Abstract
Purpose of review
The aim of this article is to review the most recent literature regarding the immunopathogenesis of pathogen-associated immune reconstitution disease and to discuss the role of immune activation and various effector molecules and cells such as macrophages, effector and regulatory T cells, and natural killer cells in immune reconstitution disease.
Recent findings
Many HIV patients receiving antiretroviral treatment develop immune reconstitution disease, which is characterized by exaggerated inflammatory immune responses to replicating or dead pathogens. In the majority of these cases, immune reconstitution disease is associated with restoration of pathogen-specific cellular immune responses involving CD4+ or CD8+ effector T cells. The precise conditions that trigger immune reconstitution disease have not yet been identified. Immune reconstitution disease patients have overt immune activation, which may be due to poor homeostatic control after the fast initial immune recovery in patients receiving antiretroviral therapy. Poor homeostatic control in immune reconstitution disease patients may be linked to unbalanced restoration of effector and regulatory T cells.
Summary
Although the precise mechanism of immune reconstitution disease is not well understood, it is probably related to rapid restoration of pathogen-specific immune responses and poor homeostatic control that promote exaggerated immunopathological responses, especially if viable pathogens or pathogen debris are present at high concentrations.
Keywords: antiretroviral treatment, HIV/AIDS, immune reconstitution, immunopathogenesis, IRD, IRIS
Introduction
A considerable number of HIV patients who are receiving antiretroviral therapy (ART) develop extensive inflammatory responses within the first weeks or months after starting treatment, a phenomenon referred to as immune reconstitution disease (IRD) [1]. Two distinct, but overlapping clinical scenarios of IRD are commonly seen: unmasking IRD and paradoxical IRD [2,3•]. In ‘unmasking’ IRD, patients with advanced immune suppression prior to ART are unable to mount an effective immune response against the viable pathogenic organisms that are present, but improving immunity after ART allows previously unrecognized pathogens to evoke an inflammatory response (unmasking). In contrast, ‘paradoxical’ IRD is the clinical worsening of an infection that was previously successfully treated and is caused by exaggerated activation of the immune system against persisting antigens present as dead organisms or debris following the initiation of ART. It is currently unknown whether the ‘unmasking’ and ‘paradoxical’ forms of IRD occur via the same pathophysiological mechanisms.
IRD has been associated with a variety of mycobacterial, viral, fungal and parasitic infections [3•,4]. We currently do not know if all inflammation associated with IRD occurs via the same mechanism, regardless of the inciting pathogen, or if distinct mechanisms exist for different opportunistic pathogens.
This review focuses on the immunopathogenesis of pathogen-associated IRD whereby patients with advanced HIV experience clinical worsening due to exaggerated inflammatory responses to occult, latent, or previously treated infections. The aim of this review is to discuss the putative role of various effector molecules and immune cells such as macrophages, T cells, regulatory T cells (Tregs), and natural killer cells in IRD associated with a variety of different pathogens.
Pathogenesis of immune reconstitution disease
Although data are limited, the inflammatory response associated with IRD appears to be cellmediated and anti-gendriven [1]. The development of IRD requires advanced HIV infection with severe immune damage, improving immunity in response to ART, the presence of inciting antigens that trigger an immune response and the apparent loss of normal homeostatic control of immune responses, resulting in an overexuberant inflammatory response. The strongest predictor for the development of IRD, however, is a low CD4+ T-cell count prior to starting ART [5,6]. In AIDS patients, damage to homeostatic control mechanisms [7], followed by rapid ART-associated restoration of pathogen-specific immune responses, could promote exaggerated inflammatory responses, especially if viable pathogens or pathogen debris are present at high concentrations. Nevertheless, it is intriguing that restoration of pathogen-specific immunity is uneventful in some patients but leads to severe immunopathology in others.
Immune correlates of immune reconstitution disease
The identification of laboratory markers that facilitate early recognition of IRD would be very useful either to prevent IRD, or to diagnose IRD and monitor treatment. Furthermore, they could provide a better insight in the immunopathogenesis of IRD, although their association with IRD may be indirect or even nonspecific.
