory effect of VP on serum deprivation-induced apoptotic cell death in H32 hypothalamic cells. (A) Light microscopy images of H32 hypothalamic cells following incubation with 20% serum (S20%); serum-free medium for 6h (SF 6h), in the presence of 10nM VP without (SF+VP), or with 1μM Gö6983 (SF+VP+Gö). (B) Caspase 3 activity in H32 hypothalamic cells incubated in serum-free conditions for 6h with or without VP (10 nM), or in the presence the genenic PKC inhibitors, BIM (100nM) or Gö6983 (1μM), added 15 min before VP. Bars represent the average ± S.E.M of the values obtained in 3 independent experiments conducted in duplicate. * p< 0.05, compared to serum-free group; # p< 0.05 compared to serum-free + VP group.
Effect of the phorbol ester, PMA, on serum deprivation induced apoptosis
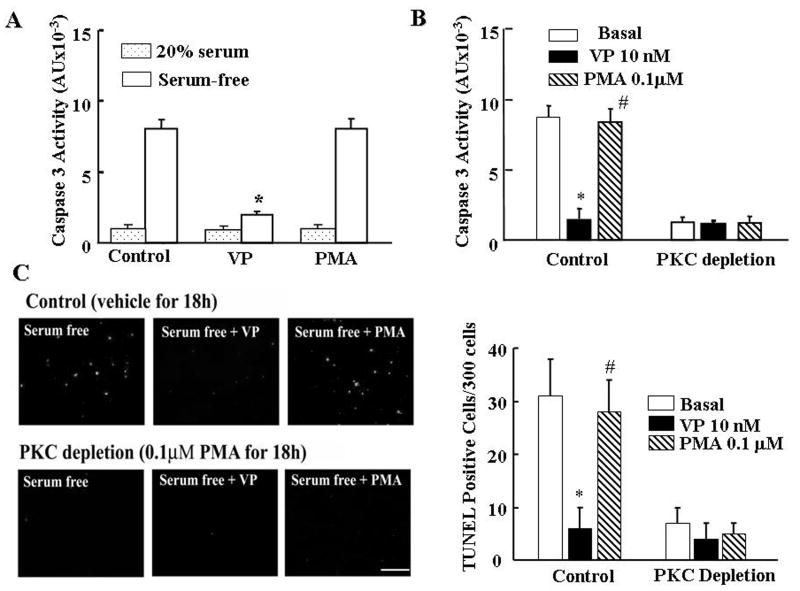
To further study the participation of PKC on the antiapoptotic action of VP we examined the effect of PKC stimulation by incubation with 100nM PMA on caspase 3 activation in the presence and in the absence of serum. Serum deprivation for 6h increased caspase 3 activity by 10-fold, and co-incubation with VP prevented this effect. Unexpectedly, incubation of the cells for 6h with PMA had no significant effect on serum-deprivation-induced caspase 3 activity (Fig 2A). Neither VP nor PMA had any effect on the low caspase 3 activity levels in the presence of 20% serum. Moreover, PKC depletion by prolonged treatment of the cells with PMA (100nM for 24h), completely blocked the stimulatory effect of serum deprivation on caspase 3 activation on its own, and had no effect on VP-induced caspase 3 inhibition (Fig 2B). In the presence of 20% serum, PKC depletion had not effect on caspase 3 activity (806±117 vs 849±125 for control and PKC depletion, respectively). Consistent with the caspase 3 data, detection of apoptotic cells using TUNEL staining revealed a marked increase in the number of stained cells following 6h serum deprivation compared with 20% serum (31±7 vs 3±3%, p<0.05) (Fig 2 C and D). As for caspase 3 activity, the increase in TUNEL staining induced by serum deprivation was completely blocked by VP, decreasing from 31±7 to 6±4%, p<0.05. Also consistent with the caspase 3 data, PMA did not prevent the increase in TUNEL staining induced by serum deprivation (Fig 2 C and D). PKC depletion following 24 h incubation with PMA prevented the increase in TUNEL labeling observed in serum free medium (Fig 2 C and D).
Fig.2.
The effect of stimulation PKC (acute-treatment with PMA) or PKC depletion (24h-PMA treatment) on serum-deprivation induced apoptosis in H32 cells. (A) Caspase 3 activity in H32 hypothalamic cells incubated in the presence of 20% serum or serum-free medium for 6h, in the presence or absence of 10nM VP or 100nM PMA. (B) Effect of PKC depletion by prolonged PMA treatment on caspase 3 activity in H32 cells. Following 24h exposure to PMA (100nM) cells were incubated in serum-free medium (Basal) in the presence or absence of 10nM VP or 100nM PMA for 6h. (C) TUNEL staining of control or PKC depleted H32 cells (PMA 100nM for 18h), following incubation for 6h in serum-free medium (Basal) in the presence or absence of 10nM VP or 100nM PMA. Scale bar, 100 μm. Bars represent the average ± S.E.M of the percent of TUNEL stained cells in a total of about 300 cells counted in 3 independent experiments conducted in duplicate. * p< 0.05, compared to Basal (serum-free) group; # p< 0.05 compared to serum-free + VP group.
