Abstract
This longitudinal study utilized a community sample of children (N=91, 45% female, 8–11 years at time 1) to investigate physiological responses (heart rate reactivity [HRR] and electrodermal responding [EDR]) during delay of gratification in relation to emotionality, self-regulation, and adjustment problems. Cluster analyses identified three profiles among children who successfully delayed: children who waited easily with low EDR and moderate HRR, children who had difficulty waiting with high EDR and moderate HRR, and children who had difficulty waiting with low EDR and low HRR. The 3 clusters and children who did not wait were compared. Children with low EDR-low HRR had the lowest self-regulation, and like the no-wait group, demonstrated the greatest baseline adjustment problems. The high EDR-moderate HRR group demonstrated highest self-regulation and increases in depression across one year. Distinct profiles among children in delay contexts point to children who are over- and under-regulated with implications for adjustment problems.
Keywords: delay of gratification, emotionality, self-regulation, adjustment problems, middle childhood
1. Introduction
1.1 Delay of gratification and self-regulation
Self-regulation plays an essential role in children’s emotional and behavioral adjustment (Rothbart, Ahadi, & Evans, 2000). Delay of gratification is a child’s ability to wait for a delayed reward in lieu of taking an immediate lesser reward. This ability is thought to require self-regulation, and structured delay of gratification laboratory tasks are commonly used to measure self-regulation. However, the ability to delay can reflect low reward-oriented approach motivation, high inhibition, high self-regulation or effortful control, or a combination of these (e.g., Eisenberg et al., 2003). Distinguishing these processes may have implications for the association between performance on delay tasks and children’s adjustment. Delay of gratification tasks are complex in that they likely elicit both affective and regulatory responses (Eisenberg et al., 2003). Physiological variables can help identify affective or motivational contributions to children’s delay behavior, clarifying children's regulatory responses. Thus, by examining profiles of physiological responses during a delay task, this study sought to clarify the affective and self-regulatory mechanisms underlying children’s ability to delay and their relation to adjustment problems in early adolescence.
Self-regulation reflects attentional, approach/avoidance, and inhibitory mechanisms (Thompson, 1990), and encompasses a child’s ability to modulate or maintain emotions and behaviors in order to achieve a particular goal (Cole, Martin, & Dennis, 2004). Children with higher levels of self-regulation may be better equipped to modulate emotions and behavior when faced with situations requiring delay of gratification, and successful delay of gratification is thought to be an important behavioral indicator of self-regulation. The ability to delay is an indicator of adaptive behaviors, including being able to inhibit inappropriate impulses and accomplish goal-oriented activities, and is associated with social competence and lower externalizing problems (Strayhorn, 2002; Mischel, Shoda, & Rodriguez, 1989). This ability to delay continues to develop throughout adolescence, and examination of delay abilities during the transition from middle childhood to early adolescence is particularly relevant given the increases in adjustment problems such as depression and externalizing problems during this period (e.g. Hankin, Abramson, Moffitt et al., 1998; Loeber & Hay, 1997).
Despite our knowledge of factors associated with delay of gratification, how and why children are able to wait for a reward needs to be explored further. There is a range in how easy or difficult it is for children to delay gratification, even when delaying successfully. These differences are behaviorally observable (e.g. some children wait calmly, while others fidget) and suggest variation in motivational or regulatory systems that lead to successful delay. Researchers have considered the inability to delay of gratification a measure of approach motivation (Mischel, et al., 1988), impulsivity (Olson et al., 1999), or poor self-control (Strayhorn, 2002). Evenden (1999) suggests that delay behavior reflects impulsivity, which is a multifaceted construct including motivational and cognitive-regulatory components. Similarly, Eisenberg et al. (2003) distinguish effortful and “reactive” control systems in delay of gratification. Reactive control involves motivational systems including approach and inhibition to reward and punishment cues, whereas effortful control is a key feature of self-regulation and involves voluntary inhibition and modulation of feelings, behaviors, and physiological reactions. When delaying gratification, both motivational and effortful control processes are likely to be at play. However, the relative contribution of motivational and effortful control processes may differ across children, with potential implications for their adjustment. Additionally, among children who delay successfully, understanding the different mechanisms of delay across children has the potential to elucidate the motivational and effortful control underpinnings of adjustment problems. For some children, successful delay may be due to low approach or high inhibition, whereas other children may succeed due to appropriate levels of effortful control.
Examination of physiological responses during delay might illuminate motivational processes underlying delay of gratification. Heart rate (HR), a measure of the number of beats per minute, is known to change in response to stressors and challenges. This HR reactivity is thought to reflect different aspects of Autonomic Nervous System (ANS) functioning. Electrodermal activity (EDA) is a measure of electrical conductivity at the skin’s surface. EDA increases with sweat gland hydration, a known correlate of sympathetic nervous system activity.
1.2 Heart rate and electrodermal activity in relation to motivational systems
HR and EDA have been linked with motivation. Fowles (1988) proposed that increases in EDA during punishment reflect activation of the behavioral inhibition system (BIS), a motivational system which produces fear and anxiety, serving to inhibit approach behaviors in response to negative consequences and cues of aversive consequences. Changes in HR during reward are thought to reflect activation of the behavioral activation system (BAS), a motivational approach system that activates behavior in response to positive incentives or active avoidance of punishment. HR responses to reward differ in children who are high in impulsivity, which is thought to reflect higher BAS approach (Colder & O’Connor, 2004). Alternatively, emotion theorists posit that HR reflects affective responses to cues, and that HR decelerates more in response to negative cues (Bradley, Codispoti, Cuthbert, & Lang, 2001). HR deceleration also represents the activation of attentional processes (Suess, Porges, & Plude, 1994). The range of HR correlates is likely due to the fact that HR reflects both ANS parasympathetic or regulatory activity and sympathetic or BAS activity (Beauchaine, 2001; Mezzacappa et al., 1998), but overall, in reward situations such as delay of gratification tasks, HR increases are likely to reflect behavioral activation or appetitive motivation (Beauchaine, 2001; Fowles, 1988). EDA reflects inhibitory sympathetic systems and indexes negative arousal, and may help clarify the role of HR as a behavioral correlate. This study examined simultaneous HR and EDA patterns as indicators of motivational and effortful control systems.
1.3 Heart rate and electrodermal activity in relation to negative emotionality and adjustment
HR and EDA activity are related to emotionality and adjustment (Fowles, Kochanska, & Murray, 2000; McManis et al., 2001; Mezzacappa et al., 1998). HR variables are consistently associated with negative emotionality. High resting HR and low HR variability predict more extreme fear and distress responses and inhibition (e.g. Fabes, Eisenberg, Karbon, Troyer, & Switzer, 1994; Huffman et al., 1998; Kagan, 1997). Shy children show a greater increase in HR during a demanding task than non-shy children (Schmidt et al., 1999), and HR longitudinally predicts higher levels of behavioral inhibition (Marshall & Stevenson-Hinde, 1998; Kagan, Reznick, Clark, Snidman, & Garcia-Coll, 1984). Low resting HR has consistently been associated with aggressive behaviors, and may reflect fearlessness and sensation seeking in children and adolescents (e.g. Raine, 2002). Overall, HR reactivity may be higher for children who are more inhibited, shy, or fearful, and lower for children who are less fearful.
