Abstract
The indole-3-carbinol (I3C) metabolite 3,3-diindolylmethane (DIM) is a proposed cancer prevention agent for various tumor types, including breast cancer. Here, we show that DIM up-regulates expression of the tumor suppressor protein BRCA1 in carcinoma and normal cell types. Up-regulation of BRCA1 was dose- and time-dependent; and it was observed at physiologically relevant micromolar and submicromolar DIM concentrations when cells were exposed for 72 hr. Treatment with the parent compound (I3C) or DIM (1 µM) protected against cell killing due to H2O2 and other oxidants; and the protection was abrogated by knockdown of BRCA1. DIM stimulated signaling by the antioxidant transcription factor NFE2L2 (NRF2) through the antioxidant response element in a BRCA1-dependent manner. We further showed that DIM rapidly stimulated phosphorylation of BRCA1 on serine-1387 and serine-1524 and that these phosphorylations are required for protection against oxidative stress. DIM-induced phosphorylation of BRCA1 on serine-1387 was dependent upon ATM. Finally, in our assay systems, H2O2-induced cell death was not due to apoptosis. However, a significant component of cell death was attributable to autophagy; and both DIM and BRCA1 inhibited H2O2-induced autophagy. Our findings suggest that low concentrations of DIM protect cells against oxidative stress via the tumor suppressor BRCA1 by several distinct mechanisms.
Keywords: indole-3-carbinol (I3C), diindolylmethane (DIM), oxidative stress, BRCA1, autophagy
INTRODUCTION
Indole-3-carbinol (I3C), a phytochemical from cruciferous vegetables is of interest because a diet rich in cruciferous vegetables is associated with a reduced risk of several tumor types such as breast cancer (1,2) and because dietary supplementation with I3C can prevent estrogen-dependent cancers (breast, cervix, endometrium) in animals (3–5). In the acid environment of the stomach, I3C undergoes hydrolysis to a number of products, including a dimeric product, 3,3’-diindolylmethane (DIM), its major active metabolite (6). DIM is acid stable and is detected in the bloodstream after oral intake of I3C or DIM (7,8).
Most studies on DIM have utilized supraphysiological concentrations (15–50 µM). These studies indicate that DIM can inhibit invasion, angiogenesis, and cell proliferation and induce apoptosis by modulating signaling pathways involving Akt, NF-κB, and FOXO3 (9–12), It can also modulate basal and estrogen-inducible gene expression (13,14); and it can induce an endoplasmic reticulum stress response (15). Like I3C, DIM can regulate estrogen metabolism by shifting the metabolism from potentially carcinogenic 16α-hydroxy derivatives to inert 2-hydroxy derivatives (16). DIM is also an androgen receptor antagonist in prostate cancer cells (17). Previous studies suggest that I3C and DIM can function as ligands for the aryl hydrocarbon receptor (18,19) and can inhibit estrogen receptor activity independently of any effect on estrogen metabolism (20).
There is limited available information on plasma levels of DIM that can be achieved through oral ingestion of I3C or DIM. In humans, after single oral doses of I3C of 400–1200 mg, the peak plasma concentrations (observed after 2–3 hr) ranged from 61–607 ng/ml of DIM (about 0.25–2.5 µM) (21). Oral administration of DIM might yield higher plasma DIM levels, since oral I3C is converted to various acid condensation products in the stomach (22). The percent conversion of I3C to its hydrolysis products and absorption is unclear, but in the study cited, no I3C was detected in plasma.
Previously, we reported that I3C up-regulates expression of the breast cancer susceptibility genes BRCA1 and BRCA2 due, in part, to an endoplasmic reticulum stress response (23). Here, we report that DIM stimulates BRCA1 signaling and expression at low concentrations and protects cells against oxidative stress in a BRCA1-dependent manner.
MATERIALS AND METHODS
Cells and culture
All cell lines except HMECs (normal human mammary epithelial cells) and Brca1-deficient mouse embryonic fibroblasts (MEFs) were obtained from the ATCC (Manassas, VA). MEFs homozygous for a deletion of Brca1 exon 11 and wild-type MEFs were generously provided by Dr. Chuxia Deng (NIDDK, Bethesda, MD) (24). All cell types except HMECs were cultured using standard techniques (23,25). HMECs were obtained from Clonetics (San Diego, CA) and grown in a defined medium containing mammary epithelial cell basal medium (ScienCell Research Laboratories, Carlsbad, CA) supplemented with 4 µg/ml bovine pituitary extract (BD Biosciences, San Jose, CA), 5 µg/ml insulin, 10 ng/ml EGF (Sigma Chemical Co., St. Louis, MO), 0.5 µg/ml hydrocortisone (Sigma), and 10−5 M isoproterenol (Sigma).
Reagents
Indole-3-carbinol was obtained from the Sigma and dissolved in DMSO prior to dilution in cell culture medium. A bioavailable formulation of diindolylmethane (BR-DIM) was generously provided by Dr. Michael Zeligs (Bioresponse, Boulder, CO). It is herein referred to as “DIM”. DIM was also dissolved in DMSO. H2O2, paraquat, nickel acetate, and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) were purchased from Sigma.
Expression vectors
The wild-type BRCA1 vector (wtBRCA1) was described earlier (25). Expression vectors encoding full-length BRCA1 with point mutations at different serine residues (S1387A, S1423A, S1457A, S1524A) were created by site-directed mutagenesis of wtBRCA1 within the pcDNA3 vector (Invitrogen, Carlsbad, CA), using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The mutations were confirmed by sequencing. A human beclin 1 cDNA [provided by Dr. G Kroemer (Institut Gustave Roussy, Villejuif, France)] and a human NFE2L2/NRF2 cDNA (Invitrogen) were inserted into the pcDNA3 vector. The green fluorescent protein-light chain 3 (GFP-LC3) vector was provided by Dr. Gabriel Lopez-Berestein (Mount Sinai School of Medicine, New York, NY) (26).
Transient transfections
Subconfluent proliferating cells were transfected overnight with the indicated vector (15 µg of DNA/100-mm dish or 5 µg/well in 6-well dishes) using Lipofectamine 2000 (Invitrogen); washed; and allowed to recover for several hours. The cells were then harvested using trypsin; seeded into 48- or 96-well dishes; allowed to attach overnight; and subjected to the indicated treatments prior to MTT assays.
siRNA treatments
Proliferating cells in 12-well dishes were treated with gene-specific siRNA or control-siRNA using siRNA Transfection Reagent (Santa Cruz Biotechnology, Santa Cruz, CA). BRCA1-siRNA (sc-29219), ATM-siRNA (sc-29761), ATR-siRNA (sc-29763), beclin 1-siRNA (sc-29797), and control-siRNA (sc-37007) were purchased from Santa Cruz. The cells were exposed to the indicated siRNA (50 nM) for at least 48 hr. For experiments lasting more than 72 hr, the cells were refed with fresh siRNA of the same type (50 nM) on the third day. The efficacy of the knockdown was confirmed by Western blotting.