PKC isoforms involved in serum-deprivation induced apoptosis and the anti-apoptotic effect of VP
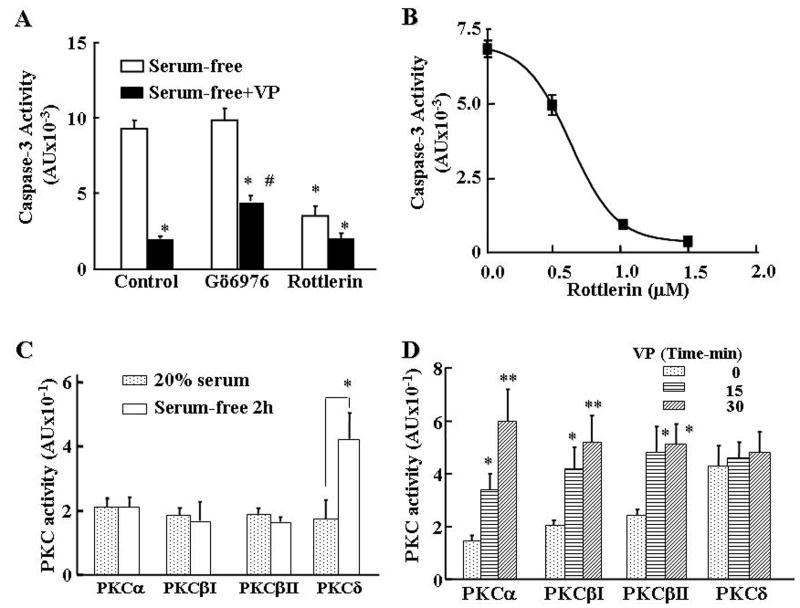
To determine whether the unexpected findings with PMA are due to differential involvement of PKC isoforms, we first examined the effect of pretreatment of H32 cells with PKC subtype specific inhibitors on the inhibitory effect of VP on serum-deprivation-induced caspase-3 activity. As shown in Fig 3A, pretreatment with the PKCα and β inhibitor, Gö6976, (100nM) for 15min partially reversed the inhibitory action of VP on serum deprivation-induced caspase-3 activity by about 35% (p<0.05), and had no effect on its own. On the other hand, the PKCδ inhibitor, rottlerin, on its own markedly reduced (∼70%) serum deprivation-induced caspase-3 activity (P<0.05), but had no effect on the protective action of VP. The dose-response for the inhibitory effect of rottlerin on serum deprivation-induced caspase-3 activity revealed an IC50 of 638nM and full inhibition with 1μM (Fig 3B). In the presence of serum, caspase 3 activity was low and showed a small but significant increase after incubation with the PKC α and β inhibitor, Go6976 (806±117 vs 1255±178 for control and Go6976, respectively, p<0.05). On the other hand, the PKCδ inhibitor, rottlerin, tended to decrease caspase 3 activity and prevented the stimulatory effect of Go6976 (659±104 vs 855±155 for rottlerin and rottlerin+Go6976, respectively). Since the latter observation suggests that serum deprivation induces apoptosis through activation of PKCδ, and that activation of PKCα and β are protective, we measured PKC activity following 2 h serum deprivation. As shown in Fig 3C, serum deprivation had a marked (4-fold) stimulatory effect on PKCδ (P<0.05). On the other hand, serum deprivation had no effect on PKCα or PKC β activity. Analysis of the effects of VP on PKC subtype activity in serum-deprived H32 cells revealed marked increases in PKCα, PKCβI and PKCβII activity at 15 and 30min incubation (Fig 3D). In contrast, the elevated levels of PKCδ activity following serum deprivation were unaffected by VP (Fig 3D).
Fig.3.
Role of PKC isoforms on the protective action of VP in serum deprivation-induced apoptosis, and serum-deprivation induced PKC isoform activities. (A) Caspase 3 activity in H32 hypothalamic cells incubated in serum-free medium for 6h with or without 10nM VP, or in the presence of the PKCα and β inhibitor, Go6976 (100nM) or the PKCδ inhibitor, rottlerin (20μM), added 15 min before VP. Bars represent the average ± S.E.M of the values in 3 independent experiments conducted in duplicate. * p< 0.05, compared with serum-free; # p< 0.05 compared with serum-free + VP. (B) The dose-response of the effect of the PKC δ inhibitor, rottlerin on serum deprivation-induced caspase 3 activity. H32 hypothalamic cells were incubated in serum-free medium for 6h with increasing doses of rottlerin. Data are the mean and SEM of data obtained in three individual experiments run in duplicate. (C) Effect of serum deprivation on PKC subtype activities. Cells were incubated in serum free medium for 2h before lysis, PKC subtype immunoprecipitation and measurement of PKC activity. (D) Effect of VP (10nM) for 15 or 30 min on PKC subtype activity in H32 cells subjected to serum-free condition for 2h. Bars represent the average ± S.E.M of three experiments conducted in duplicate. * p< 0.05 and ** p<0.01 compared to the corresponding 0 min VP treatment group.
