Abstract
Serum levels of pro-(vascular endothelial growth factor [VEGF]) and anti-(thrombospondin-1 [TSP]) angiogenic cytokines were prospectively measured in a phase II trial of chemoimmunotherapy (CIT) for chronic lymphocytic leukemia (CLL) patients(n=56). Pretreatment VEGF levels were lower among patients who achieved complete remission (CR) or nodular partial remission (nPR) relative to those with partial remission (PR) or stable/progressive disease (median 122.0 pg/ml vs. 246.8 pg/ml; p=0.03). VEGF:TSP ratio was lower (anti-angiogenic phenotype) among patients who achieved CR/nPR. The pretreatment VEGF:TSP ratio also correlated with overall survival (p=0.008). A pro-angiogenic profile appears associated with diminished response and inferior survival in CLL patients receiving CIT.
Keywords: Chronic lymphocytic leukaemia; angiogenesis; therapy; VEGF, prognostic factors
INTRODUCTION
Combination chemotherapy has significantly improved response rates and progression-free survival (PFS) for younger patients with chronic lymphocytic leukaemia (CLL) (Eichhorst, et al 2006). More recent studies suggest that combining the anti-CD20 monoclonal antibody, rituximab, with chemotherapy improves response rates and PFS.(Hallek 2008, Kay, et al 2007a, Keating, et al 2005) Although overall response rates with such chemoimmunotherapy (CIT) are ≥ 90% (Byrd, et al 2003, Hallek 2008, Kay, et al 2007a, Keating, et al 2005), multi-centre trials suggest that only 40–50% of patients experience a complete remission (CR).(Byrd, et al 2003, Hallek 2008) Importantly, the extent of remission has been shown to predict survival, with those achieving a CR after CIT having a longer time to salvage therapy and overall survival(OS).(Kay, et al 2007a, Keating, et al 2005)
Clinical and biological characteristics that predict response to therapy can identify patients who are more or less likely to benefit from a given type of treatment. In addition to their value in patient counseling, such biological markers can (i) provide insight into specific mechanisms of treatment resistance (ii) identify alternative pathways that may be targeted to enhance treatment efficacy, and (iii) identify individuals who may benefit from a modified or alternative treatment strategy. To date, few biological parameters have consistently identified CLL patients unlikely to benefit from CIT (e.g. 17p- by fluorescent in situ hybridization [FISH]).(Kay, et al 2007a) Thus there remains a need for biomarkers that can identify the subset of CLL patients who are unlikely to respond to CIT and who may benefit from an alternative or targeted treatment strategy.
Angiogenesis is an important regulator of the malignant potential of solid tumours and haematological malignancies.(Shanafelt and Kay 2006) CLL patients have been found to have detectable levels of pro and anti-angiogenic cytokines in the serum and abnormal neovascularization in the marrow and lymph nodes. In vitro studies also found that CLL B-cells synthesize and secrete both pro and anti-angiogenic cytokines (Kay, et al 2002) and possess a VEGF-based autocrine pathway (Kay, et al 2002) that facilitates leukemia cell survival partly through up-regulation of anti-apoptotic proteins including MCL and XIAP. Interactions between CLL B-cells and their microenvironment were found to alter the secretion of angiogenic factors that result in enhanced leukemic B-cell resistance to apoptosis.(Kay, et al 2007b) Inter-patient variation in markers of angiogenesis also appear to have potential prognostic implications (reviewed in (Shanafelt and Kay 2006)). Although several small studies suggest that pro-angiogenic cytokine levels(Gora-Tybor, et al 2002, Smolej, et al 2007) and marrow micro-vessel density(Molica, et al 2007) decrease among responding patients after purine nucleoside analogue-based treatment, no study has yet evaluated the ability of pretreatment angiogenic cytokine levels to predict response. We hypothesized that serum levels and ratios of pro- versus anti-angiogenic cytokines would predict response to CIT. To this end, we prospectively measured angiogenic cytokine levels at study entry among patients enrolled in our phase II trial of pentostatin, cyclophosphamide, and rituximab(PCR)(Kay, et al 2007a) to test this hypothesis.