Reporter assays
The NQO1-ARE-Luc reporter contains the antioxidant response element (ARE) of NAD(P)H dehydrogenase quinone 1 (NQO1) driving a minimal promoter upstream of luciferase (27). GST-α1-Luc contains the luciferase gene under the control of a promoter segment containing 940-bp up-stream of the transcription start site of mouse GST-α1. x-CT-Luc contains the luciferase gene under the control of a 235-bp segment up-stream of the transcription start site of mouse cystine/glutamate transporter. These reporters were generously provided by Dr. Filiberto Cimino (Universita di Napoli Federico II, Naples, Italy) (28). Reporter assays were performed as described earlier (23,25). Briefly, cells in 48-well dishes were transfected overnight with the indicated expression vector(s) [0.25 µg/well] and reporter (0.25 µg/well), using Lipofectamine 2000. They were then washed, allowed to recover for several hr, treated as indicated, and harvested for luciferase measurements. Luciferase values were normalized to the control conditions and expressed as means ± SEMs of quadruplicate wells. Transfection efficiency was monitored using the Galacto-Star Mammalian Reporter Gene Assay System (Applied Biosystems, Foster City, CA). Each experiment was performed at least twice to assure that the findings were reproducible.
Treatment with oxidants
Subconfluent proliferating cells in 48-well or 96-well dishes were treated with different doses of H2O2, paraquat, or nickel acetate for 24 hr (unless otherwise stated) in the presence of DIM or vehicle (DMSO) and assayed for MTT dye reduction, a measure of mitochondrial viability (29).
MTT assays
After the indicated treatment, cells in 48- or 96-well dishes were tested for MTT dye conversion. Cell viability was calculated as the amount of dye conversion relative to sham-treated control cells and expressed as means ± SEMs of ten replicate wells. At least two independent experiments were performed to assure reproducibility of the findings.
Autophagy assays
Monodansylcadaverine (MDC) staining
Autophagic vacuoles were detected based on MDC staining (30). After the indicated treatments, cells cultured on glass coverslips were incubated with 0.05 mM MDC for 60 min at 37°C; fixed in 4% paraformaldehyde (15 min) and washed twice with PBS. The glass coverslips were mounted onto slides using Geltol as mounting medium. Quantitative determination of MDC-positive cells was performed immediately after preparation, using a Nikon Mikrophot-FXA with a 356 nm excitation filter and a 545 nm barrier filter. Cells were manually scored as positive if they contained significant staining for autophagic vacuoles, as compared with the large majority of untreated control cells. The percentage of MDC positive cells was determined by counting of 1000 cells per culture and plotted as means ± SEMs based on three experiments.
Western blotting
Western blotting was performed as described earlier (23,25). Briefly, aliquots of whole cell lysate (100 µg protein) were separated using a 12% SDS-polyacrylamide gel. The proteins were transferred to a nitrocellulose membrane, which was incubated with primary antibody overnight at 4°C, followed by the addition of horseradish peroxidase-linked secondary antibody (Santa Cruz) at 1:2000. Protein bands were visualized using the enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL). The primary antibodies were: rabbit polyclonal anti-BRCA1 (Santa Cruz, sc-642, 1:500); mouse monoclonal anti-BRCA2 (Santa Cruz, sc-56033, 1:500); mouse monoclonal α-actin (Santa Cruz, sc-1616, 1:500); anti-phospho-BRCA1 (S1387) [Millipore (Billerica, MA), AB3257, 1:1,000]; anti-phospho-BRCA1 (S1423) [Millipore, AB3259, 1:500]; anti-phospho-BRCA1 (S1457) [Millipore, 07–007, 1:500]; anti-phospho-BRCA1 (S1524) [Cell Signaling Technology (Danvers, MA), #9009, 1:500]; mouse monoclonal anti-ATM (Santa Cruz, sc-73615), rabbit polyclonal anti-ATR (Santa Cruz, sc-21848); rabbit polyconal anti-beclin 1 (Sigma); rabbit polyconal anti-LC3 [Novus Biologicals (Littleton, CO), AB3257, 1:200]. Equal protein loading was confirmed by immunoblotting for α-actin. Colored markers (BioRad, Hercules, CA) were used as molecular size standards.
Semi-quantitative RT-PCR
Semi-quantitative RT-PCR was performed as described before (23,25). The cycle number was adjusted so that all reactions fell into the linear range of amplification. The forward and reverse primers (product sizes) were: BRCA1: 5'-TTGCGGGAGGAAAATGGGTAGTT A-3'; 3'-TGTGCCAAGGGTGAATGATGAAG-5' (285-bp); MGST1: 5'-ACTGCGCTGGCTTTGGC AAG-3'; 3'-AGATCCGAGCACCTACAAAG-5' (200-bp); β-actin: 5'-TGTTACCAACTGGGACG ATA-3'; 3'-TGTTACCAACTGGGACGATA-5' (764-bp); and β2-macroglobulin (β-MG): 5'-CTCGC CTACTCTCTCTTTCT-3'; 5'-TGTCGGATGGATGAAACCCAG-3' (136-bp).
Cell cycle analysis
Cells were harvested using trypsin, washed with PBS, and fixed in cold 70% ethanol. The samples were then treated with RNase A, stained with propidium iodide (100 mg/ml), and analyzed by FACSort (Becton Dickson, San Jose, CA), using the ModFit software (Verity Softwarehouse, Topsham, ME). At least 20,000 events were collected and analyzed.
Statistical methods
Where appropriate, comparisons were made using two-tailed Student’s t-tests.
RESULTS
DIM stimulates BRCA1 expression
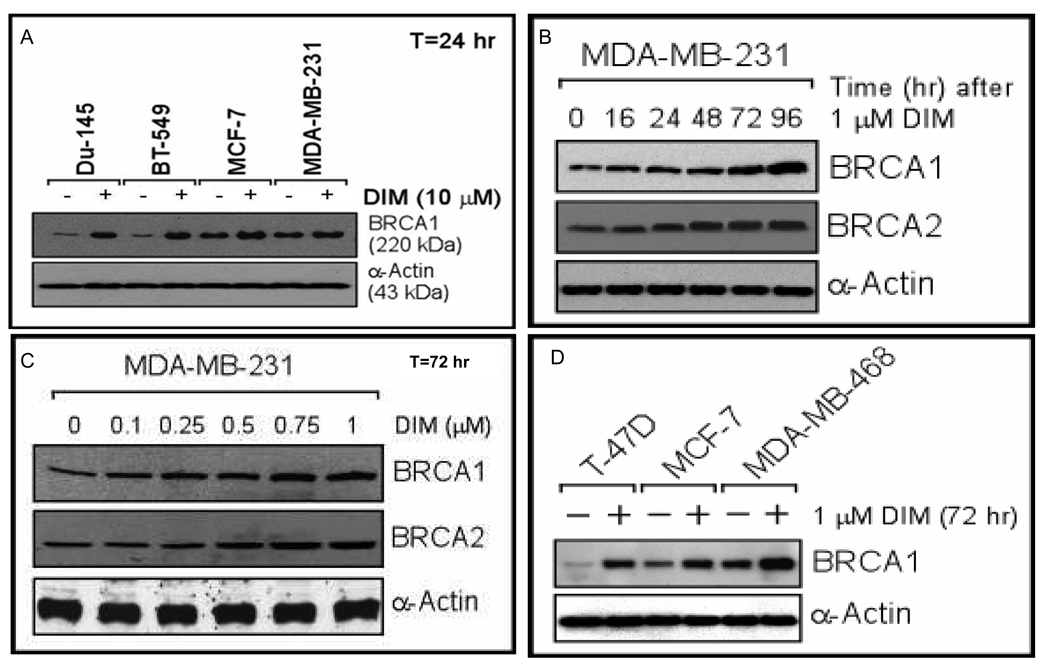
We found that DIM (10 µM x 24 hr) stimulated BRCA1 protein expression in various carcinoma cell types, including prostate (DU-145) and breast (BT549, MCF-7, MDA-MB-231) [Fig. 1A]. Similar results were observed in two additional breast cancer cell lines (BT474, MDA-MB-453), a lung cancer cell line (A549), and a cervical cancer cell line (HeLa) [Supplemental Fig. 1A]. It appeared that cell lines with low BRCA1 expression exhibited more stimulation than those with high basal expression. In DU-145 and MDA-MB-231 cells, stimulation of BRCA1 protein expression was dose-dependent (24 hr exposure) and time-dependent (10 µM DIM) [Supplemental Figs. 1B–1E]. At 10 µM of DIM, increased BRCA1 protein levels were first observed after 8 hr. Densitometry corresponding to these Western blots is provided in Supplemental Fig. 2.