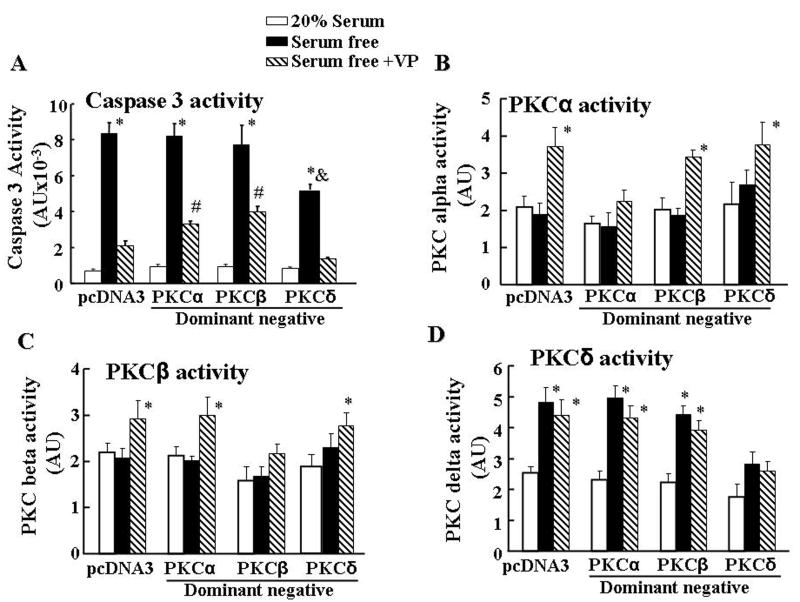
To further examine the differential involvement of PKCα and β, and PKCδ on the effects of serum deprivation and VP on apoptosis, we transfected H32 cells with PKCα, PKCβ or PKCδ dominant negative mutants before subjecting them to serum deprivation in the presence and absence of VP. Consistent with the effect of PKC inhibitors, transfection of PKCα or PKCβ dominant negative mutant significantly reduced the inhibitory action of VP on serum deprivation-induced caspase-3 activity (32% and 38%, respectively, P<0.05). On the other hand, PKCδ dominant negative mutant, had no effect on the inhibitory action of VP, but reduced (48%) serum deprivation-induced caspase-3 activity (P<0.05) (Fig 4A). To verify the effectiveness of the PKC dominant negative mutants, cells were transfected with PKCα, PKCβ or PKCδ dominant negative mutants, subjected to serum deprivation in the presence and absence of VP, and then examined for PKC isoform activity. As shown in Fig 4B, PKCα dominant negative mutant blocked VP-induced PKCα activity, but had no effect on VP-induced PKCβ activity. On the other hand, PKCβ dominant negative mutant only blocked VP-induced PKCβ activity (Fig 4C), while PKCδ dominant negative mutant only blocked serum free-induced PKCδ activity (Fig 4D).
Fig.4.
Effect of PKC subtype dominant negative mutants on the protective effect of VP on caspase 3 activity (A), PKCα (B), PKCβ (C) and PKCδ (D) activities. PKCα, PKCβ or PKCδ dominant negative mutants or the empty vector (pcDNA 3) were transfected into H32 cells. Twenty four hours after transfection, cells were incubated in 20% serum, or serum-free medium with or without 10nM VP (Serum-free+VP). Bars represent the mean ± S.E.M of three experiments conducted in duplicate. * p< 0.05, compared to 20% serum in the pcDNA3 control group; # p< 0.05, compared to serum-free + VP in the pcDNA3 group. & p< 0.05 compared to serum-free in the pcDNA3 group.
Effect of the phorbol ester, PMA, on PKC isoform activity
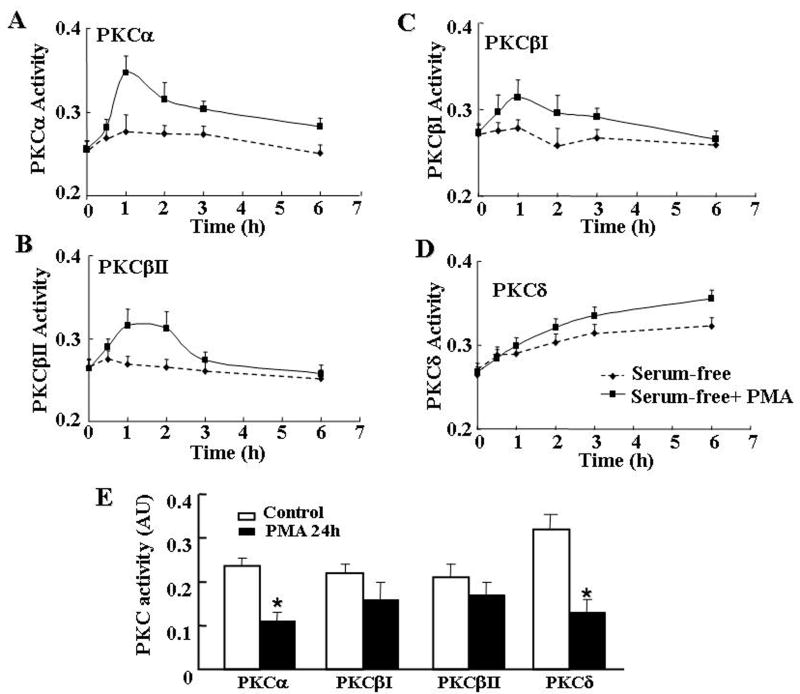
To clarify the paradoxic effects of PMA on caspase 3 activity, we examined the time course of PMA in PKC subtype activity. Incubation of H32 cells with PMA resulted in transient increases in PKCα (Fig 5A) and PKCβI (Fig 5B) and βII (Fig 5C) activity, with levels reaching a maximum from 1 to 2 hours, gradually declining by 3 hours, and reaching levels not significantly different from basal by 6 hours. However, PKCδ activity showed a sustained increase up to 6 hours incubation with PMA (Fig 5D). A more prolonged exposure of the cells to PMA (24 h) significantly reduced PKCα and markedly decreased PKCδ activity (P<0.01) (Fig 5E). PKCβI and βII showed a tendency to decrease but the effect was not statistically significant (Fig 5E).