METHODS
Between March 2002 and July 2005, 64 eligible patients were enrolled in a phase II trial of PCR as initial therapy for CLL. Approval for this study was obtained by the Mayo Clinic and the Ohio State University institutional review boards with informed consent of participants obtained in accord with the Declaration of Helsinki. Details of the study, treatment schedule, and supportive care measures were previously reported.(Kay, et al 2007a) Briefly, patients received pentostatin(2 mg/m2), cyclophosphamide(600 mg/m2), and rituximab(375 mg/m2) intravenously on day 1 once every 21 days for 6 cycles. For the first cycle only, rituximab was given on a thrice weekly schedule as previously described.(Kay, et al 2007a)
To test our hypothesis that serum levels of angiogenic cytokines would predict response to CIT, we examined serum levels of pro-(vascular endothelial growth factor [VEGF] and fibroblast growth factor [bFGF]) and anti-(thrombospondin-1 [TSP]) angiogenic cytokines for each patient immediately prior to treatment. VEGF (isoform 165) and bFGF were measured using Quantikine kits (R&D Systems, Minneapolis, MN) and TSP using the Accucyte assay (CytImmune Sciences Inc, Rockville, MD) according to the manufacturer’s instructions. Other molecular and biological prognostic parameters, including CD38, ZAP-70, IGHV mutation status, and recurrent cytogenetic abnormalities, as assessed by FISH were also performed on baseline study samples as previously reported.(Kay, et al 2007a)
To evaluate the relationship between serum angiogenic cytokine levels and Rai stage(0–II vs. III–IV), known prognostic factors(CD38, ZAP-70, IGVH mutation, FISH) or clinical outcome, the Wilcoxon rank sum test or Kruskal-Wallis test was used depending on whether continuous variables were being compared between 2 groups or more than 2 groups. OS and time to salvage treatment (TTT) were calculated from the first day of PCR therapy. TTT was defined as the time from the first date of PCR therapy to the date of initiation of next therapy. Univariate Cox models were used to assess the correlation of serum angiogenic cytokine values with OS and TTT. Overall, statistical significance was declared for p<0.05. As the comparisons were hypothesis generating in nature, a correction for multiple comparisons was not employed.
RESULTS/DISCUSSION
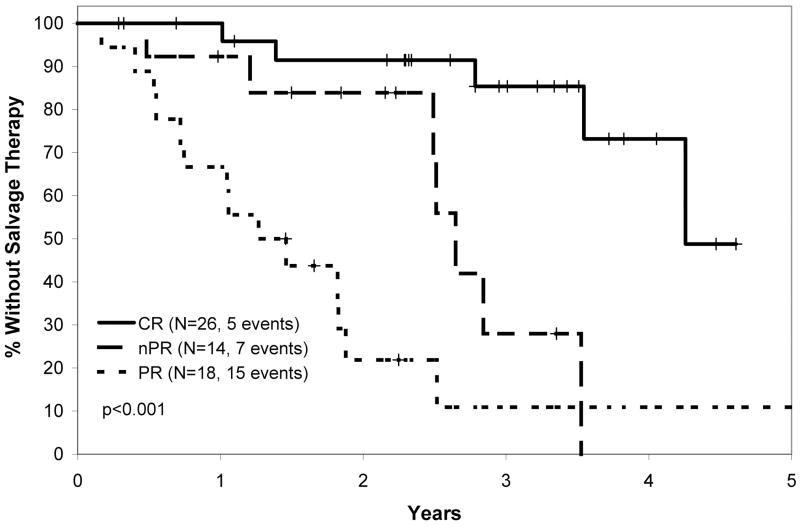
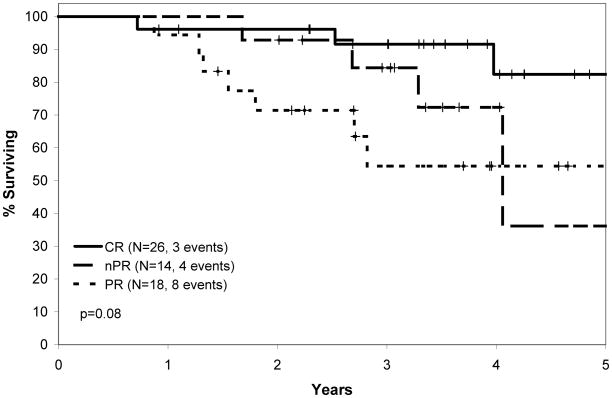
The overall response rate in the PCR clinical trial was 91%, with 41% CR, 22% nodular partial remission (nPR), and 28% partial remission (PR).(Kay, et al 2007a) With respect to conventional prognostic parameters (CD38, ZAP-70, IGHV mutation status, FISH), no characteristic other than the presence of 17p- predicted response to treatment.(Kay, et al 2007a) Median TTT after PCR for all patients was 34 months. Median TTT was approximately 51 months among patients achieving CR, 32 months among patients achieving nPR, and 16 months among patient attaining a PR (Figure 1A). This result was not unexpected and was similar in trends to the findings of other CIT trials demonstrating a relationship between response and time to progression.(Keating, et al 2005, Tam, et al 2008) On updated long-term analysis of OS after PCR, the median survival was 66 months for all treated patients. Median survival by response type is shown in Figure 1B.
