Abstract
Background
Sudden cardiac death remains a leading cause of mortality despite advances in medical treatment for the prevention of ischemic heart disease and heart failure. Recent studies showed a benefit of ICD implantation, but appropriate shocks for ventricular tachyarrhythmias were only noted in a minority of patients during 4-5 years of follow-up. Accordingly, better risk stratification is needed to optimize patient selection. In this regard microvolt T-wave alternans (TWA) has emerged as a potentially useful measure of arrhythmia vulnerability, but it has not been evaluated previously in a prospective randomized trial of ICD therapy.
Methods and Results
This investigation was a prospective substudy of the SCD-HeFT trial including 490 patients at 37 clinical sites. TWA tests were classified by blinded readers as + (37%), - (22%), or indeterminate (41%) by standard criteria. The composite primary endpoint was the first occurrence of any of the following events: sudden cardiac death, sustained ventricular tachycardia/fibrillation or appropriate ICD discharge. During a median follow-up of 30 months, there were no significant differences in event rates between TWA + or − patients (Hazard ratio 1.24, p=0.56, [CI 0.60, 2.59]), or TWA − and non − (+ and indeterminate) subjects (Hazard ratio 1.28, p=0.46, CI [0.65, 2.53]). Similar results were obtained including or excluding patients randomized to amiodarone in the analyses.
Conclusions
TWA testing did not predict arrhythmic events or mortality in SCD-HeFT, although a small reduction of events (20-25%) among TWA − patients cannot be excluded given the sample size of this study. Accordingly, these results suggest that TWA is not useful to help make clinical decisions regarding ICD therapy among patients with heart failure and left ventricular systolic dysfunction.
Keywords: Defibrillation, Electrocardiography, Heart Failure, T-wave alternans
Sudden cardiac death remains a leading cause of mortality despite advances in medical treatment for the prevention of ischemic heart disease and heart failure. Recent studies of the implantable defibrillator (ICD) showed significant reductions in all cause and sudden death mortality in certain high risk cohorts of patients.1-8 Two studies in particular are most responsible for the dramatic increase in ICD use for primary prevention. Specifically, the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) evaluated patients with symptomatic heart failure (NYHA II-III) and left ventricular systolic dysfunction,6 whereas MADIT II evaluated patients with coronary artery disease and ischemic cardiomyopathy.5 Both studies showed a benefit of ICD implantation, but appropriate shocks for ventricular tachyarrhythmias were only noted in a minority of patients during 4-5 years of follow-up. This suggests that many patients with current indications for ICD implantation may not benefit from this invasive therapy and that better risk stratification is needed to optimize patient selection and the cost effectiveness of this therapy.9-11
Microvolt T-wave alternans (TWA) is a noninvasive test of arrhythmia vulnerability. The results of previous observational studies showed that TWA predicts ICD shocks or arrhythmic events in diverse patient populations,12-17 including those with heart failure and ischemic cardiomyopathy.18-21 This has led to the hypothesis that TWA can be used to identify patients most likely to benefit from ICD implantation.20 However, the value of TWA for risk stratification has not been evaluated previously in a large randomized trial of ICD therapy. Accordingly, the present study was designed to test prospectively the hypothesis that TWA is a clinically important risk stratifier in a subgroup of the SCD-HeFT cohort.
Methods
This investigation was a prospective substudy of the SCD-HeFT trial. In SCD-HeFT, 2,521 subjects were randomized in equal proportions to receive ICD therapy, amiodarone or placebo.6 In the TWA substudy, 490 SCD-HeFT patients were enrolled at 37 clinical sites. All patients gave written informed consent and this study was approved by the Institutional Review Board at each participating site. In addition to the inclusion/exclusion criteria for the main trial, the only other inclusion criteria were the presence of sinus rhythm on the day of evaluation and the ability to walk on a treadmill.22 Clinical sites who participated in the substudy were encouraged to enroll all patients who were enrolled in the main study at that site, with the exception of those with absolute contraindications as noted above. This approach was intended to simulate clinical practice and provide the most accurate assessment of the utility of TWA in this population, avoiding the potential selection bias of excluding patients due to ambient ventricular arrhythmias (i.e. frequent PVC's), deconditioning, or heart failure status.
