Abstract
A single gene deletion causes lack of leptin and obesity in B6.V-Lepob (obese) mice compared to wild-type C57BL/6J (B6) mice. This study compared the phenotype of nociception and supraspinal antinociception in obese and B6 mice by testing two hypotheses: 1) microinjection of cholinomimetics or an adenosine receptor agonist, but not morphine, into the pontine reticular formation (PRF) is antinociceptive in B6 but not obese mice, and 2) leptin replacement in obese mice attenuates differences in nociceptive responses between obese and B6 mice. Adult male mice (n=22) were implanted with microinjection guide tubes aimed for the PRF. The PRF was injected with neostigmine, carbachol, nicotine, N6-p-sulfophenyladenosine (SPA), morphine, or saline (control) and latency to paw withdrawal (PWL) from a thermal stimulus was recorded. B6 and ob mice did not differ in PWL following saline microinjection into the PRF. Neostigmine, carbachol, and nicotine caused PWL to increase significantly in B6 but not obese mice. An additional 15 obese mice were implanted with osmotic pumps that delivered leptin for seven days. Leptin replacement in obese mice restored the analgesic effect of PRF neostigmine to the level displayed by B6 mice. The results show for the first time that leptin significantly alters supraspinal cholinergic antinociception.
Perspective
This study specifies a brain region (the pontine reticular formation), cholinergic neurotransmission, and a protein (leptin) modulating thermal nociception. The results are relevant for efforts to understand the association between obesity, disordered sleep, and hyperalgesia.
Keywords: acetylcholine, adenosine, morphine, obesity, sleep, pain
Introduction
B6.V-Lepob (obese) mice differ from wild type C57BL/6J (B6) mice by a single nonsense mutation at the ob gene22, 53. This mutation results in the inability of obese mice to produce the protein leptin. Obese mice exhibit many phenotypic differences from congenic B6 mice including altered metabolic function, respiratory abnormalities34, and disrupted sleep14, 25. Human obesity is associated with increased complaints of pain21, 30. This association encouraged the present study aiming to determine whether the nociceptive phenotype was differentially expressed in B6 and obese mice.
The brain regions and molecules that link obesity and nociception are complex and poorly understood. Administration of opioid, cholinergic, and adenosinergic drugs to the pontine reticular formation (PRF) alters nociceptive responses of cat23, 44 and rat50. Therefore, the present study was designed to determine whether PRF administration of opioid, cholinergic, and adenosinergic drugs alters latency of paw withdrawal from a thermal stimulus as a function of mouse genotype and leptin replacement in obese mice. Portions of these results have been presented in abstract form47-49.
Methods
Surgery, Recovery, and Behavioral Conditioning
All experiments and procedures were approved by the University of Michigan Committee on Use and Care of Animals. Adult male B6 (n=13) and obese (n=9) mice from Jackson Laboratory (Bar Harbor, ME) were anesthetized with 2–3% isoflurane (Abbott Laboratories, North Chicago, IL) in 100% oxygen and placed in a David Kopf (Tujunga, CA) model 926 stereotaxic frame with model 923 mouse head holder and anesthesia mask. Isoflurane levels were reduced to 1.3–1.8% and maintained throughout the surgery. To enable access to the PRF, mice were implanted with one 26-gauge stainless steel guide tube (Plastics One, Roanoke, VA) aimed for coordinates 4.72 mm posterior, 0.65 mm lateral, and 5.60 mm ventral to bregma36. Dental acrylic was applied to the guide tube and two stainless steel screws were placed in the skull to provide an anchor surface for the acrylic. Additional obese mice (n=15) also were each implanted with an Alzet (Cupertino, CA) model 1007D osmotic pump set to deliver leptin at 15μg/day for seven days. All mice were allowed to recover for seven days following surgery, during which time they were handled and conditioned daily to being placed in a Plexiglas chamber. Animals were housed individually in a constantly illuminated and temperature regulated room with free access to food, water, and bedding.
