Abstract
BACKGROUND
Deferral for travel to malaria-endemic areas excludes many blood donors in the United States. Most transfusion-transmitted malaria is associated with lengthy residence in malaria-endemic areas rather than routine travel. This study compares the impact of existing deferral requirements to the risk that a presenting donor with malaria travel history harbors malaria parasites under current and hypothetical alternate regulations.
STUDY DESIGN AND METHODS
Deferred donors from six blood centers were sampled to estimate a national cohort of donors deferred annually for malaria travel to different geographic regions. Risk for malaria infection following travel to each region, and distribution of incubation periods for each malaria species were estimated for U.S. travelers. Region-specific travel risks were used to estimate the risk that a presenting blood donor with malaria travel might asymptomatically harbor malaria parasites at different intervals following return to the United States.
RESULTS
Travel to Africa presents risk for malaria infection >1000 times that of travel to malaria-endemic parts of Mexico, yet Mexico accounts for >10 times as many deferred donors. Shortening the deferral period from 12 to 3 months for travelers to Mexico increases the risk of collecting a contaminated unit by only 1 unit per 57 years (sensitivity analysis, 1 every 29 - 114 years), at annual gain of >56,000 donations.
CONCLUSION
This study provides the first systematic appraisal of the U.S. requirements for donor qualification regarding travel to malarial areas. Consideration should be given to relaxing the guidelines for travel to very low-risk areas such as Mexico.
Keywords: Plasmodium, malaria, blood donor, deferral, malaria travel, transfusion transmitted disease
INTRODUCTION
The risk of transmitting malaria infection to a blood recipient depends on the likelihood that presenting blood donors are incubating new infections or silently carrying chronic infections. Local transmission is uncommon in the United States, so risk for malaria infection in blood donors is largely limited to individuals with a history of travel to or residency in a locale endemic for malaria. Each year ≈ 28 million U.S. residents travel to a malaria-endemic country,1,2 accounting for most of the ≈ 1,500 cases of malaria diagnosed annually within the U.S. For both U.S.-born and foreign-born travelers, the predominant species of parasite is Plasmodium falciparum, and the source country is typically in sub-Saharan Africa.
The rate of transfusion-transmitted malaria (TTM) in the U.S. has diminished significantly over the last several decades, from roughly 1 to 1.5 cases per 106 units of whole blood or packed red cells transfused in the late 1960s and early 1970s to < 0.1 per 106 transfusions between 1990 and 2005.3,4 In the absence of an FDA-approved malaria screening test, the exclusion of presenting donors who might present risk of malaria infection has been the primary safeguard against transfusion of Plasmodium-contaminated blood products. Blood collection centers are governed by the recommendations/requirements of the Food and Drug Administration (FDA) and voluntarily by the standards of AABB (formerly the American Association of Blood Banks), which currently stipulate a one-year deferral period for travelers to malaria-endemic areas, and a three-year deferral period for former residents of malaria-endemic countries and for those who report a history of malaria infection.5,6 The reliance on detailed travel histories, however, draws criticism for being complicated, contributing to donor and staff errors, and having very low specificity.7 Furthermore, the policy has a significant negative impact on operations and blood availability due to the sheer number of donors deferred, estimated recently as 150,000 donors annually.8 Of additional significance, donors who are temporarily deferred may return less frequently than non-deferred donors, compounding the impact on blood availability.9
No systematic analysis of the tradeoffs associated with the malaria deferral requirements has been performed, and, indeed, it is unclear to what extent the donor qualification requirements have contributed to the drop in rates of TTM in the U.S. The significant impact on blood availability, especially in light of estimates sharply lowering the size of the population eligible to donate,10 argues for close scrutiny of the impact and presumed benefits associated with the current requirements, particularly related to U.S. civilian travelers. This manuscript estimates the risk of a malaria-infected donor presenting to donate under current and hypothetical alternate travel deferral guidelines. We report risk separately by geographic destination of travel, since malaria transmission intensity and, consequently, risk, vary widely across regions.
MATERIALS AND METHODS
Source of data on presenting US blood donors deferred for travel to malaria-endemic areas
Six blood centers participating in the Retrovirus Epidemiology Donor Study - II (REDS-II) program sponsored by the National Heart, Lung and Blood Institutes (NHLBI) provided data for this analysis (see list in Table 1 legend). These centers represent geographically and demographically diverse populations and collectively account for more than 8% of annual blood collections in the U.S.11 Each center retrieved blood donation records from the first sixty donors deferred for malaria travel every other month for 2006. Data were recorded on donor demographics, date of presentation, dates of travel in malaria-endemic regions, and the destination country(ies) with malaria risk. Countries were grouped into regions according to the classification used by the U.S. Department of Commerce's Office of Travel and Tourism Industries (OTTI). Deferral records lacking the destination country or the date that deferral began were excluded from the analysis. Collection and analysis of donor data was approved by the IRB of each participating center.
Table 1.
Malaria travel deferrals at 6 REDS-II Blood Centers, 2006
| BCP | BCW | HOX | ITxM | NEARC | SARC | Total | |
|---|---|---|---|---|---|---|---|
| # travel deferrals | 2,761 | 2,128 | 1,122 | 1,622 | 3,570 | 1,804 | 13,007 |
| # allogeneic donations, 2006 | 123,140 | 195,479 | 92,276 | 159,815 | 405,083 | 302,989 | 1,278,782 |
| Travel deferral / 1,000 donations | 22.4 | 10.9 | 12.1 | 10.1 | 8.8 | 5.9 | 10.2 |
BCP = Blood Centers of the Pacific (San Francisco, CA)
BCW = Blood Center of Wisconsin (Milwaukee, WI)
HOX = Hoxworth Blood Center/University of Cincinnati Academic Health Center (Cincinnati, OH)
ITxM = the Institute for Transfusion Medicine (Pittsburgh, PA)
NEARC = American Red Cross New England Region (Dedham, MA)
SARC = American Red Cross Southern Region (Douglasville, GA)
Estimate of Annual Deferrals of U.S. Blood Donors for Malaria-Risk Travel to Different Regions
The distribution of destination countries by OTTI region for the six REDS-II centers was used to determine weighted estimates of the annual number of malaria travel deferrals associated with each region for the six centers and for U.S. donors overall. Wald type 95% confidence intervals were approximated (standard errors were approximated using weights and assuming distribution of travel deferrals by region for U.S. donors was the same as the distribution for the six centers). Annual data on allogeneic donations and malaria travel deferrals were recorded and summed across all centers, and each center's contribution to national estimates was weighted by its share of the collective REDS-II malaria travel deferrals. Based on their aggregate contribution of 8.64% of US allogeneic donations,11 the blood centers' estimates for region-specific deferrals were multiplied by 11.6 to extrapolate to the U.S. overall. Because no deferrals were recorded for travel to Oceania (Vanuatu, Solomon Islands, and Papua New Guinea), we multiplied Oceania's travelers by the same ratio of malaria-travel deferred donors to U.S. travelers that applied to all other geographic regions collectively (1.76%). When a donor reported more than one trip with a visit to a malaria-endemic area, the most recent trip was chosen for analysis. When more than one geographic region was visited within the trip used for data analysis, the donor's visit was allocated to the geographic region of higher risk for malaria infection.