TWA testing was conducted using submaximal treadmill exercise to achieve a heart rate of 120 bpm for at least 2 minutes.23,24 β-adrenergic blockers were withheld for at least 24 hours before the study, because these agents suppress TWA and increase the number of indeterminate TWA tests due to chronotropic incompetence.25 Careful skin preparation including mild abrasion was performed in order to reduce the skin-electrode impedance. Special high resolution electrodes (High-Res ™, Cambridge Heart, Inc, Bedford MA) were used to minimize noise. Electrocardiographic leads were placed at the standard precordial lead positions (V1-V6) and in an orthogonal X,Y,Z configuration, as described previously.23,24 TWA was measured with the CH2000 system (Cambridge Heart, Inc, Bedford, MA) utilizing a spectral method of analysis (D10 algorithm) designed to allow detection of alternans in the microvolt range of amplitude.
TWA was prospectively defined as positive when it was sustained for at least one minute with an onset heart rate < 110 bpm, alternans amplitude ≥ 1.9 μV, and alternans ratio (signal to noise ratio) ≥ 3 in the vector magnitude lead, any orthogonal lead or 2 consecutive precordial leads (TWA+). TWA was defined as negative if the criteria for a positive test were not met, if there was no significant alternans for one minute while the heart rate was ≥ 105 bpm, and if the tracing was not obscured by noise and had <10% ectopic beats (TWA-). Otherwise, TWA was considered indeterminate.20,24,25 When an indeterminate test was obtained, investigators were instructed to repeat the test the same day when possible. Data were analyzed by 2 experienced readers who were blinded with respect to the clinical data and randomization status of the patient. The arrhythmic or mortality risk of patients with TWA indeterminate results is reported to be similar to TWA positive patients and these groups are often combined for analsyis.24,26 Therefore, comparisons of TWA non-negative (positive or indeterminate) with TWA negative patients were also performed.
Patients were followed clinically as part of SCD-HeFT every 3 months. The composite primary endpoint for this substudy was the first occurrence of any of the following events: sudden cardiac death, sustained ventricular tachycardia/fibrillation or appropriate ICD discharge. Sudden cadiac death was defined as death within one hour of the onset of symptoms or during sleep without any other identified cause. An appropriate ICD discharge was a shock delivered for a persistent ventricular tachyarrhythmia. Of note, ICD pulse generators were uniformly programmed for a single zone (rate cutoff 188 bpm or 320 msec) with prolonged detection (18/24 intervals) to reduce the incidence of shocks for self terminating arrhythmias. The primary hypothesis was that patients who are TWA+ or non-negative are more likely to experience a primary endpoint event than those who are TWA-. The secondary hypothesis was that patients who are TWA + and receive an ICD are more likely to have appropriate ICD discharges than those who are TWA - and receive an ICD. All endpoints, including the classification of death and ICD discharges, were adjudicated by event committees blinded to the results of TWA testing. Moreover, the interpretation of the results of TWA testing was completed and the database closed, while the primary randomization of the main trial was still intact and before any endpoint analyses were performed. For this reason, it was unknown at the time of enrollment if patients randomized to drug therapy were receiving amiodarone or placebo. Since amiodarone is reported to decrease TWA amplitude,27 subjects on this medication were initially excluded from the primary endpoint analyses prospectively. For completeness, however, results are also provided for the entire substudy population, including those randomized to amiodarone.
The sample size required for this study was calculated as follows. Of the enrolled patients it was assumed that 45% of studies would be TWA + and that TWA would have a sensitivity of 70% for sudden death or tachyarrhythmic events (i.e., of the patients who experienced an event, 70% would be TWA +). The annual rate of sudden cardiac death was assumed to be 5%, all patients would be followed for a minimum of 2.5 years after enrollment, and the tachyarrhythmic event rate for triggering a defibrillator discharge in the ICD arm would be twice the underlying sudden death rate. Combining all of these assumptions resulted in the projection of a three-fold increased number of events over 2.5 years in the TWA + group compared to the TWA − group (29% vs. 10%). Given these assumptions, 138 patients with interpretable studies from the ICD and placebo arms combined were required to provide 90% power for detecting a difference of this magnitude for the primary hypothesis. Assuming 20% of patients would have studies with indeterminate results and allowing for 5% withdrawals or loss to follow-up, the total sample size required was 175 patients.