Quantification of Nociception
Thermal nociceptive threshold was measured using an IITC Life Sciences Model 336 Plantar Simulator Analgesia Meter (Woodland Hills, CA) following a Hargreaves paw withdrawal protocol19. Mice were placed in individual Plexiglas chambers on a raised, tempered glass surface. A light box beneath the glass surface provided a radiant heat source as a 4×6 mm light beam. The adjustable heat source was set at 40% active intensity and 10% idle intensity (to allow aiming of the beam when inactivated). To test for thermal nociceptive threshold, the light was focused on the plantar surface of one hind paw. The light and timer were activated simultaneously and stopped when the mouse reacted by moving its paw away from the thermal stimulus. The time to the nearest 0.01 s between turning on the light and the reaction of the mouse was recorded as the paw withdrawal latency (PWL). A cutoff time of 15 s for the light beam was maintained throughout all experiments to ensure no injury to the paw. Measurements were taken at least 30 s apart and alternated between right and left hind paws to prevent habituation to the stimulus. PWL measurements were expressed as a percent change from pre-microinjection baseline values in the form of percent maximum potential effect (%MPE)4 using the following equation:
Percent MPE provides a quantitative index of nociception while accounting for individual differences between mice, as well as stimulus cutoff time.
Experimental Procedure
At the beginning of each experiment mice were allowed one h to habituate to the chambers before 5 baseline PWL measurements were taken, each 5 min apart. The PRF was injected with 50 nL of either 0.9% saline (vehicle control) or drug using a 31 gauge microinjector (Plastics One, Roanoke, VA) connected to a Hamilton syringe via polyethylene tubing (PE 20). Three PWL measurements were taken at each of six time points, occurring at 10, 20, 30, 60, 90, and 120 min post injection. Drug solutions (10 mM) microinjected included the adenosine A1 receptor agonist N6-p-sulfophenyladenosine (SPA; 222.7 ng), the acetylcholinesterase inhibitor neostigmine bromide (151.6 ng), the cholinergic receptor agonist carbachol (91.4 ng), the mu opioid agonist morphine sulfate (379.4 ng), and the nicotinic acetylcholine receptor agonist nicotine base (81.1 ng). Repeated microinjections into the same mouse were separated by at least 5 days. Obese mice used for the leptin replacement portion of the study received only one microinjection of either saline or neostigmine on the seventh day of leptin replacement. After PWL testing, obese mice were anesthetized and the osmotic pump was removed. Blood was collected from a tail snip, and the serum was separated and frozen for later verification of leptin delivery.
Histological Confirmation of Microinjection Sites
Mice were deeply anesthetized and decapitated 2–5 days after the final microinjection experiment. For the group of obese mice that received leptin, a final blood collection was made immediately after decapitation. Serum was separated and frozen for a subsequent leptin assay. Brains were quickly removed, frozen, and sliced in 40 μm coronal sections. Sections were mounted serially onto glass slides, fixed with hot (80° C) paraformaldehyde vapor, and stained with cresyl violet. Stereotaxic coordinates of each microinjection site were obtained by comparing the stained sections to a mouse brain atlas36.
Assay for Leptin
Delivery of leptin to obese mice implanted with osmotic pumps was verified using a leptin ELISA kit (Crystal Chem Inc.; Downers Grove, IL). Blood collected on day 7, the last day of leptin treatment, was used to verify presence of leptin. Blood collected on day 9, two days after pump removal, was used to verify degradation of leptin. Positive and negative control samples from B6 and obese mice, respectively, were also tested in each assay.
Statistical Analyses
All %MPE data were evaluated by two-way analysis of variance (ANOVA; drug by time and strain by time) for repeated or random measures. Additional inferential statistics included Student’s t-test and Tukey Kramer post hoc multiple comparisons test. All tests used P < 0.05 as an indication of significance. Statistical tests were performed in consultation with the University of Michigan Center for Statistical Consultation and Research using Statistical Analysis System v9.1.3 (SAS Institute, Inc., Cary, NC) and GBStat (Dynamic Microsystems, Inc., Silver Spring, MD). Data are reported as mean ± standard error of the mean (SEM).