Fig. 1. DIM up-regulates BRCA1 expression.
A. Subconfluent proliferating cells were incubated with 10 µM DIM (or DMSO) for 24 hr and Western blotted for BRCA1 and α-actin (loading control). B–D. Cells were treated with DIM for the indicated times using the indicated concentrations and harvested for Western blotting for BRCA1, BRCA2, and α-actin.
In MDA-MB-231 cells exposed to a low dose of DIM (1 µM), BRCA1 (and BRCA2) protein levels continued to increase for four days (Fig. 1B); and in cells exposed to DIM for 72 hr, increased BRCA1/2 levels were detected at ≤ 0.5 µM DIM (Fig. 1C). Other breast cancer cell lines (T47D, MCF-7, MDA-MB-468) also showed increases in BRCA1 protein after a 72 hr exposure to 1 µM DIM (Fig. 1D). MDA-MB-231 cells treated with DIM for 72 hr showed dose-dependent increases in BRCA1 mRNA at ≥ 0.5 µM DIM [Supplemental Fig. 1F]. These findings suggest that low, physiologically relevant concentrations of DIM stimulate BRCA1 expression.
I3C and DIM protect breast cancer cells against oxidative stress
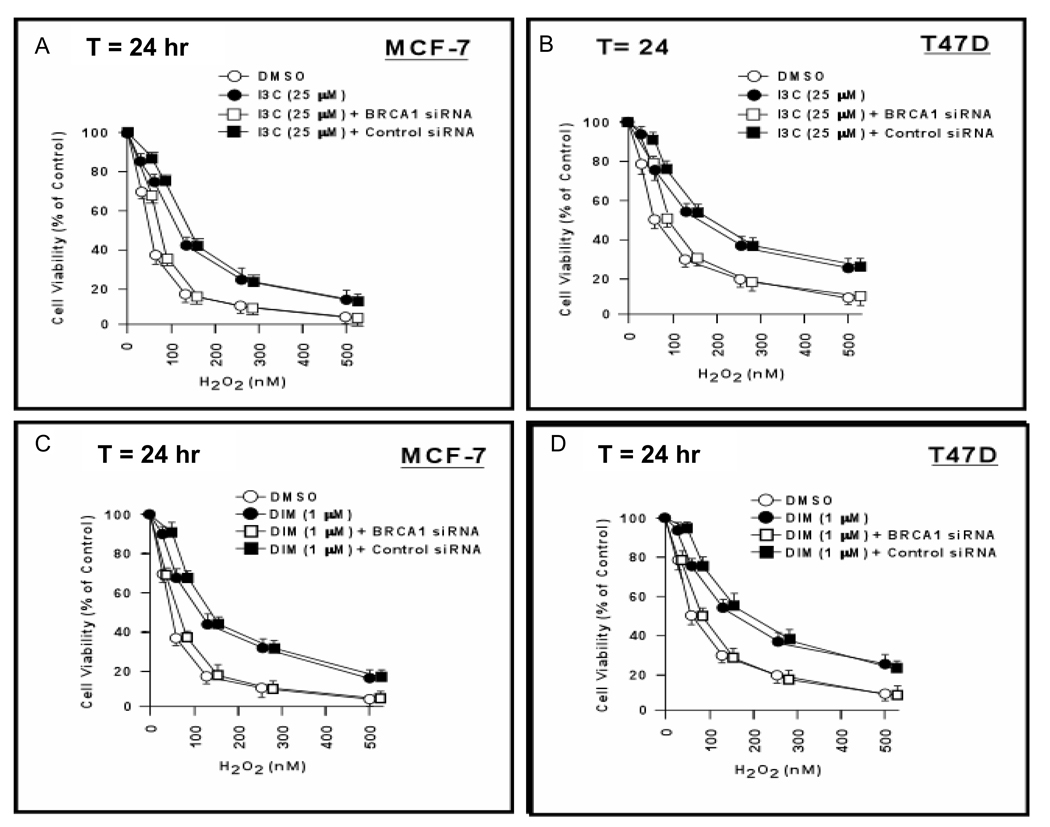
MCF-7 and T47D cells were incubated with I3C (25 µM) or vehicle (DMSO) plus H2O2 for 24 hr and tested for cell viability using MTT assays. Without H2O2, 25 µM I3C has no effect on cell viability, using MTT assays (23). I3C enhanced the survival of MCF-7 and T47D cells at all but the lowest dose of H2O2 (P < 0.001–0.01) [Figs. 2A-2B]. To test the role of BRCA1 in I3C cell protection, cells were pre-treated with BRCA1-siRNA or control-siRNA for 48 hr before incubation with I3C/H2O2. BRCA1-siRNA (but not control-siRNA) abrogated I3C-mediated cell protection (P < 0.01). We next tested the effect of DIM (1 µM) on sensitivity to H2O2. Like I3C, DIM protected both cell lines against H2O2 (P < 0.001–0.01); and BRCA1-siRNA (but not control-siRNA) abrogated DIM-mediated cell protection (P < 0.01) [Figs. 2C-2D]. The efficacy of BRCA1 knockdowns is illustrated in Supplemental Fig. 3.
Fig. 2. I3C and DIM protect breast carcinoma cells against oxidative stress.
A,B. MCF-7 or T47D cells were pre-treated with BRCA1-siRNA, control-siRNA (50 nM × 48 hr), or no siRNA; incubated with I3C (25 µM) or vehicle (DMSO) plus H2O2 for 24 hr; and harvested for MTT assays. Cell viability values are means ± SEMs of 10 wells. C,D. MCF-7 or T47D cells were pre-treated with siRNAs; incubated with DIM (1 µM) plus the indicated dose of H2O2 for 24 hr; and assayed for cell viability.
DIM also protected DU-145 cells against H2O2; and it protected MDA-MB-231 cells against nickel acetate, an agent whose toxicity is mostly due to oxidative stress (31) [Supplemental Figs. 4A–4B]. DIM protected MDA-MB-231 cells against paraquat, an herbicide that causes toxicity via generation of superoxide ions (32) [Supplemental Figs. 4C–4D]; and it protected MDA-MB-231 cells against H2O2 in a BRCA1-dependent manner (P < 0.001) [Supplemental Fig. 4E].