Fig.5.
Time course of the effect of the phorbol ester, PMA, on PKC α (A), PKCβII (B) PKCβI (C) or PKCδ (D) isoforms activity H32 cells were incubated in serum free medium with or without PMA (100nM) for up to 6h. (E) Effect of prolonged incubation with PMA (100nM) on PKC isoform activities. After 24h incubation with vehicle (Control) or PMA in the presence of 20% serum, cells were serum-deprived for 6h. Bars represent the mean ± S.E.M of the values obtained in three individual experiments conducted in duplicate. * p<0.05 compared to corresponding Control group.
Activation of PKC and MAP kinase mediate VP-induced phosphorylation-inactivation of pro-apoptotic protein Bad
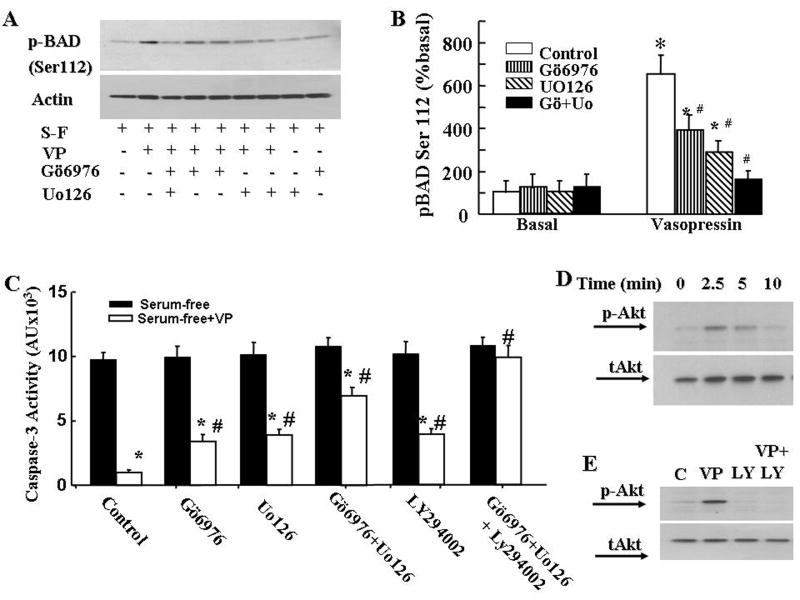
We showed previously that the antiapoptotic actions of VP involve phosphorylation-inactivation of the pro-apoptotic protein, Bad, and that this was partially mediated via the activation of the MAPK/ERK/RSK pathway. To determine the involvement of PKC on VP-induced Bad phosphorylation, we measured phospho-Bad levels by Western blot. Phospho-Bad (Ser112) levels were low in serum deprived cells, and increased following incubation of the cells with 10nM VP. Pretreatment of cells with the PKC inhibitor Gö6976, reduced VP-induced Bad phosphorylation by about 50% (p< 0.05, Fig 5A). The MEK inhibitor UO126 reduced VP-induced Bad phosphorylation by 65%, p< 0.05). Co-incubation of the cells with both the PKC inhibitor Gö6976 and the MEK inhibitor UO126, completely blocked the stimulatory effect of VP on Bad phosphorylation (Fig 6 A and B). On the other hand, co-incubation with Gö6976 and UO126 reduced (∼75%, p<0.05), but did not prevent the inhibitory effect of VP on caspase-3 activity (Fig 6C).
Fig.6.
Effect of signaling transduction inhibitors on the effects of VP on Bad phosphorylation (A and B), caspase 3 activity (C), and effects of VP on Akt phosphorylation (D and E). (A) H32 cells were incubated in serum-free medium for 2 h (control) before addition of 10nM VP, in the presence or absence of the PKCα and β inhibitor, Gö6976, or/and the MEK inhibitor, UO126. Gö6976 and UO126 were added 15 min before VP. Western blot of phosphor-BAD (Ser112) was conducted as described in Methods. (B) Data represent the mean ± S.E.M of three experiments conducted. * p< 0.05, compared to corresponding basal group, # p< 0.05 compared to control of VP treatment group. (C) Caspase 3 activity in H32 hypothalamic cells incubated in serum-free conditions for 6h with or without VP (10nM), or in the presence of the PKCα and β inhibitor, Gö6976, the MEK inhibitor, UO126, the PI3K inhibitor, LY294002 or their combination. Inhibitors were added 15min before VP. Bars represent the mean ± S.E.M of three experiments conducted in duplicate. * p< 0.05, compared to serum-free group; # p< 0.05 compared to control of serum-free+VP group. (D) Western blot showing the time course of Akt phosphorylation (p-Akt) in the presence of 10nM VP in H32 cells pre-incubated in serum-free medium for 2 h. (E) Western blot for phosphor-Akt in H32 cells preincubated in serum-free medium for 2 h (control) before addition of 10nM VP, or in the presence of the PI3K inhibitor, LY294002, added 15 min before VP.