Estimated Risk for Malaria Infection in U.S. Travelers to Different Regions
We combined data on imported malaria cases in 2005 from the U.S. Centers for Disease Control12 with data from OTTI1 and the World Tourism Organization2 on travel patterns for U.S. residents during 2005 to develop a malaria risk estimate for U.S. travelers to different geographic regions. Briefly, OTTI data are derived from a self-administered survey completed by outbound air passengers departing from the United States.13 WTO data reflect tourist arrival counts at national borders by country of origin.2 For each region, the estimated number of travelers is limited to those who visited a country endemic for malaria. Based on our review of travel patterns and distribution of malaria risk within countries, we estimated the proportion of travelers to each region that may have gone to malaria-endemic parts of one or more countries. The risk for pre-symptomatic malaria infection at various time intervals was derived by multiplying the rate of malaria acquisition for each region by a geographic region-specific measure that itself combines the proportion of all imported malaria cases attributable to each of the four human Plasmodia (spp. falciparum, vivax, malariae, and ovale) for 2000-2005 and the particular incubation periods for each species in US travelers. See Appendix for analytical approach and data used in calculations. The external data sources1,2,12 provided population data, not sample data, hence no statistical analysis was performed.
Residual Risk for Malaria Infection in Blood Donors and Infections Interdicted by Donor Deferral
For each geographic region, we estimated the number of malaria infections that might be expected in a group of equivalent size and travel history as our deferral cohort. For this, we imputed to the presenting donors the same risk for malaria infection as borne by US travelers overall to each respective region. Residual risk is intended to estimate the number of donors per year who might be accepted for donation while infected with Plasmodium parasites. The two other outcomes for malaria infections are that they become symptomatic before donor presentation (leading to self-deferral) or they are interdicted by the deferral requirement itself. For these calculations, actual donor return behavior was applied to each region, along with the data in the Appendix. Estimates for residual risk are shown for the current deferral period of one year and under hypothetical alternate scenarios of a three-month deferral period and with no deferral at all, for each geographic region. To ensure conservative estimates, calculations assume that all donors appear to donate on the first day of eligibility. Because the residual risk estimates are primarily dependent on known population data (i.e., travel patterns to endemic areas, and incubation periods of the different malaria species in U.S. civilians) and not samples, sensitivity analysis is preferable to confidence intervals for capturing uncertainty in the model.
Sensitivity Analysis
To determine the sensitivity of our residual risk estimates to imprecision in the risk for malaria infection, we repeated our calculations with different proportions of travelers to each region assumed to have traveled to malaria-endemic areas. For Africa and Oceania, baseline estimates (based on CDC travel guidelines during the relevant time period14) assume large majorities of travelers visited at-risk areas, while only 20% of visitors to Mexico were assumed to travel to endemic areas. For all other regions, 50% of all travelers were assumed to engage in travel that would trigger a deferral at presentation to donate. Separate tables were created with these proportions adjusted both up and down for all regions.
RESULTS
Malaria Travel Deferrals
The six REDS-II centers reported a total of 13,007 deferrals for travel to malaria-endemic areas by US residents in 2006 (Table 1). The rate of malaria travel deferrals varied significantly, and the aggregate figure of 10.2 deferrals/1,000 donations is consistent with other estimates.15
Geographic area visited for donors deferred for malaria travel and extrapolation to U.S. annually
Of 2,108 malaria travel deferrals included in this analysis (97.6% of donors sampled), the majority were recorded for donors who visited low-risk areas (Table 2). Mexico constituted 41% of malaria travel deferrals, and the Caribbean and Central America accounted for another 13% and 22%, respectively. By contrast, relatively few donors were deferred for travel to high-risk areas like Africa (3.7%) and Oceania (0 deferrals). The number of deferred donors sampled from each blood center represented a sample varying from 10% (NEARC) to 32% (HOX) of annual malaria travel deferrals, representing in total 16.2% of the 13,007 malaria travel deferrals reported by the six centers. Extrapolation to the U.S. overall yields an estimated 150,537 travel deferrals annually. Counting the Caribbean, the Americas contribute 117,857 of the national total, with Mexico alone contributing > 61,000 lost donors annually. Considering that the average donor contributes 1.7 donations annually,15 this represents >100,000 donations lost from travelers to Mexico versus <9,500 due to travel to the riskiest large region, Africa.
Table 2.
Distribution of Regions Visited for Malaria Travel Deferrals and Projected Number of Donors Deferred for Travel to each Region (columns sum to 100%), 6 REDS-II Blood Centers, 2006; N (%)
| BCP | BCW | HOX | ITxM | NEARC | SARC | Total (%) | Weighted annual estimates for 6 REDS-II Blood Centers, 2006 (95% CI) | Projected Annual Number of U.S. Donors Deferred Nationally (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Africa | 16 (4.8) | 6 (1.7) | 13 (3.6) | 14 (3.9) | 11 (3.1) | 18 (5.2) | 78 (3.7) | 475.6 (364.4, 586.8) | 5,500 (4,215 - 6,785) |
| Asia | 100 (29.9) | 27 (7.6) | 37 (10.4) | 44 (12.3) | 46 (12.8) | 69 (19.9) | 323 (15.3) | 2120.7 (1901.9,2339.5) | 24,524 (21,995 - 27,054) |
| Caribbean | 9 (2.7) | 37 (10.5) | 59 (16.6) | 88 (24.5) | 63 (17.5) | 24 (6.9) | 280 (13.3) | 1630.9 (1434.7, 1827.0) | 18,859 (16,592 - 21,127) |
| Central America | 75 (22.5) | 41 (11.6) | 87 (24.4) | 74 (20.6) | 97 (26.9) | 88 (25.4) | 462 (21.9) | 2896.4 (2650.0,3142.8) | 33,494 (30,645 - 36,343) |
| Middle East | 2 (0.6) | 6 (1.7) | 4 (1.1) | 7 (1.9) | 6 (1.7) | 12 (3.5) | 37 (1.8) | 219 (142.8, 295.2) | 2,533 (1,652-3,413) |
| N. America (Mexico) | 120 (35.9) | 229 (64.9) | 148 (41.6) | 118 (32.9) | 131 (36.4) | 124 (35.8) | 870 (41.3) | 5317.7 (5026.5, 5608.8) | 61,494 (58,127-64,860) |
| Oceania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 (0.0, 19.7) | 123*(0-319) |
| South America | 12 (3.6) | 7 (2.0) | 8 (2.2) | 14 (3.9) | 6 (1.7) | 11 (3.2) | 58 (2.8) | 346.7 (251.3,442.1) | 4,009 (2,907-5,112) |
| Total | 334 | 353 | 356 | 359 | 360 | 346 | 2,108 | 13,007 | 150,537 |
Ratio of Oceania deferrals to Oceania malaria travel assumed to be equal to ratio of overall deferrals to overall malaria travel. Thus, projected Oceania travel deferred donors is this fraction of Oceania travelers, instead of 0.