DIM stimulates antioxidant response element (ARE)-dependent signaling
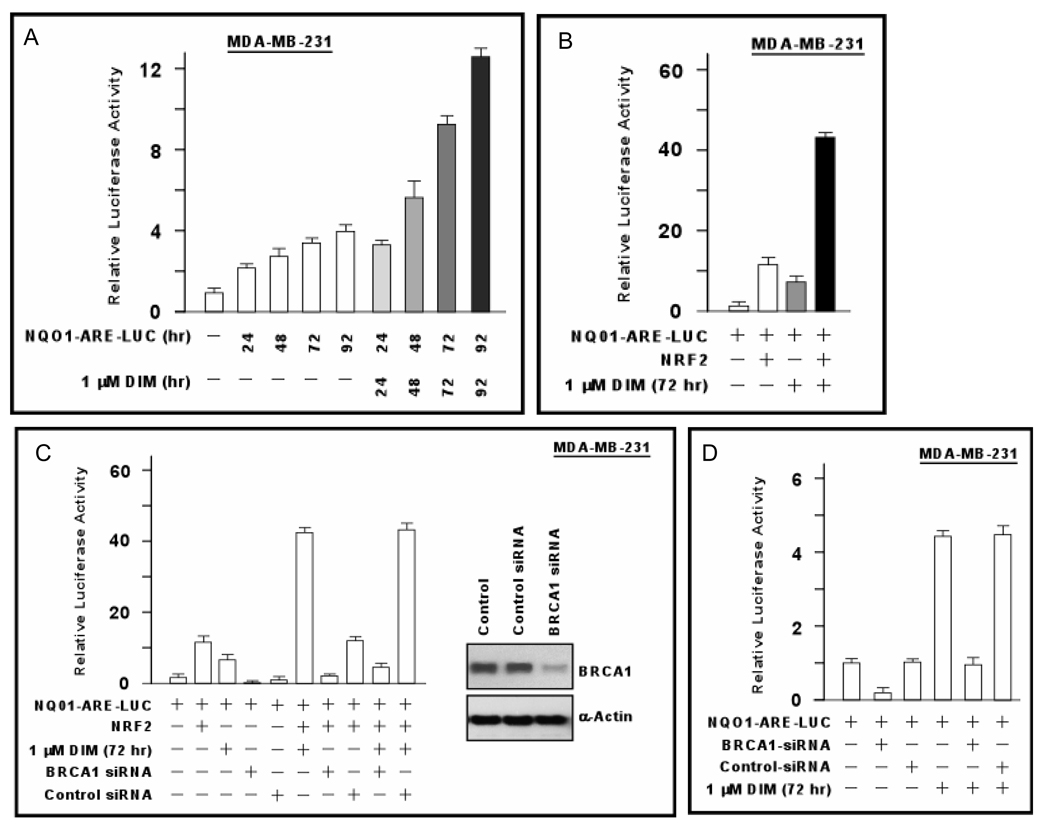
In response to oxidative stress, the transcription factor NFE2L2 (NRF2) dissociates from its cytoplasmic inhibitor, translocates to the nucleus, and activates transcription of genes containing an ARE in their promoters (33). We used a luciferase reporter driven by the NQO1-ARE (27) to determine if DIM regulates antioxidant signaling. Because DIM stimulates BRCA1 expression in a time-dependent manner, we tested exposure times from 24–96 hr. DIM (1 µM) stimulated NQO1-ARE-Luc activity in MDA-MB-231 at all time points (P < 0.01) [Fig. 3A]. The fold-stimulation relative to cells incubated without DIM was about three-fold at 72–96 hr in this experiment, although it was up to 7-fold in other experiments. In cells co-transfected with an NRF2 expression vector, NRF2 stimulated NQO1-ARE-Luc by 10-fold; and DIM enhanced NRF2-stimulated reporter activity by another 4-fold (P < 0.001) [Fig. 3B].
Fig. 3. DIM stimulates antioxidant response element signaling.
A. MDA-MB-231 cells were transfected overnight with the NQO1-ARE-Luc reporter; incubated ± DIM (1 µM) for the indicated time; and assayed for luciferase activity. Luciferase values are expressed relative to control conditions (0 DIM) as means ± SEMs of quadruplicate wells. B. MDA-MB-231 were transfected with NQO1-ARE-Luc ± NRF2 expression vector; treated ± DIM (1 µM × 72 hr); and assayed for luciferase activity. C,D. MDA-MB-231 cells were treated with BRCA1-siRNA, control-siRNA, or no siRNA for 48 hr; transfected with NQO1-ARE-Luc ± NRF2; treated ± DIM (1 µM × 72 hr); and assayed for luciferase activity. The BRCA1 knockdown is demonstrated by Western blotting in panel C.
We tested the role of BRCA1 in DIM-stimulated NRF2 activity by pre-treating MDA-MB-231 cells with BRCA1-siRNA and determining the effect of DIM (1 µM × 72 hr) on NRF2-stimulated NQO1-ARE-Luc activity. BRCA1-siRNA (but not control-siRNA) inhibited NQO1-ARE-Luc activity due to NRF2 alone (P < 0.001) and reduced the (DIM+NRF2)-stimulated reporter to less than that of NRF2 alone (P < 0.001) [Fig. 3C]. BRCA1-siRNA also reduced the basal reporter activity and blocked the DIM-stimulated activity (P < 0.001) [Fig. 3D].
We tested the ability of DIM to stimulate two additional ARE-containing reporters, GST-α1-Luc and x-CT Luc (28). NRF2 stimulated GST-α1-Luc activity by about (6–7.5)-fold in MDA-MB-231 and T47D cells; and DIM (1 µM) gave an additional 5-fold stimulation (P < 0.001) [Supplemental Fig. 5A]. The DIM stimulation of NRF2 activity was abrogated by BRCA1-siRNA (P < 0.001). The ability of NRF2 to stimulate GST-α1-Luc activity without DIM was also attenuated by BRCA1-siRNA (P < 0.001). Similar results were obtained using the c-CT-Luc reporter; and DIM caused dose-dependent increases in the mRNA for MGST1, another ARE-regulated gene (34) [Supplemental Figs. 5B–5C], suggesting that DIM stimulates ARE signaling in a BRCA1-dependent manner.
To further examine the role of endogenous BRCA1 in NRF2 signaling, we tested wild-type (Brca1+/+) vs Brca1-deficient (Brca1−/−) MEFs (24). In wild-type cells, NRF2 and DIM (1 µM × 72 hr) robustly stimulated NQO1-ARE-Luc activity; and the combination of (DIM+NRF2) gave much greater stimulation than either agent alone (P < 0.001) [Supplemental Fig. 5D]. In contrast, Brca1−/− cells showed no DIM-induced stimulation of reporter activity; and the stimulation by NRF2 was less than that observed in Brca1+/+ cells.