Measures of EDA range from those measuring general sympathetic arousal (skin conductance level, number of nonspecific skin conductance responses) to those that measure sympathetic activation in response to specific stimuli (number of skin conductance orienting responses). EDA has been shown to correlate with emotionality, particularly fearfulness. Skin conductance level was positively correlated with fearfulness in 4-year-olds (Fowles et al., 2000), and skin conductance reactivity was positively related to facial distress and negatively related to helping behavior (Fabes et al., 1994). However, EDA may be less related to affective valence and more related to arousal, as EDA increases for both unpleasant and pleasant stimuli (Lang, Greenwald, Bradley, & Hamm, 1993). Much research on EDA has focused on conduct problems and criminal behavior (e.g. Herpertz et al., 2001). Children with externalizing disorders demonstrate lower baseline EDA levels (Van Goozen et al., 2000), fewer nonspecific electrodermal responses, and fewer electrodermal responses to orienting and startle paradigms (Beauchaine et al., 2001; Herpertz et al., 2001). Overall, higher EDA seems to be associated with general arousal and sometimes with distress under conditions of threat, whereas lower EDA is associated with externalizing problems.
2.4 Advantages of studying heart rate and electrodermal response profiles
Studying multiple physiological responses might help illuminate the complexity of associations among emotionality, self-regulation, and adjustment. For instance, effortful control, a dimension of self-regulation, and reward motivation both relate to adjustment problems. Children who show high BAS activity, or high levels of reward motivation, are higher in impulsivity (e.g. Colder & O’Connor, 2004, Oosterlaan & Sergeant, 1998). The combination of high BAS or impulsivity and low levels of effortful control predicts externalizing problems (e.g. Eisenberg et al., 2005; Lengua et al., 1998). BIS sensitivity has been shown to predict better behavior in the classroom setting, presumably because these children are motivated to avoid potential punishment (e.g. Blair, 2003). However, these children are also rated as being less on task in the classroom (Blair, 2003), and low attentional control combined with high inhibition may contribute to shyness and internalizing problems (e.g. Eisenberg et al., 2005). However, to better understand the relation among physiology, emotionality, self-regulation, and adjustment it is important to study patterns of activity of multiple physiological measures. In contrast to examining a single physiological measure, patterns of reactivity across multiple physiological measures may provide a more accurate picture of reactivity (Bauer et al., 2002). Hence, this study examined patterns of HR reactivity and EDA simultaneously using a profile approach, potentially providing more rich information than the separate consideration of these indices. Patterns of HR and EDA among children who successfully wait during a delay of gratification task might identify distinct contributions of motivation and effortful control to the ability to delay that might have implications for children’s adjustment. Prior research with EDA has consistently associated higher EDA with arousal, and as such we hypothesize that higher EDA will be associated with difficulty with delay in this study. Previous research has shown a number of different associations between HR variables, motivation, and effortful or attentional processes. HR decreases during a delay task might reflect an increase in attention to reward (e.g. Suess, Porges, & Plude, 1994), or a negative emotional response (e.g. Bradley, Codispoti, Cuthbert, & Lang, 2001), while increases might reflect behavioral activation (e.g. Beauchaine, 2001) or shyness (e.g. Schmidt et al., 1999). As such, we did not have specific directional hypotheses about associations with HR reactivity and delay task performance or difficulty.
In this exploratory study, physiological profiles were used to examine the roles that motivation and self-regulation played in children’s successful delay of gratification. The first goal was to identify patterns of HR and electrodermal response (EDR) during a delay of gratification task to explore physiological patterns underlying successful delay. The second goal was to explore whether the physiological profiles that emerged among children who delayed successfully were related to measures of emotionality, self-regulation, and adjustment problems. We also assessed whether these measures were different in children who did not successfully delay gratification compared to the children who did succeed in delaying gratification. Finally, we examined whether delay profiles would predict relative changes in adjustment problems across one year, as children transitioned into early adolescence.
2. Method
2.1 Participants
Participants were 91 children (ages 8–11 at time 1) and their mothers. The sample was drawn from a community sample (N=101). Families were recruited to participate through the 3rd–5th grade classrooms in five public schools in the urban area surrounding the university. Families were recruited via information forms sent home with the students. One child per family was asked to participate. Children with developmental disabilities (except learning disabilities) and families not fluent in English were excluded from the study to ensure understanding of the questionnaire measures. Of the 101 families in the sample, 10 were missing either physiological or observational data, usually as a result of equipment failures, resulting in complete data for 91 families. An additional 6 families did not participate in the follow-up assessment and were missing time 2 outcome data, resulting in a sample size of 85 for longitudinal analyses.
Demographic information was obtained via mother response to structured interview items collected at time one. Responses to “What is your child’s gender?” and “What is your child’s date of birth?” were used to obtain information about gender and age. The sample of 91 families consisted of 55% male and 45% female children. Children were 8–11 years at time 1 (M = 9.92, SD = .82 years; exact age calculated with date of birth and date of interview). Mothers were provided with close-ended response options for the question “What is your child’s ethnic or racial background?” to provide information regarding ethnicity. The ethnic distribution of the sample was: 34% African American, 5% Asian American, 54% European American, and 7% other or multiple ethnic/racial backgrounds. Mothers were provided with close-ended response options for the question “What is your current marital status?” Twenty-four percent of the children lived in single-parent households. The modal level of maternal education was completion of college or professional/technical school. The distribution of income was flat with 33% of the sample earning less than $30,000, 33% between $31,000 and $70,000, and 33% exceeding $71,000 per year.
Participants with complete data (n=85) were compared to those who were missing any data (n=16) on time 1 demographic variables and questionnaire measures of emotionality, self-regulation, and adjustment. The participants with complete data did not differ from those with missing data on child age, sex, ethnicity, or family income. Nor did they differ on child fear, irritability, attention regulation, inhibitory control, or impulsivity. Participants missing data demonstrated higher levels of conduct problems (t[99] = 2.47, p<.05, M missing = 6.47, M no-missing = 4.42) and depression (t[99] = 2.22, p<.05, M missing = 5.70, M no-missing = 4.11). Thus, some bias in outcome measures might have been introduced due to missing data.
2.2 Procedure
Data were collected using structured, scripted 2-hour interviews and tasks that were conducted in the families’ homes. After confidentiality was explained, mothers signed informed consent forms, and children signed assent forms. Mothers and children were interviewed by trained interviewers in separate rooms to ensure the privacy of responses. Interviewers read scripted instructions and all questionnaire items to the participants. All questionnaire responses were in discrete, multiple-choice format. Following the child interview, a delay of gratification task was administered and videotaped. Heart rate (HR) and electrodermal responding (EDR) were recorded during this delay of gratification task. Families were interviewed a second time approximately one year after their first interview (range = 11.24–16.92 months, M=12.65, SD=1.19 months). Families received $40 ($50 if two parents participated) compensation for participating in the interview at time 1, and compensation increased by $10 at time 2.
2.3 Measures
Descriptive statistics for all study variables are presented in Table 1.
Table 1.