Several serological indicators of IRD have been described since it was first recognized. In particular, plasma levels of the pro-inflammatory cytokine IL-6 are elevated during IRD, irrespective of the provoking pathogen and responding immune cells [1,8]. The source of IL-6 in IRD is not precisely known but it may be derived from activated macrophages [1]. We might anticipate that levels of other serum markers of inflammation, such as C-reactive protein (CRP) and other pro-inflammatory cytokines such as TNF-α), IL-1, IL-8, will also be increased in IRD but prospective studies are needed to assess their predictive value in IRD. Although increased serum levels of soluble CD30 (sCD30) have been associated with higher HIV viral loads and history of cytomegalovirus (CMV)-associated IRD [9], high sCD30 levels are probably a better indicator of ineffective ART in general than of IRD [10]. Increased levels of IFN-γ, human interferon-inducible protein 10 (IP-10) and mono-kine induced by IFN-γ (MIG) have been reported in patients who developed Mycobacterium tuberculosis IRD after starting ART [11]. High levels of IL-2 and IL-7 cytokines were also detected in Mycobacterium avium complex (MAC) IRD patients (Seddiki et al., unpublished data). These two cytokines, which are known for their role in high cell turnover, may certainly play a role in the exuberant T-cell responses observed in these patients (Fig. 1).
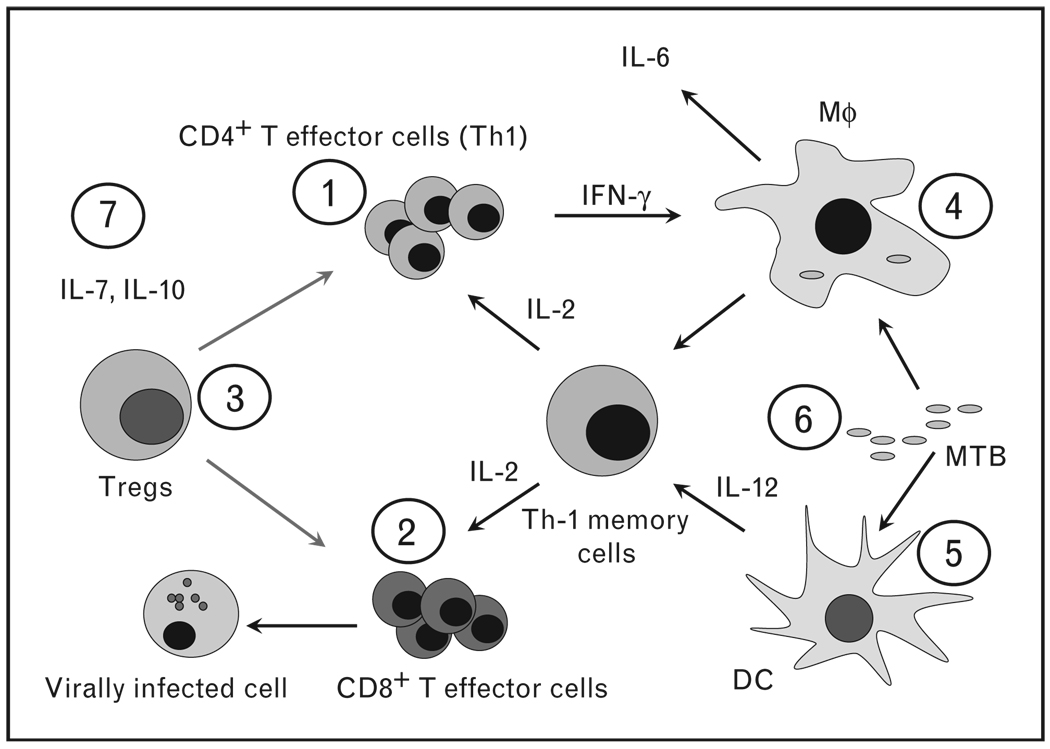
Figure 1. The immunopathogenesis of immune reconstitution disease is not precisely known but there are indications that various players are involved.