RESULTS
All Microinjection Sites were Localized to the PRF
Figure 1 shows that all microinjection sites were localized to the PRF. Average ± SEM stereotaxic coordinates of the microinjection sites were 4.92 ± 0.05 mm posterior, 0.75 ± 0.07 mm lateral, and 4.61 ± 0.08 mm ventral to bregma for B6 mice. Microinjection coordinates for obese mice that did not undergo leptin treatment were 4.85 ± 0.04 mm posterior, 0.87 ± 0.07 mm lateral, and 4.99 ± 0.08 mm ventral to bregma. Microinjection coordinates for obese mice that received leptin replacement were 4.94 ± 0.02 mm posterior, 0.73 ± 0.08 mm lateral, and 4.94 ± 0.07 mm ventral to bregma.
Figure 1.

All microinjection sites were localized to pontine reticular formation regions comprised of the oral (PnO) and caudal (PnC) pontine reticular nucleus36. Microinjection sites for B6 mice (black dots, n=13), obese mice (green dots, n=8), and obese mice that received leptin (purple dots, n=15) are shown on five coronal drawings of the mouse brainstem. Numbers at the lower right of each drawing indicate distance (mm) posterior to bregma. The top left figure shows a typical cresyl violet stained tissue section with an arrow pointing to the microinjection site. The top right figure represents a sagittal view of the mouse brain with vertical lines indicating the anterior to posterior range of the microinjection sites. Brain drawings were modified from the Paxinos and Franklin mouse brain atlas36.
Cholinomimetics and SPA Produced an Antinociceptive Response in B6 Mice
Figure 2 (left column, A-E) shows %MPE as a function of time following microinjection of five drugs into the PRF. Consistent with previous findings in cat23, two-way ANOVA revealed no significant change in %MPE following microinjection of morphine (Fig. 2A). Nicotine administration also did not significantly alter %MPE in B6 mice (Fig. 2B). ANOVA revealed that %MPE was significantly altered as a function of drug following microinjection of SPA (Fig. 2C; F=5.53; df=1,119; P<0.05), carbachol (Fig. 2D; F=7.54; df=1,119; P<0.05), and neostigmine (Fig. 2E; F=18.37; df=1,107; P<0.001). Microinjection of SPA (Fig. 2C; F=4.04; df=5, 119; P<0.01) and carbachol (Fig. 2D; F=2.74; df=5,119; P<0.05) produced a significant time-dependent change in %MPE. ANOVA also revealed a significant time by drug interaction following microinjection of SPA (Fig. 2C; F=4.04; df=5,119; P<0.01). These interactions derive from the drug effects dissipating over time. Post hoc Tukey Kramer test comparing drug and saline %MPE values revealed that SPA (Fig. 2C) significantly (P<0.05) increased %MPE values at 20 and 30 min post injection, and that carbachol (Fig. 2D) significantly (P<0.05) increased %MPE values at 10, 60, and 90 min post injection. Tukey Kramer test also showed that neostigmine (Fig. 2E) increased %MPE values at all time points except 90 min post injection. Consistent with hyperalgesia reported for some obese humans21, 30 and rats17, mean PWL in s was significantly (P<0.01) less for obese (5.31 s) than for B6 (7.0 s) mice following PRF microinjection of saline.
Figure 2.
Percent maximum potential effect (%MPE) values following microinjections into the pontine reticular formation of B6 and B6.V-Lepob (obese) mice. Graphs showing data from B6 and obese mice for each drug are presented side by side to facilitate comparison. The %MPE is a measurement of change from baseline (no injection) values. Higher %MPE values indicate a longer delay in moving the paw away from the stimulus, consistent with decreased nociception. Saline microinjections (vehicle control) produced %MPE values around 0, indicating little change from baseline (no injection) values. Asterisks indicate a significant difference from saline at designated time points.