Means, standard deviations and ranges for study variables.
| M | SD | Min | Max | |
|---|---|---|---|---|
| Behavioral and Physiological Measures During Delay: |
||||
| Difficulty with delay | 2.35 | 1.20 | 1.00 | 4.00 |
| Non-specific EDR (per minute) |
7.11 | 5.13 | 0.10 | 24.00 |
| HR change from baseline | −1.00 | 6.67 | −18.86 | 20.03 |
| Questionnaire Measures: | ||||
| Fearfulness | −0.01 | 0.85 | −1.88 | 1.77 |
| Irritability | −0.03 | 0.79 | −2.19 | 1.44 |
| Attention regulation | −0.08 | 0.79 | −2.47 | 1.59 |
| Inhibitory control | 0.09 | 0.76 | −1.57 | 1.81 |
| Impulsivity | 0.02 | 0.73 | −2.04 | 1.46 |
| Adjustment Measures: | ||||
| Time 1 Depression | 4.52 | 3.34 | 0.00 | 20.00 |
| Time 1 Conduct Problems | 4.94 | 3.44 | 0.00 | 15.50 |
| Time 2 Depressiona | 3.65 | 2.64 | 0.00 | 18.00 |
| Time 2 Conduct Problemsa | 4.36 | 3.41 | 0.00 | 19.00 |
Note: n = 91
n = 85
2.3.1 Delay of gratification
Children’s ability to delay gratification was assessed using a variation of a delay task that was intended to increase older children’s motivation or interest in the task. Most delay tasks have children decide to wait for more of a reward vs. receiving less if they fail to wait (e.g., 1 marshmallow now or as many as you want later). For older children, we anticipated that choice of a prize from a range of prizes would be more appealing. Children were told that they would receive a small prize as appreciation for their participation in the study. They were shown an opaque box containing an unknown prize. Then they were shown a prize-box containing a variety of prizes ranging in size and desirability. Showing them a range of prizes was designed to insure that the children would identify at least one prize that was sufficiently appealing to them that they would be motivated to wait. They were instructed that they could have the unknown prize immediately, which could be any of the prizes just displayed, including an undesirable one. Alternatively, if they waited for the interviewers to complete their “paperwork,” they could have the prize of their choice. The wait period was 10 minutes. The average delay time for the whole sample was 8.03 minutes (SD = 3.59). Sixty-five of the 91 children waited the entire 10-minute delay period. The average delay time for the children who did not wait the entire delay period (n=26) was 2.19 minutes (SD = 2.55). Eight of the children who did not wait had a delay time of < 30 sec, and another 5 children delayed for < 60 sec. Thus, half of the children who did not wait had delay times of < 60 sec., and as a result, there was less opportunity to obtain reliable physiological and behavioral measures on them.
Because the delay task was administered at the end of a lengthy interview, the delay time was 10 minutes (compared to the more typical 20-minute delay used in this age group). Waiting for 10 minutes was not expected to be particularly difficult for children of this age. Therefore, an observational rating of behaviors indicating difficulty with delay was also included. Raters were graduate and undergraduate research assistants who were trained in the behavioral rating system. Ratings of behaviors indicating difficulty delaying, including boredom, fidgeting, focus on the prize box, inquiring about the prize, annoyance, facial grimaces, and body tension, were made from video recordings of the task. Ratings were based on the number of occurrences of each of these behaviors during the 10 minute delay and the intensity of behaviors indicating difficulty. An overall difficulty waiting rating score was based on the number and intensity of behaviors, and scores indicated no difficulty with delay behaviors (0), mild difficulty with delay (1 = 1 to 5 behaviors of low intensity), moderate difficulty with delay (2 = 7 to 21 behaviors with low intensity or 1 to 5 behaviors with high intensity), or high difficulty with delay (3 = more than 21 low intensity behaviors or 6 or more high intensity behaviors). Children who did not wait the entire delay period were assigned a value of 4. Inter-rater reliability (kappa) for this rating was .83.
2.3.2 Physiological measures
Heart rate (HR) and electrodermal responding (EDR) were measured during the delay of gratification task. HR was measured by attaching a photo-electric plethysmograph (Biopac Systems Inc., Santa Barbara, CA) to the distal phalange of the child’s ring finger. EDR was measured with two sensors placed on distal phalanges of the middle and pointer finger. Gel was used on the EDR sensors to assure that skin contact was maintained. To minimize movement artifacts, sensors were applied to the non-dominant hand. Children were reminded to keep their hand still and asked to place their hand on a plastic mat that had an illustration of a hand on it. Biopac’s MP 100MSW was used to record HR and EDR directly onto the hard drive of a laptop computer, and the data were scored using AcqKnowledge, version 3.2.6. HR was sampled at a frequency of .5 Hz and EDR was sampled at 1 Hz. Data collection was monitored continuously and extraneous events or artifacts were flagged for removal during data scoring. Outlying data points were checked and verified minute-by-minute if they were > 3 SD from the group mean. HR and non-specific fluctuations in EDR were calculated from data collected during the task. Resting HR was calculated using a baseline recording of 60s taken after stable recordings were obtained. During this baseline period the interviewers asked the child to sit quietly for a minute. The mean HR of the baseline recording was subtracted from the mean HR during the 10-minute delay period to obtain HR reactivity during delay. Non-specific fluctuations in EDR per minute were calculated as the number responses during the delay time divided by delay time. Responses were counted if an increase of .05 or more μSeimens occurred in a 1–3 second time period.
2.3.3 Emotionality and self-regulation
Mother and children’s report on questionnaire measures of children’s negative emotionality and self-regulation included the fear, irritability, and attention regulation subscales of the Early Adolescent Temperament Questionnaire (EATQ; Capaldi & Rothbart, 1992), and the impulsivity and inhibitory control subscales of the Child Behavior Questionnaire (CBQ, Rothbart et al., 2001). Items assess a range of temperamental characteristics (e.g., “When someone tells me to stop doing something, it is very easy for me to stop,” “I feel scared when I enter a darkened room at home”) Each item has responses ranging from 1 (“Almost always untrue”) to 5 (“Almost always true”). Mother and child reports of temperament and adjustment were sought to partially address the effects of reporter bias on the observed associations. Combining reporters has been suggested to capture differing perspectives of behavior (e.g., Bird, Gould, & Staghezza, 1993; Hinshaw & Park, 1999) and would also reduce the number of statistical tests conducted. Although there are limitations to combining reporters (e.g. Tein, Roosa, & Michaels, 1994), including modest to moderate correlations across reporters and loss of information from differing perspectives, others have suggested that the practice results in substantial reduction in distortion due to bias and an increase in statistical power (Biesanz & West, 2004; Hoyt, 2000) and can produce a more reliable estimate of the construct being rated, increasing the generalizability of the findings (Cook & Goldstein, 1993). Based on previous confirmatory factor analyses with this sample, mother and child report on the questionnaire measures were combined (Lengua, 2002). Subscale scores within reporter were calculated as the mean-weighted sum of the items on a subscale, then mother and child report scores were averaged. The reliability of the combined measures was assessed using a composite alpha, calculated taking into account the Cronbach’s alpha, variance for each contributing scale, and the covariance between the scales. Internal consistency reliabilities for mother and child report of fearfulness were .66 and .65, respectively, and the correlation between mother and child report of fearfulness was .37 (p < .001). The composite alpha for the combined mother and child report of fearfulness was .66. Alphas for mother and child reports of irritability were .77 and .70, respectively, and the subscales were correlated .24 (p < .05). The composite alpha for irritability was .74. Alphas for mother- and child-report attention regulation were .79 and .63, respectively, and the subscales were correlated .27 (p < .01). The composite alpha for attention regulation was .76. Alphas for mother- and child-report impulsivity were .72 and .69, respectively, and the subscales were correlated .11 (n.s.). The composite alpha for impulsivity was .70. Alphas for mother- and child-report inhibitory control were .79 and .60, respectively, and the subscales were correlated .24 (p < .01). The composite alpha for inhibitory control was .67.