CD4 T cells (1) are involved in mycobacterial and other granulomatous immune reconstitution disease (IRD) whereas CD8+ T cells (2) are more frequently associated with viral IRD. IRD could be the consequence of unbalanced reconstitution of overactivated T cells and regulatory T cells (Tregs) (3). Direct activation of monocytes (4) and dendritic cells (5) during immune reconstitution, in particular by living or dead mycobac-teria or antigenic debris could be a possibility (6). Antigen load (6) during immune restoration may be a determining factor as well. Finally, the cytokine environment (7) during immune restoration, IL-7 and IL-10 in particular, both important in T-cell homeostasis, could have a pivotal role in the IRD. MTB, Mycobacterium tuberculosis; Mϕ, macrophage.
Genetic predisposition
IRD has been associated with distinct human leucocyte antigen (HLA) profiles and regulatory cytokine gene polymorphisms. For instance, it was shown several years ago that patients with CMV retinitis or encephalitis IRD frequently carry HLA-B44 and an ancestral haplotype HLA-A2, B44, DR4 [12]. In addition, patients who have experienced herpes virus IRD rarely carry IL-12B-3’UT whereas patients with mycobacterial IRD rarely carry TNF-α-308*2 and IL-6–174*G [13]. As these alleles are linked to low cytokine production, these observations tend to confirm the role of the Th1 cytokine IL-12 in CMV IRD [14] and the role of the pro-inflammatory cytokines IL-6 and TNF-α in mycobacterial IRD. These and other genetic markers may be useful for identifying patients who have genetic susceptibility to IRD.
Role of effector T cells
T cells play important roles in host defense and immunopathology associated with mycobacterial, viral, fungal, and parasitic infections. Although IRD is often associated with CD4+ Th1-mediated immunopathology, mainly mycobacterial infections, there are indications that both CD4+ and CD8+ effector T-cells are involved in IRD pathogenesis. Indeed, preliminary data from microarray analysis of gene expression are in support of the important role of T-cell activation in IRD pathogenesis (P.R. Bohjanen, unpublished results). We hypothesize that the effectors cells involved in IRD are similar to the effector cells that play a role in the normal immune responses to specific pathogens (Table 1) [11, 14–19,20•,21–51] but, in IRD, these responses are exaggerated.
Table 1.
Effector mechanisms in immune restoration disease
| Pathogen | Immunopathology | Effector cells and cytokines | References |
|---|---|---|---|
| Bacteria | |||
| Mycobacterium tuberculosis (MTB) |
Lymphadenopathy, pulmonary manifestations, systemic inflammatory responses etc. |
CD4 T cells, macrophages?, Tregs?, LPS? |
[11,15–19,20•] |
| Mycobacterium avium complex (MAC) |
Lymphadenitis, pulmonary granulomatous responses |
CD4 T cells? | [21,22] |
| Mycobacterium leprae | Borderline disease and type 1 reactions | CD4 T cells? | [23] |
| Other mycobacteria | Lymphadenopathy, pulmonary manifestations, | CD4 T cells? | [24] |
| Viruses | |||
| Herpes simplex virus (HSV) | Dermatological manifestations | CD8 T cells, IL-6 | [25,26] |
| Varicella zoster virus (VZV) | Dermatological manifestations | CD8 & CD4 T cells | [27,28] |
| Human herpes virus 8 (HHV-8) | Kaposi sarcoma flare | CD8 T cells?, IFN-γ, TNF-α | [29–32] |
| Cytomegalovirus (CMV) | CMV retinitis, immune recovery uveitis | CD8 T cells, NK cells, CD4 cells (IFN-γ), IL-6 (macrophages), IL-12, Treg deficiency? |
[14,33–37] |
| Hepatitis B virus (HBV) | Inflammatory flares and elevated liver enzymes | CD8 T cells, Tregs? | [2,38] |
| Hepatitis C virus (HCV) | Inflammatory flares and elevated liver enzymes | T cells, cytokines, Tregs? | [38,39] |
| JC virus (JCV) | Progressive multifocal leukoencephalopathy (PML) | CD8 T cells | [40–43] |
| Fungi | |||
| Cryptococcus neoformans | Central nervous system cryptococcosis | CD4 T cells, IFN-γ, macrophages | [44–46] |
| Pneumocystis carinii | Pneumonia | CD8 T cells, CD4 T (Th2), IL-4, IL-5, IL-6 |
[47,48] |
| Parasites | |||
| Leishmania spp. | Visceral, cutaneous and mucosal leishmaniasis, post kala-azar dermal leishmaniasis |
CD4 T cells and IFN-γ?, macrophages? | [49] |
| Schistosoma mansoni | Colitis and polyposis, granuloma formation around eggs |
CD4 T cells? | [50,51] |
IL, interleukin; LPS, lipopolysaccharide; NK, natural killer; Th2, T helper type 2; TNF, tumour necrosis factor; Tregs, regulatory T cells.
