Abstract
Objective
To compare the effect of unmodified cows’ milk and iron supplemented formula milk on psychomotor development in infants from inner city areas when used as the main milk source.
Design
Double blind, randomised intervention trial.
Setting
Birmingham health centre.
Subjects
100 infants, mean age 7.8 months (range 5.7 to 8.6 months), whose mothers had already elected to use unmodified cows’ milk as their infant’s milk source.
Intervention
Changing to an iron supplemented formula milk from enrolment to 18 months of age, or continuing with unmodified cows’ milk.
Main outcome measures
Developmental assessments using Griffiths scales at enrolment and at 18 and 24 months.
Results
85 participants completed the trial. There were no significant differences in haemoglobin concentration between the two groups at enrolment, but by 18 months of age 33% of the unmodified cows’ milk group, but only 2% of the iron supplemented group, were anaemic (P<0.001). The experimental groups had Griffiths general quotient scores that were not significantly different at enrolment, but the scores in both groups declined during the study. By 24 months the decrease in the mean scores in the unmodified cows’ milk group was 14.7 whereas the decrease in the mean scores in the iron supplemented group was 9.3 (P<0.02, 95% confidence interval 0.4 to 10.4). Mean subquotient scores were considerably lower in the unmodified cows’ milk group at 24 months; significantly so for personal and social scores (P<0.02, −5.4 to 17.2).
Conclusion
Replacing unmodified cows’ milk with an iron supplemented formula milk up to 18 months of age in infants from inner city areas prevents iron deficiency anaemia and reduces the decline in psychomotor development seen in such infants from the second half of the first year.
Key messages
Iron deficiency anaemia is common in infants from inner cities who are given unmodified cows’ milk in the first year of life
Giving an infant iron supplemented formula milk instead of cows’ milk not only prevents anaemia but reduces the decline in developmental performance observed in those given only cows’ milk
An iron supplemented formula milk rather than cows’ milk should be provided free of charge for infants up to the age of 18 months who are living in inner cities and who are not receiving breast milk
Introduction
Iron deficiency anaemia—that is, a haemoglobin concentration <110.0 g/l—still occurs in 10 to 30% of preschool children living in inner cities in the United Kingdom.1,2 There is a well established association between iron deficiency anaemia and developmental delay, and randomised studies providing oral iron supplements suggest that this may be causal.3–8 We have previously shown that iron deficiency anaemia in infants and toddlers receiving unmodified cows’ milk as their main milk source is eliminated by changing to an iron supplemented formula milk between 6 and 18 months of age.9 Our study aimed to address an additional and pragmatic question: does randomisation to receive an iron supplemented formula milk between 6 and 18 months of age lead to an additional developmental advantage compared with continuing receipt of unmodified cows’ milk? Detailed haematological and nutritional data from the study have already been published.9 We now present the developmental outcomes.
Subjects and methods
Recruitment
Our keyworker (AD) received the names of all infants aged 6-8 months (567 identified) living in an inner city area of Birmingham from health visitors dealing with that area. AD visited the families, and the parents of only those infants whose mothers had already changed their infant’s diet to unmodified cows’ milk (n=116) were asked to consider including their infant in the study. All mothers were given both verbal and written explanations of the study.
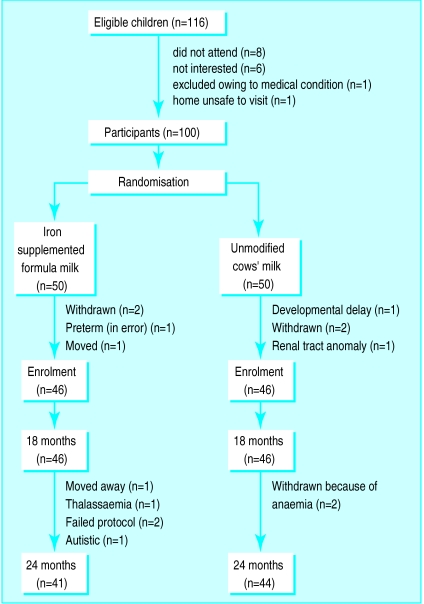
The mean age of infants at recruitment (47 boys and 53 girls) was 7.8 months (range 5.7 to 8.6 months). The population was 75% Caucasian, 24% AfroCaribbean, and 2% Asian (Indians). We excluded all preterm infants. Figure 1 shows the withdrawals and losses of participants from the study.
Figure 1.
Withdrawals and losses from study
The participants lived in a socially deprived area with poor housing, high unemployment, and poor public amenities—locally there was only one bank and no large supermarkets. The small local shops were expensive and had limited stocks of food, particularly fresh fruit and vegetables.