2.3.4 Child adjustment problems
Both mother and child reports of adjustment problems were obtained. Mothers completed the Child Behavior Checklist (CBCL; Achenbach, 1991). The CBCL for children ages 6 to 18 is a 113-item questionnaire measure designed to assess a range of psychological problems (i.e. “Feels too guilty,” “Destroys things belonging to others”). Each item has a three-point response range, including 0 (“Not at all true”), 1 (“Somewhat or sometimes true”), and 2 (“Very true”). Subscales of conduct problems (17-items, alpha=.78) and depression (12-items, alpha=.64) were scored based on rationally and empirically derived subscales using the CBCL (Lengua, Sadowski, Friedrich & Fisher, 2001). Child report of conduct problems was measured by the delinquent behavior and aggressive behavior subscales of the Youth Self Report (Achenbach, 1991), which has similar items to the CBCL (i.e., “I am shy,” “My moods and feelings change suddenly”) and the same response scale. The 25-item scale had an alpha of .82. Depression was assessed using the Children’s Depression Inventory (CDI; Kovacs, 1992) which contains 27 items, each of which consists of three statements. For each item, the individual is asked to select the statement that best describes his or her feelings for the past two weeks (i.e., “I like myself,” “I do not like myself,” “I hate myself”; items are scored 0, 1, and 2, respectively). The CDI had an alpha of .80 in the current sample. Mothers’ and children’s report of conduct problems and depression were correlated .35 and .13, respectively, and the composite alpha for the measures of conduct problems and depression aggregated across reporter were .85 and .78, respectively.
3. Results
3.1 Physiological response to delay and children’s sex, age and racial or ethnic background
First, we examined whether physiological responses to delay were correlated with children’s sex, age or racial or ethnic background to identify potential covariates for subsequent analyses. To examine differences by race, 3 dummy coded variables were created representing African-American children (n=32) vs. all others, Asian-American children (n=4) vs. all others, and other ethnic/racial backgrounds (n=6) vs. all others. Boys demonstrated more non-specific EDR (M = 8.17, SD = 5.52) than girls (M = 5.69, SD = 4.37, t[89] = 2.29, p<.05). There were no other significant sex, age or racial/ethnic differences on the physiological measures.
3.2 Physiological response to delay and emotionality, self-regulation, and adjustment
The correlations of physiological responses to the delay task with ratings of children’s difficulty with delay and measures of emotionality, self-regulation, and adjustment are presented in Table 2. Difficulty-with-delay ratings were related to more non-specific fluctuations in EDR during the delay task, but were not related to HR reactivity during the task. Difficulty-with delay ratings were also related to higher levels of time 1 conduct problems. Non-specific EDR was related to higher fearfulness. Greater increases in HR during the delay compared with baseline HR were related to higher attention regulation. Measures of emotionality and self-regulation were moderately correlated with each other and significantly related to conduct problems and depression at both time 1 and time 2.
Table 2.
Correlations among physiological, behavioral and questionnaire measures of emotionality, self-regulation, and adjustment problems.
| 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 1.0 | 11. a | 12. a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioral and Physiological Measures During Delay: |
|||||||||||
| 1. Non-specific EDR | .05 | .30** | .21* | .18 | .16 | .03 | −.14 | .15 | .16 | .17 | .13 |
| 2. HR Reactivity | -- | −.19 | −.14 | −.06 | .27** | .10 | −.14 | −.09 | −.17 | .06 | .16 |
| 3. Difficulty with Delay | -- | .08 | .24* | −.08 | −.28** | .23* | .19 | .24* | .06 | .19 | |
| Emotionality & Self- regulation: |
|||||||||||
| 4. Fearfulness | -- | .56** | −.26** | −.27** | .14 | .39** | .31** | .08 | .15 | ||
| 5. Irritability | -- | −.25** | −.45** | .46** | .36** | .45** | .31** | .41** | |||
| 6. Attention regulation | -- | .52** | −.41** | −.33** | −.32** | −31* | −.20* | ||||
| 7. Inhibitory control | -- | −.46** | −.46** | −.51** | −.28** | −.43** | |||||
| 8. Impulsivity | -- | .16 | .28** | .06 | .24* | ||||||
| Adjustment Problems: | |||||||||||
| 9. Time 1 Depression | -- | .55** | .41** | .37** | |||||||
| 10. Time 1 Conduct Problems | -- | .35** | .69** | ||||||||
| 11. Time 2 Depressiona | -- | .45** | |||||||||
| 12. Time 2 Conduct Problemsa | -- |
p<.05
p<.01
n = 91
Sample size for correlations with Time 2 outcomes n = 85
3.3 Physiological profiles
A cluster analysis was conducted to generate subgroups based on physiological profiles of children who waited the entire 10-minute delay period (N=65). First, the physiological variables (HR reactivity and the number of non-specific EDRs during the delay task) were standardized. Ward’s method (Aldenderfer & Blashfield, 1984), a hierarchical agglomerative clustering procedure, was used with these standardized physiological variables. The squared Euclidean distance was used to combine children into similar groups. Two, three and four cluster solutions were compared, and because the four-cluster solution resulted in a cluster with a small number of cases (n=3), we did not proceed to examine a greater number of clusters. A 3-cluster solution was selected as having the smallest number of distinct groups with unique patterns of physiological responses, without collapsing across distinct patterns of physiological responses.
Differences in the distribution of children in the clusters across sex, age or racial background were examined. There were no differences in the composition of the groups across sex (χ2 (3, 91) = 3.51, n.s.), age (F(3,87)=0.94, n.s.), or racial background (χ2 (3, 91) = 1.41, n.s.). Therefore, these variables were not examined further.