Full antiapoptotic actions of VP involve BAD phosphorylation and activation of the PI3K/Akt pathway
The above results suggest that Bad phosphorylation only partially mediates the antiapototic actions of VP and that the full protective effect requires an additional pathway independent of PKC and MAP kinase activation. To determine the involvement of the PI3K/Akt pathway, we examined the effect of PI3 kinase inhibitor, LY294002, on VP-induced caspase 3 inhibition in serum deprived H32 cells. As shown in Fig 6C, 10μM LY294002 had no effect on serum deprivation induced caspase 3 activation on its own but it reduced the protective effect of VP by 35%, P<0.05. The PI3K inhibitor, wortmanin, had a similar effect, reducing the inhibitory effect of VP on caspase 3 activity by 38%, p<0.05 (Data not shown). Western blot analysis of phospho-Akt levels revealed a transient increase in Akt phosphorylation following incubation with 10nM VP, with a marked increase by 2.5 min, starting to decline by 5 min, and returning to basal levels after 10min incubation (Fig 6D). The effect of VP on phospho-Akt levels was completely suppressed by addition of LY294002 to the cells 15 min prior to VP (Fig 6E).
Discussion
The main purpose of this study was to investigate the role of PKC, a major signaling pathway initiated by VP V1 receptor activation, on the protective effects of VP on serum deprivation-induced apoptosis. We previously showed that activation of the ERK/RSK pathway plays an important role in mediating the antiapoptotic action of VP, but this pathway can not fully account for the ability of VP to phosphorylate (and inactivate) the proapoptotic protein, Bad, or preventing caspase 3 activation. Since the major signaling pathway of V1 receptors is activation of PLC and PKC, we sought to test the hypothesis that in addition to MAP kinase signaling, activation of PKC plays an important role mediating the antiapoptotic actions of VP. The results of the present study demonstrate differential roles of PKC subtypes on serum deprivation-induced apoptosis and the protective effect of VP, with PKCδ mediating the proapoptotic effect of serum deprivation, and PKCα and β contributing to the antiapoptotic actions of VP. In addition, we show that the full protective effect of VP requires a Bad-independent pathway, mediated by the PI3 kinase/protein kinase B (Akt) pathway. Ongoing studies show that VP partially prevents nutrient deprivation-induced apoptosis in primary cultures of hypothalamic or cortical neurons (Chen and Aguilera, 2009). Since VP is released within the brain during stress, the peptide could act as a neuroprotective agent in some conditions. The neuronal cell line H32 contains endogenous VP receptors and provides a good experimental model to study the mechanisms by which VP exerts it neuroprotective actions.
It is known that PKC is involved in signal transduction associated with cell proliferation and differentiation, as well as apoptosis (Ohno and Nishizuka, 2002; Nakajima, 2006). PKC activation promotes neuronal survival in sympathetic and sensory neurons and reduces serum-deprivation-induced death of cerebellar granule neurons (Zirrgiebel et al., 1995; Tanaka and Koike, 2001). Moreover, PKC inhibitors induce apoptosis in cortical and cerebellar granule neurons, as well as in neuronal cell lines (Heasley and Johnson, 1989; Koh et al., 1995; Zirrgiebel et al., 1995). Consistent with a role of PKC mediating the protective effects of VP, the present experiments showed that generic PKC inhibitors, at concentrations about 10-fold the IC50, reduced the protective effect of VP on serum deprivation induced caspase-3 activation. However, the inability of activation of PKC by the phorbol ester, PMA, to mimic the protective effects of VP did not support this hypothesis. Moreover PKC depletion following 24h exposure of the cells to PMA did not induce apoptosis as expected but had the opposite effect preventing serum deprivation induced caspase activation. The latter findings indicate that the involvement of PKC on the antiapoptotic actions of VP is complex and suggest the involvement of differential activation of PKC subtypes. In mammals, the PKC enzyme family is heterogeneous and comprises at least twelve known isoforms divided into three major subsets according to their sensitivity to the second messengers Ca2+ and diacylglycerol (DAG) (Poole et al., 2004). The conventional PKCs (α, β and γ) are regulated by both Ca2+ and DAG, while the novel PKCs (δ, ε, η, θ, μ and υ) are insensitive to Ca2+ but respond to DAG, and the atypical PKCs (ξ, λ and ζ) are regulated by neither DAG nor Ca2+. Several PKC isoforms are commonly co-expressed in the same cell, where they are believed to have different functions (Lenz et al., 2002).