DIM stimulates BRCA1 signaling
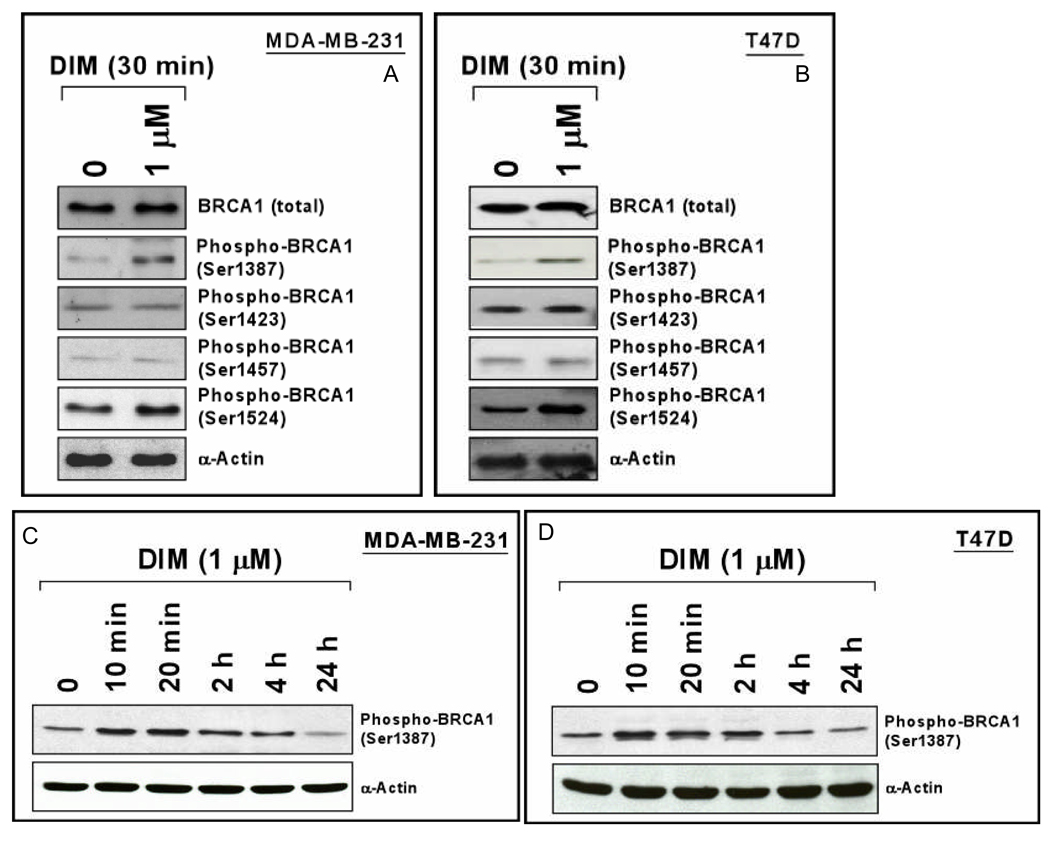
Although DIM (1 µM) may take more than 24 hr to increase BRCA1 protein levels, a 24 hr exposure conferred BRCA1-dependent protection against H2O2, suggesting that some DIM effects occur before BRCA1 levels increase. We tested the effect of DIM on BRCA1 phosphorylation by Western blotting using phospho-specific antibodies in MDA-MB-231 and T47D cells. A 30 min exposure to DIM caused increased phosphorylation of BRCA1 on S1387 and S1524, but not on S1423 or S1457 (Figs. 4A-4B). The DIM-induced phosphorylation of BRCA1 on S1387 was time-dependent (Figs. 4C-4D), with high levels of phosphorylation observed at short times (10–20 min) and reduction in phosphorylation from 2–24 hr. Ataxia-telangiectasia mutated (ATM) and A-T and Rad3-related (ATR) are PI3 kinase family proteins that phosphorylate BRCA1 and other substrates in response to DNA damage (see Discussion). We tested the effect of knocking down these proteins on phospho-BRCA1 (S1387) levels. ATM-siRNA had little effect on basal phosphorylation but blocked DIM-induced phosphorylation in MDA-MB-231 and T47D cells (Supplemental Fig. 6); while ATR-siRNA had no effect on DIM-induced phosphorylation (data not shown).
Fig. 4. DIM stimulates BRCA1 signaling.
A–D. Cells were treated with DIM (1 µM) for the indicated time and harvested to detect total or phosphorylated BRCA1 by Western blotting.
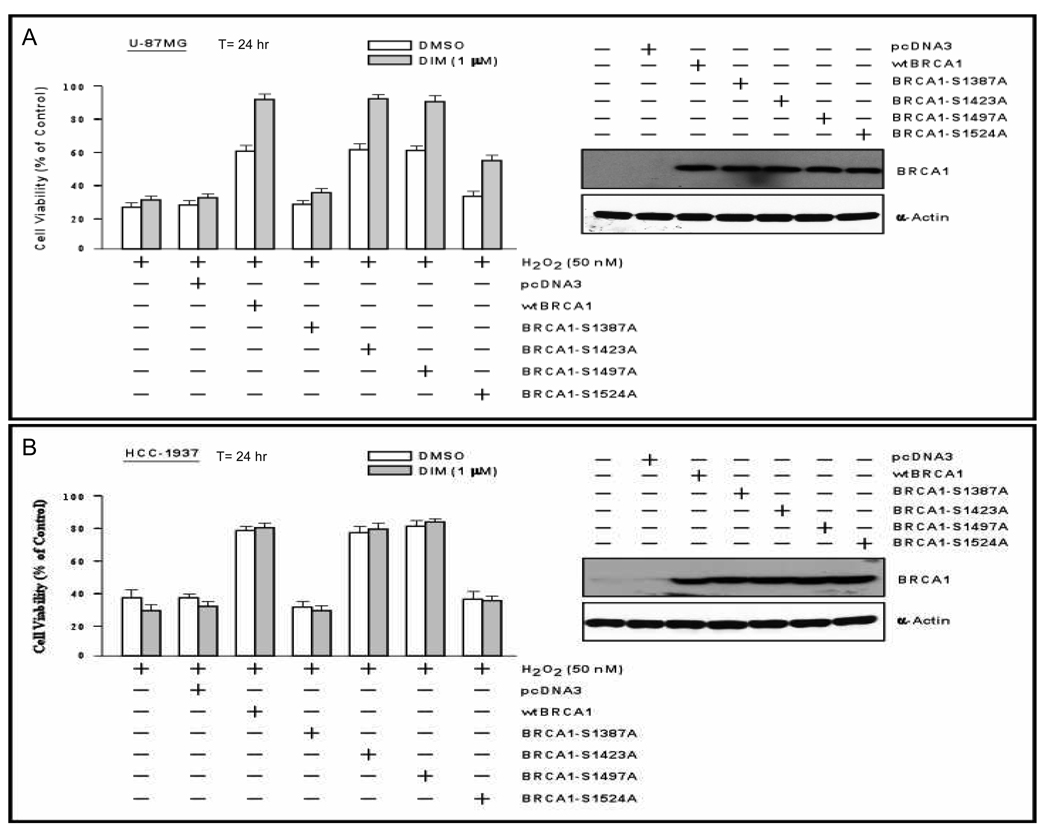
We tested the significance of these findings using a cell line with no detectable endogenous BRCA1 (U87MG glioma cells). The cells were transfected with wtBRCA1 or expression vectors for BRCA1 proteins with alanine mutations of different serine residues and tested for sensitivity to H2O2 (50 nM for 24 hr) ± DIM. Expression of the mutant BRCA1 proteins was confirmed by Western blotting. Consistent with the absence of endogenous BRCA1, DIM did not significantly protect untransfected cells or cells transfected with empty pcDNA3 vector. wtBRCA1 alone conferred significant protection, as did the BRCA1 mutants S1423A and S1457A (P < 0.001) [Fig. 5A]. However, S1387A and S1524A conferred little or no protection. DIM conferred additional protection of cells transfected with wtBRCA1, S1423A, S1457A, and S1524A but little protection of cells transfected with S1387A. These findings suggest that DIM-induced phosphorylation of S1387 and to some extent S1524A contribute to cell protection.