Mycobacterium tuberculosis-associated immune reconstitution disease
Most tuberculosis (TB)-IRD develops within the first 3 months of ART and can occur as ‘unmasking’ or ‘paradoxical’ IRD [52]. TB-IRD occurs during the period when redistribution of memory T cells occurs [53]. Patients with TB-IRD have restored skin test responses to TB antigens and increased numbers of TB-specific CD4 T cells [11]. These findings suggest that Th1 cells are not only important in protection and TB granuloma formation but also in TB-IRD [16,54]. Similar to TB-IRD, Th1 cells may also be involved in IRD associated with nontuberculous mycobacterial infections (Table 1).
Viral immune reconstitution disease
Most patients with viral IRD involving CMV, hepatitis B virus (HBV), hepatitis C virus (HCV), JC virus, varicella zoster virus (VZV), herpes simplex virus (HSV) and human herpes virus-8 (HHV-8) also have restored T-cell responses [34]. In viral infections, CD8+ T cells in particular are involved in protection and immunopathology. These cells are probably also implicated in viral IRD associated with CMV [34], HSV and VZV [27,28], JCV [41], HHV-8 [30,32] and HBV and HCV IRD [39]. The precise contribution of the different T-cell subsets in viral IRD pathogenesis, however, is much less clear than in mycobacterial infections (Table 1). For instance, interaction between CD4+ and CD8+ T cells appears to be pivotal in CMV end-organ disease development [35] and both CD4+ and CD8+ T cells contribute to the formation of viral vesicles in HSV and VZV skin lesions [55].
Cryptococcal immune reconstitution disease
Cryptococcus neoformans is the fungal pathogen most commonly associated with IRD [46]. The high susceptibility of HIV-1 patients to C. neoformans suggests that CD4 T cells are important in protection against C. neoformans. Antigen-stimulated CD4+ T cells produce IFN-γ that activates phagocytosis of C. neoformans by macrophages, and CD4+ T cells have a direct cytotoxic effect on C. neoformans [44]. The precise effector mechanisms involved in cryptococcal IRD are not known but it is likely that CD4 T cells and IFN-γ are involved.
Pneumocystis pneumonia immune reconstitution disease
CD8 T cells are the primary effector cells responsible for host tissue inflammatory damage in Pneumocystis pneumonia (PCP), and alveolar macrophages, CD4+ T cells, CD8+ T cells, and even γδT cells have important roles in PCP defense [47]. Recent data from animal models provide evidence that CD4+ T cells, and in particular, Th2 cytokines such as IL-4, IL-5 and IL-6 contribute to respiratory disease in PCP-associated IRD, whereas CD8 T cells modulate the CD4 T-cell- and eosinophil-mediated pulmonary pathology [48,56].
Schistosome immune reconstitution disease
A few cases of IRD have been described in HIV patients co-infected with parasitic helminths such as Schistosoma mansoni [50]. These patients developed eosinophilia, enteritis or colonic inflammatory polyposis after antire-troviral treatment. The immunopathology of schistosomiasis is mainly linked to the granulomatous immune response around trapped eggs in host tissues [57]. HIV infection appears to reduce egg excretion [58], possibly as the result of reduced granuloma formation. ART may restore granuloma formation around trapped eggs resulting in IRD. As granuloma formation around the eggs requires functional CD4+ T cells [59], it is likely that these cells play a role in schistosome IRD. Other parasitic infections such as leishmaniasis, strongyloidiasis, cryptosporidiosis and toxoplasmosis have also been associated with IRD [50].