The use of PKC subtype-specific inhibitors and dominant negative mutants, as well as the measurement of PKC subtype activity in the present experiments, revealed converse roles of PKC subtypes on cell survival in H32 neuronal cells. Firstly, we show that activation of PKCδ mediates the pro-apoptotic effects of serum deprivation. This was first suggested by the attenuating effect of the PKCδ selective inhibitor, rottlerin, at concentrations in the range of its IC50 for PKCδ, on serum deprivation-induced caspase-3 activity. The marked induction of PKCδ (without changes in PKCα and PKCβ) by serum deprivation, as well as the protective effects of PKCδ knock out by the dominant negative mutant on serum-deprivation-induced apoptosis confirmed that PKCδ plays a critical role on the pro-apoptotic actions of serum deprivation in H32 cells. These data are in agreement with previous reports showing that PKCδ can act as a pro-apoptotic protein kinase in a number of cells, including neurons (Spitaler M et al., 1999; Cross et al., 2000; Bright et al., 2004). PKCδ induced apoptosis involves tyrosine phosphorylation and activation by death signals, translocation to different cellular compartments, including mitochondria and nucleus, and association with apoptosis related proteins (Majumder et al., 2000; Matassa et al., 2001; Brodie and Blumberg, 2003; Murriel et al., 2004; Reyland, 2007). The involvement of these mechanisms of action of PKCδ in mediating the proapoptotic actions of serum deprivation in H32 neuroendocrine cells remain to be elucidated.
Secondly, the ability of VP to markedly activate PKCα and PKCβ in serum deprived cells, in conjunction with the attenuating effects of selective PKCα and PKCβ inhibitors and dominant negative mutants to reduce the effect of VP strongly suggest that activation of PKCα and PKCβ plays an important role mediating the antiapoptotic actions of VP. The present findings are in keeping with studies in other cell lines showing that PKCα and PKCβ, as well as PKCε, PKCλ and PKCξ inhibit cell death (Spitaler M et al., 1999; Cross et al., 2000; Bright et al., 2004; Nakajima, 2006). In addition, the present demonstration that VP prevents the activation of caspase-3 activity by serum deprivation without affecting activation levels of PKCδ indicate that activation of PKCα and PKCβ can overcome the pro-apoptotic actions of PKCδ. The demonstration that PKCδ mediates serum deprivation induced apoptosis in conjunction with the fact that this PKC subtype is predominantly depleted following prolonged long-term exposure of the cells to PMA (24h), explained the unexpected observation that PMA treatment for 24h prevented serum deprivation induced apoptosis rather than blocking the protective effect of VP. The fact that in the presence of serum the PKCδ inhibitor, rottlerin, prevented the increase in caspase 3 activity induced by 6h incubation with the PKCα/β inhibitor, Go6976, is also in agreement with an antiapoptotic role of PKCα/β, antagonizing proapoptotic actions of prevailing levels of PKCδ activity.
The data also provide evidence that the mechanism by which VP-induced PKCα and PKCβ activation promotes cell survival involves phosphorylation, and presumably inactivation of the proapoptotic protein Bad. In its dephosphorylated state, Bad interacts with anti-apoptotic proteins, Bcl-2 and Bcl-XL, resulting in release of mitochondrial cytochrome c. Phosphorylation of Bad at the serine residue 112 allows binding of Bad to the scaffolding protein 14-3-3, preventing its interaction with the antiapoptotic proteins Bcl-2 and Bcl-XL, mitochondrial damage, and the release of cytochome c (Zha J et al., 1996). We have previously shown that transactivation of the MAP kinase pathway by VP, leading to activation of RSK is critical but not sufficient for Bad phosphorylation and protection against serum deprivation induced cell death (Chen et al., 2008). Since we have shown that activation of PKCα is part of the mechanism by which VP transactivate the EGF receptor leading to phosphorylation of ERK in H32 cells (Volpi S. et al., 2004), it is possible that PKC mediates the antiapoptotic effect of VP indirectly by activation of the MAP kinase pathway. However, the present demonstration that the combination of the PKCα and PKCβ inhibitor, Gö6976 and MAP kinase inhibitor, UO126, completely obliterate the effect of VP on Bad phosphorylation indicates that both pathways can independently mediate Bad phosphorylation. It is noteworthy that in spite of totally inhibition of Bad phosphorylation at Ser 112, the combined inhibition of the MAP kinase and PKC pathways only partially blocked the inhibitory effects of VP on caspase 3 activation. This suggests that the full antiapoptotic action of VP involves an additional antiapoptotic pathway, possibly in a Bad-independent manner.