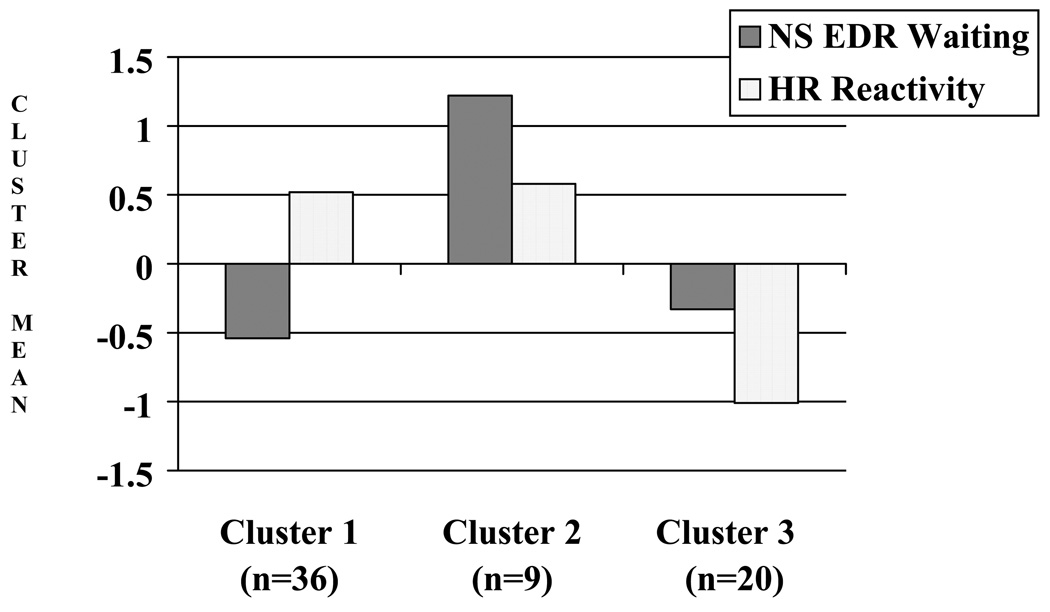
The overall sample mean on non-specific EDR during the delay was 7.11 (SD = 5.13, range = 0.10–24.00). The sample mean on HR reactivity was −1.00 (SD = 6.67, range = −18.86–20.03). The pattern of differences among the clusters are depicted in Figure 1. The first cluster represents the majority of the children who waited during the delay (n=36; 39%) with a moderately low level of non-specific EDRs (standardized: M=−0.54, SD=0.46; unstandardized: M=4.22, SD=2.34) and a moderately high increase in average HR during the delay period compared with baseline (standardized: M=0.52, SD=0.57; unstandardized: M=2.51, SD=3.75). The second cluster (n=9; 10%) consisted of children who had a high level of non-specific EDRs (standardized: M=1.22, SD=0.43; unstandardized: M=13.23, SD=2.21) and moderately high HR reactivity (standardized: M=0.58, SD=0.46; unstandardized: M=2.94, SD=3.06). The third cluster (n=20; 22%) consisted of children who had a moderately low level of non-specific EDR (standardized: M=−0.33, SD=.72; unstandardized: M=5.32, SD=3.70) and low HR reactivity (standardized: M=−1.01, SD=0.76; unstandardized: M=−7.62, SD=5.02).
Figure 1.
Mean standardized HR reactivity and EDR for each cluster.
3.4 Group differences
Group comparisons were conducted across the 3 groups emerging from the cluster analyses together with the “no-wait” group of children who did not wait the entire delay period. The 4 groups were compared on ratings of difficulty waiting and measures of emotionality, self-regulation, and adjustment problems. Mean differences across groups were tested using one-way ANOVA. Eta-squared indicated the proportion of variance in each variable accounted by the group differences, and significant group differences were explored using the Tukey HSD test (see Table 3). The 4 groups differed significantly on observational ratings of difficulty waiting during delay. Cluster 1 demonstrated the lowest level of difficulty waiting, whereas Clusters 2 and 3 demonstrated higher levels of difficulty waiting, and the no-wait group demonstrated the highest level of difficulty. Post-hoc tests showed that Cluster 1 was significantly different from the other 3 groups. The no-wait group was significantly higher on difficulty waiting than Clusters 2 and 3, which did not differ from each other.
Table 3.
Group differences on emotionality, self-regulation and adjustment problems.
| No-Wait n= 26 M (SD) |
Cluster 1 n= 36 M (SD) |
Cluster 2 n= 9 M (SD) |
Cluster 3 n= 20 M (SD) |
F1 | p | Partial Eta2 |
|
|---|---|---|---|---|---|---|---|
| Physiological Measures: | |||||||
| Non-specific EDR | 11.13 (5.94)2 | 4.22 (2.34) | 13.23 (2.21) | 5.32 (3.70) | 23.763 | .001 | .30 |
| HR Reactivity | −1.72 (7.88)2 | 2.51 (3.75) | 2.94 (3.06) | −7.62 (5.02) | 17.073 | .001 | .30 |
| Difficulty with Delay | 2.88 (0.43)ad | 1.57 (0.74) abc | 2.00 (0.93) bd | 2.10 (0.85)cd | 15.82 | .001 | .25 |
| Emotionality & Self-regulation: | |||||||
| Fearfulness | −0.12 (0.83) | −0.01 (0.77) | −0.03 (0.85) | 0.16 (0.97) | 0.42 | ns. | .04 |
| Irritability | −0.12 (0.86) | 0.05 (0.64) | −0.25 (0.88) | −0.01 (0.90) | 0.47 | ns. | .02 |
| Attention regulation | 0.15 (0.77) | 0.11 (0.76) | 0.24 (0.77) a | −0.31 (0.85) a | 3.05 | .05 | .07 |
| Inhibitory control | −0.06 (0.77) | 0.22 (0.75) | 0.39 (0.74) a | −0.17 (0.84) a | 2.74 | .05 | .09 |
| Impulsivity | −0.17 (0.79) | 0.00 (0.68) | −0.31 (0.78) | 0.25 (0.80) | 2.21 | .10 | .04 |
| Adjustment Problems: | |||||||
| T1 Depression | 5.60 (4.26)a | 3.42 (2.51)ab | 3.83 (2.08) | 4.68 (3.72)b | 2.72 | .05 | .09 |
| T1 Conduct problems | 5.25 (4.14)a | 3.50 (2.08)ab | 4.56 (3.06) | 5.63 (3.38)b | 2.69 | .05 | .07 |
| T2 Depression4 | 3.72 (2.25) | 3.32 (2.77) | 4.88 (3.75) | 2.78 (2.28) | 2.23 | .10 | .03 |
| T2 Conduct problems4 | 4.86 (4.29) | 3.63 (2.26) | 4.00 (2.55) | 4.06 (3.05) | 1.79 | ns. | .05 |
Groups with corresponding superscripts differed significantly.
Degrees of freedom for F are (3, 87) for time 1 and (3, 81) for time 2.
Tests of homogeneity of variance indicate that the variance for the no-wait group was significantly greater than for the other 3 groups. These data are included for descriptive purposes, but are not considered comparable to the physiological data for the other groups given that for many children in this group, the data are based on < 60 seconds of physiological recordings.
These mean differences across the groups were significant because the cluster analyses identified clusters that maximized the differences on the physiological variables.
Sample size for longitudinal analyses was 85.
To further explore differences among the groups on behaviors indicating difficulty with delay, the groups were compared on the individual coded behaviors. The groups differed on fidgeting (F[3, 87]=4.56, p<.01) and facial grimaces (F[3, 87]=5.62, p<.01), with cluster 2 showing the highest level of fidgeting and facial grimaces. The groups also differed on boredom/annoyance (F[3, 87]=3.28, p<.05) and body tensing (F[3, 87]=2.86, p<.05), with group 3 demonstrating the highest levels of boredom/annoyance and tensing. There were no differences on the other behaviors coded.