Risk for malaria infection in US travelers
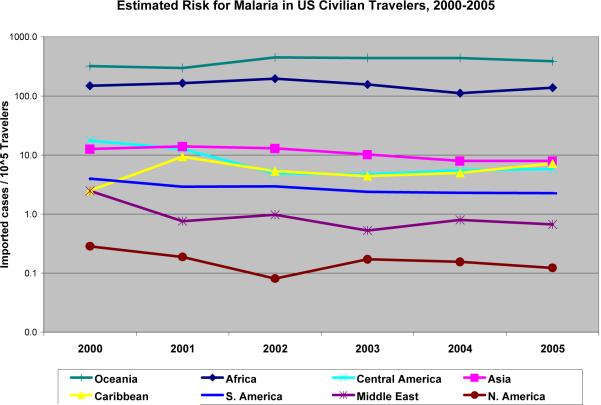
The CDC reported area of travel for 856 of 870 cases of malaria diagnosed in US civilians for 2005.12 Adjusting for potential exposure to malaria, we estimate aggregate risk for malaria infection in U.S. travelers to be 10.04 per 105 travelers (Table 3), with the risk varying widely across geographic areas. Compared to travel to malarious parts of Mexico, travel to Africa represents an 1,100-fold higher risk for malaria illness. Figure 1 shows considerable stability in both the absolute risks conferred by each region and the magnitude of differences between them.
Table 3.
Imported malaria in U.S. civilian travelers to different regions and risk for pre-symptomatic infection up to one-year post-return, 2005
| U.S. civilian travelers to endemic countries | Estimated %of travelers visiting areas endemic for malaria | US civilian travelers to deferrable areas of endemic countries | Imported malaria cases | Rate of Malaria Infection per 105 U.S. civilian travelers to deferrable areas of endemic countries | Pre-symptomatic malaria prevalence per 105 U.S. travelers to each region up to 1 year post-return | Relative risk to acquire malaria infection | Relative risk for malaria infection 12 months post-return | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| return | 30 days | 90 days | 180 days | 1 year | ||||||||
| AFRICA | 556,170 | 80% | 444,936 | 611 | 137.32 | 119.36 | 18.94 | 7.97 | 3.552 | 0.911 | 1116 | 309 |
| ASIA | 2,465,981 | 50% | 1,232,991 | 98 | 7.95 | 7.13 | 3.56 | 2.02 | 1.011 | 0.189 | 65 | 64 |
| CARIBBEAN | 1,063,981 | 50% | 531,991 | 38 | 7.14 | 6.17 | 0.68 | 0.22 | 0.105 | 0.030 | 58 | 10 |
| C. AMERICA | 1,756,000 | 50% | 878,000 | 51 | 5.81 | 5.21 | 2.68 | 1.52 | 0.767 | 0.142 | 47 | 48 |
| MIDDLE EAST | 598,383 | 50% | 299,192 | 2 | 0.67 | 0.59 | 0.20 | 0.11 | 0.052 | 0.010 | 5 | 3 |
| MEXICO | 20,325,000 | 20% | 4,065,000 | 5 | 0.12 | 0.11 | 0.06 | 0.03 | 0.016 | 0.003 | 1 | 1 |
| OCEANIA | 7,755 | 90% | 6,980 | 27 | 386.85 | 346.62 | 173.12 | 98.27 | 49.350 | 9.118 | 3145 | 3090 |
| S. AMERICA | 2,125,713 | 50% | 1,062,857 | 24 | 2.26 | 2.02 | 0.93 | 0.52 | 0.263 | 0.049 | 18 | 17 |
| Total | 28,898,983 | 8,521,945 | 856 | 10.04* | 8.79 | 2.11 | 1.04 | 0.50 | 0.11 | |||
Shaded cells on bottom row reflect weighted average of prevalence based on region-specific travel patterns and risks.
Figure 1.
Recent Estimates of Malaria Risk for U.S. Civilian Travelers, by Geographic Region
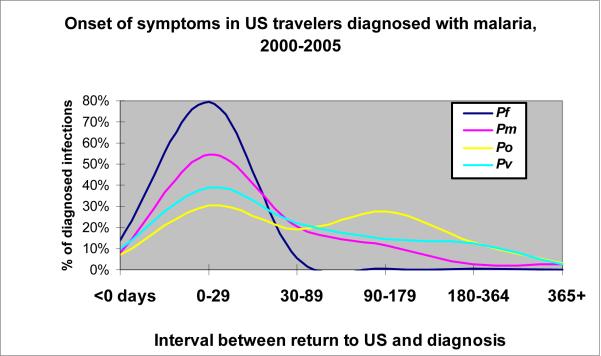
The likelihood that a traveler might have undiscovered (i.e., asymptomatic) malaria infection at any point following return to the U.S. varies directly with his or her risk for acquiring infection and inversely with the interval following return. As shown in Figure 2 (supporting data in the Appendix), U.S. residents with P. falciparum infections develop symptoms quickly, with > 90% having symptom onset within a month and only 1% becoming symptomatic > 3 months post-return. By contrast, the other 3 species are associated with a more delayed onset of symptoms, with ≈ 3% occurring beyond 12 months following return to the U.S. Based on these data, and the respective share of imported malaria from each region that is due to each species, at the end of 12 months a traveler to malaria-endemic parts of Africa has an estimated risk for malaria infection of 0.91 per 105 travelers, or 1 per 109,000 travelers. By contrast, a traveler to Mexico has an estimated risk for malaria infection one year following return of 0.003 per 105, or one per 33 million travelers.
Figure 2.
Distribution of onset in imported malaria cases in US residents, 2000-2005, by Plasmodium species. Pf = Plasmodium falciparum, Pv = P. vivax, Pm = P. malariae, Po = P. ovale.