Role of regulatory T cells
Tregs maintain homeostasisby suppressing other immune cells and thereby preventing collateral damage from inflammatory responses. The balance between allowing the immune response to clear infections and preventing immunopathology is delicate, and it is possible that IRD is the result of an unbalanced immune reconstitution of effector and regulatory T cells in patients receiving ART. In addition, Tregs could be defective in either numbers or function in maintaining homeostasis in patients with IRD. Interestingly, CD4+CD25+CD127lo FoxP3+ Tregs have been found to expand significantly in HIV-1 infected patients who developed mycobacterial IRD after ART, compared with healthy controls and to HIV+ patients who did not develop IRD (Seddiki, personal communication). Furthermore, the ratio of Tregs to effector/memory subsets was higher in IRD-patients (Seddiki et al. unpublished). High IL-2 levels, found in IRD patients, probably promote the survival of Tregs. The suppressive function of Tregs, however, was found to be compromised in patients with IRD, suggesting that these Tregs were unable to function efficiently (Seddiki et al., unpublished data). This defect was correlated with the failure of Tregs to suppress the secretion of a number of inflammatory cytokines and chemokines including IL-6, IL-4, TNF-α and IFN-γ. In contrast, IL-10 production was found to be relatively low in some patients with mycobacterial IRD, suggesting that an imbalance in inflammatory and regulatory cytokines might explain the aberrant immune responses observed (personal communication).
An interesting view on the possible role of Tregs and systemic bacterial lipopolysaccharides (LPS) in TB IRD was published recently [20•]. Subjects that do not develop IRD could have normal Tregs or have developed tolerance (anergy) to persistent LPS/tubercle antigens. In contrast, those individuals with defective Tregs or those with enormous plasma LPS could be vulnerable to IRD.
Role of natural killer cells
Natural killer cells belong to the innate immune system and have the capacity to be activated by virus-infected cells. Stimulation of natural killer cells, however, is partially dependent on cytokines produced by activated CD4 T cells such as IL-2, IFN-γ and IL-15. The activity of natural killer cells is determined by the expression of cell-surface molecules (killer immunoglobulin-like receptors; KIRs), which activate or inhibit their function. Patients with CMV IRD were indeed found to carry significantly more activating KIRs encoded by the KIR genes 3DS1 and 2DS5 than controls [36], suggesting that natural killer cells may have a role in herpes IRD.
The role of macrophages
Macrophages require intact Th1-type responses to be activated and establish immunity to chronic intracellular bacterial infections like TB and chronic intracellular parasitic infections such as leishmaniasis. Consequently, these infections are well known opportunistic infections associated with HIV. Most patients with TB-IRD have restored TB-specific CD4 T cells [11] but little is known about macrophage function in these patients. Inappropriate activation of macrophages could contribute to the immunopathogenesis of TB IRD [18,19] and Leishmania IRD.
Role of leukotrienes
Leukotrienes are inflammatory mediators released by mast cells. Interestingly, several cases of IRD, associated with urticarial vasculitis, secondary syphilis and tuberculosis were successfully treated with the leukotriene receptor antagonist montelukast, a drug used to treat asthma, suggesting that leukotrienes maybe involved in the immunopathogenesis of some types of IRD [60,61].
Predictors of immune reconstitution disease
Since the first cases of IRD, studies have tried to identify clinical and laboratory predictors of IRD. A low CD4 count ( < 100 cells/µl) at the initiation of treatment has been identified as one of the best predictors of IRD. Other parameters, however, such as a history of more frequent opportunistic infections, higher CD8 counts and lower hemoglobin levels were also recognized as being predictors of IRD associated with MAC, CMV, MTB and Cryptococcus [62]. The authors suggest that the higher number of CD8 cells may reflect a higher level of immune activation. As CD8+ T cells are mainly implicated in viral infections, the predictive power of CD8 T-cell count can probably be improved when used in the context of the prediction of viral IRD.
Conclusion
IRD is the result of an exaggerated cellular immune response to living or dead pathogens or debris. IRD is associated with restoration of pathogen-specific effector T cells and regulatory T cells. Tregs may be suppressed, however, by the disrupted cytokine environment because of impairment of the homeostatic control mechanisms. Apart from this fact, it is not clear which factors or combination of factors trigger IRD. These factors could be pathogenrelated (antigen load), genetic or immune related such as for instance the diversity of pathogen-specific T cells during immune restoration. Although data are still very scarce, macrophages and natural killer cells are also suspected to play a role in IRD.