Figure 1.
Figure 1A: Time to Salvage Therapy After PCR (pentostatin, cyclophosphamide, rituximab) Therapy.
Time from first date of PCR therapy to the date of next therapy based on response to PCR. Log rank p value shown.
Figure 1B: Overall Survival After PCR Therapy.
Time from first date of PCR therapy to death based on response to PCR. Log rank p value shown.
Overall, 56 of 64(87%) patients underwent baseline angiogenic serum cytokine evaluation (Table I). To better evaluate the presence of angiogenic switching in CLL B-cells, ratios of VEGF:TSP and bFGF:TSP were calculated for all patients, with higher ratios suggestive of a pro-angiogenic phenotype. We next evaluated the relationship between VEGF, bFGF, TSP, VEGF:TSP ratio, and bFGF:TSP ratio and other prognostic parameters. Angiogenic cytokine levels and the VEGF:TSP and bFGF:TSP ratios had no relationship with disease stage at study entry. The median VEGF:TSP ratio was significantly lower (anti-angiogenic) in IGHV mutated patients compared to unmutated patients (p=0.008). In addition, levels of bFGF and bFGF:TSP were significantly correlated with FISH defects (p=0.01, 0.04, respectively); a more pro-angiogenic phenotype was observed among patients with trisomy 12. Although these findings reinforce the concept that more aggressive disease in CLL based on prognostic parameters can be associated with a more pro-angiogenic phenotype, no significant relationships were found between baseline angiogenic cytokine values and CD38 or ZAP-70 status.
Table 1.
Baseline Serum Levels of Pro and Anti-Angiogenic Cytokines Table shows the median VEGF(pg/ml), bFGF(pg/ml), and thrombospondin levels(pg/ml) for patients enrolled on phase II trial of PCR as first line treatment for CLL(N=56). Additional columns show the ratio of VEGF:TSP and bFGF:TSP where smaller ratios indicate a more “anti-angiogenic” phenotype and higher ratios indicate a more “pro-angiogenic” phenotype.
| N | VEGF median (Q1, Q3) | bFGF median (Q1, Q3) | TSP median (Q1, Q3) | VEGF:TSP median (Q1, Q3) | bFGF:TSP median (Q1, Q3) | |
|---|---|---|---|---|---|---|
| All patients | 56 | 172 (93, 378) | 6.6 (1.6, 24.2) | 1395 (771, 3715) | 0.0914 (0.0524, 0.2654) | 0.0036 (0.0009, 0.0161) |
| IGHV Mutation Status | ||||||
| Mutated | 16 | 151 | 4.5 | 1708 | 0.05931 | 0.0022 |
| Unmutated | 39 | 226 | 7.4 | 1277 | 0.12911 | 0.0054 |
| CD38 Status | ||||||
| Negative | 37 | 177 | 3.7 | 1455 | 0.0867 | 0.0029 |
| Positive | 19 | 154 | 8.4 | 1277 | 0.1103 | 0.0061 |
| FISH | ||||||
| 13q- | 20 | 137 | 2.42 | 1982 | 0.0789 | 0.00133 |
| Normal | 6 | 184 | 11.52 | 1284 | 0.1479 | 0.00953 |
| +12 | 13 | 226 | 35.52 | 1434 | 0.1291 | 0.01713 |
| 11q- | 12 | 152 | 2.22 | 1836 | 0.1014 | 0.00263 |
| 17p- | 3 | 253 | 5.72 | 1137 | 0.1867 | 0.00503 |
| ZAP-70 | ||||||
| Negative | 33 | 154 | 5.7 | 1991 | 0.0867 | 0.0028 |
| Positive | 17 | 119 | 8.4 | 973 | 0.1103 | 0.0062 |
| Response | ||||||
| CR | 24 | 1114 | 7.1 | 1278 | 0.08475 | 0.0043 |
| nPR | 13 | 2454 | 7.8 | 1991 | 0.07875 | 0.0062 |
| PR | 14 | 2374 | 3.1 | 1385 | 0.17185 | 0.0023 |
| Stable or progressive disease | 5 | 2534 | 15.7 | 1355 | 0.18675 | 0.0063 |
mutated vs. unmutated; p=0.008(Wilcoxon rank sum)
FISH p=0.01(Kruskal-Wallis)
FISH p=0.04(Kruskal-Wallis)
CR or nPR vs PR, stable or progressive disease; p=0.03(Wilcoxon rank sum); CR vs nPR, PR, stable or progressive disease; p=0.02(Wilcoxon rank sum)
CR or nPR vs. PR, stable or progressive disease; p=0.04(Wilcoxon rank sum); CR vs nPR, PR, stable or progressive disease; p=0.18(Wilcoxon rank sum)