Fig. 5. Protection against H2O2 by DIM plus different phosphorylation site mutants of BRCA1.
U87MG (A) or HCC1937 (B) cells were transfected overnight with the indicated expression vector; harvested using trypsin; inoculated into 96-well dishes; allowed to attach overnight; treated with DIM or vehicle (DMSO) plus H2O2 (50 nM) for 24 hr; and tested for cell viability using MTT assays. Values are means ± SEMs of 10 replicate wells. Western blots for total BRCA1 for the different transfection conditions are provided.
DIM does not protect BRCA1 mutant breast cancer cells against H2O2
We similarly studied HCC1937, a breast cancer cell line homozygous for mutant BRCA1 (5382insC) (35). DIM failed to protect HCC1937 cells against H2O2; but wtBRCA1 conferred significant protection (P < 0.001) [Fig. 5B]. Here, (DIM+wtBRCA1) did not confer additional protection beyond that of wtBRCA1 alone. The S1387A and S1524A mutations abrogated BRCA1 protection against H2O2, while the S1423A and S1457A mutants retained full protection activity (P < 0.001). These results are similar to those obtained in U87MG, except that none of the BRCA1 proteins allowed additional protection by DIM. Explanations for this difference between U87MG and HCC1937 are considered in the Discussion.
DIM stimulates BRCA1 signaling and expression in normal mammary epithelial cells
We tested the effects of DIM on normal human mammary epithelial cells (HMECs) and MCF-10A, an immortal line of non-tumor human mammary epithelial cells (36). DIM caused phosphorylation of BRCA1 on S1387, with a peak effect by 20 min (Supplemental Figs. 7A–7B). Over the time period studied (0–4 hr), total BRCA1 protein levels were unchanged. At longer times, DIM caused increases in total BRCA1 protein levels that first occurred at 8–16 hr and continued over 24–48 hr (Supplemental Figs. 7C–7D). Finally, DIM protected both cell types against H2O2 (P < 0.01) [Supplemental Figs. 7E–7F].
DIM blocks autophagy caused by H2O2
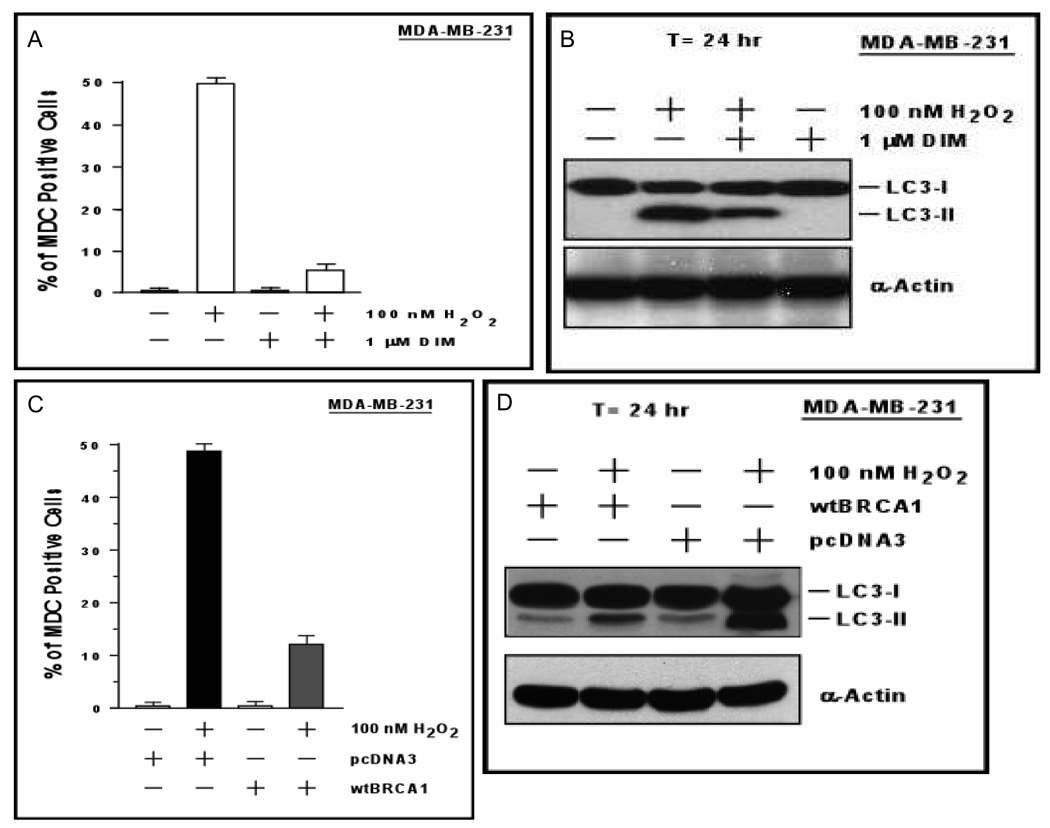
Based on flow cytometric detection of cells with sub-G1 DNA content, H2O2 caused little or no apoptosis in MDA-MB-231 or T47D cells at a dose that should kill a large majority of cells (200 nM) [Supplemental Fig. 8]. As a positive control, the DNA-damaging agent adriamycin yielded a large peak of cells with apoptotic sub-G1 DNA content. We quantified the autophagy rate by staining the cells with monodansylcadaverine (MDC), a dye that stains autophagic vacuoles. MDA-MB-231 cells were treated with H2O2 (100 nM) ± DIM for 24 hr and assayed for the presence of MDC-positive autophagic vacuoles. H2O2 caused an increase in the percentage of cells exhibiting autophagy, from < 2% to nearly 50%; and most of this increase was blocked by DIM (P < 0.001) [Fig. 6A]. H2O2 also caused conversion of a portion of the cytoplasmic LC3 (LC3-I) to membrane-associated LC3 (LC3-II) [Fig. 6B], a marker of autophagy (37). The H2O2-induced conversion of LC3-I into LC3-II was reduced by DIM treatment. wtBRCA1 also inhibited H2O2-induced autophagy (P < 0.001) [Fig. 6C] and attenuated the conversion of LC3-I to LC3-II (Fig. 6D). Photomicrographs of MDC-stained cells corresponding to Figs. 6A and 6C are provided in Supplemental Fig. 9–10. We measured autophagy by a second method in which MDA-MB-231 cells were transfected with a GFP-LC3 expression vector and examined for formation of puncta (autophago-somes) (38). These assays gave results similar to those in Figs. 6A and 6C (data not shown).
Fig. 6. Contribution of autophagy to DIM and BRCA1 protection against oxidative stress.
A. MDA-MB-231 cells cultured on coverslips were treated ± DIM (1 µM) and ± H2O2 (100 nM) for 24 hr and assayed for MDC staining of autophagic vacuoles. Values are means ± SEMs of three experiments. B. MDA-MB-231 cells were treated ± DIM (1 µM) and ± H2O2 (100 nM) for 24 hr and Western blotted for LC3 or actin. C. Autophagy assays were performed as in panel A, except that the cells were transfected with wtBRCA1 or pcDNA3 vector and treated with H2O2 but not DIM. D. MDA-MB-231 cells were transfected with wtBRCA1 or pcDNA3; exposed to H2O2 for 24 hr; and Western blotted as in panel B.