Since the PI3K/protein kinase B (Akt) pathway has been shown to inactivate several pro-apoptotic molecules (Song et al., 2005), we sought to investigate the role of this pathway on the protective actions of VP in H32 cells. Here we demonstrate that VP induces a rapid but transient phosphorylation of Akt in serum deprived H32 cells. In addition, PI3 kinase inhibitors partially blocked the inhibitory action of VP on caspase 3 activity, and simultaneous blockade of PKC, MAP kinase and PI3 kinase pathways completely prevented the effect of VP on caspase 3. These data strongly suggest a role for the PI3/Akt pathway. One of the mechanisms by which Akt activation prevents apoptosis is inactivation of Bad by phosphorylation at Ser136 (Datta et al., 1997). Because of problems with high background using Ser136 phospho-Bad antibodies for western blots in H32 cells, in these experiments it was not possible to determine whether VP phosphorylates Bad at Ser136. It is possible that the lack of complete blockade of caspase 3 activation after blocking Ser112 phosphorylation (by the combination of PKC and MAP kinase inhibitors) is due to Ser136 phosphorylation by Akt. However, this is unlikely since we have shown that the phosphorylation inactive mutant, in which both Ser112 and 136 were replaced by alanine (Bad 2SA) serving as a dominant negative mutant of Bad, is also unable to completely block the ability of VP to prevent caspase 3 activation (Chen et al., 2008). It has been shown that Akt can prevent apoptosis by increasing the threshold for the onset of several apoptotic pathways, by increasing the expression of antiapoptotic proteins, such as Bcl-2 (Hovelmann et al, 2007). However, this does not appear to be the case in H32 cells, since recent experiments have shown that VP does not increase Bcl-2 and Bcl-XL antiapoptotic proteins up to 6 h incubation (Chen and Aguilera, unpublished observations). Alternatively, activation of Akt by VP could prevent cell death by modulating the apoptotic process at the post-mitochondrial level, by directly inhibiting caspase 9 and 3 activation at a step after cytochrome c release. In this regard, it has been shown that Akt regulates cell survival and apoptosis at a post-mitochondrial level without altering expression levels of endogenous, Bcl2, Bcl-x, or Bax (Zhou et al., 2000). In addition to the above discussed pathways, activation of PLC by VP results in increases of inositol phosphates, and consequent release of calcium from endoplasma reticulum (Birnbaumer, 2000). Previous studies indicating that inhibitors of calcium calmodulin dependent kinase reduced the ability of VP to inhibit serum deprivation-induced caspase-3 activity suggest that calcium is also involved in the mechanisms mediating the antiapoptotic effects of VP (Chen et al., 2008). However, the exact role of calcium in this process remains to be elucidated.
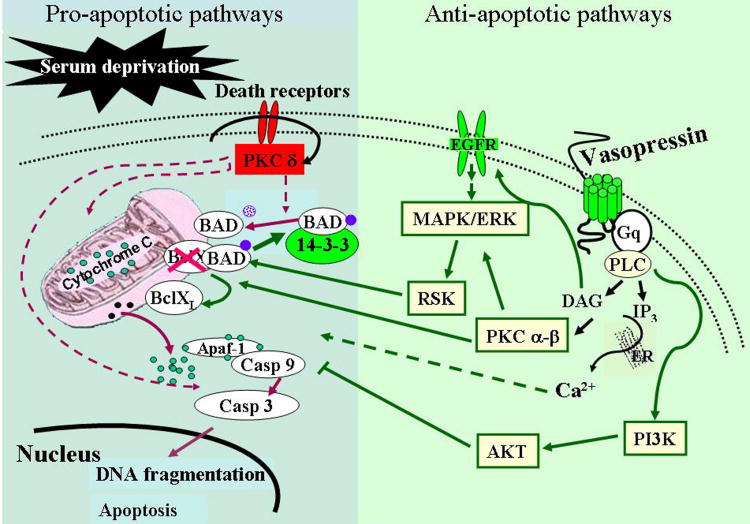
In summary, the present experiments reveal differential roles of PKC subtypes on serum deprivation-induced apoptosis and the protective effect of VP, with PKCδ mediating the proapoptotic effect of serum deprivation, and PCKα and β contributing to the antiapoptotic actions of VP (Fig. 7). The combined effects of PKC α and β and Erk/Rsk MAP kinase activation are responsible for the ability of VP to phosphorylate (and inactivate) the proapoptotic protein Bad. On the other hand, Bad phosphorylation by VP alone is not sufficient to prevent apoptosis, and the full protective effect of VP on serum deprivation-induced caspase 3 (and apoptosis) in H32 cells involves a Bad-independent pathway mediated by activation of the PI3 kinase/Akt pathway.
Fig.7.
Diagram of the proposed mechanisms mediating serum deprivation induced apoptosis, and the protective effect of VP in the hypothalamic cell line H32. The proapoptotic patways are depicted on the left light blue panel and red arrows and the antiapoptotic pathways initiated by VP in the right green panel. Serum deprivation activates PKCδ leading to caspase activation and apoptotic cell death. Activation of PLC coupled V1 VP receptors by VP results in IP3 and DAG formation and consequent activation of PKCα and β, as well as transactivation of EGF receptor/MEK/ERK/RSK cascade. RSK and PKC α and β mediate phosphorylation of BAD causing its inactivation and preventing cytochrome c release and apoptotic cell death. In addition, activation of Akt by VP contributes to the protective effect of VP probably by modulating the apoptotic process at the post-mitochondrial level inhibiting caspase 9 and 3 activation.
Acknowledgments
This work was supported by the Intramural Research Program of National Institute of Child Health and Human Development, NIH.