Morphine, Cholinomimetics, and SPA had No Effect on Thermal Nociception in Obese Mice
Figure 2 (right column, F-J) reports %MPE as a function of time and drug for obese mice. As observed in the B6 mice, microinjection of morphine (Fig. 2F) produced no significant change in %MPE. Obese mice also showed no significant increase in %MPE caused by microinjection of nicotine (Figs. 2G), SPA (Fig. 2H), or carbachol (Fig. 2I). Following neostigmine administration (Fig. 2J) there was a significant time by drug effect (F=3.97; df=5,107; P<0.01). Post hoc Tukey Kramer analysis revealed that %MPE was significantly (P<0.05) increased by neostigmine at 10 min post injection.
Leptin Replacement in Obese Mice Restored the Antinociceptive Effect of PRF Neostigmine Administration
To determine whether leptin replacement restores the antinociceptive response to neostigmine, another group of obese mice (n=15) was given continuous leptin for one week via osmotic pumps and the PRF was then microinjected with either neostigmine or saline. Figure 3 shows the %MPE (mean +SEM) following PRF neostigmine administration to 9 B6 mice and 11 obese mice that received leptin replacement. There was no significant difference (t=0.29; P>0.05) for B6 mice (49.85 +11.3) compared to obese mice that received leptin (46.33 +3.9). Thus, leptin replacement in obese mice restored %MPE to levels displayed by B6 mice. Seven days of leptin replacement also reduced the body weight of obese mice by approximately 21% compared to pre-leptin treatment weight (Table 1). Measures of leptin and body weight confirmed leptin delivery for all 15 obese mice (Table 1).
Figure 3.
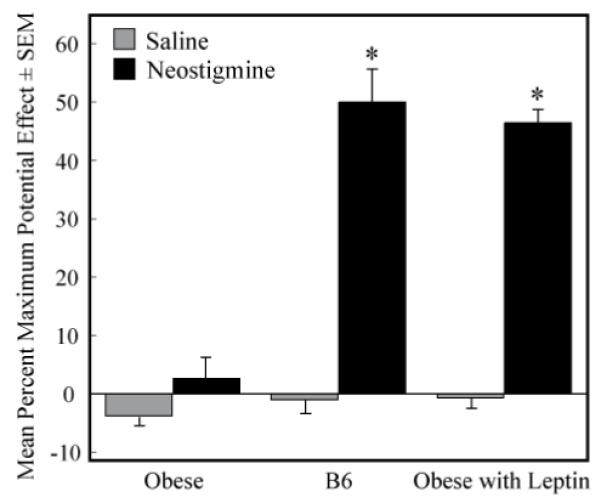
Leptin replacement restored the antinociceptive effect of pontine reticular formation neostigmine in obese mice. Graph shows %MPE values averaged over the course of 2 h following microinjection of saline or neostigmine for obese mice, B6 mice, and obese mice that received leptin replacement. Asterisks indicate a significant difference (P<0.05) between response to neostigmine and saline.
Table 1.
Leptin replacement was verified using an ELISA. Leptin day 7 values indicate leptin concentrations (ng/mL) on the last day of leptin treatment. Leptin day 9 values were tested to verify elimination of leptin 2 days after removal of the osmotic pump. Leptin replacement also decreased body weight in obese mice, as expected
| Leptin ± SEM (ng/mL) | %Decrease in Body Weight ± SEM | |
|---|---|---|
| B6 Control | 5.24 ± 0.50 | ---- |
| Obese Control | 0.03 ± 0.01 | ---- |
| Obese leptin day 7 | 24.50 ± 1.36 | 20.82 ± 2.05 |
| Obese leptin day 9 | 0.02 ± 0.01 | 21.89 ± 2.11 |
Discussion
The results show for the first time that microinjection of cholinomimetics and an adenosine A1 receptor agonist, but not morphine, into the PRF of B6 mouse modulates thermal antinociception. Cholinomimetics and the adenosine A1 receptor agonist did not produce a significant antinociceptive response in obese mice. These differences in nociceptive phenotype are of interest relative to the fact that B6.V-Lepob and B6 mice differ by lack of the leptin-producing ob gene. The finding that leptin replacement in obese mice restored the thermal antinociceptive response to neostigmine supports the interpretation that the protein leptin can modulate supraspinal cholinergic antinociception. The results are discussed in relation to the emerging understanding of a significant interaction between obesity, sleep disruption, and pain perception.