To further test the contribution of autophagy to H2O2-mediated cell killing. we knocked-down or over-expressed the essential autophagy gene beclin 1. In MDA-MB-231 cells, beclin 1-siRNA increased cell survival by ≥ two-fold at each dose of H2O2 (P < 0.001); while a wt-beclin 1 expression vector reduced cell survival (Supplemental Fig. 11A), suggesting that autophagy contributed to H2O2-induced cell death in our assays. Finally, we showed that DIM causes dose-dependent inhibition of beclin 1 expression in MDA-MB-231 cells (Supplemental Fig. 11B). wtBRCA1 similarly inhibited beclin 1 expression and blocked the H2O2-induced stimulation of beclin 1 expression (Supplemental Fig. 11C). DIM also inhibited beclin 1 expression in T47D cells (Supplemental Fig. 11D); and wtBRCA1 blocked the H2O2-induced increase in beclin 1 protein (Supplemental Fig. 11E). Densitometry showing the effect of DIM and wtBRCA1 on beclin 1 levels is provided in Supplemental Fig. 12. Thus, DIM and BRCA1 may render cells resistant to autophagy, in part, through beclin 1.
DISCUSSION
We showed that low doses of DIM (1 µM) stimulate BRCA1 expression and protect cells against oxidative stress, in part, through BRCA1. Protection against oxidative stress by DIM (and I3C) is consistent with the previous finding that BRCA1 protects against oxidative stress (25). The BRCA1 protection was attributed, in part, to stimulation of an antioxidant response via NRF2, a redox-sensitive transcription factor that stimulates expression of phase II detoxifying enzymes and antioxidant genes through the ARE (33,39). We identified several mechanisms by which DIM protects against oxidative stress, including the BRCA1-dependent stimulation of NRF2 activity. DIM stimulated several NRF2-regulated promoters, including NQO1 (an oxidoreductase), GST-α1 (a glutathione S-transferase), and x-CT (cystine/glutamate transporter). NRF2 appears to be a target for some dietary agents proposed for cancer prevention (39), although this was not demonstrated previously for DIM or I3C.
Interestingly, DIM stimulated BRCA1 phosphorylation on S1387 and S1524 before any increase in BRCA1 protein levels. DIM-induced phosphorylation of S1387 was observed after 10 min and showed a time course suggestive of a signaling event. In cell lines lacking functional BRCA1, S1387 and S1524 were required for protection against oxidative stress by exogenous BRCA1. While the significance of these phosphorylations is not clear, prior studies suggest that these sites are phos-phorylated by ATM and/or ATR in response to ionizing or UV radiation (40,41). ATM was required for DIM-induced phosphorylation of BRCA1 on S1387, suggesting that DIM signals through ATM.
In U87MG cells (which do not express BRCA1), DIM conferred additional protection against H2O2 beyond that afforded by exogenous BRCA1; but BRCA1 with an S1387A mutation failed to support DIM-mediated protection. In contrast, HCC1937 cells, which express mutant BRCA1, showed no additional protection from DIM beyond that due to exogenous BRCA1. It is possible that the endogenous mutant BRCA1 interferes with the transfected BRCA1 proteins, that the relevant phosphorylations cannot be induced or enhanced by DIM in this cell type, or that the protection by exogenous BRCA1 is already maximal and cannot increase further.
While the mechanism by which DIM induces BRCA1 phosphorylation is unclear, DIM and I3C can each activate an endoplasmic reticulum stress response (unfolded protein response) that is BRCA1-dependent; and this response is required for I3C stimulation of BRCA1 expression (15,23). We speculate that DIM induces low grade stress signaling that directly or indirectly leads to rapid phosphorylation of BRCA1. One possibility is that DIM interacts with one or more stress-responsive proteins and the signal is transmitted to BRCA1. While DIM and/or I3C can bind to several proteins (eg., aryl hydrocarbon receptor, estrogen receptor, and androgen receptor) (17–20), relatively little is known about how DIM interacts with cell proteins or other macromolecules.
Another interesting finding was that H2O2 caused little or no apoptosis. Reactive oxygen species, including H2O2, can cause different combinations of autophagy, apoptosis, and/or necrosis in a cell line and stimulus dependent manner (42–44). Autophagy is a process by which cells degrade macromolecules and organelles that can lead to cell survival (eg., under austere conditions in tumors) or cell death in different contexts. In our assays, H2O2-induced autophagy was cytotoxic and DIM/BRCA1 inhibition of autophagy was cytoprotective, as evidenced by the following: 1) inhibition of autophagy by knockdown of beclin 1 (45) increased cell survival; 2) DIM and wtBRCA1 inhibited H2O2-induced autophagy and at the same time protected cells against cytotoxicity; and 3) we detected little or no H2O2-induced apoptosis. Since the effects of oxidative stress are variable in terms of autophagy vs apoptosis, we cannot say whether DIM/BRCA1 inhibition of autophagy would be cytoprotective in a different context (eg., starvation rather than oxidative stress).
H2O2 caused a large increase in the percentage of cells undergoing autophagy that was inhibited by DIM or wtBRCA1. Consistent with this finding, H2O2 caused conversion of LC3-I to LC3-II, a marker of autophagy (37); and the conversion of LC3-I to LC3-II was attenuated by DIM or wtBRCA1. Consistent with the idea that the protective effect of DIM is mediated by BRCA1, wtBRCA1 blocked the H2O2-induced up-regulation of beclin 1. These findings link DIM and BRCA1 to regulation of autophagy.
Low doses of DIM also stimulated BRCA1 signaling and expression and protected against oxidative stress in normal or non-tumor-derived human mammary epithelial cells. These findings are important because normal cell types are presumably the main target for cancer prevention. Since we did not test whether H2O2 causes and DIM blocks autophagy in these cell types, we cannot say whether the observed protection reflects autophagy inhibition.
Our findings have implications for understanding chemoprevention and its limitations. Since DIM’s protective effects occurred at physiologic concentrations, it is reasonable to speculate that DIM blocks carcinogenesis, in part, by enabling normal cells to mount a more effective antioxidant response. This antioxidant response may include an increased ability of cells to repair oxidative DNA damage, since BRCA1 contributes to various DNA repair processes (46). Tumor cells often exhibit oxidative stress due to impaired antioxidant defenses. Thus, the presence of pre-existing cancer cells that are protected by antioxidants is a possible explanation for the mixed results obtained in clinical studies using antioxidants to prevent cancer (47–49). Here, the ability of DIM to promote survival of oxidatively stressed tumor cells could limit its activity as a chemoprevention agent.
Autophagy is regarded as a double-edged sword. On the one hand, autophagy is a tumor suppressor mechanism that can serve as a second type of programmed cell death replacing apoptosis. On the other hand, it can be utilized as a survival mechanism by tumor cells in nutrient limiting conditions (50). As noted above, we do not know whether DIM inhibition of autophagy will extend to other cell types and other causes of autophagy (eg., starvation). Nor do we know the extent to which autophagy inhibition by DIM is the consequence of a reduced stimulus for autophagy due to an increased cellular antioxidant response. Thus, at present, it is difficult to assess the contribution of autophagy as a target for DIM-mediated chemoprevention.
Acknowledgments
Financial support: This research was supported, in part, by United States Public Health Service grants RO1-CA80000, RO1-CA104546, and R01-CA82599 [to EMR] and by a grant from the Susan G. Komen Breast Cancer Foundation (PDF0403044) [to EMR/JK Rih].