Abbreviations
- VP
vasopressin
- MAPK
mitogen activated protein kinase
- ERK
extracellular signal-regulated kinases
- CaMK
Ca2+/calmodulin dependent kinase
- PKC
protein kinase C
- Akt
Protein Kinase B
- GPCR
G protein-coupled membrane receptor
- EGFR
epidermal growth factor receptors
References
- Alescio-Lautier B, Paban V, Soumireu-Mourat B. Neuromodulation of memory in the hippocampus by vasopressin. Eur J Pharmacol. 2000;405:63–72. doi: 10.1016/s0014-2999(00)00542-2. [DOI] [PubMed] [Google Scholar]
- Bhave SV, Malhotra RK, Wakade TD, Wakade AR. Survival of chick embryonic sensory neurons in culture is supported by phorbol esters. J Neurochem. 1990;54:627–632. doi: 10.1111/j.1471-4159.1990.tb01917.x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M. Vasopressin receptors. Trends Endocrinol Metab. 2000;11:406–410. doi: 10.1016/s1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- Bright R, Raval A, Dembner J, Pérez-Pinzón MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Chen J, Aguilera G. The Neuronal Protective Effect of Vasopressin; 90th Annunal Meeting of the Endocrine Society; Washinton, DC. 2009. [Google Scholar]
- Chen J, Volpi S, Aguilera G. Anti-apoptotic actions of vasopressin in H32 neurons involve MAP kinase transactivation and Bad phosphorylation. Exp Neurol. 2008;211:529–538. doi: 10.1016/j.expneurol.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM. PKC-delta is an apoptotic lamin kinase. Oncogene. 2000;19:2331–2337. doi: 10.1038/sj.onc.1203555. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley LE, Johnson GL. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989;264:8646–8652. [PubMed] [Google Scholar]
- Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol. 1994;14:302–321. [PubMed] [Google Scholar]
- Koh JY, Wie MB, Gwag BJ, Sensi SL, Canzoniero LM, Demaro J, Csernansky C, Choi DW. Staurosporine-induced neuronal apoptosis. Exp Neurol. 1995;135:153–159. doi: 10.1006/exnr.1995.1074. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wotjak CT, Neumann ID, Engelmann M. Release of vasopressin within the brain contributes to neuroendocrine and behavioral regulation. Prog Brain Res. 1998;119:201–220. doi: 10.1016/s0079-6123(08)61571-x. [DOI] [PubMed] [Google Scholar]
- Lenz JC, Reusch HP, Albrecht N, Schultz G, Schaefer M. Ca2+-controlled competitive diacylglycerol binding of protein kinase C isoenzymes in living cells. J Cell Biol. 2002;159:291–302. doi: 10.1083/jcb.200203048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait SJ, O'Carroll AM, Brownstein MJ. Molecular biology of vasopressin receptors. Ann N Y Acad Sci. 1995;771:273–292. doi: 10.1111/j.1749-6632.1995.tb44688.x. [DOI] [PubMed] [Google Scholar]
- Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J Neurosci. 2001;21:2929–2938. doi: 10.1523/JNEUROSCI.21-09-02929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- McCann SM, Franci CR, Antunes-Rodrigues J. Hormonal control of water and electrolyte intake and output. Acta Physiol Scand Suppl. 1989;583:97–104. [PubMed] [Google Scholar]
- Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- Nakajima T. Signaling cascades in radiation-induced apoptosis: roles of protein kinase C in the apoptosis regulation. Med Sci Monit. 2006;12:RA220–224. [PubMed] [Google Scholar]
- Ohno S, Nishizuka Y. Protein kinase C isotypes and their specific functions: prologue. 132. 2002;4:509–511. doi: 10.1093/oxfordjournals.jbchem.a003249. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacology Biochemistry and Behavior. 2005;82:223–227. doi: 10.1016/j.pbb.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Poole AW, Pula G, Hers I, Crosby D, Jones ML. PKC-interacting proteins: from function to pharmacology. Trends Pharmacol Sci. 2004;25:528–535. doi: 10.1016/j.tips.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase Cdelta and apoptosis. Biochem Soc Trans. 2007;35:1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaler M, Wiesenhofer B, Biedermann V, Seppi T, Zimmermann J, Grunicke H, Hofmann J. The involvement of protein kinase C isoenzymes alpha, epsilon and zeta in the sensitivity to antitumor treatment and apoptosis induction. Anticancer Res. 1999;19:3969–3976. [PubMed] [Google Scholar]
- Tanaka S, Koike T. Activation of protein kinase C delays apoptosis of nerve growth factor-deprived rat sympathetic neurons through a Ca(2+)-influx dependent mechanism. Neurosci Lett. 2001;313(1-2):9–12. doi: 10.1016/s0304-3940(01)02193-0. 2001 Nov 2. 313:9-12. [DOI] [PubMed] [Google Scholar]
- Thibonnier M. Signal transduction of V1-vascular vasopressin receptors. Regul Pept. 1992;38:1–11. doi: 10.1016/0167-0115(92)90067-5. [DOI] [PubMed] [Google Scholar]
- Volpi S, Soh JW, Aguilera G. Activation of GAGA binding protein to the vasopressin V1b receptor promoter involves VP-induced MAP kinase phosphorylation though transactivation of the EGF receptor. 86th Annual Meeting of The Endocrine Society; New Orleans, Louisiana. 2004. [Google Scholar]
- Volpi S, Liu Y, Aguilera G. Vasopressin increases GAGA binding activity to the V1b receptor promoter through transactivation of the MAP kinase pathway. J Mol Endocrinol. 2006;36:581–590. doi: 10.1677/jme.1.01995. [DOI] [PubMed] [Google Scholar]
- Wakade AR, Wakade TD, Bhave SV, Malhotra RK. Demonstration of adrenergic and dopaminergic receptors in cultured sympathetic neurons--their coupling to cAMP but not to the transmitter release process. Neuroscience. 1988;27:1021–1028. doi: 10.1016/0306-4522(88)90206-0. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D. Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]