The groups differed significantly on attention regulation and inhibitory control (see Table 3). Cluster 2 demonstrated the highest levels of attention regulation and inhibitory control. Cluster 1 and the no-wait group demonstrated moderate levels on both variables. Cluster 3 demonstrated the lowest levels of both. Post-hoc tests showed that Clusters 2 and 3 differed significantly on both variables. A trend towards a difference among the groups on impulsivity suggested a similar pattern, with Cluster 2 demonstrating the lowest level of impulsivity and Cluster 3 demonstrating the highest.
The groups demonstrated significant differences on depression and conduct problems at time 1 (see Table 3). Cluster 1 demonstrated the lowest levels of depression and conduct problems. Cluster 2 demonstrated a moderate level of problems. Cluster 3 demonstrated moderate depression and a high level of conduct problems. The no-wait group demonstrated the depression and conduct problems similar to that of Cluster 3. Cluster 1 differed significantly from Cluster 3 and the no-wait group. The groups did not differ significantly on time 2 adjustment measures, although there was a trend towards a difference among the groups on time 2 depression, with Cluster 2 demonstrating the highest level of depression. The effect sizes for all group differences were modest.
Because of the modest to moderate correlations between mother and child report measures of temperament and adjustment, the patterns of differences among groups were examined separately across reporters to identify possible differences in the pattern of findings. Significant group differences emerged only for child report attention regulation, mother and child report of conduct problems, and child report of depression. There were trends toward significant differences on mother report of attention regulation and inhibitory control and child report of inhibitory control and impulsivity. The pattern of differences that emerged was very similar to the pattern when reporters were combined. Cluster 2 demonstrated the highest levels of self-regulation; cluster 1 demonstrated moderate levels of self-regulation or levels equivalent to cluster 2, depending on the measure; and cluster 3 and the no-wait group demonstrated lower levels of self-regulation. Cluster 3 and the no-wait group had the highest levels of conduct problems and depression, and group 1 had the lowest levels of both.
Skin conductance level (SCL) and change in SCL are alternative and potentially equally meaningful measures of electrodermal responding (EDR) in this study. We reanalyzed the data replacing nonspecific EDR with SCL mean and change. The pattern of findings for the cluster analyses and group differences were largely the same in both cases. SCL and SCL change were correlated .63 and .23 with nonspecific EDR, respectively. For both SCL and SCL change, 3-cluster solutions were tenable, and demonstrated a nearly identical pattern as when nonspecific EDR was used. Variations among the patterns were in the distribution of subjects in the clusters (i.e., the n’s for the clusters differed somewhat), and in the magnitude of the EDR differences across clusters (e.g., for cluster 2 nonspecific EDR was 1.22 SDs above the mean, SCL was 0.84 SDs above the mean, and SCL change was 1.12 SDs above the mean). Also, the pattern of differences across groups in the temperament and adjustment measures was nearly identical with the exception that the group differences on impulsivity were nonsignificant.
Finally, we examined whether group status predicted rank order changes in depression and conduct problems from time 1 to time 2 using multiple regression. Three dummy codes were used to represent the 4 groups. Children were coded as belonging to the “no wait” group vs. all others, Cluster 2 vs. all others, and Cluster 3 vs. all others. Child age, sex and time 1 levels of both depression and conduct problems were controlled in these analyses (see Table 4). Neither child age nor sex was related to changes in children’s depression or conduct problems. Time 2 depression was predicted by both time 1 depression and conduct problems. Children in Cluster 2 demonstrated relative increases in depression across one year. Conduct problems demonstrated a high degree of stability, and group membership did not predict relative changes across one year.
Table 4.
Predicting rank order changes in depression and conduct problems from group status.
| Time 2 Depression | Time 2 Conduct Problems | |||||
|---|---|---|---|---|---|---|
| β at entry |
β final step |
ΔR2 | β at entry |
β final step |
ΔR2 | |
| Step 1: | .22** | .49** | ||||
| Age | .14 | .15 | .01 | .07 | ||
| Sex | −.13 | 13 | .11 | .01 | ||
| Time 1 Depression | .24* | .21* | .04 | .04 | ||
| Time 1 Conduct Problems | .25* | .28* | .65** | .66** | ||
| Step 2: | .05t | .01 | ||||
| No wait group | −.03 | .02 | ||||
| Cluster 2 | .20* | −.04 | ||||
| Cluster 3 | −.14 | −.08 | ||||
n= 85;
p < .10
p < .05
p < .01
4. Discussion
Delay of gratification is a commonly used measure of self-regulation, and the ability to delay is consistently shown to predict children’s adjustment (Rothbart et al., 2000). The processes underlying delay likely include a combination of reward or approach motivation and effortful control (Eisenberg et al., 2003). Effortful control continues developing through middle childhood and adolescence, and the processes underlying the ability to delay may be increasingly important for positive adjustment as children transition to adolescence. The results of this study highlight differences in physiological and behavioral profiles among children who waited for a preferred prize in a delay task and suggest different underlying reasons for delaying gratification with implications for early adolescent adjustment.
Physiological measures allowed the identification of distinct responses to a delay task. Consistent with other studies, EDR was associated with greater fearfulness (e.g., Fowles et al., 2000), and it appeared that some children, those with elevated EDR, experienced the delay period with greater negative sympathetic arousal. HR reactivity during the delay was related to higher attention regulation, but was not related to behavioral observations of difficulty with delay. An increase in HR during the delay relative to baseline may have indicated a child actively attending to the task of waiting, or bringing effortful control or regulatory processes online (e.g. Eisenberg et al., 2005). For instance, respiratory sinus arrhythmia (RSA) suppression, that is a reduction in the normal HR respiratory rhythm or vagal tone that results in higher HR, during threat or challenge is indicative of active efforts to deal with the challenge (Berntson, Cacioppo, & Fieldstone, 1996) and is associated with better self-regulation and adjustment (Calkins & Dedmon, 2000). Similarly, HR increases as children engage in inhibitory control tasks (Wolfe & Bell, 2004). Alternatively, a decrease in HR during the task might indicate focused attention on the prize, which would increase difficulty delaying (Peake, Hebl, & Mischel, 2002). A decrease in HR might also reflect low effortful control, less engagement in effortful control processes, or low autonomic arousal. Unfortunately, we cannot completely disentangle these different possibilities in this study, however, by using a profile approach, we were able to identify different patterns of response to a delay task.
By examining patterns of EDR and HR reactivity together, we identified three profiles of children who successfully delayed gratification but demonstrated unique patterns of response and unique patterns of questionnaire reports of self-regulation and adjustment. The first cluster demonstrated moderately low EDR and moderately high HR reactivity, suggesting that those children experienced relatively little negative arousal and shored up moderate levels of self-regulation to successfully delay. This group had average levels of attention regulation and inhibitory control, demonstrated the lowest behavioral difficulty during the delay, and had the lowest levels of conduct problems and depression. Thus, this group appeared to be well-regulated and well-adjusted. This pattern would be consistent with the suggestion that moderate levels of reactivity and regulation, compared to over- or under-regulation, are associated with better adjustment (Eisenberg & Fabes, 1992).