To evaluate whether elevated levels of pro-angiogenic cytokines may identify patients less likely to respond to CIT or have a shorter clinical benefit, we evaluated the relationship between baseline serum VEGF, bFGF, TSP, VEGF:TSP ratio, and bFGF:TSP ratio and response to PCR therapy. The baseline serum VEGF level was significantly lower among patients who achieved a CR or nPR than those attaining PR, stable disease or progressive disease (median 122.2 pg/ml vs. 246.8 pg/ml; p=0.03). The VEGF:TSP ratio was also significantly lower (anti-angiogenic phenotype) among patients who achieved CR or nPR relative to those with a PR, stable disease or progressive disease (median 0.08 vs. 0.19; p=0.04). No difference in response to PCR therapy was observed based on baseline serum bFGF levels, TSP levels, or bFGF:TSP ratios.
Finally, we evaluated the relationship of baseline serum VEGF, bFGF, TSP, VEGF:TSP ratio, and bFGF:TSP ratio with TTT and OS. The VEGF:TSP ratio was significantly correlated with OS (p=0.008, Hazard Ratio =1.26 [95% confidence interval: 1.06–1.49]). This correlation with OS persisted in two factor multivariate Cox models adjusting for stage, absolute lymphocyte count at the time of study entry, IGHV mutation status, CD38 status, FISH results, or ZAP-70 status (all p<0.01). No other significant relationships between baseline angiogenic cytokine levels and TTT or OS were observed.
The present analysis has several important limitations. Angiogenesis is a complex process regulated by an intricate balance of numerous pro- and anti-angiogenic molecules.(Ribatti, et al 2007) In this regard, our use of 2 pro-angiogenic and 1 anti-angiogenic cytokines is a gross simplification of this continuum where other unmeasured cytokines undoubtedly play an important role in this process. Future studies exploring the relationship between response to treatment and angiogenic phenotype using more comprehensive profiling of angiogenic cytokines will be enlightening. Since the relationship between the VEGF:TSP ratio and response is not absolute, the lack of a relationship between baseline VEGF:TSP and time to progression in this small series is not surprising. Given the lack of association with TTT however, it is surprising that the VEGF:TSP ratio was associated with OS. Although we are unable to explain this unexpected result, it is possible that patients with more angiogenic phenotypes relapse at the same rate as those with less angiogenic phenotypes but are less likely to respond to salvage therapy when relapse occurs.
In summary, the results of this prospective analysis are consistent with the hypothesis that a relationship exists between pre-treatment serum angiogenic cytokine levels and response to CIT in patients with CLL. Specifically, a pro-angiogenic phenotype (higher pro-angiogenic factors and lower anti-angiogenic factors) at the time of treatment correlated with a poorer response to CIT and a lower likelihood of achieving a CR or nPR. This finding supports previous in vitro studies suggesting that angiogenic cytokines are important for the survival of CLL B cells and that these cytokines may up-regulate levels of anti-apoptotic proteins known to influence response to therapy.(Shanafelt and Kay 2006) Combining anti-VEGF therapy with chemotherapy has been proven to prolong OS in patients with colon and non-small cell lung cancer and to improve PFS in patients with metastatic breast cancer(Hurwitz, et al 2004; Sandler, et al 2006). While additional studies are needed to confirm the present findings with other CIT regimens and in larger patient populations, the results suggest that a pro-angiogenic profile is associated with diminished response and inferior survival in CLL patients receiving CIT. Furthermore, the findings suggest that the addition of anti-angiogenic therapy and, in particular, anti-VEGF therapy may enhance the efficacy of CIT in CLL patients and encourage further clinical testing of this strategy.