Residual Risk for Malaria Infection in Blood Donors and Infections Interdicted by Donor Deferral
We would expect an estimated 13.45 malaria infections per year from 150,537 people with the same travel history as our malaria travel deferral cohort (Table 4a). Based on actual intervals of return behavior by region, an estimated >11 infections would have onset of symptoms preceding the individual's presentation to donate, with the deferral requirement itself interdicting the collection of blood from 2 donors with pre-symptomatic infection. Under current policy, we estimate that an aggregate 0.16 donors might still harbor pre-symptomatic infection 12 months following return to the U.S., constituting a residual risk for collecting one parasitemic unit of blood every 6 years. Virtually all of this residual risk attaches to Africa, Asia, and Central America, either due to a high risk for malaria infection associated with travel there or to receiving large numbers of travelers. For Mexico, our cohort of >61,000 deferred donors would be expected to acquire 0.08 infections annually, with residual risk virtually nil (0.002, or one per 500 years) after twelve months.
Table 4a.
Sensitivity Analysis -- Infections interdicted by deferral requirements and residual risk using best estimates of malaria risk in travelers
| 12 month deferral | 3 month deferral | No deferral | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malaria rate per 105 travelers | Estimated Infections | Deferred donations | Interdicted infections* | Residual risk† | Deferred donations* | Interdicted infections* | Residual risk† | Increase in risk (infected donors accepted /yr)‡ | Deferred donations* | Interdicted infections* | Residual risk† | Increase in risk (infected donors accepted /yr)‡ | |
| AFRICA | 137.32 | 7.55 | 5,500 | 0.79 | 0.05 | 1,586 | 0.65 | 0.44 | 0.388 | 0 | 0 | 6.56 | 6.515 |
| ASIA | 7.95 | 1.95 | 24,524 | 0.43 | 0.05 | 8,087 | 0.28 | 0.49 | 0.447 | 0 | 0 | 1.75 | 1.701 |
| CARIBBEAN | 7.14 | 1.35 | 18,859 | 0.12 | 0.01 | 6,748 | 0.10 | 0.04 | 0.035 | 0 | 0 | 1.16 | 1.158 |
| C. AMERICA | 5.81 | 1.95 | 33,494 | 0.52 | 0.05 | 12,932 | 0.36 | 0.51 | 0.461 | 0 | 0 | 1.75 | 1.698 |
| MID-EAST | 0.67 | 0.02 | 2,533 | 0.00 | 0.00 | 552 | 0.00 | 0.00 | 0.003 | 0 | 0 | 0.01 | 0.015 |
| MEXICO | 0.12 | 0.08 | 61,494 | 0.02 | 0.00 | 28,098 | 0.02 | 0.02 | 0.018 | 0 | 0 | 0.07 | 0.066 |
| OCEANIA | 386.85 | 0.48 | 123 | 0.13 | 0.01 | 49 | 0.09 | 0.12 | 0.110 | 0 | 0 | 0.43 | 0.415 |
| S. AMERICA | 2.26 | 0.09 | 4,009 | 0.02 | 0.00 | 1,185 | 0.01 | 0.02 | 0.019 | 0 | 0 | 0.08 | 0.079 |
| Total | 13.45 § | 150,537 | 2.02 | 0.16 | 59,235 | 1.51 | 1.65 | 1.480 | 0 | 0 | 11.81 | 11.647 | |
Calculations based on actual intervals between donor return and donor presentation to donate
Calculations assume all donors present to donate on first day of eligibility
Increase in risk compared to existing 12-month deferral
We estimate that 11 of these infections would manifest clinically prior to presentation to donate.
Were the deferral length hypothetically shortened from 12 to 3 months, estimated residual risk increases ten-fold to 1.65 parasitemic donors who might qualify annually to donate. Assuming no changes in return patterns, there are 59,235 donors whose presentation occurs within this 3-month period and, thus, are still deferred, meaning an additional >90,000 donors are newly qualified to donate. Mexico alone adds 33,396 donors with marginal risk of 0.018 (one infected unit per 57 years). Imputing an average 1.7 donations per year to these donors implies the potential for gain of >56,700 units annually with vanishingly small incremental risk. Removing the deferral altogether increases estimated risk to unacceptable levels for many regions, but for Mexico and South America (0.07 and 0.08 malaria-infected donors accepted per year) the residual risk barely surpasses that for Africa (0.05 infected donors) under existing guidelines.
Sensitivity Analysis
Assuming that the true risk for malaria infection amongst U.S. travelers is lower (Table 4b) or higher (Table 4c) than the estimates shown in Table 3 does not dramatically alter the relative risks across the different regions. The range in risks for Mexico under a 3-month deferral period is an additional contaminated unit every 29 to 114 years, and for no deferral at all ranges from 7.6 to 30.3 years, with attendant gains annually of >33,000 and >61,000 donors, respectively.
Table 4b.
Sensitivity Analysis -- Infections interdicted by deferral requirements and residual risk using lower estimates of malaria risk in travelers
| 12 month deferral | 3 month deferral | No deferral | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malaria rate per 105 travelers | Estimated Infections | Deferred donations | Interdicted infections* | Residual risk† | Deferred donations* | Interdicted infections* | Residual risk† | Increase in risk (infected donors accepted /yr)‡ | Deferred donations* | Interdicted infections* | Residual risk‡ | Increase in risk (infected donors accepted /yr)‡ | |
| AFRICA | 122.06§ | 6.72 | 5,502 | 0.70 | 0.04 | 1,586 | 0.58 | 0.39 | 0.345 | 0 | 0 | 5.84 | 5.793 |
| ASIA | 5.30 | 1.30 | 24,532 | 0.29 | 0.03 | 8,089 | 0.19 | 0.33 | 0.298 | 0 | 0 | 1.17 | 1.134 |
| CARIBBEAN | 4.76 | 0.90 | 18,865 | 0.08 | 0.00 | 6,750 | 0.07 | 0.03 | 0.023 | 0 | 0 | 0.78 | 0.772 |
| C. AMERICA | 3.87 | 1.30 | 33,505 | 0.35 | 0.03 | 12,936 | 0.24 | 0.34 | 0.308 | 0 | 0 | 1.16 | 1.133 |
| MID-EAST | 0.45 | 0.01 | 2,533 | 0.00 | 0.00 | 552 | 0.00 | 0.00 | 0.002 | 0 | 0 | 0.01 | 0.010 |
| MEXICO | 0.06 | 0.04 | 61,513 | 0.01 | 0.00 | 28,107 | 0.01 | 0.01 | 0.009 | 0 | 0 | 0.03 | 0.033 |
| OCEANIA | 366.49 | 0.28 | 76 | 0.07 | 0.01 | 30 | 0.05 | 0.07 | 0.064 | 0 | 0 | 0.25 | 0.243 |
| S. AMERICA | 1.51 | 0.06 | 4,011 | 0.01 | 0.00 | 1,186 | 0.01 | 0.01 | 0.013 | 0 | 0 | 0.05 | 0.053 |
| Total | 10.60 | 150,537 | 1.51 | 0.12 | 59,235 | 1.14 | 1.18 | 1.062 | 0 | 0 | 9.29 | 9.171 | |
Calculations based on actual intervals between donor return and donor presentation to donate
Calculations assume all donors present to donate on first day of eligibility
Increase in risk compared to existing 12-month deferral
Lower rates of malaria are derived by assuming higher proportions of U.S. travelers to each region visit malaria-endemic areas: Africa (90%), Oceania (95%), Mexico (40%), all other regions (75%)
Table 4c.