The second cluster of children demonstrated high EDR and moderate HR reactivity. Thus, they appear to have been negatively aroused while waiting but shored up adequate levels of self-regulation to successfully delay. This group had the highest levels of attention regulation and inhibitory control. They demonstrated difficulty while waiting but had moderate levels of adjustment problems. In addition, this group of children demonstrated increases in depression across one year. This appears to be consistent with children characterized as over-controlled, who have been found to demonstrate higher levels of internalizing problems (e.g. Murray & Kochanska, 2002, Rubin, Stewart, & Coplan, 1995). High EDR and significantly higher attention regulation and inhibitory control indicate that these children show high levels of both reactive and effortful control (e.g. Eisenberg et al., 2005). This cluster represents about 10 percent of the children in our entire sample, which is similar to the proportion of children who have been described in other studies as highly inhibited (e.g. Caspi & Silva, 1995; Kagan & Snidman, 1999). This cluster may also account for the lack of observed zero-order correlation between HR reactivity and difficulty with delay, as these children showed an increase in HR, suggesting higher effortful control, but still demonstrated significant difficulty waiting.
The third cluster demonstrated average levels of EDR and very low HR reactivity. They demonstrated difficulty with delay, had the lowest levels of attention regulation and inhibitory control, and the highest level of adjustment problems. This group might be characterized as under-aroused (Van Goozen et al., 2000) or under-controlled (Eisenberg et al., 2001; Murray & Kochanska, 2002), both of which are related to adjustment problems, particularly externalizing problems. The very low HR reactivity in this group may be reflective of low autonomic arousal (e.g. Raine, 2002) or may reflect less use of effective attentional strategies during the task. This group was very similar to the group of children who did not wait during the delay on their levels of conduct problems and depression. Interestingly, this group demonstrated lower levels of attention regulation and inhibitory control than the children who did not wait. Thus, despite waiting for the entire 10-minute delay period, these children appear to be poorly regulated and demonstrate problems similar to those who were unable to wait. There are a number of possible reasons for why these children were able to wait. In addition to the possibility that they were highly motivated by the possibility of choosing their own prize, these children might have been responding to the presentation of a variety of prizes and seen one or more that they did not want to receive. These children might also have been responding to social desirability cues and been able to wait because they thought that they should.
Recall that the rating of difficulty waiting, rather than delay time, was the key measure of delay in this study. A particularly interesting finding is that the second and third clusters were behaviorally indistinguishable in terms of their difficulty-with-delay ratings. However, their physiological and self-regulation profiles suggest different reasons for their difficulty, with one group likely characterized as overcontrolled and the other as undercontrolled or underaroused (e.g. Van Goozen, 2000). A closer look at the specific behaviors that contributed to higher difficulty waiting scores revealed that children in cluster 2 demonstrated more fidgeting and facial grimaces, whereas the children in cluster 3 demonstrated more body tensing and boredom/annoyance. Fidgeting and facial grimaces may be reflective of the negative arousal experienced by children who demonstrated higher levels of EDR during the task. In a relatively neutral situation (waiting), these children show signs of BIS activation that the majority of children do not show. Both physiologically and behaviorally, these children demonstrated higher levels of anxiety and inhibition that place them at risk for increases in internalizing problems, which we observed in our follow-up analyses. This cluster was not rated as being significantly more fearful, suggesting that their physiological responses reflect BIS/anxiety rather than a fear response (Fowles & Kochanska, 2000). In contrast, the body-tensing and boredom/annoyance behaviors that were observed more in children in cluster 3 may reflect a qualitatively different type of difficulty waiting. These children may be focused on the reward and struggling to refrain from taking it. Alternatively, they may be underaroused and have trouble waiting due to disinterest during the task.
Thus, among children who waited the entire delay period there were children who had difficulty waiting. However, the reasons for their difficulty appeared to differ, and these differences had implications for the children’s adjustment. It is useful to look within groups of children who wait during delay tasks to better understand processes underlying delay and the relation of delay to adjustment. Children who demonstrate greater difficulty with delay may do so for different reasons. One group (Cluster 3) showed an expected pattern of low self-regulation and higher adjustment problems and was very similar to the children who did not wait the entire 10 minutes. However, another group (Cluster 2) demonstrated very high levels of self-regulation and negative arousal that were associated with moderate levels of adjustment problems but increases in depression over time. These findings support research that shows that both negative emotionality and self-regulation contribute to adjustment (Lengua, 2003) and that taking both into account may differentiate the types of problems children are likely to develop (Eisenberg et al., 2003; Rubin et al., 1995).
There were some limitations of this study. First, HR reactivity is difficult to interpret given that HR measures reflect both sympathetic and parasympathetic activity. Utilizing pre-ejection period, a measure of sympathetic influence on cardiac activity, and RSA, a measure of parasympathetic influence, might provide clarification of the roles of reward-oriented motivation and effortful control, respectively, in children’s delay behaviors. The delay of gratification task used was a relatively short time period for the age group, and a longer delay task (e.g. 20 minutes) might have resulted in fewer children waiting the entire time, particularly among children who had difficulty waiting and HR and EDR decreases (i.e. cluster 3). However, it is likely that the underlying physiological responses during delay would have been similar in a longer time period, resulting in similar interpretation of findings. Additionally, we did not collect information on physical factors that might have influenced physiological variables, such as body weight or Body Mass Index. The relatively small sample size might have limited power to detect modest effects. On the other hand, the combination of physiological, behavioral, and questionnaire measures was a strength of the study, providing information from multiple methods that illuminated differences among children who delayed successfully and those who did not wait. Some children who were distinguishable on physiological responses were similar on behavioral ratings of difficulty waiting but demonstrated unique patterns of effortful control and adjustment. In contrast, some children who waited were similar on adjustment measures to those who did not wait.
Evidence from this study might clarify the motivational and regulatory processes associated with delaying gratification and may inform theoretical models of the development of adjustment problems during the developmental transition of early adolescence. In particular, it offers evidence of motivational and regulatory systems playing different underlying roles among children who exhibited similar behaviors. Among children who demonstrated difficulty while waiting for a prize, there were children who showed low levels of effortful control and higher adjustment problems and children who showed high levels of effortful control and moderate adjustment problems. This pattern suggests distinct pathways to similar outcomes of successful delay and adjustment problems. Considering information from physiological, observational and questionnaire measures can be useful in illuminating pathways to children’s adjustment problems.
Acknowledgements
This research was supported by a Royalties Research Fund Award from the University of Washington and by NIMH Grant #R29MH57703. The authors thank the families who participated in this study, and Ted Beauchaine, Lara Embry, Erica Kovacs and Maureen Zalewski for their contribution to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anna C. Wilson, Email: longann@ohsu.edu.
Liliana J. Lengua, Email: liliana@u.washington.edu.