Power calculation—
We performed a power calculation, which showed that if 47 participants were allocated to each dietary group this would provide a study power of 95% at a significance level of 5% for a difference in haemoglobin concentration of 7.5 g/l between groups.
Study design
After recruitment we randomised the infants in the pharmacy department at Birmingham Children’s Hospital by random numbers in blocks of four to receive either an iron supplemented formula milk or to continue on unmodified cows’ milk. We gave the results of randomisation to AD who was therefore unblinded. At 18 months, those infants randomised to change to an iron supplemented formula milk were transferred back to cows’ milk, and both groups continued on the cows’ milk until 24 months of age. Serial haematological, anthropometric, and developmental assessments using the Griffiths scales were made at enrolment and at 18 and 24 months of age.10 We excluded those participants whose haemoglobin concentration decreased to <90 g/l and referred them to their general practitioner.
We supplied the iron supplemented formula milk free of charge, and we gave those mothers whose infants remained on the cows’ milk a monthly payment equivalent to the cost of 500 ml cows’ milk daily. Mothers from both groups on income support were still entitled to claim free cows’ milk with milk tokens. However, as not all parents were in receipt of income support, and therefore not entitled to the cows’ milk, the cows’ milk group received funding to purchase 500 ml cows’ milk per day. Table 1 lists the nutrient content of the cows’ milk and iron supplemented formula milk.
Table 1.
Nutrient composition of unmodified cows’ milk and iron supplemented formula (0.28 MJ of energy per 100 ml each)
| Nutrient per 100 ml reconstituted feed | Unmodified cows’ milk11 | Iron supplemented formula |
|---|---|---|
| Protein (g) | 3.2 | 2.1 |
| Fat (g) | 3.9 | 3.1 |
| Carbohydrate (g) | 4.8 | 8.0 |
| Sodium (mg) | 55 | 31 |
| Potassium (mg) | 140 | 89 |
| Calcium (mg) | 115 | 72 |
| Magnesium (mg) | 11.0 | 7.1 |
| Phosphorus (mg) | 92 | 58 |
| Iron (mg) | 0.05 | 1.2 |
| Copper (μg) | Trace | 43 |
| Zinc (mg) | 0.4 | 0.4 |
| Chloride (mg) | 100 | 58 |
| Vitamin A (re) (μg) | 52 | 80 |
| Carotene (g) | 21 | 24 |
| Vitamin D (μg) | 0.03 | 1.1 |
| Thiamin B-1 (mg) | 0.04 | 0.04 |
| Riboflavin B-2 (mg) | 0.17 | 0.15 |
| Nicotinamide (mg) | 0.1 | 0.65 |
| Vitamin C (mg) | 1 | 10 |
| Vitamin E (tc) (mg) | 0.09 | 0.48 |
| Vitamin B-6 (mg) | 0.06 | 0.04 |
| Vitamin B-12 (μg) | 0.4 | 0.2 |
| Folic acid (μg) | 6.0 | 7.0 |
| Pantothenic acid (mg) | 0.35 | 0.36 |
| Biotin (μg) | 1.9 | 3.0 |
re=retinol equivalent; tc=tocopherol.
Developmental assessments
The Griffiths scale calculates an overall developmental score (general quotient), which is the mean of five subscales: locomotor, personal and social, hearing and speech, eye and hand coordination, and performance (manipulation and precision).10
Five trained and experienced observers performed the Griffiths scales. The observers were blinded to the group randomisations.
Statistical analyses—
We performed statistical analyses with either paired and unpaired Student’s t tests, χ2 tests, or Fisher’s exact tests, and analysis of variance.
Ethical approval—
We obtained ethical approval from the South Birmingham Health Authority’s ethics committee. We obtained informed written consent from caregivers.
Results
Withdrawals and losses
Some data points were missing due to intercurrent illness in a participant, transiently being unable to locate children, or insufficient volume of blood for assay. Out of 269 contacts, a developmental score was unavailable on 11 occasions (3%).
During the course of the study some children were found to have conditions that led their caregivers to withdraw them from the study. This was either because recommended treatment (for example, diet for an infant with renal disease) interfered with the study or because the diagnosis made continuing participation impractical (fig 1).
Sociodemographic characteristics of the study groups—
After randomisation we found no significant difference between the two groups (table 2).
Table 2.