Sensitivity Analysis -- Infections interdicted by deferral requirements and residual risk using higher estimates of malaria risk in travelers
| 12 month deferral | 3 month deferral | No deferral | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malaria rate per 105 travelers | Estimated Infections | Deferred donations | Interdicted infections* | Residual risk† | Deferred donations* | Interdicted infections* | Residual risk† | Increase in risk (infected donors accepted /yr)‡ | Deferred donations* | Interdicted infections* | Residual risk† | Increase in risk (infected donors accepted /yr)‡ | |
| AFRICA | 156.94§ | 8.63 | 5,496 | 0.90 | 0.05 | 1,585 | 0.74 | 0.50 | 0.444 | 0 | 0 | 7.50 | 7.440 |
| ASIA | 15.90 | 3.90 | 24,508 | 0.87 | 0.09 | 8,081 | 0.56 | 0.99 | 0.894 | 0 | 0 | 3.49 | 3.400 |
| CARIBBEAN | 14.29 | 2.69 | 18,847 | 0.24 | 0.01 | 6,743 | 0.21 | 0.08 | 0.070 | 0 | 0 | 2.33 | 2.315 |
| C. AMERICA | 11.62 | 3.89 | 33,472 | 1.04 | 0.10 | 12,923 | 0.72 | 1.02 | 0.922 | 0 | 0 | 3.49 | 3.394 |
| MID-EAST | 1.34 | 0.03 | 2,531 | 0.01 | 0.00 | 551 | 0.00 | 0.01 | 0.005 | 0 | 0 | 0.03 | 0.029 |
| MEXICO | 0.25 | 0.15 | 61,452 | 0.04 | 0.00 | 28,079 | 0.03 | 0.04 | 0.035 | 0 | 0 | 0.14 | 0.132 |
| OCEANIA | 409.60 | 0.92 | 224 | 0.24 | 0.02 | 88 | 0.17 | 0.23 | 0.211 | 0 | 0 | 0.82 | 0.801 |
| S. AMERICA | 4.52 | 0.18 | 4,007 | 0.04 | 0.00 | 1,185 | 0.02 | 0.04 | 0.038 | 0 | 0 | 0.16 | 0.158 |
| Total | 20.39 | 150,537 | 3.36 | 0.28 | 59,235 | 2.46 | 2.90 | 2.618 | 0 | 0 | 17.95 | 17.668 | |
Calculations based on actual intervals between donor return and donor presentation to donate
Calculations assume all donors present to donate on first day of eligibility
Increase in risk compared to existing 12-month deferral
Higher rates of malaria are derived by assuming lower proportions of U.S. travelers to each region visit malaria-endemic areas: Africa (70%), Oceania (85%), Mexico (10%), all other regions (25%).
DISCUSSION
Given the challenges to developing a screening assay suitable for FDA licensure, prevention of TTM in the U.S. will continue to rely on exclusion of donors who present risk for harboring malaria parasites. With both an increasing demand for blood11,16 and shrinking pool of eligible donors,10 reason compels the industry to seek balance in efforts to enhance both blood safety and blood availability.
While this analysis has focused on the risk for malaria in U.S. civilian travelers who present to donate, data from the U.S. and elsewhere clearly show that the risk for TTM lies primarily with those who have a history of long-term residency in malaria-endemic areas. Recent reviews of TTM in the U.S. implicate only 1 routine U.S. civilian traveler in 31 TTM cases occurring since 1980 where an infected donor was identified.3,4 The other 30 either grew up in malarious areas or were U.S-born residents with long-term tenure overseas. The predominance of foreign-born residents also prevails in recent TTM cases in England17 and Canada.18 Typically, the implicated donor grew up or lived extensively in Africa, and in some cases met existing deferral guidelines. The salient factor in this regard is that an individual who has lived under repeated exposure to malaria parasites will typically, over time, develop sufficient immune response that clinical, but not sterilizing immunity is established.19 That is, he or she might feel and appear healthy enough to donate blood, even with frank Plasmodium parasitemia. Individuals with such a history of malaria can harbor malaria parasites for years, and represent a different population than that of (presumably naïve) U.S. civilian travelers whose incubation periods are shown in Figure 2. Acknowledgment of the risk from this population stimulated recent changes in France, where blood donors born or raised in endemic countries now require serological testing regardless of the interval since their last potential exposure to malaria.20
The important role of these “semi-immune” donors is one of three patterns evident in TTM cases in the U.S.3,4 Another is that an estimated 60 to 70% of cases represent a failure of the screening process. That is, the donor should have been deferred under existing requirements at the time, but was mistakenly allowed to donate. A final pattern is the disproportionate role of P. malariae in TTM in the U.S., especially among donors who met existing deferral requirements. Despite representing only 4% of single-species infections of imported malaria among U.S. civilians in recent years, P. malariae accounts for two-thirds of TTM cases where the donor was properly qualified for donation. Put another way, 25% of cases of TTM in the U.S. can be attributed to a Plasmodium species known for its indolent nature and asymptomatic carriage that can last decades.21 The biological constraints posed by partial clinical immunity to malaria and by silent carriage of less-virulent Plasmodium parasites will continue to pose a risk for TTM unless or until the U.S. adds serologic, antigenic, or nucleic acid detection assays to its arsenal of infectious disease screening tests. Some countries have implemented serological tests for those with travel or residence risk as a means to shorten the deferral period,20,22,23 and the FDA has sought guidance on whether selective screening in the U.S. might shorten the deferral period without compromising safety.24
Our analysis provides valuable input to the discussion of how blood availability might be enhanced without undermining blood safety. Combining data on risks for malaria with the relevant incubation periods in U.S. civilian travelers with malaria illness, we developed well-informed estimates of the risks incurred by shortening the deferral period for presenting blood donors with routine malaria travel risk. For donors with travel to Mexico, we conclude that >56,000 donations per year might be collected at the marginal added risk of one parasitemic donor accepted from every 29 to 114 years under a 3-month deferral period. Abolishing the deferral entirely for travel to Mexico allows for recovery of >100,000 donations annually at risk of an additional parasitemic unit of blood collected every 7 to 30 years. To ensure that any changes in guidelines be at the minimum risk-neutral, the incremental risk associated with relaxation of deferrals for travel to low-risk areas could be more than offset by permanently deferring donors who report a previous history of malaria infection, which would have very small incremental donor loss.15,20 Although the FDA has suggested a donor “re-entry” scheme contingent on negative serological results 4 months after return to the U.S., 24 our data indicate that by that point in time risk for latent falciparum malaria is virtually nil and that for vivax malaria is reduced by > 70%. Where the underlying risk for malaria acquisition is exceptionally small, as in Mexico, serology-contingent re-entry adds very little value in terms of risk protection, while conferring attendant downsides of higher costs, greater complexity, and donor loss from false positive results.