All data are presented as mean ± SD, except where noted. Continuous variables were compared with t-tests or analysis of variance and discrete variables were compared with the conventional chi square test or Fisher's exact test. Event free survival was calculated using the Kaplan-Meier method.28 The significance of differences in event-free survival between groups was assessed using a stratified Cox proportional-hazards regression model,29 stratifying the analysis based on the patients' randomized treatment assignment in the main trial. Relative risks were expressed as hazard ratios with 95% confidence intervals (CI) and were calculated using the Cox model. A p value < 0.05 was considered statistically significant. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The patient population evaluated was typical of those with mild to moderate heart failure, and they were very similar to the overall SCD-HeFT population. Specifically, the cohort in this substudy (n=490) was 76% male with a mean age of 59±12 yrs and left ventricular ejection fraction of 24±7%. Ischemic heart disease was present in 49% of subjects and 71% had NYHA Class II heart failure functional status at the time of enrollment. The baseline QRS duration was 115.6±30.9 msec and 31% of subjects had QRS prolongation (> 120 msec). This cohort was well treated medically with 96% receiving angiotensin converting enzyme inhibitors or angiotensin receptor blockers and 74% were receiving beta adrenergic blockers prior to TWA testing. Diuretics were used by 83% of subjects and 64% were receiving digoxin. Of note, none of these characteristics differed from the larger population randomized in SCD-HeFT. The patients enrolled in this substudy represent 45±22% of all patients enrolled in the main study at these 37 clinical sites. There were 146 patients randomized to amiodarone therapy, 178 randomized to placebo therapy and the remaining 166 subjects were randomized to ICD implantation. There were no statistically significant differences in any clinical characteristics among the therapy assignments.
TWA results were classified as positive in 37% of subjects, negative in 22% and indeterminate in 41%. The indeterminate rate was higher than expected, but independent of randomization assignment. Specifically, 38% of placebo patients, 40% of ICD patients and 45% of amiodarone patients had indeterminate TWA results (p=0.36). Similarly, the frequencies of TWA + or − results were also independent of randomization assignment. During a median follow-up period of 30 months, the primary endpoint was observed in 75 subjects, including 27 subjects with sudden death, 3 with resuscitated cardiac arrest, 40 with sustained VT and 25 with appropriate ICD discharge. Of note, patients could experience more than one tachyarrhythmia event (e.g. VT leading to ICD discharge), but they reached the primary endpoint with the first such episode. During the follow-up period, 81 subjects died from any cause. The distribution of deaths and events comprising the composite endpoint grouped by TWA classification is shown in Table 1.
Table 1.
Distribution of Clinical Events Contributing to Primary and Secondary Endpoints
| TWA Classification | ||||
|---|---|---|---|---|
| Positive | Negative | Indeterminate | Total | |
| Number of Patients | 182 | 135 | 173 | 490 |
| Sudden Cardiac Death | 7% (12) | 4% (6) | 5% (8) | 5% (26) |
| Resuscitated Cardiac Arrest due to Ventricular Arrhythmia | 1% (2) | 0% (0) | <1% (1) | <1% (3) |
| Sustained VT | 8% (14) | 7% (9) | 10% (17) | 8% (40) |
| Appropriate ICD Discharge | 5% (10) | 1% (2) | 8% (13) | 5% (25) |
| All-Cause Death (Cardiac and Non-Cardiac) | 18% (33) | 12% (16) | 19% (32) | 17% (81) |
| Any of: Sudden Death, Resuscitated Cardiac Arrest, Sustained VT, or Appropriate ICD Discharge | 17% (31) | 12% (16) | 16% (28) | 15% (75) |
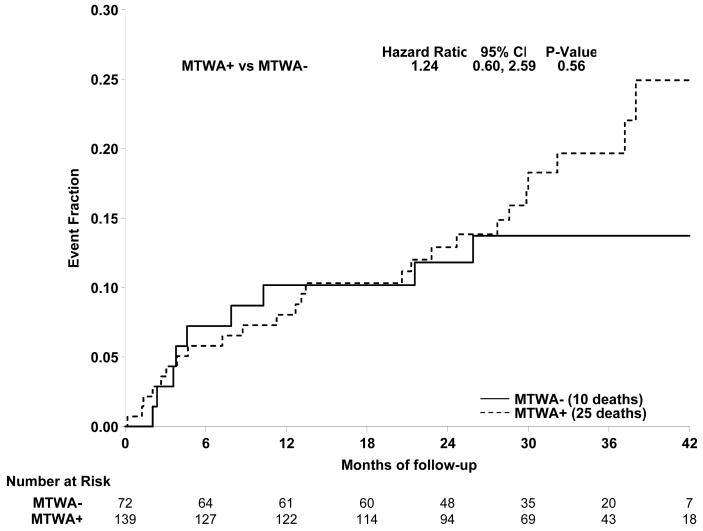
As noted above, the drug randomization of patients was unknown at the time of enrollment. The patients randomized to amiodarone therapy (n=146) were excluded from the initial analysis prospectively. Accordingly, the prespecified primary analysis was restricted to patients randomized to placebo or ICD therapy. These results are shown in Figure 1A; there was no significant difference in event free survival between TWA + and − pts (HR=1.24, p=0.56, 95% CI [0.60, 2.59]). Similarly, event free survival did not differ between TWA − and TWA non − (+ or Indeterminate) subjects (HR=1.28, p=0.46, CI [0.65, 2.53]) (Figure 1B). It is noteworthy that the curves were largely overlapping for at least one year, with TWA − patients having an event rate of 10.2% at 12 months (i.e. 89.8% event free survival).