Characteristics of two study groups
| Variable | Iron supplemented formula milk (n=50) | Unmodified cows’ milk (n=50) |
|---|---|---|
| Maternal age at leaving education (years): | ||
| 16 | 38 | 41 |
| 17-18 | 9 | 6 |
| >18 | 3 | 3 |
| Mothers’ age (years): | ||
| <20 | 2 | 3 |
| 20-25 | 25 | 20 |
| 26-30 | 16 | 22 |
| 31-35 | 6 | 3 |
| >36 | 1 | 2 |
| Home ownership: | ||
| Own home | 2 | 1 |
| Council owned house | 18 | 20 |
| Council flat | 30 | 29 |
| Income: | ||
| Income support | 31 | 28 |
| Family credit | 11 | 12 |
| Parental smoking | 34 | 31 |
| No of siblings: | ||
| 1 | 19 | 16 |
| 2 | 8 | 11 |
| 3 | 3 | 5 |
| 4 | 2 | 1 |
| >4 | 2 | 0 |
Haematology
At enrolment there were no statistically significant differences in mean haemoglobin concentration between the two groups; 16% of the cows’ milk group and 13% of the iron supplemented formula milk group were already anaemic.
By 18 months, 2% of the iron supplemented formula milk group and 33% of the cows’ milk group were anaemic (P<0.0001). At 24 months, when the iron supplemented formula milk group had been returned to cows’ milk for 6 months, 26% of the cows’ milk group were anaemic but none of the iron supplemented formula milk group were anaemic (P=0.0017). Similar changes occurred in mean corpuscular volume and ferritin concentration: there was no difference at enrolment, but at 18 and 24 months there were significantly lower values in the cows’ milk group.9
Developmental outcomes
Interobserver differences—
We found no statistically significant differences when the developmental scores for the different observers were compared by analysis of variance for both iron supplemented formula milk and cows’ milk groups.
Griffiths general quotient scores—
At enrolment there were no significant differences between the two groups. Scores in each group declined during the study (table 3, fig 2). By 18 months the mean general quotient score had decreased by 8.3 (P=0.002) and 6.7 (P=0.02) points in the cows’ milk and iron supplemented formula milk groups respectively.
Table 3.
Analysis of developmental scores for unmodified cows’ milk and iron supplemented formula milk groups from enrolment to 18 months (46 and 46 infants respectively) and from enrolment to 24 months (44 and 42 infants respectively)
| Enrolment mean | Enrolment-18 months
|
Enrolment-24 months
|
||||
|---|---|---|---|---|---|---|
| Mean difference | P value (95% CI) for difference between groups* | Mean difference | P value (95% CI) for difference between groups* | |||
| General quotient | ||||||
| Unmodified cows’ milk | 109.2 | −8.3 | 0.66 (−3.1 to 6.1) | −14. 7 | 0.036 (0.4 to 10.4) | |
| Iron supplemented formula | 111.5 | −6.7 | −9.3 | |||
| Locomotor | ||||||
| Unmodified cows’ milk | 114.5 | −7.2 | 0.47 (−4.7 to 10.1) | −20.0 | 0.98 (−4.1 to 12.2) | |
| Iron supplemented formula | 115.3 | −4.4 | −15.9 | |||
| Personal and social | ||||||
| Unmodified cows’ milk | 113.4 | −12.9 | 0.31 (−3.6 to 11.2) | −19.0 | 0.02 (1.2 to 16.8) | |
| Iron supplemented formula | 112.0 | −9.1 | −10.0 | |||
| Eye and hand coordination | ||||||
| Unmodified cows’ milk | 110.8 | −12.1 | 0.56 (−5.9 to 10.6) | −16.4 | 0.28 (−4.2 to 14.2) | |
| Iron supplemented formula | 108.8 | −9.7 | −11.3 | |||
| Hearing and speech | ||||||
| Unmodified cows’ milk | 109.4 | −6.7 | 0.49 (−4.6 to 7.5) | −13.5 | 0.1 (−1.5 to 12.8) | |
| Iron supplemented formula | 106.5 | −5.3 | −7.8 | |||
| Performance (manipulation and precision) | ||||||
| Unmodified cows’ milk | 109.4 | −4.8 | 0.21 (−7.1 to 8.8) | −7.1 | 0.09 (−1.3 to 15.0) | |
| Iron supplemented formula | 105.7 | −3.9 | −0.2 | |||
Change in scores between groups.