Supraspinal Cholinergic Antinociception
For B6 mice, time course measures of %MPE showed that PRF microinjection of SPA, carbachol, and neostigmine caused significant antinociceptive responses (Fig. 2). PRF administration of morphine and nicotine did not alter the time course of %MPE. These results are similar to the effects of morphine, cholinomimetics23, and SPA44 microinjected into homologous regions of cat PRF. Similar drug effects observed in mouse, cat, and ongoing studies of rat50, support the interpretation that the present thermal antinociceptive effects of SPA, carbachol, and neostigmine are not species-specific.
Neostigmine prevents the breakdown of the endogenous neurotransmitter ACh. This mechanism of action makes the antinociceptive effects of neostigmine particularly relevant for understanding the role of the PRF in thermal nociception. Spinally administered neostigmine has been used in patients with promising antinociceptive results11. Intranasal nicotine also has been shown to reduce post operative pain in women recovering from uterine surgery15. In contrast to B6 mice, the congenic line of obese mice showed no significant enhancement of antinociceptive behavior following microinjection of cholinomimetics or SPA (Fig. 2). These results are in line with data showing that obese mice have a differential sleep response to PRF neostigmine administration compared to B6 mice14.
Microinjection of morphine in obese and B6 mice (Figs. 2A & 2F) produced no change in nociceptive response when compared with microinjection of saline. This finding is consistent with previous data from cat23, and suggests that the PRF is not a brain region that contributes to the pain relieving effect of morphine. Morphine administered to the PRF has been shown to decrease acetylcholine release in the PRF29 and to inhibit the rapid eye movement (REM) phase of sleep27.
The PRF is comprised of the pontine reticular nucleus, oral part (PnO) and caudal part (PnC)36. The present focus on the PRF as a brain region modulating supraspinal thermal antinociception derives, in part, from the facts that pain and sleep are inversely related, and that both cholinergic28, 45 and adenosinergic10 neurotransmission in the PRF regulates sleep. Sleep disruption is a leading complaint of patients experiencing pain and pain patients frequently exhibit disrupted sleep8, 24. Depriving healthy subjects of one night of REM sleep causes a hyperalgesic response to nociceptive stimuli37. The foregoing points are clinically relevant because opioids disrupt the sleep/wake cycle and decrease the amount of REM sleep7, 27.
Obese humans have disordered sleep5, 20, 39, 40, 46 and diet-induced obese mice show altered sleep patterns that are reversed with weight loss18. Rat models of metabolic syndrome52 are hyperalgesic for acute17 and chronic32 pain and have disrupted sleep33. The foregoing evidence and the present results support the view that the PRF is one brain region modulating the association between obesity, disordered sleep, and nociception.