References
- Achenbach TM. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Aldenderfer MS, Blashfield RK. Cluster Analysis. In: Lewis-Beck MS, editor. Quantitative Applications in the Social Sciences: Sage series # 07–044. New York, NY: Sage; 1984. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behavior: Advantages of a multisystem approach. Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning on psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating Conduct Disorder from Attention Deficit/Hyperactivity Disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Fieldstone A. Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biological Psychology. 1996;44:1–17. doi: 10.1016/s0301-0511(96)05197-6. [DOI] [PubMed] [Google Scholar]
- Biesanz JC, West SG. Towards understanding assessments of the Big Five: Multitrait-multimethod analyses of convergent and discriminant validity across measurement occasion and type of observer. Journal of Personality. 2004;72:845–876. doi: 10.1111/j.0022-3506.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- Blair C. Behavioral inhibition and behavioral activation in young children: Relations with self-regulation and adaptation to preschool in children attending Head Start. Developmental Psychobiology. 2003;42:301–311. doi: 10.1002/dev.10103. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: Longitudinal evidence from a birth cohort. Child Development. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Colder CR, O’Conner RM. Gray’s reinforcement sensitivity model and child psychopathology: Laboratory and questionnaire assessment of the BAS and BIS. Journal of Abnormal Child Psychology. 2004;32:435–451. doi: 10.1023/b:jacp.0000030296.54122.b6. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child developmental research. Child Development. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cook WL, Goldstein MJ. Multiple perspectives on family relationships: A latent variables model. Child Development. 1993;1993:1377–1388. doi: 10.1111/j.1467-8624.1993.tb02958.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA. Emotion, regulation, and the development of social competence. In: Clark MS, editor. Emotion and social behavior. Review of personality and social psychology. Vol. 14. Newbury Park, CA: Sage; 1992. pp. 119–150. [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL, Fabes RA, Losoya SH, Valienta C, Reiser M, Cumberland A, Shepard SA. The relations of problem behavior status to children's negative emotionality, effortful control, and impulsivity: Concurrent relations and prediction of change. Developmental Psychology. 2005;41:193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Fabes RA, Smith CL, Reiser M, Shepard SA, Losoya SH, Guthrie IK, Murphy BC, Cumberland AJ. The relations of effortful control and ego control to children’s resiliency and social functioning. Developmental Psychology. 2003;39:761–776. doi: 10.1037/0012-1649.39.4.761. [DOI] [PubMed] [Google Scholar]
- Evenden J. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Karbon M, Troyer D, Switzer G. The relations of children’s emotion regulation to their vicarious emotional responses and comforting behaviors. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Psychophysiology and psychopathology: A motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Kochanska G. Temperament as a moderator of pathways to conscience in children: The contribution of electrodermal activity. Psychophysiology. 2000;37:788–795. [PubMed] [Google Scholar]
- Fowles DC, Kochanska G, Murray K. Electrodermal activity and temperament in preschool children. Psychophysiology. 2000;37:777–787. [PubMed] [Google Scholar]
- Gray J. The neuropsychology of anxiety: an enquiry into the functions and the septohippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell K. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Wenning B, Mueller B, Qunaibi M, Sass H, Herpertz-Dahlmann B. Psychophysiological responses in ADHD boys with and without Conduct Disorder: Implications for adult antisocial behavior. Journal of American Academy of Child and Adolescent Psychiatry. 2001;40:1222–1230. doi: 10.1097/00004583-200110000-00017. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Park T. Research problems and issues: Toward a more definitive science of disruptive behavior disorders. In: Quay HC, Hogan AE, editors. Handbook of Disruptive Behavior Disorders. New York, NY: Kluwer Academic/Plenum Publishers; 1999. pp. 593–620. [Google Scholar]
- Hoyt WT. Rater bias in psychological research: When is it a problem and what can we do about it? Psychological Methods. 2000;5:64–86. doi: 10.1037/1082-989x.5.1.64. [DOI] [PubMed] [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing K, Relyea N, Simons R. Observational, Physiological, and Self-Report Measures of Children’s Anger: Relations to Reactive versus Proactive Aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Kagan J. Temperament and the reactions to unfamiliarity. Child Development. 1997;68:139–143. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Kagan J, Snidman N. Early childhood predictors of adult anxiety. Journal of Biological Psychiatry. 1999;46:1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lengua LJ. The contribution of emotionality and self-regulation to the understanding of children’s response to multiple risk. Child Development. 2002;73:144–161. doi: 10.1111/1467-8624.00397. [DOI] [PubMed] [Google Scholar]
- Lengua LJ. Associations among emotionality, self-regulation, adjustment problems and positive adjustment in middle childhood. Journal of Applied Developmental Psychology. 2003;24:595–618. doi: 10.1016/j.appdev.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Sadowski CA, Friedrich WN, Fisher J. Rationally and empirically derived dimensions of children’s symptomatology: Expert ratings and confirmatory factor analyses of the CBCL. Journal of Consulting and Clinical Psychology. 2001;69:683–698. [PubMed] [Google Scholar]
- Lengua LJ, West SG, Sandler IN. Temperament as a predictor of symptomatology in children: Addressing contamination of measures. Child Development. 1998;69:164–181. [PubMed] [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Stevenson-Hinde J. Behavioral Inhibition, Heart Period, and Respiratory Sinus Arrhythmia in Young Children. Psychobiology. 1998;33:283–292. [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: Verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38:222–231. [PubMed] [Google Scholar]
- Mezzacappa E, Kindlon D, Saul JP, Earls F. Executive and motivational control of performance task behavior and autonomic heart-rate regulation in children: Physiologic validation of two-factor solution inhibitory control. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:525–531. [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Murray KT, Kochanska G. Effortful control: Factor structure and relation to externalizing and internalizing behaviors. Journal of Abnormal Child Psychology. 2002;30:503–514. doi: 10.1023/a:1019821031523. [DOI] [PubMed] [Google Scholar]
- Olson SL, Schilling EM, Bates JE. Measurement of impulsivity: Construct coherence, longitudinal stability, and relationship with externalizing problems in middle childhood and adolescence. Journal of Abnormal Child Psychology. 1999;27:151–165. doi: 10.1023/a:1021915615677. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Sergeant JA. Effects of reward and response cost on response inhibition in AD/HD, disruptive, anxious, and normal children. Journal of Abnormal Child Psychology. 1998;26:161–174. doi: 10.1023/a:1022650216978. [DOI] [PubMed] [Google Scholar]
- Peake PK, Hebl M, Mischel W. Strategic attention deployment for delay of gratification in working and waiting situations. Developmental Psychology. 2002;38:313–326. doi: 10.1037//0012-1649.38.2.313. [DOI] [PubMed] [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology and Psychiatry. 2002;43:417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Reznick JS, Kagan J, Snidman N, Gersten M, Baak K, Rodenberg A. Inhibited and uninhibited children: A follow-up study. Child Development. 1986;57:660–680. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional and personality development. 5th ed. New York: Wiley; 1998. pp. 105–176. [Google Scholar]
- Rubin KH, Stewart SL, Coplan RJ. Social withdrawal in childhood: Conceptual and empirical perspectives. Advances in Clinical Child Psychology. 1995;17:157–196. [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Strayhorn JM. Self-control: Theory and research. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:7–16. doi: 10.1097/00004583-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Suess PA, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Tein J, Roosa MW, Michaels M. Agreement between parent and child reports on parental behaviors. Journal of Marriage and the Family. 1994;56:341–355. [Google Scholar]
- Van Goozen SH, Matthys W, Cohen-Kettenis PT, Buitelaar JK, Van Engeland H. Hypothalamic-Pituitary-Adrenal axis and autonomic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. Working memory and inhibitory control in early childhood: Contributions from physiology, temperament, and language. Developmental Psychobiology. 2004;44:68–83. doi: 10.1002/dev.10152. [DOI] [PubMed] [Google Scholar]