Figure 1.
A. Comparison of primary event rates for the TWA+ (broken line) and TWA- (solid line) patients among subjects randomized to ICD or Control (primary endpoint of the study). There was no significant difference between event rates for the 2 groups (HR =1.24, p=0.56). B. Comparison of event rates for the TWA non − (broken line) and TWA − (solid line) patients in the ICD and Control groups. Again event rates did not differ significantly for the groups (HR = 1.28, p=0.46).
Several preplanned secondary analyses were performed, including an evaluation of TWA to predict the composite endpoint of appropriate ICD discharge or sudden death in the ICD cohort. Kaplan Meier analysis showed no significant differences between TWA − and TWA non − groups (p=0.64). The event rates at 36 months were 21.7% for TWA- subjects and 25.2% for TWA non − subjects. Analyses of all cause mortality based on TWA results in the placebo, ICD or combined groups also showed no statistically significant differences for any comparisons.
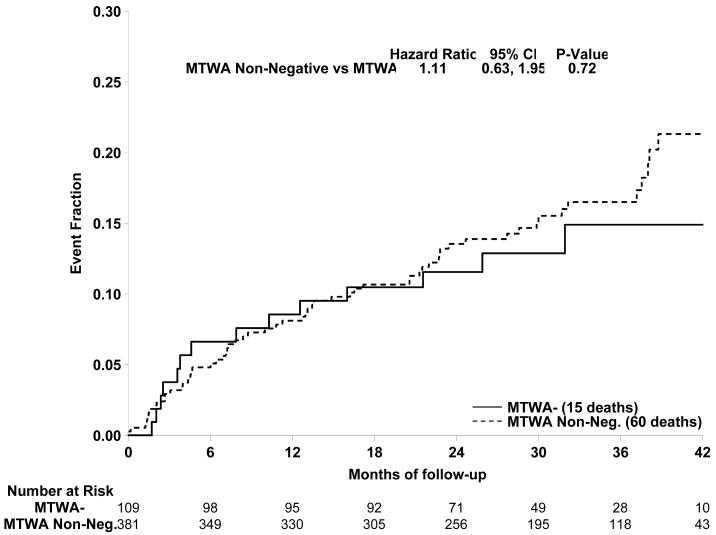
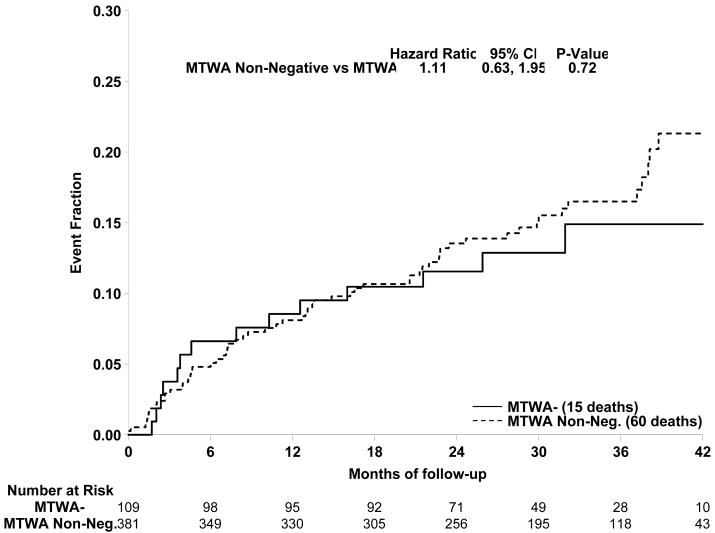
Subjects randomized to amiodarone therapy were excluded from the primary analyses. However, as noted above, the TWA results of these subjects did not differ from those in the placebo or ICD arms of SCD-HeFT. Accordingly, further analyses were performed including the amiodarone group. Results comparing TWA - and TWA non - subjects for the entire cohort (490 patients) are presented in Figure 2. Again, there was no statistical difference in event free survival between groups (HR=1.11, p=0.72, 95% CI [0.63, 1.95]). The event rate at 36 months for the TWA − group was 14.9%, whereas the event rate for the TWA non − group was 16.5%. Analysis of TWA + and − patients, excluding the indeterminate subjects, also showed no significant difference (HR =1.17, p=0.61, CI [0.63, 2.18]).