Relaxing the deferral period for Mexico, without the requirement of negative serology, would bring the deferral criteria into balance with known risks and benefits. Data from U.S. travelers over recent (Figure 1) and longer time horizons clearly show that travel risks for malaria are most strongly associated with trips to Sub-Saharan Africa. Recent evidence from the U.S. and elsewhere overwhelmingly implicate semi-immune donors of African origin in the great majority of cases of TTM.3,4,17,18,25,26 Yet, our data clearly show that while travelers to Mexico incur a risk for malaria infection three orders of magnitude lower than those to Africa, they represent > 10 times as many deferrals.
Triage of malaria risk across different destinations has been adopted by travel medicine physicians, some of whom now tailor their recommendations for malaria chemoprophylaxis according to the degree of risk inherent in travel to a given region.27 In areas of lowest malaria endemicity, the risks for side effects and serious adverse reactions from prophylactic antimalarials can outweigh the risk for malaria illness and --especially for vivax malaria-- fatal malaria infections.28 To be sure, recommendations might be strengthened to accommodate lengthy travel periods and in any case should be tailored to an individual's specific plans. 29 The geographic disparities in malaria risk are further highlighted by the fact that travelers to several regions -including the Caribbean and parts of South and Southeast Asia—are more likely to report fever due to dengue infection than due to malaria.30,31 Even so, not all public health viewpoints consider the absence of recommendations for travel chemoprophylaxis an indication that deferral is unwarranted.4 We believe this manuscript provides evidence to support a donor deferral policy that relaxes the current twelve-month deferral period for non-immune travelers to some locales where risk for infection is extremely low.
This study is subject to some potential limitations. First, the 6 REDS-II blood centers might not be representative of US blood centers overall in terms of donor travel patterns. We believe, however, that the degree of geographic and demographic diversity across the six centers provides a reasonable basis for extrapolating to U.S. donors overall. That the results of both numbers of deferrals and geographic destinations varied significantly across the centers supports this conclusion. In any case, since blood centers do not routinely collect and report data on travel destinations of deferred donors, our data represent the best effort to systematically estimate risk from a cohort of donors deferred for travel to malarious areas. Our risk calculations include only those travelers who visited countries endemic for malaria, an approach that that is more rigorous than other analyses that also include visits to non-endemic countries when estimating travel risk to different regions.32, 33 Moreover, concerns about sample representativeness are somewhat mitigated in that the main driver of residual risk estimates is the 1000-fold difference in traveler risk between the least- and most-risky destinations. Though the countrywide estimates of malaria travel deferrals or the distribution across destination regions might vary somewhat under different sampling methods, any such differences would be unlikely to undermine the basic conclusions of this manuscript.
Second, our analysis does not take into account possible changes in the presenting donor population under new deferral requirements. We limited our risk estimates to those donors who are deferred from donating under the existing rules, assuming a static population of deferred donors for the purpose of estimating the risk that this group might pose at 0, 3 or 12 months following return to the U.S. Unless the risk for malaria is associated with an individual's decision to attempt to donate, one might expect increase in risk to increase in a linear fashion with the size of the presenting donor population.
A third potential limitation is that historical data might not reflect future patterns of malaria risk, such that our estimates of risk for TTM from donors with travel to different regions might not hold going forward. Figure 1, however, shows a significant amount of stability in both the absolute and relative risks of acquiring malaria by U.S. civilian travelers in recent years, and there is no reason to a priori assume that changes in the future epidemiology of malaria will represent dramatic departures rather than typical variation. In any case, the rapid public health response following a recent malaria outbreak in the Bahamas34 indicates the ability to adequately address future contingencies.
Finally, our risk estimates in U.S. travelers would be strengthened were we to know with certainty the proportion of travelers to each country that visited areas with malaria risk. Outside of Sub-Saharan Africa, few countries (East Timor, Vanuatu, and French Guiana) are deemed endemic throughout their national boundaries. For Africa and Oceania, the CDC Yellow Book presents sufficient detail to conclude that a large majority of travel to each region would incur risk and, thus, trigger a travel deferral for presenting donors. For Mexico, Yellow Book information was supplemented by one participating blood center's (NEARC) review of Blood Donation Records (BDRs), performed to identify non-deferrable travel to Mexico; this review found that ~ 20% of donors reporting travel to Mexico within the prior 3 years visited endemic areas. For the remaining regions, we assumed that an estimate of 50% of travelers incurring risk for malaria was reasonable. Even if these assumptions are not entirely accurate, it is noteworthy that the sensitivity analysis in Tables 4b and 4c demonstrates that the absolute and relative risks do not vary dramatically under altered assumptions.
Uncertainty in the travel risk estimates also exists to the extent that CDC surveillance data are less than 100% sensitive for malaria infections diagnosed in the U.S. One recent paper estimates that the national malaria surveillance system --from which the data in this manuscript is derived-- might under-report actual malaria cases by 25%.35 Whether the under-reporting identified is associated with geographic location is unclear, but it is likely that estimated risks for one or more locations would be slightly higher with a surveillance system that detects all cases. Counterbalancing this effect is a conservative assumption embedded in the risk estimates presented here: That all donors present to donate on the first day of eligibility under current and alternate deferral requirements. For example, of the deferred donors who would be recovered by shortening the deferral period from 12 to 3 months, their risk for asymptomatically harboring malaria parasites was estimated as if all donors would present on day 91, the first day of eligibility. Because the risk for asymptomatic parasitemia diminishes with time, using the “worst-case” assumption rather than actual interval between return to the U.S. and presentation to donate inflates the risk to a “theoretical maximum” under a three-month deferral period. This conservative assumption likely exerts a powerful effect, perhaps best reflected in the gap between estimated residual risk under current deferral requirements for donors with malaria travel (1 infection per 6 years) and empirical observation (1 case of TTM due to routine travelers in the last >25 years).