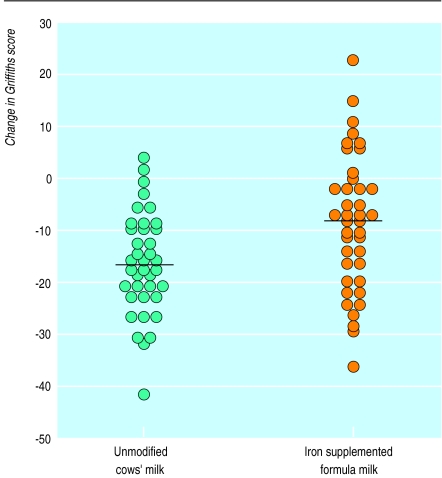
Figure 2.
Changes in Griffiths general quotient score between enrolment and 24 months of age in infants receiving unmodified cows’ milk or iron supplemented formula milk (P=0.02)
By 24 months there had been a further decrease of 6.4 points in the mean score of the cows’ milk group (P<0.001), whereas a decrease of only 2.6 points had occurred in the iron supplemented formula milk group. The decrease in general quotient score between enrolment and 24 months was significantly greater in the cows’ milk group than in the iron supplemented formula milk group (14.7 versus 9.3 respectively, P<0.02; 95% confidence interval 0.4 to 10.4) (fig 2).
Subquotient scores
At enrolment there were no significant differences in mean subquotient scores between the two study groups, but they declined in both groups throughout the study (table 3, fig 3).
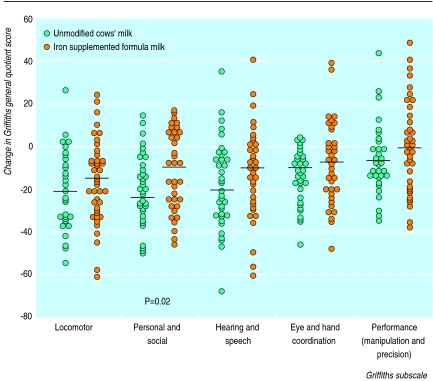
Figure 3.
Changes in Griffiths subscales between enrolment and 24 months of age
The decrease in subquotient scores from enrolment was consistently greater in the cows’ milk group than in the iron supplemented formula milk group, both at 18 and 24 months, and in all subscales. However, only the decrease in personal and social skills between enrolment and 24 months was significantly greater in the cows’ milk group than the iron supplemented formula milk group (P=0.02).
Developmental scores and haematological status—
There was no significant linear correlation between haemoglobin concentration and general quotient scores at 24 months. However, those participants allocated cows’ milk were significantly clustered towards both a lower haemoglobin concentration and lower general quotient score than those receiving iron supplemented formula milk, who were clustered significantly towards both a higher haemoglobin concentration and general quotient score. Thus, of the 24 participants with both a haemoglobin concentration <120 g/l and general quotient score <100 at 24 months, 20 had received cows’ milk. In contrast, 13 of the 16 with a haemoglobin concentration >120 g/l and a general quotient score >100 had received iron supplemented formula milk (P<0.0001).
Growth and nutrient intakes—
Both groups grew satisfactorily on both the iron supplemented formula milk and the cows’ milk.9
Discussion
Our study shows that in a population of socioeconomically deprived infants, changing from unmodified cows’ milk to an iron supplemented formula milk from 7 to 18 months of age prevented iron deficiency anaemia at 24 months, and significantly reduced the decline in psychomotor performance seen in those infants randomised to continue on cows’ milk.
Nutritional basis of the observed effects—
In contrast to other randomised studies, we chose to look at a realistic and practical dietary intervention.6,7 We have previously shown that this intervention prevents the development of anaemia, but the precise nutritional basis of the developmental advantage in the group receiving iron supplemented formula milk is uncertain. The intakes of the two groups also differed substantially in nutrients other than iron.9 However, the strength of the recognised association between iron deficiency anaemia and developmental delay, and the scale of the difference in iron status between the two groups, lead us to suggest that it is the disparity in iron status between the two groups that is the most plausible explanation for the observed difference in developmental performance.
Comparison with previous studies—
Our findings support previous studies of supplementation with oral iron in children with iron deficiency anaemia, where an improvement in developmental performance was noted.6,7,12 Moffatt and colleagues conducted a similar longitudinal cohort study to our own and showed a developmental advantage at 9 and 12 months, which was no longer detectable at 15 months of age.8 The transient nature of the effect may have been due to differences in the timing, duration, and mildness of the iron deficiency compared with our study group.
Basis of the developmental advantage—
The Griffiths scale has been well validated, and the subscales provide useful insights into the basis of the differences in developmental scores.10,13 In our study the major difference was in the personal and social subscale. This supports the view that iron deficiency anaemia may exert its effects on developmental performance by alterations in affect, thereby making a child clingy, lethargic, irritable, and listless,5 and leading to impaired learning skills.