Figure 2.
Comparison of primary event rates for the TWA non - (broken line) and TWA- (solid line) patients among all 490 subjects in this study. There was no significant difference between event rates for the 2 groups (HR =1.11, p=0.72).
Previous studies reported that TWA is less predictive of arrhythmic events in the presence of QRS prolongation or bundle branch block.19,30 Accordingly, the primary and secondary endpoints were analyzed with subjects with QRS prolongation (≥ 120 msec) excluded. These retrospective analyses again showed no significant predictive value of TWA among subjects with narrow QRS duration. Finally, analyses were performed to assess the predictive value of TWA separately among patients with ischemic and nonischemic etiologies of left ventricular systolic dysfunction. Again, there were no significant predictive values of TWA among subject with either etiology of heart failure. In the ischemic subgroup (n=240) the Hazard Ratio was 0.72 (p=0.41, CI [0.34, 1.55]) comparing TWA − and TWA non - subjects, whereas in the nonischemic subgroup (n=250) the Hazard Ratio was 1.67 (p=0.21, CI [0.71, 3.99]).
Discussion
The present study was the first prospective evaluation of TWA to predict arrhythmia vulnerability in a randomized trial for ICD therapy for prevention of all cause mortality. The primary results of this study showed that TWA was not a useful test to predict arrhythmic events in SCD-HeFT. Specifically, TWA results did not predict life threatening ventricular arrhythmias, appropriate shocks or all cause mortality in this cohort.
Multiple previous studies of different cohorts showed a better predictive value of TWA for arrhythmic events. There are several possible explanations for these discrepant findings. Several previous studies enrolled some low risk patients, such as those undergoing evaluation of supraventricular arrhythmias or those with coronary artery disease and a preserved left ventricular ejection fraction.12,13,17 Such patients have a low risk of arrhythmic events or abnormal TWA results, so this will tend to overestimate the negative predictive value of the test. Other single center studies are subject to selection bias if consecutive patients are not enrolled. In addition, the uncontrolled use of ICD therapy may bias results since ICD shocks are a major component of endpoint events. In the present study, sites were encouraged to enroll all patients in sinus rhythm who could walk on a treadmill and ICD implantation was determined randomly to minimize selection bias. In addition, there was uniform programming of devices with a high heart rate cutoff, (188 bpm), long detection intervals and shock therapy only to minimize therapy for self terminating events. Interestingly, a recent large study also showed a disappointing negative predictive value for TWA among high risk patients with left ventricular systolic dysfunction.31
The rate of indeterminate findings was higher than reported with previous studies of TWA. This occurred despite on-site training of each center with technical support during the initial studies, as well as recommending repeating indeterminate tests the same day when possible. Moreover, less than 10% of indeterminate results were due to technical problems, such as noise or protocol violations. Rather, frequent ectopy or an inadequate heart rate response were the primary reason for the indeterminate studies. The cause(s) of this high indeterminate rate is unclear. The number of centers in this study was much larger than previous studies that were conducted at one or only several highly experienced sites.12-13, 15-23 Moreover, sites were encouraged to enroll all patients, even if frequent ectopy or deconditioning was present that increases the likelihood of an indeterminate study, whereas many previous studies excluded such patients by design to reduce the indeterminate rate. As such, we feel that our results are more representative of the application of this technology to the general heart failure population and to physicians using this test for risk stratification.