In summary, we present the first systematic evaluation of the relative impact and estimated benefit (in terms of infectious units interdicted) of malaria travel deferral guidelines currently in use in the United States. We believe this analysis demonstrates that serious consideration should be given to modifying the current requirements, in that the adverse impact (i.e., donor loss) falls most heavily on those donors with the lowest risk for malaria infection. Assuming that a donor reports no history of malaria illness and no history of long-term residence in malaria-endemic areas, we think that many travel-deferred donors could be safely accepted on a shorter timetable than the current 12-month period. Specifically, we would urge that travelers to malarious parts of Mexico be accepted for donation at an interval no longer than three months following return to the U.S.
While the low and declining rate of TTM in the United States is indeed something to celebrate, this phenomenon has been accompanied by significant impact on blood availability. Whether the success in recent decades is due to or independent of the deferral requirements for routine U.S. travelers has been subject to little scrutiny. Our analysis strongly supports the position that deferral requirements could be relaxed for at least Mexico, significantly enhancing blood availability with de minimis increase in risk.
ACKNOWLEDGEMENTS
The authors thank the staff at all six participating blood centers. Without their help, this study would not have been possible. The Retrovirus Epidemiology Donor Study (REDS)- II is presently the responsibility of the following persons: Blood Centers: 1) American Red Cross Blood Services, New England Region: R. Cable, J. Rios, R. Benjamin; 2) American Red Cross Blood Services, Southern Region/Emory University: C.D. Hillyer, K.L. Hillyer, J.D. Roback; 3) Blood Center of Wisconsin: J. Gottschall, A.E. Mast; 4) Hoxworth Blood Center, University of Cincinnati Academic Health Center: R.A. Sacher, S.L. Wilkinson, P.M. Carey; 5) Regents of the University of California/Blood centers of the Pacific/BSRI: E.L. Murphy, M.P. Busch, B. Custer; 6) The Institute for Transfusion Medicine (ITxM)/LifeSource Blood Services: D. Triulzi, R. Kakaiya, J. Kiss; Central Laboratory: Blood Systems Research Institute: M.P. Busch, P. Norris; Coordinating Center: Westat, Inc.: J Shulman, M. King; National Heart, Lung, and Blood Institute, NIH: G.J. Nemo, S. Glynn; Steering Committee Chairman: R.Y. Dodd. The authors state that they have no conflicts of interest.
Financial support: This study was supported by contracts N01HB47168; N01HB47169; N01HB47170; N01HB47171; N01HB47172; N01HB47174; N01HB47175; N01HB57181 from the National Heart, Lung, and Blood Institute.
Appendix: Data for computing pre-symptomatic malaria infection in U.S. travelers (Table 3) and residual risk in donors (Tables 4a-4c) at different times following travel to various geographic regions
The estimates for pre-symptomatic infection in U.S. travelers and for residual risk in Tables 4a-4c are derived from 1) the incubation periods for imported malaria in US civilians, by Plasmodium species, 2) the relative contribution of each Plasmodium species to the cases of imported malaria originating in each geographic region, and, 3) the estimated number of malaria infections expected from a cohort of travelers to each region that is the size of the estimated number of donors deferred for travel to that region. A weighted measure is developed for each region to estimate the probability that a parasitemic traveler from that region has an infection that has yet to become symptomatic by 1 month, 3 months, 6 months, and 12 months post-return to the United States. The data for interval between return to U.S. and onset of symptoms, for single-species Plasmodium infection (multiple infections and infections of unknown species are excluded) in US civilian travelers, are shown here*:
| Interval | P. falciparum | P. malariae | P. ovale | P. vivax | Total |
| < 0 Days | 14.0% | 8.0% | 7.2% | 9.9% | 12.7% |
| 0 to 29 days | 79.7% | 54.5% | 30.4% | 39.0% | 69.1% |
| 30 to 89 days | 5.4% | 20.5% | 18.8% | 21.8% | 9.6% |
| 90 to 179 days | 0.5% | 11.6% | 27.5% | 14.3% | 4.4% |
| 180 to 364 days | 0.3% | 2.7% | 13.0% | 12.3% | 3.2% |
| > 365 days | 0.2% | 2.7% | 2.9% | 2.7% | 0.9% |
Thus, a traveler who returns to the U.S. infected with P. vivax, for example, is estimated to have 2.7% probability of remaining asymptomatically parasitemic for a full year in calculating residual risk. The risk that a traveler or presenting donor might be parasitemic at a given point in time takes account of the differences in incubation periods by species and the proportion of each species responsible for imported malaria from each region, shown below*:
| Region | N | % due to P. f | % due to P. m | % due to P. o | % due to P. v |
| Africa | 3004 | 83% | 5% | 4% | 9% |
| Asia | 482 | 13% | 4% | 2% | 81% |
| Caribbean | 134 | 93% | 1% | 1% | 5% |
| Central America | 251 | 10% | 3% | 1% | 85% |
| Middle East | 12 | 50% | 0% | 8% | 42% |
| Mexico | 33 | 12% | 3% | 0% | 85% |
| Oceania | 121 | 14% | 2% | 2% | 81% |
| South America | 136 | 21% | 3% | 1% | 75% |
| Weighted totals | 66% | 4% | 3% | 27% | |
We estimate residual risk of a parasitemic donor at T, time interval following return to U.S. from any given region as follows:
The estimate for residual risk (under existing guidelines) for malaria infection in a donor who traveled to Africa and presents on Day 366 following return, is shown below. These estimates assume that travelers to Africa who present to donate have risk for acquiring malaria equivalent to overall US travelers to Africa, such that out of a cohort of 5,500 travelers to Africa we expect 7.55 infections annually.
Data provided courtesy of Dr. Paul Arguin of the Centers for Disease Control, reporting the data in Tables 4 and 5 of 6 years of malaria surveillance summaries by US Civilian vs. Foreign Civilian status.