Implications of the study—
We acknowledge that it is difficult to quantify precisely the developmental advantage in the infants receiving iron supplemented formula milk, but neverthless believe that this study has a number of important implications. Firstly, it confirms the well recognised observation that socioeconomic deprivation places infants at increased risk of adverse developmental outcomes.14,15 Secondly, this developmental deficit seems, in part, to be nutritionally mediated. Thirdly, iron deficiency anaemia is common in high risk populations,14 and both this and the developmental disadvantage are susceptible to a simple intervention: the provision of an iron supplemented formula milk in place of cows’ milk.
Breast milk is clearly the milk of choice for the developing infant.16 Our study suggests that in those mothers who find breast feeding impractical, iron supplemented formula milk seems to be effective and acceptable, and benefits high risk infants and children up to the age of at least 18 months.15
Acknowledgments
We thank the health visitors at Nechells Health Centre for their help, Dr P Davies for his statistical advice, and Dr M Huntley for advice on the Griffiths scales.
Footnotes
Funding: Farley Health Products.
Conflict of interest: None.
References
- 1.Dalman PR. Nutritional anaemias in childhood. In: Suskind RM, Lweinter-Suskind L, editors. Textbook of paediatric nutrition, 2nd ed. New York: Raven; 1993. [Google Scholar]
- 2.Gregory JR, Collins DL, Davies PSW, Hughes JM, Clarke PC. National diet and nutrition survey: children aged 1½ to 4½ years. 1. Report of the diet and nutrition survey. London: HMSO; 1995. [Google Scholar]
- 3.Lawson M. Iron. Nutritional and physiological significance. Report of the British Nutrition Foundation task force. London: Chapman and Hall; 1995. Iron in infancy and childhood. [Google Scholar]
- 4.Lansdown R, Wharton BA. Iron. Nutrition and physiological significance. Report of the British Nutrition Foundation’s task force. London: Chapman and Hall; 1995. Iron and mental and motor behaviour in children. [Google Scholar]
- 5.Pollitt E. Iron deficiency and cognitive function. Ann Rev Nutr. 1993;13:521–537. doi: 10.1146/annurev.nu.13.070193.002513. [DOI] [PubMed] [Google Scholar]
- 6.Aukett MA, Parkes YA, Scott PH, Wharton BA. Treatment with iron increases weight gain and psychomotor development. Arch Dis Child. 1986;61:849–857. doi: 10.1136/adc.61.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idjradinata P, Pollitt E. Reversal of developmental delays in iron deficiency in infants treated with iron. Lancet. 1993;341:1–4. doi: 10.1016/0140-6736(93)92477-b. [DOI] [PubMed] [Google Scholar]
- 8.Moffatt MEK, Longstaffe S, Besant J, Dureski C. Prevention of iron deficiency and psychomotor decline in high-risk infants through use of iron-fortified infant formula: a randomised clinical trial. J Pediatr. 1994;125:527–534. doi: 10.1016/s0022-3476(94)70003-6. [DOI] [PubMed] [Google Scholar]
- 9.Daly A, Macdonald A, Aukett A, Williams J, Wolff A, Davidson J, et al. Prevention of anaemia in inner city toddlers by an iron supplemented cows’ milk. Arch Dis Child. 1996;75:9–16. doi: 10.1136/adc.75.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths R. The abilities of babies. London: University of London Press; 1954. [Google Scholar]
- 11.Holland B, Welch AA, Unwin ID, Buss DH, Paul AA, Southgate DAT. McCance and Widdowson’s the composition of foods, 5th ed. Cambridge: Royal Society of Chemistry, Ministry of Agriculture, Fisheries, and Food; 1991. [Google Scholar]
- 12.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anaemia and iron therapy effects on infant developmental test performance. Pediatrics. 1987;79:981–995. [PubMed] [Google Scholar]
- 13.Ramsay M, Fitzhardinge PM. A comparative study of two developmental scales: the Bayley and the Griffiths. Early Hum Dev. 1977;1:151–157. doi: 10.1016/0378-3782(77)90016-0. [DOI] [PubMed] [Google Scholar]
- 14.Aylward GP. The relationship between environmental risk and developmental outcome. Dev Behav Pediatr. 1992;13:222–229. [PubMed] [Google Scholar]
- 15.Booth IW, Aukett MA. Iron deficiency anaemia in infancy and early childhood. Arch Dis Child. 1997;76:549–554. doi: 10.1136/adc.76.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall D. Health for children, 3rd ed. Oxford: Oxford University Press; 1996. [Google Scholar]