In the present study, TWA measurements were performed after withholding beta blockers for 24-36 hours. This strategy is employed because full beta blockade markedly reduces TWA magnitude, out of proportion to the effect of these drugs on mortality or ICD shocks, and increases the number of indeterminate tests due to a blunted heart rate response with exercise.25 Of note, other drugs with no impact on mortality, such as amiodarone and procainamide,27,32 also decrease TWA magnitude and prevalence suggesting that they also would reduce the prognostic value of TWA. For these reasons, beta blockers and antiarrhythmic drugs are often13,19-23,31 but not always15-18 withheld when measuring TWA. A study comparing the predictive value of TWA on and off beta blockers has not been performed.
This study should be interpreted in light of certain methodologic limitations. The number of patients studied was relatively small, so we cannot rule out that the hazard ratios observed would truly represent a 20-25% reduction of arrhythmic event among TWA negative patients. However, the number of patients studied was larger and the duration of follow-up was longer than most previous studies of TWA. Moreover, the 10.2% primary event rate at one year among TWA- subjects makes it very unlikely that TWA testing would be useful to stratify patients for ICD implantation. This substudy included 19% of the entire SCD-HeFT population, so we cannot exclude that different results would have been observed in other patients in the main study. However, 37 clinical sites participated in this substudy and patient characteristics were very similar to the main study. Consequently, it is very likely that these were representative patients and results. Another potential limitation is that this study was performed on a very restricted group of patients with systolic dysfunction and NYHA II or III CHF symptoms. Accordingly, we cannot extrapolate these results to other groups of patients, such as those with more preserved ejection fractions or without symptomatic heart failure. Finally, TWA studies were performed after withholding beta blockers for 24-36 hours as noted above. While this strategy is commonly employed in clinical studies of TWA, it is unknown if the predictive accuracy of this test is affected by reducing beta blockade transiently.
In summary, in a cohort of 490 subjects enrolled in SCD-HeFT, TWA testing did not predict arrhythmic events or mortality. Accordingly, these results suggest that TWA should not be used to make clinical decisions regarding ICD therapy among patients who meet “SCD-HeFT Criteria” for implantation (i.e. symptomatic heart failure and left ventricular systolic dysfunction). Further prospective studies of TWA, particularly with prespecified or randomized allocation of ICD therapy,33 are needed to define better the role of this test for risk stratification of sudden death.
Acknowledgments
Funding Sources: This study was supported by NIH and Cambridge Heart Inc, Bedford, MA.
Footnotes
Conflict of Interest Disclosures:
| Michael R Gold: | Research Grant Cambridge Heart, Consultant: Medtronic, Boston Scientific, St Jude |
| John I Ip: | None |
| Otto Costantini: | Consultant: Boston Scientific, St Jude |
| Jeanne Poole: | Research Grant HAT, Biotronik, Speaker Bureau Boston Scientific, Sorin, Medtronic, Biotronik |
| Steven McNulty: | None |
| Daniel B Mark: | Research Grant: Medtronic, Consultant Medtronic |
| Kerry L Lee: | Research Grant Cambridge Heart, Honoraria Medtronic |
| Gust H Bardy | Research Grant NIH, Medtronic, Philips, Laerdal, Ownership Interest Cameron Health, Consultant Philips, Boston Scientific, Institution/Employer Seattle Institute for Cardiac Research |
Clinical Trial Registration: NCT 0000609
References
- 1.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. Multicenter Automatic Defibrillator Implantation Trial Investigators. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G, Multicenter Unsustained Tachycardia Trial Investigators A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 4.Connolly S, Gent M, Roberts R, Dorian P, Roy D, Sheldon R, Mitchell L, Green M, Klein G, O'Brien B. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 8.Kadish A, Dyer A, Daubert J, Quigg R, Estes NA, Anderson K, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders W, Schaechter A, Levine J, Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 9.Buxton AE. Sudden death after myocardial infarction--who needs prophylaxis, and when? N Engl J Med. 2005;352:2638–2640. doi: 10.1056/NEJMe058085. [DOI] [PubMed] [Google Scholar]