REFERENCES
- 1.Office of Travel and Tourism Industries, United States Department of Commerce . U.S. resident travel abroad historical visitation - outbound, 1995-2005. Washington, D.C.: Available at http://tinet.ita.doc.gov/view/f-2005-11-001/index.html. [Google Scholar]
- 2.World Tourism Organization . Yearbook of Tourism Statistics: Data 2001-2005. WTO; Madrid, Spain: 2007. [Google Scholar]
- 3.Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–8. doi: 10.1056/NEJM200106283442603. [DOI] [PubMed] [Google Scholar]
- 4.Parise ME. Traveler's malaria, locally-transmitted malaria and transfusion-transmitted malaria in the United States [monograph on the Internet] U.S. Food and Drug Administration, Center for Biologics Evaluation and Research (CBER), Food and Drug Administration; Rockville (MD): 2006. Available at http://www.fda.gov/cber/blood/malaria071206mp.htm. [Google Scholar]
- 5.Zoon K. Memorandum to all registered blood establishments. Dept. of Health and Human Services, Food and Drug Administration; Jul 26, 1994. Recommendation for deferral of donors for malaria risk. [Google Scholar]
- 6.Standards for blood banks and transfusion services. 25th ed. AABB; Bethesda, MD: 2008. [PubMed] [Google Scholar]
- 7.Stramer SL. Issues with Malaria Screening in the US [monograph on the Internet] U.S. Food and Drug Administration, Center for Biologics Evaluation and Research (CBER), Food and Drug Administration; Rockville (MD): 2006. Available at http://www.fda.gov/cber/blood/malaria071206ss.htm. [Google Scholar]
- 8.Spencer BR, Custer B, Kakaiya RM, Hillyer KL, Wilkinson SL, Gottschall JL, Steele WR. Low risk for malaria transmission from presenting blood donors excluded for travel to Mexico [abstract] Transfusion. 2006;46 Suppl:27A. [Google Scholar]
- 9.Custer B, Chinn A, Hirschler NV, Busch MP, Murphy EL. The consequences of temporary deferral on future whole blood donation. Transfusion. 2007;47:1514–23. doi: 10.1111/j.1537-2995.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 10.Riley W, Schwei M, McCullough J. The United States' potential blood donor pool: estimating the prevalence of donor-exclusion factors on the pool of potential donors. Transfusion. 2007;47:1180–88. doi: 10.1111/j.1537-2995.2007.01252.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitaker BI, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. Department of Health and Human Services; Washington (DC): 2006. [Google Scholar]
- 12.Thwing J, Skarbinski J, Newman RD, Barber AM, Mali S, Roberts JM, Slutsker L, Arguin PM. Malaria surveillance - United States, 2005. MMWR. 2007;56:23–40. [PubMed] [Google Scholar]
- 13.Office of Travel and Tourism Industries, United States Department of Commerce . Survey of International Air Travelers (In-Flight Survey) program. Washington, D.C.: Available at http://tinet.ita.doc.gov/research/programs/ifs/description.html. [Google Scholar]
- 14.Centers for Disease Control . Health information for international travel, 2005-2006. US Department of Health and Human Services, Public Health Service, CDC; Atlanta, GA: 2005. [Google Scholar]
- 15.Leiby DA, Nguyen ML, Notari EP. Impact of donor deferrals for malaria on blood availability in the United States. Transfusion. 2008;48:2222–28. doi: 10.1111/j.1537-2995.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan M, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 17.Kitchen AD, Barbara JAJ, Hewitt PE. Documented cases of post-transfusion malaria occurring in England: a review in relation to current and proposed donor-selection guidelines. Vox Sang. 2005;89:77–80. doi: 10.1111/j.1423-0410.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 18.Slinger R, Giulivi A, Bodie-Collins M, Hindieh F, John RS, Sher G, Goldman M, Ricketts M, Kain KC. Transfusion-transmitted malaria in Canada. Canadian Medical Assoc J. 2001;164:377–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Schofield L, Mueller I. Clinical immunity to malaria. Curr Molecular Med. 2006;6:205–21. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 20.Garraud O, Assal A, Pelletier B, Danic B, Kerleguer A, David B, Joussemet M, de Micco P. Overview of revised measures to prevent malaria transmission by blood transfusion in France. Vox Sang. 2008;95:226–31. doi: 10.1111/j.1423-0410.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 21.Vinetz JM, Li J, McCutchan TF, Kaslow DC. Plasmodium malariae infection in an asymptomatic 74-year-old Greek woman with splenomegaly. N Engl J Med. 1998;338:367–71. doi: 10.1056/NEJM199802053380605. [DOI] [PubMed] [Google Scholar]
- 22.Kitchen AD, Lowe PHJ, Lalloo K, Chiodini PL. Evaluation of a malarial antibody assay for use in the screening of blood and tissue products for clinical use. Vox Sang. 2004;87:150–5. doi: 10.1111/j.1423-0410.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 23.Seed CR, Kitchen A, Davis TME. The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria. Transfus Med Rev. 2005;19:229–40. doi: 10.1016/j.tmrv.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 24.FDA Issue Summary to Blood Products Advisory Committee Meeting; FDA; Bethesda, MD. September 11, 2008; 2008. Available at http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4379B2_1.htm (accessed September 5, 2008) [Google Scholar]
- 25.Bruneel F, Thellier M, Eloy O, Mazier D, Boulard G, Danis M, Bédos JP. Transfusion-transmitted malaria. Intensive Care Med. 2004;20:1851–2. doi: 10.1007/s00134-004-2366-6. [DOI] [PubMed] [Google Scholar]
- 26.Frey-Wettstein M, Maier A, Markwalder K. Münch U. A case of transfusion transmitted malaria in Switzerland (ltr) Swiss Med Wkly. 2001;131:320. doi: 10.4414/smw.2001.09720. [DOI] [PubMed] [Google Scholar]
- 27.Schlagenhauf P, Petersen E. Malaria chemoprophylaxis: strategies for risk groups. Clin Micriobiology Rev. 2008;21:466–72. doi: 10.1128/CMR.00059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behrens RH, Carroll B, Beran J, Bouchaud O, Hellgren U, Hatz C, Jelinek T, Legros F, Mühlberger N, Myrvang B, Siikamäki H, Visser L. The low and declining risk of malaria in travelers to Latin America: is there still an indication for chemoprophylaxis? Malaria J. 2007;6:114–20. doi: 10.1186/1475-2875-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LH, Wilson ME, Schlagenhauf P. Prevention of malaria in long-term travelers. JAMA. 2006;296:2234–44. doi: 10.1001/jama.296.18.2234. [DOI] [PubMed] [Google Scholar]
- 30.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E. Fever in returned travelers: Results from the GeoSentinel surveillance network. Clin Infect Dis. 2007;44:1560–68. doi: 10.1086/518173. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS. Spectrum of disease and relation to place of exposure among ill returned travelers. NEJM. 2006;354:119–30. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 32.Askling HH, Nilsson J, Tegnell A, Janzon R, Ekdahl K. Malaria risk in travelers. Emerging Infect Dis. 2005;11:436–41. doi: 10.3201/eid1103.040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leder K, Black J, O'Brien D, Greenwood Z, Kain KC, Schwartz E, Brown G, Torresi J. Malaria in travelers: A review of the Geosentinel Surveillance Network. Clin Inf Dis. 2004;39:1104–12. doi: 10.1086/424510. [DOI] [PubMed] [Google Scholar]
- 34.CDC Malaria - Great Exuma, Bahamas, May-June 2006. MMWR. 2006;55:1013–16. [PubMed] [Google Scholar]
- 35.Hwang J, McClintock S, Patrick Kachur S, Slutsker L, Arguin P. Comparison of national malaria surveillance system with the national notifiable diseases surveillance system in the United States. J Publ Hlth Management Pract. doi: 10.1097/PHH.0b013e31819d816a. in press. [DOI] [PubMed] [Google Scholar]