Acknowledgements
Luc Kestens is coordinator of the EC funded project on the immunopathogenesis of tuberculosis immune reconstitution inflammatory syndrome (TBIRIS). Paul R. Bohjanen is the principal investigator of a project funded through the Tibotec REACH Initiative to study the pathogenesis of HIV IRIS in sub-Saharan Africa.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 527).
- 1.French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:16–21. doi: 10.1007/s11904-007-0003-z. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, French M. Immune reconstitution disease: recent developments and implications for antiretroviral treatment in resource-limited settings. Current Opinion in HIV & AIDS. 2007;2:339–345. doi: 10.1097/COH.0b013e3281a3c0a6. [DOI] [PubMed] [Google Scholar]
- 3. Dhasmana DJ, Dheda K, Ravn P, et al. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and management. Drugs. 2008;68:191–208. doi: 10.2165/00003495-200868020-00004. This is an excellent general overview of the various aspects of IRD.
- 4.Murdoch DM, Venter WD, Van RA, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 6.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 7.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol Cell Biol. 2007;85:6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 8.Stone SF, Price P, Keane NM, et al. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 9.Keane NM, Price P, Lee S, et al. An evaluation of serum soluble CD30 levels and serum CD26 (DPPIV) enzyme activity as markers of type 2 and type 1 cytokines in HIV patients receiving highly active antiretroviral therapy. Clin Exp Immunol. 2001;126:111–116. doi: 10.1046/j.1365-2249.2001.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghi M, Susal C, Daniel V, et al. Short communication: decreasing soluble CD30 and increasing IFN-gamma plasma levels are indicators of effective highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2007;23:886–890. doi: 10.1089/aid.2006.0228. [DOI] [PubMed] [Google Scholar]
- 11.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 12.Price P, Keane NM, Stone SF, et al. MHC haplotypes affect the expression of opportunistic infections in HIV patients. Hum Immunol. 2001;62:157–164. doi: 10.1016/s0198-8859(00)00239-1. [DOI] [PubMed] [Google Scholar]
- 13.Price P, Morahan G, Huang D, et al. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16:2043–2047. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Schrier RD, Song MK, Smith IL, et al. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina. 2006;26:165–169. doi: 10.1097/00006982-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Ruhwald M, Ravn P. Immune reconstitution syndrome in tuberculosis and HIV-co-infected patients: Th1 explosion or cytokine storm? AIDS. 2007;21:882–884. doi: 10.1097/QAD.0b013e3280b079c8. [DOI] [PubMed] [Google Scholar]
- 16.Saunders BM, Frank AA, Orme IM, Cooper AM. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol. 2002;216:65–72. doi: 10.1016/s0008-8749(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 17.Stone SF, Price P, French MA. Immune restoration disease: a consequence of dysregulated immune responses after HAART. Curr HIV Res. 2004;2:235–242. doi: 10.2174/1570162043351345. [DOI] [PubMed] [Google Scholar]
- 18.French MA. The immunopathogenesis of mycobacterial immune restoration disease. Lancet Infect Dis. 2006;6:461–462. doi: 10.1016/S1473-3099(06)70530-8. [DOI] [PubMed] [Google Scholar]
- 19.Van den BR, Vanham G, Raes G, et al. Mycobacterium-associated immune reconstitution disease: macrophages running wild? Lancet Infect Dis. 2006;6:2–3. doi: 10.1016/S1473-3099(05)70302-9. [DOI] [PubMed] [Google Scholar]
- 20. Shankar EM, Vignesh R, Murugavel KG, et al. Immune reconstitution inflammatory syndrome in association with HIV/AIDS and tuberculosis: Views over hidden possibilities. AIDS Res Ther. 2007;4:29. doi: 10.1186/1742-6405-4-29. This paper presents a challenging view on the possible role of LPS in tuberculosis IRD.