The Role of Leptin in Antinociception
Leptin was discovered in 199453 and has been widely reported to function as a satiety factor involved in metabolic regulation of energy expenditure and energy input16. Leptin receptors are localized to the arcuate nucleus38 and ventromedial hypothalamus12, further demonstrating that leptin regulates feeding and energy balance16. Studies of obese mice have provided insight into the roles of leptin. Obese mice have deficiencies in brain development1, immunology26, breathing34, and endothelial function51, and show disrupted sleep14, 25. Replacement of leptin has been found to restore partial or complete function associated with leptin deficiencies. Leptin levels have also been shown to be influenced by the duration of sleep41. In particular, there exists a correlation in obese humans between short sleep duration and decreased levels of leptin43. The present results show that leptin replacement for seven days restored the antinociceptive effect of PRF neostigmine such that the %MPE response of obese mice to neostigmine was no different from the response of B6 mice (Fig. 3). Leptin replacement for seven days was modeled after previous studies showing that leptin normalized breathing in leptin deficient mice34. The Table 1 data show that leptin replacement produced serum leptin levels in ob mice that were about five-times greater than levels of leptin in B6 mice. The mechanisms contributing to this five-fold increase in leptin are not known. These data encourage future studies to determine whether leptin delivered to normal B6 mice can enhance thermal antinociceptive responses.
The mechanisms by which leptin altered supraspinal cholinergic antinociception are unknown. Administering neostigmine into the PRF decreases the degradation of ACh released from the terminals within the PRF. These ACh releasing terminals originate from cholinergic neurons localized to the laterodorsal and pedunculopontine tegmental nuclei42. Leptin can modulate cholinergic function by altering expression of choline acetyltransferase13, the enzyme responsible for synthesis of ACh. It remains to be determined whether replacing leptin in obese mice increases the production of acetylcholine.
Limitations and Conclusions
Publication of the mouse genome documents a 99% homology with the human genome31, indicating that genotypic and phenotypic studies of mice can provide novel insights into human disorders6. One limitation for studies using the B6.V-Lepob mouse is that the mutation causing the obese mouse phenotype is rarely present in humans. Some forms of human obesity are associated with reduced leptin receptor affinity for leptin and elevated serum levels of leptin35. Studies quantifying the relationship between serum and cerebrospinal fluid levels of leptin show that defective transport of leptin across the blood-brain barrier can be more important than decreased leptin receptor availability or affinity2, 3. Thus, it should be clear that the link between obesity and nociception is multifactorial and cannot be attributed to leptin alone. Despite these limitations, loss of the influence of leptin may account for some of the phenotypic similarities between obese mice and humans43, 46. The results encourage future studies designed to examine nociceptive responses in the db/db, mouse which has a mutation in the lepr gene that encodes for the leptin receptor9.
ACKNOWLEDGEMENTS
This research was supported by National Institute of Health grants HL65272, HL57120, HL40881, MH45361, and the Department of Anesthesiology. We thank Dr. A. Parlow of the National Hormone and Peptide Program at NIDDK for mouse leptin, Professor E.F. Domino for providing the nicotine base, and K. Welch of the University of Michigan Center for Statistical Analysis and Research for help with statistical analyses. S. Jiang, M.A. Norat, and C.J. Van Dort provided expert assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaroke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, DiPalma CR, Farrell CL. Transport of leptin across blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava HN, Zhao GM. Effect of nitric oxide synthase inhibition on tolerance to the analgesic action of D-Pen2, D-Pen5 enkephalin and morphine in the mouse. Neuropeptides. 1996;30:219–223. doi: 10.1016/s0143-4179(96)90067-0. [DOI] [PubMed] [Google Scholar]