REFERENCES
- 1.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham S, Marshall J, Mettlin C, Rzepka T, Nemoto T, Byers T. Diet in the epidemiology of breast cancer. Am J Epidemiol. 1982;116:68–75. doi: 10.1093/oxfordjournals.aje.a113403. [DOI] [PubMed] [Google Scholar]
- 3.Bradlow HL, Michnovicz J, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12:1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- 4.Kojima T, Tanaka T, Mori H. Chemoprevention of spontaneous endometrial cancer in female donryu rats by indole-3-carbinol. Cancer Res. 1994;54:1446–1449. [PubMed] [Google Scholar]
- 5.Jin L, Qi M, Chen DZ, Anderson A, Yang GY, Arbeit JM, et al. Indole-3-carbinol prevents cervical cancer in human papilloma virus type 16 (HPV16) transgenic mice. Cancer Res. 1999;59:3991–3997. [PubMed] [Google Scholar]
- 6.Rogan EG. The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo. 2006;20:221–228. Review. [PubMed] [Google Scholar]
- 7.Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, et al. Physiological modeling of formulated and crystalline 3,3'-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32:632–638. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- 8.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3'-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 9.Kong D, Banerjee S, Huang W, Li Y, Wang Z, Kim HR, et al. Mammalian target of rapamycin repression by 3,3'-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res. 2008;68:1927–1934. doi: 10.1158/0008-5472.CAN-07-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 11.Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3'-Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun. 2006;340:718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 12.Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3–3'-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr. 2003;133(7 Suppl):2448S–2455S. doi: 10.1093/jn/133.7.2448S. Review. [DOI] [PubMed] [Google Scholar]
- 13.Mulvey L, Chandrasekaran A, Liu K, Lombardi S, Wang XP, Auborn KJ, et al. Interplay of genes regulated by estrogen and diindolylmethane in breast cancer cell lines. Mol Med. 2007;13:69–78. doi: 10.2119/2006-00038.Mulvey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter TH, Liu K, Ralph W, Jr, Chen D, Qi M, Fan S, et al. Diindolylmethane alters gene expression in human keratinocytes in vitro. J Nutr. 2002;132:3314–3324. doi: 10.1093/jn/132.11.3314. [DOI] [PubMed] [Google Scholar]
- 15.Sun S, Han J, Ralph WM, Chandrasekaran A, Liu K, Auborn KJ, et al. Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro. Cell Stress Chaperones. 2004;9:76–87. doi: 10.1379/CSC-2R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3'-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 17.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3'-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 18.Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–1136. doi: 10.1016/0006-2952(93)90258-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen I, Safe S, Bjeldanes L. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochem Pharmacol. 1996;51:1069–1076. doi: 10.1016/0006-2952(96)00060-3. [DOI] [PubMed] [Google Scholar]
- 20.Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. 2000;130:2927–2931. doi: 10.1093/jn/130.12.2927. [DOI] [PubMed] [Google Scholar]
- 21.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3'-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 22.De Kruif CA, Marsman JW, Venekamp JC, Falke HE, Noordhoek J, Blaauboer BJ, et al. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem Biol Interact. 1991;80:303–315. doi: 10.1016/0009-2797(91)90090-t. [DOI] [PubMed] [Google Scholar]
- 23.Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94:407–426. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, et al. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 25.Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang HJ, et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64:7893–7909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 26.Akar U, Ozpolat B, Mehta K, Fok J, Kondo Y, Lopez-Berestein G. Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol Cancer Res. 2007;5:241–249. doi: 10.1158/1541-7786.MCR-06-0229. [DOI] [PubMed] [Google Scholar]
- 27.Bloom D, Dhakshinamoorthy S, Jaiswal AK. Site-directed mutagenesis of cysteine to serine in the DNA binding region of Nrf2 decreases its capacity to up-regulate antioxidant response element-mediated expression and antioxidant induction of NAD-(P)H:quinone oxidoreductase 1 gene. Oncogene. 2002;21:2191–2200. doi: 10.1038/sj.onc.1205288. [DOI] [PubMed] [Google Scholar]
- 28.Faraonio R, Vergara P, Di Marzo D, Pierantoni MG, Napolitano M, Russo T, et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 29.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 30.Biederbick A, Kern HF, Elsässer HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- 31.Costa M, Salnikow K, Sutherland JE, Broday L, Peng W, Zhang Q, et al. The role of oxidative stress in nickel and chromate genotoxicity. Mol Cell Biochem. 2002;234–235:265–275. Review. [PubMed] [Google Scholar]
- 32.Smith LL. Mechanism of paraquat toxicity in lung and its relevance to treatment. Hum Toxicol. 1987;6:31–36. doi: 10.1177/096032718700600105. Review. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 34.Kelner MJ, Bagnell RD, Montoya MA, Estes LA, Forsberg L, Morgenstern R. Structural organization of the microsomal glutathione S-transferase gene (MGST1) on chromosome 12p13.1–13.2. Identification of the correct promoter region and demonstration of transcriptional regulation in response to oxidative stress. J Biol Chem. 2000;275:13000–13006. doi: 10.1074/jbc.275.17.13000. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson GE, Chen TT, Stastny VA, Virmani AK, Spillman MA, Tonk V, et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998;58:3237–3242. [PubMed] [Google Scholar]
- 36.Tait L, Soule HD, Russo J. Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6087–6094. [PubMed] [Google Scholar]
- 37.Asanuma K, Tanida I, Shirato I, Ueno T, Takahara H, Nishitani T, et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 2003;17:1165–1167. doi: 10.1096/fj.02-0580fje. [DOI] [PubMed] [Google Scholar]
- 38.Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM. The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy. 2005;1:23–36. doi: 10.4161/auto.1.1.1495. [DOI] [PubMed] [Google Scholar]
- 39.Gopalakrishnan A, Tony Kong AN. Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem Toxicol. 2008;46:1257–1270. doi: 10.1016/j.fct.2007.09.082. Review. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, O'Donnell AH, Kim ST, Kastan MB. Phosphorylation of serine 1387 in Brca1 is specifically required for the Atm-mediated S-phase checkpoint after ionizing irradiation. Cancer Res. 2002;62:4588–4591. [PubMed] [Google Scholar]
- 41.Gatei M, Zhou BB, Hobson K, Scott S, Young D, Khanna KK. Ataxia telangiectasia mutated (ATM) kinase and ATM and Rad3 related kinase mediate phosphorylation of Brca1 at distinct and overlapping sites. In vivo assessment using phospho-specific antibodies. J Biol Chem. 2001;276:17276–17280. doi: 10.1074/jbc.M011681200. [DOI] [PubMed] [Google Scholar]
- 42.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 44.Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta. 2008;1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009. Review. [DOI] [PubMed] [Google Scholar]
- 45.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 46.Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25:5864–5874. doi: 10.1038/sj.onc.1209874. Review. [DOI] [PubMed] [Google Scholar]
- 47.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100 doi: 10.1093/jnci/djn148. 773-3. [DOI] [PubMed] [Google Scholar]
- 48.Engel RH, Evens AM. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11:300–312. doi: 10.2741/1798. Review. [DOI] [PubMed] [Google Scholar]
- 49.Cabello CM, Bair WB, 3rd, Wondrak GT. Experimental therapeutics: targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022–1037. Review. [PubMed] [Google Scholar]
- 50.Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009 Jan 27; doi: 10.1007/s10495-008-0307-5. [Epublished ahead of print] [DOI] [PubMed] [Google Scholar]