- 21.Appelberg R. Pathogenesis of Mycobacterium avium infection: typical responses to an atypical mycobacterium? Immunol Res. 2006;35:179–190. doi: 10.1385/IR:35:3:179. [DOI] [PubMed] [Google Scholar]
- 22.Riddell J, Kaul DR, Karakousis PC, et al. Mycobacterium avium complex immune reconstitution inflammatory syndrome: Long term outcomes. J Transl Med. 2007;5:50. doi: 10.1186/1479-5876-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ustianowski AP, Lawn SD, Lockwood DN. Interactions between HIV infection and leprosy: a paradox. Lancet Infect Dis. 2006;6:350–360. doi: 10.1016/S1473-3099(06)70493-5. [DOI] [PubMed] [Google Scholar]
- 24.Bell HC, Heath CH, French MA. Pulmonary Mycobacterium celatum immune restoration disease: immunopathology and response to corticosteroid therapy. AIDS. 2005;19:2047–2049. doi: 10.1097/01.aids.0000191228.36797.5b. [DOI] [PubMed] [Google Scholar]
- 25.Lehloenya R, Meintjes G. Dermatologic manifestations of the immune reconstitution inflammatory syndrome. Dermatol Clin. 2006;24:549–570. doi: 10.1016/j.det.2006.06.007. vii. [DOI] [PubMed] [Google Scholar]
- 26.Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J Infect Dis. 2001;184:1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- 27.Domingo P, Torres OH, Ris J, Vazquez G. Herpes zoster as an immune reconstitution disease after initiation of combination antiretroviral therapy in patients with human immunodeficiency virus type-1 infection. Am J Med. 2001;110:605–609. doi: 10.1016/s0002-9343(01)00703-3. [DOI] [PubMed] [Google Scholar]
- 28.Feller L, Wood NH, Lemmer J. Herpes zoster infection as an immune reconstitution inflammatory syndrome in HIV-seropositive subjects: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:455–460. doi: 10.1016/j.tripleo.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Alessandri G, Fiorentini S, Licenziati S, et al. CD8+CD28− T lymphocytes from HIV-1-infected patients secrete factors that induce endothelial cell proliferation and acquisition of Kaposi’s sarcoma cell features. J Interferon Cytokine Res. 2003;23:523–531. doi: 10.1089/10799900360708641. [DOI] [PubMed] [Google Scholar]
- 30.Connick E, Kane MA, White IE, et al. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin Infect Dis. 2004;39:1852–1855. doi: 10.1086/426078. [DOI] [PubMed] [Google Scholar]
- 31.Lambert M, Gannage M, Karras A, et al. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood. 2006;108:3871–3880. doi: 10.1182/blood-2006-03-014225. [DOI] [PubMed] [Google Scholar]
- 32.Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS. 2005;19:635–644. doi: 10.1089/apc.2005.19.635. [DOI] [PubMed] [Google Scholar]
- 33.Stone SF, Price P, French MA. Cytomegalovirus (CMV)-specific CD8+ T cells in individuals with HIV infection: correlation with protection from CMV disease. J Antimicrob Chemother. 2006;57:585–588. doi: 10.1093/jac/dkl049. [DOI] [PubMed] [Google Scholar]
- 34.Stone SF, Price P, Tay-Kearney ML, French MA. Cytomegalovirus (CMV) retinitis immune restoration disease occurs during highly active antiretroviral therapy-induced restoration of CMV-specific immune responses within a predominant Th2 cytokine environment. J Infect Dis. 2002;185:1813–1817. doi: 10.1086/340636. [DOI] [PubMed] [Google Scholar]
- 35.Bronke C, Palmer NM, Jansen CA, et al. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. J Infect Dis. 2005;191:873–880. doi: 10.1086/427828. [DOI] [PubMed] [Google Scholar]
- 36.Price P, Witt C, de Santis D, French MA. Killer immunoglobulin-like receptor genotype may distinguish immunodeficient HIV-infected patients resistant to immune restoration diseases associated with herpes virus infections. J Acquir Immune Defic Syndr. 2007;45:359–361. doi: 10.1097/QAI.0b013e31805b82a1. [DOI] [PubMed] [Google Scholar]
- 37.Aandahl EM, Michaelsson J, Moretto WJ, et al. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai SL. Immunopathogenesis of viral hepatitis B and C. Changgeng Yi Xue Za Zhi. 1999;22:159–170. [PubMed] [Google Scholar]