- 5.Bjorvatn B, Sagen IM, Oyane N, Waage S, Fetveit A, Pallesen S, Ursin R. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16:66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 6.Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, Oesch F, Zabel B. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–132. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- 7.Bonafide CP, Aucutt-Walter N, Divittore N, King T, Bixler EO, Cronin AJ. Remifentanil inhibits rapid eye movement sleep but not the nocturnal melatonin surge in humans. Anesthesiology. 2008;108:627–633. doi: 10.1097/ALN.0b013e3181684bc3. [DOI] [PubMed] [Google Scholar]
- 8.Bonica JJ. The Management of Pain. I. Lea & Febiger; Philadelphia: 1990. [Google Scholar]
- 9.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 10.Coleman CG, Baghdoyan HA, Lydic R. Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J Neurochem. 2006;96:1750–1759. doi: 10.1111/j.1471-4159.2006.03700.x. [DOI] [PubMed] [Google Scholar]
- 11.Collins JG. Spinally administered neostigmine--something to celebrate. Anesthesiology. 1995;82:327–328. doi: 10.1097/00000542-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr., Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Di Marco A, Demartis A, Gloaguen I, Lazzaro D, Delmastro P, Ciliberto G, Laufer R. Leptin receptor-mediated regulation of cholinergic neurotransmitter phenotype in cells of central nervous system origin. Eur J Biochem. 2000;267:2939–2944. doi: 10.1046/j.1432-1033.2000.01308.x. [DOI] [PubMed] [Google Scholar]
- 14.Douglas CL, Bowman GN, Baghdoyan HA, Lydic R. C57BL/6J and B6.V-LEPOB mice differ in the cholinergic modulation of sleep and breathing. J Appl Physiol. 2005;98:918–929. doi: 10.1152/japplphysiol.00900.2004. [DOI] [PubMed] [Google Scholar]
- 15.Flood P, Daniel D. Intranasal nicotine for postoperative pain treatment. Anesthesiology. 2004;101:1417–1421. doi: 10.1097/00000542-200412000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56:S38–46. doi: 10.1111/j.1753-4887.1998.tb01685.x. discussion S54-75. [DOI] [PubMed] [Google Scholar]
- 17.Geisser ME, Wang W, Smuck M, Koch LG, Britton SL, Lydic R. Nociception before and after exercise in rats bred for high and low aerobic capacity. Neurosci Letts. 2008;443:37–40. doi: 10.1016/j.neulet.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased by weight gain and decreased by weight loss in mice. Sleep. 2008;31:627–633. doi: 10.1093/sleep/31.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 20.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rossler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 21.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007;8:430–436. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 23.Kshatri AM, Baghdoyan HA, Lydic R. Cholinomimetics, but not morphine, increase antinociceptive behavior from pontine reticular regions regulating rapid-eye-movement sleep. Sleep. 1998;21:677–685. doi: 10.1093/sleep/21.7.677. [DOI] [PubMed] [Google Scholar]
- 24.Lamberg L. Chronic pain linked with poor sleep; exploration of causes and treatment. JAMA. 1999;281:691–692. [PubMed] [Google Scholar]
- 25.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lord G. Role of leptin in immunology. Nutr Rev. 2002;60:S35–38. doi: 10.1301/002966402320634913. discussion S68-84, 85-37. [DOI] [PubMed] [Google Scholar]
- 27.Lydic R, Baghdoyan HA. Neurochemical mechanisms mediating opioid-induced REM sleep disruption. In: Lavigne G, Sessle BJ, Choinière M, Soja PJ, editors. Sleep and Pain. International Association for the Study of Pain (IASP) Press; Seattle: 2007. pp. 99–122. [Google Scholar]
- 28.Lydic R, Douglas CL, Baghdoyan HA. Microinjection of neostigmine into the pontine reticular formation of C57BL/6J mouse enhances rapid eye movement sleep and depresses breathing. Sleep. 2002;25:835–841. doi: 10.1093/sleep/25.8.835. [DOI] [PubMed] [Google Scholar]