Acknowledgments
Support through grants from the National Cancer Institute(CA95241-06 to N.E. Kay; CA 116237 to N.E. Kay; CA 113408 to T.D. Shanafelt) and Gabrielle’s Angel Foundation for Cancer Research(T.D. Shanafelt) are gratefully acknowledged.
Footnotes
Author Contributions: Concept: Tait Shanafelt, John Byrd, Clive Zent, Tim Call, Michael Grever, Thomas Lin, Neil Kay
Designed research: Tait Shanafelt, John Byrd, Clive Zent, Tim Call, Michael Grever, Thomas Lin, Neil Kay
Performed research: Tait Shanafelt, John Byrd, Clive Zent, Tim Call, Charla Secreto, Michael Grever, Thomas Lin, Neil Kay
Analyzed and interpreted data: Tait Shanafelt, John Byrd, Betsy LaPlant, Clive Zent, Tim Call, Michael Grever, Thomas Lin, Neil Kay
Wrote the paper: Tait Shanafelt, John Byrd, Clive Zent, Tim Call, Michael Grever, Thomas Lin, Neil Kay
Conflict of Interest Disclosure: Drs. Shanafelt and Kay receive research support from Hospira and Genentech/Biogen. Dr. Kay has served as a consultant for Genentech/Biogen.
References
- Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, Vardiman JW, Rai K, Schiffer CA, Larson RA. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, Siehl S, Jager U, Bergmann M, Stilgenbauer S, Schweighofer C, Wendtner CM, Dohner H, Brittinger G, Emmerich B, Hallek M. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- Gora-Tybor J, Blonski JZ, Robak T. Cladribine decreases the level of angiogenic factors in patients with chronic lymphocytic leukemia. Neoplasma. 2002;49:145–148. [PubMed] [Google Scholar]
- Hallek M. Immunochemotherapy with Fludarabine (F), Cyclophosphamide (C), and Rituximab (R) (FCR) Versus Fludarabine and Cyclophosphamide (FC) Improves Response Rates and Progression-Free Survival (PFS) of Previously Untreated Patients (pts) with Advanced Chronic Lymphocytic Leukemia (CLL) Blood. 2008;112 Abstract #325. [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Kay NE, Bone ND, Tschumper RC, Howell K, Geyer S, Dewald G, Hanson C, Jelinek D. B-CLL cells are capable of synthesis and secretion of both pro- and anti-angiogenic molecules. Leukemia. 2002;16:911–919. doi: 10.1038/sj.leu.2402467. [DOI] [PubMed] [Google Scholar]
- Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, Tschumper R, Bone ND, Dewald GW, Lin TS, Heerema NA, Smith L, Grever MR, Byrd JC. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007a;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an “angiogenic switch”. Leuk Res. 2007b;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M, O’brien S, Albitar M, Lerner S, Plunkett W, Giles F, Andreeff M, Cortes J, Faderl S, Thomas D, Koller C, Wierda W, Detry M, Lynn A, Kantarjian H. Early Results of a Chemoimmunotherapy Regimen of Fludarabine, Cyclophosphamide, and Rituximab as Initial Therapy for Chronic Lymphocytic Leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Molica S, Montillo M, Ribatti D, Mirabelli R, Tedeschi A, Ricci F, Veronese S, Vacca A, Morra E. Intense reversal of bone marrow angiogenesis after sequential fludarabine-induction and alemtuzumab-consolidation therapy in advanced chronic lymphocytic leukemia. Haematologica. 2007;92:1367–1374. [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Leukemia. 2007;21:44–52. doi: 10.1038/sj.leu.2404402. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Kay NE. The clinical and biologic importance of neovascularization and angiogenic signaling pathways in chronic lymphocytic leukemia. Semin Oncol. 2006;33:174–185. doi: 10.1053/j.seminoncol.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Smolej L, Andrys C, Krejsek J, Belada DZ, Zak P, Siroky O, Maly J. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are elevated in peripheral blood plasma of patients with chronic lymphocytic leukemia and decrease after intensive fludarabine-based treatment. Vnitr Lek. 2007;53:1171–1176. [PubMed] [Google Scholar]
- Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]