- 10.Mark Daniel B, Nelson Charlotte L, Anstrom Kevin J, Al-Khatib Sana M, Tsiatis Anastasios A, Cowper Patricia A, Clapp-Channing Nancy E, Davidson-Ray Linda, Poole Jeanne E, Johnson George, Anderson Jill, Lee Kerry L, Bardy Gust H, for the SCD-HeFT Investigators Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2006;114:135–142. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 11.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 13.Gold MR, Bloomfield DM, Anderson KP, El-Sherif NE, Wilber DJ, Groh WJ, Estes NA, 3rd, Kaufman ES, Greenberg ML, Rosenbaum DS. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol. 2000;36:2247–2253. doi: 10.1016/s0735-1097(00)01017-2. [DOI] [PubMed] [Google Scholar]
- 14.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005;46:75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. J Am Coll Cardiol. 2003;41:2220–2224. doi: 10.1016/s0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 16.Hohnloser SH, Klingenheben T, Li YG, Zabel M, Peetermans J, Cohen RJ. T wave alternans as a predictor of recurrent ventricular tachyarrhythmias in ICD recipients: prospective comparison with conventional risk markers. J Cardiovasc Electrophysiol. 1998;9:1258–1268. doi: 10.1111/j.1540-8167.1998.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda T, Saito H, Tanno K, Shimizu H, Watanabe J, Ohnishi Y, Kasamaki Y, Ozawa Y. T-wave alternans as a predictor for sudden cardiac death after myocardial infarction. Am J Cardiol. 2002;89:79–82. doi: 10.1016/s0002-9149(01)02171-3. [DOI] [PubMed] [Google Scholar]
- 18.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS, Fontaine JM. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–463. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Rashba EJ, Osman AF, MacMurdy K, Kirk MM, Sarang S, Peters RW, Shorofsky SR, Gold MR. Influence of QRS duration on the prognostic value of T wave alternans. J Cardiovasc Electrophysiol. 2002;13:770–775. doi: 10.1046/j.1540-8167.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 20.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT., Jr Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 21.Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung ES, Menon S, Nallamothu BK, Chan PS. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:1820–1827. doi: 10.1016/j.jacc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 22.Rashba EJ, Osman AF, Macmurdy K, Kirk MM, Sarang S, Peters RW, Shorofsky SR, Gold MR. Exercise is superior to pacing for T wave alternans measurement in subjects with chronic coronary artery disease and left ventricular dysfunction. J Cardiovasc Electrophysiol. 2002;13:845–850. doi: 10.1046/j.1540-8167.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 23.Kavesh NG, Shorofsky SR, Sarang SE, Gold MR. Effect of heart rate on T wave alternans. J Cardiovasc Electrophysiol. 1997;8:987–993. doi: 10.1111/j.1540-8167.1998.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 24.Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol. 2002;13:502–512. doi: 10.1046/j.1540-8167.2002.00502.x. [DOI] [PubMed] [Google Scholar]
- 25.Rashba EJ, Cooklin M, MacMurdy K, Kavesh N, Kirk M, Sarang S, Peters RW, Shorofsky SR, Gold MR. Effects of selective autonomic blockade on T wave alternans in humans. Circulation. 2002;105:837–842. doi: 10.1161/hc0702.104127. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman ES, Bloomfield DM, Steinman RC, Naverow PB, Constantini O, Cohen RJ, Bigger JT. “Indeterminate” microvolt T-wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;48:1399–1404. doi: 10.1016/j.jacc.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Groh WJ, Shinn TS, Engelstein EE, Zipes DP. Amiodarone reduces the prevalence of T wave alternans in a population with ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 1999;10:1335–9. doi: 10.1111/j.1540-8167.1999.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–81. [Google Scholar]
- 29.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 30.Morin DP, Zacks ES, Mauer AC, Ageno S, Janik M, Markowitz SM, Mittal S, Iwai S, Shah K, Lerman BB, Stein KM. Effect of bundle branch lock on microvolt T-wave alternans and electrophysiologic testing in ischemic cardiomyopathy. Heart Rhythm. 2007;4:904–912. doi: 10.1016/j.hrthm.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Cantillon DJ, Stein KM, Markowitz SM, Mittal S, Shah BK, Morin DP, Zacks ES, Janik M, Ageno S, Mauer AC, Lerman BB, Iwai S. Predictive value of microvolt T-wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol. 2007;50:166–173. doi: 10.1016/j.jacc.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 32.Kavesh NG, Shorofsky SR, Sarang SE, Gold MR. The effect of procainamide on T-wave alternans. J Cardiovasc Electrophysiol. 1999;10:649–654. doi: 10.1111/j.1540-8167.1999.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 33.Klingenheben T. Microvolt T-wave alternans for arrhythmia risk stratification in left ventricular dysfunction: Which patients benefit? J Am Coll Cardiol. 2007;50:174–175. doi: 10.1016/j.jacc.2007.05.002. [DOI] [PubMed] [Google Scholar]