- 29.Lydic R, Keifer JC, Baghdoyan HA, Becker L. Microdialysis of the pontine reticular formation reveals inhibition of acetylcholine release by morphine. Anesthesiology. 1993;79:1003–1012. doi: 10.1097/00000542-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Marcus DA. Obesity and the impact of chronic pain. Clin J Pain. 2004;20:186–191. doi: 10.1097/00002508-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Mouse Genome Sequencing Consortium: Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 32.Muncey A, Salleus A, Baghdoyan HA, Koch LG, Britton SL, Lydic R. Rats bred for low intrinsic aerobic running capacity recover more slowly from chronic pain compared to rats bred for high intrinsic aerobic capacity. FASEB Journal. 2008;22:945–949. [Google Scholar]
- 33.Muncey A, Salleus A, Baghdoyan HA, Koch LG, Britton SL, Lydic R. Rats bred for low intrinsic aerobic running capacity exhibit decreased and more disrupted sleep compared to those bred for high intrinsic aerobic running capacity. Sleep. 2008;31(Suppl):A27. Abstr. [Google Scholar]
- 34.O’Donnell CP, Tankersley CG, Polotsky VP, Schwartz AR, Smith PL. Leptin, obesity, and respiratory function. Respir Physiol. 2000;119:163–170. doi: 10.1016/s0034-5687(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 35.Ogier V, Ziegler O, Mejean L, Nicolas JP, Stricker-Krongrad A. Obesity is associated with decreasing levels of the circulating soluble leptin receptor in humans. Int J Obes Relat Metab Disord. 2002;26:496–503. doi: 10.1038/sj.ijo.0801951. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- 37.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 38.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 39.Seicean A, Redline S, Seicean S, Kirchner HL, Gao Y, Sekine M, Zhu X, Storfer-Isser A. Association between short sleeping hours and overweight in adolescents: results from a US Suburban High School survey. Sleep Breath. 2007;11:285–293. doi: 10.1007/s11325-007-0108-z. [DOI] [PubMed] [Google Scholar]
- 40.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–363. [PubMed] [Google Scholar]
- 41.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 42.Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. Kluwer Academic/Plenum Publishers; New York: 2005. [Google Scholar]
- 43.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanase D, Baghdoyan HA, Lydic R. Microinjection of an adenosine A1 agonist into the medial pontine reticular formation increases tail flick latency to thermal stimulation. Anesthesiology. 2002;97:1597–1601. doi: 10.1097/00000542-200212000-00036. [DOI] [PubMed] [Google Scholar]
- 45.Van Dort C, Baghdoyan HA, Lydic R. Adenosine A1 and A2A Receptors in Mouse Prefrontal Cortex Modulate Acetylcholine Release and Behavioral Arousal. J Neuroscience. 29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vgontzas AN, Lin H-M, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, Bixler EO. Short sleep duration and obesity: the role of emotional stress and sleep disturbance. Int J Obes. 2008;32:801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Baghdoyan HA, Lydic R. Nociceptive responses following pontine reticular formation microinjection of cholinomimetics, the adenosine A1 receptor agonist N6-p-sulfophenyladenosine, and morphine differ between C57BL/6J and B6.V-Lepob mice. Soc Neurosci Abstr. 2006 248.4. [Google Scholar]
- 48.Wang W, Baghdoyan HA, Lydic R. Leptin increases antinociceptive responses in B6.V-Lepob mice following microinjection of neostigmine into the pontine reticular formation. Sleep. 2007;30:0009. [Google Scholar]
- 49.Wang W, Watson SL, Baghdoyan HA, Lydic R. Nociception is decreased by cholinomimetics and the adenosine A1 receptor agonist N6-p-sulfophenyladenosine, but not morphine, microinjected into the pontine reticular formation of C57BL/6J mouse. Sleep. 2006;29:0012. [Google Scholar]
- 50.Watson SL, Watson CJ, Baghdoyan HA, Lydic R. Microinjection of hypocretin-1 into the pontine reticular nucleus, oral part (PnO) of Sprague-Dawley rat decreases response to nociceptive input. J Pain. 2008;9(Suppl 2):7. [Google Scholar]
- 51.Winters B, Mo Z, Brooks-Asplund E, Kim S, Shoukas A, Li D, Nyhan D, Berkowitz DE. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep(ob)) mice. J Appl Physiol. 2000;89:2382–2390. doi: 10.1152/jappl.2000.89.6.2382. [DOI] [PubMed] [Google Scholar]
- 52.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]