- 39.John M, Flexman J, French MA. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Koralnik IJ. New insights into progressive multifocal leukoencephalopathy. Curr Opin Neurol. 2004;17:365–370. doi: 10.1097/00019052-200406000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Vendrely A, Bienvenu B, Gasnault J, et al. Fulminant inflammatory leukoence-phalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol (Berl) 2005;109:449–455. doi: 10.1007/s00401-005-0983-y. [DOI] [PubMed] [Google Scholar]
- 42.Safdar A, Rubocki RJ, Horvath JA, et al. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clin Infect Dis. 2002;35:1250–1257. doi: 10.1086/344056. [DOI] [PubMed] [Google Scholar]
- 43.Gray F, Bazille C, dle-Biassette H, et al. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol. 2005;11(Suppl 3):16–22. doi: 10.1080/13550280500511741. [DOI] [PubMed] [Google Scholar]
- 44.Zheng CF, Ma LL, Jones GJ, et al. Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood. 2007;109:2049–2057. doi: 10.1182/blood-2006-03-009720. [DOI] [PubMed] [Google Scholar]
- 45.Lawn SD, Bekker LG, Myer L, et al. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 46.Shelburne SA, III, Darcourt J, White AC, Jr, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 47.Wright TW, Gigliotti F, Finkelstein JN, et al. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest. 1999;104:1307–1317. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhagwat SP, Gigliotti F, Xu H, Wright TW. Contribution of T cell subsets to the pathophysiology of Pneumocystis-related immunorestitution disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1256–L1266. doi: 10.1152/ajplung.00079.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawn SD, Wilkinson RJ. Immune reconstitution disease associated with parasitic infections following antiretroviral treatment. Parasite Immunol. 2006;28:625–633. doi: 10.1111/j.1365-3024.2006.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn SD. Immune reconstitution disease associated with parasitic infections following initiation of antiretroviral therapy. Curr Opin Infect Dis. 2007;20:482–488. doi: 10.1097/QCO.0b013e3282a6463d. [DOI] [PubMed] [Google Scholar]
- 51.Lawn SD. Schistosomiasis and immune reconstitution disease. AIDS. 2007;21:1986–1987. doi: 10.1097/QAD.0b013e32828e4f8c. [DOI] [PubMed] [Google Scholar]
- 52.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 53.Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4+ lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103:1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85:103–111. doi: 10.1038/sj.icb.7100027. [DOI] [PubMed] [Google Scholar]
- 55.Morizane S, Suzuki D, Tsuji K, et al. The role of CD4 and CD8 cytotoxic T lymphocytes in the formation of viral vesicles. Br J Dermatol. 2005;153:981–986. doi: 10.1111/j.1365-2133.2005.06849.x. [DOI] [PubMed] [Google Scholar]
- 56.Swain SD, Meissner NN, Harmsen AG. CD8 T cells modulate CD4 T-cell and eosinophil-mediated pulmonary pathology in pneumocystis pneumonia in B-cell-deficient mice. Am J Pathol. 2006;168:466–475. doi: 10.2353/ajpath.2006.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson MS, Mentink-Kane MM, Pesce JT, et al. Immunopathology of schis-tosomiasis. Immunol Cell Biol. 2007;85:148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mwanakasale V, Vounatsou P, Sukwa TY, et al. Interactions between Schistosoma haematobium and human immunodeficiency virus type 1: the effects of coinfection on treatment outcomes in rural Zambia. Am J Trop Med Hyg. 2003;69:420–428. [PubMed] [Google Scholar]
- 59.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27:265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 60.Hardwick C, White D, Morris E, et al. Montelukast in the treatment of HIV associated immune reconstitution disease. Sex Transm Infect. 2006;82:513–514. doi: 10.1136/sti.2005.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipman MC, Carding SK. Successful drug treatment of immune reconstitution disease with the leukotriene receptor antagonist, montelukast: a clue to pathogenesis? AIDS. 2007;21:383–384. doi: 10.1097/QAD.0b013e328011cb38. [DOI] [PubMed] [Google Scholar]
- 62.Robertson J, Meier M, Wall J, et al. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis. 2006;42:1639–1646. doi: 10.1086/503903. [DOI] [PubMed] [Google Scholar]