Abstract
The antiviral drug tenofovir (TFV) is orally administered as the fumarate salt of its disoproxil prodrug (TFV disoproxil fumarate (TDF)). TFV is a di-anion at physiological pH and, as a result, has poor lipid membrane permeability. Administration of the lipophilic and cell permeable prodrug, TFV disoproxil, enhances the oral absorption of TFV. In order to determine if oral administration of TDF also increases distribution to sites of viral infection, the plasma and circulating lymphoid cell pharmacokinetics of TFV and its phosphorylated metabolites were assessed following a single oral TDF or subcutaneous TFV administration at doses yielding equivalent plasma exposures to TFV in macaques. Despite TFV disoproxil’s lack of plasma stability and undetectable levels in the first plasma samples taken, oral administration of TDF resulted in 7.9-fold higher peripheral blood mononuclear cell exposures to the active metabolite, TFV-diphosphate. The apparent plasma terminal half-life (t1/2) of TFV was also longer following oral TDF relative to subcutaneous TFV administration (median t1/2 of 15.3 and 3.9 h, respectively), suggesting broader distribution to cells and tissues outside of the central plasma compartment. In conclusion, the disoproxil pro-moiety not only enhances the oral absorption of TFV but also tissue and lymphoid cell loading. These results illustrate that administration of even a fleeting prodrug can increase target tissue loading and gives valuable insight for future prodrug development.
Keywords: HIV, prodrug, nucleotide, tenofovir
Introduction
Tenofovir (TFV) is widely used in the treatment of human immunodeficiency virus (HIV) infection and has recently received regulatory approval for hepatitis B virus therapy. TFV is orally administered as the fumarate salt of its lipophilic and cell permeable prodrug, TFV disoproxil (TDF; Figure 1). TFV is an acyclic nucleotide analog of 2’-deoxyadenosine monophosphate (dAMP). Following two phosphorylation steps, TFV-diphosphate (DP) competes with endogenous 2’-deoxyadenosine triphosphate (dATP) for incorporation by virally encoded reverse transcriptases. Once incorporated, TFV serves as an obligate chain-terminator of elongating viral DNA.
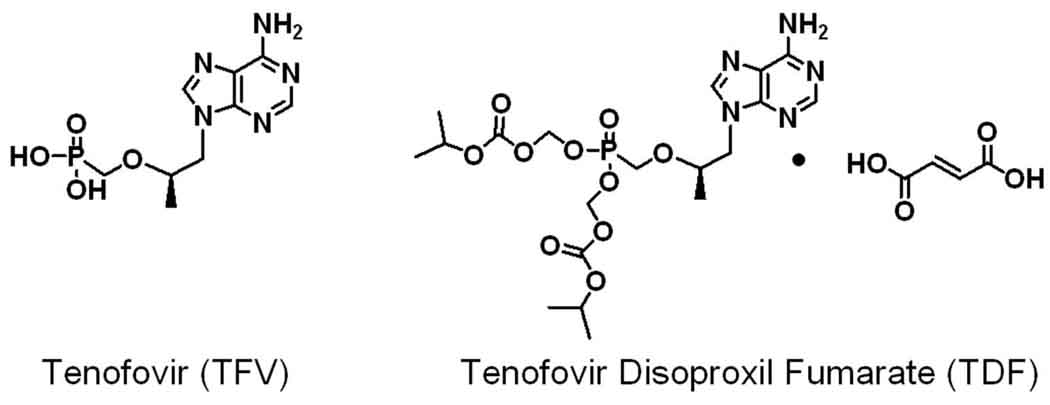
Figure 1.
Structure of TFV and the fumarate salt of its orally bioavailable disoproxil prodrug (TDF).
TFV disoproxil has >500-fold greater anti-HIV activity than TFV in vitro.1 However, TFV disoproxil is not observed in plasma at the earliest collected time-points following administration to patients2,3 and, therefore, might be assumed to not affect the loading of lymphoid cells and tissues with the active metabolite, TFV-DP. TFV disoproxil has poor stability in human plasma with a reported half-life of less than 1 min.4 The lack of detectable TFV disoproxil in plasma may also be related to degradation during intestinal absorption, where it is affected by intestinal efflux transport and hydrolase activity.5,6
In order to determine if administration of TDF has an effect on TFV distribution, we investigated the plasma pharmacokinetic profile of TFV and intracellular levels of TFV and its phosphorylated metabolites in peripheral blood mononuclear cells (PBMC) following single administration of subcutaneous TFV or oral TDF at doses resulting in equivalent plasma exposures to TFV in macaques. The findings reported here illustrate that oral administration of TDF results in efficient delivery of TFV into the systemic circulation and increases the loading of lymphoid cells with TFV-DP.
Experimental Section
Materials
TDF, TFV, TFV-MP and TFV-DP were provided by Gilead Sciences, Inc. (Foster City, CA). Stable isotope-labeled [13C15N] 2’-deoxyguanosine-TP (dGTP) and dATP were purchased from Spectra Stable Isotopes (Andover, MA). The analytical internal standards adefovir, GS-9148 (a nucleotide analog with the chemical name [5-(6-amino-purin-9-yl)-4-fluoro-2,5-dihydro-furan-2-yloxymethyl)phosphonic acid]) and GS-9148-MP were obtained from Gilead Sciences, Inc. and 2-chloro-adenosine-TP (Cl-ATP) from Sigma-Aldrich (St. Louis, MO). All other reagents were the highest grade available from Sigma-Aldrich.
Animals
Rhesus macaques (Macaca mulatta) from the type D retrovirus-free and simian T-cell-lymphotropic virus type 1-free colony at the California National Primate Research Center (CNPRC) were used in these studies. The animals were housed in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and were receiving a standard commercial primate diet. Animals were handled in strict accordance with the Guide for the Care and Use of Laboratory Animals7 and the protocol was reviewed by the Institutional Animal Care and Use Committee (IACUC) at the University of California at Davis. The animals had previously been infected with non-pathogenic SIVmac1A11 (as described previously)8 and were aviremic and healthy. Animals were between 17 and 25 months of age (3 to 4.3 kg) at the time of the initial pharmacokinetic experiments (aimed at determining doses resulting in equivalent plasma exposures), and approximately 40 to 43 months of age (4.2 to 8 kg) during the 2nd and definitive experiment. For the drug administrations and blood collections, animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, NJ) at 10 mg/kg injected intramuscularly.
Drug administration
For subcutaneous administration, TFV powder (isolated as the free base) was suspended in distilled water, dissolved by adding approximately 2 equivalents of NaOH, volume adjusted and buffered with RPMI-1640 media to a final concentration of 60 mg/ml and filter sterilized (0.2 µm filter; Nalgene, Rochester, NY). TFV solution was administered at final doses of 2.5 to 10 mg/kg subcutaneously into the backs of the monkeys. For oral administration, TDF powder was dissolved in RPMI-1640 media at a concentration of 8 mg/ml, and was administered via orogastric intubation at doses of 20 to 30 mg/kg body weight.
Plasma and PBMC Sample Collection
Blood (approximately 3 mL) was collected by venipuncture at 0, 0.5, 1, 2, 4, 8 and 24 h post-dose into Vacutainer tubes containing EDTA as an anticoagulant (BD Biosciences, San Jose, CA). Plasma was separated by spinning samples for 10 min at 900 × g. Supernatants were stored at −80 °C until further processing and analysis. At select time points (2, 4, 8 and 24 h post-dose) 8 to 10 mL of blood was collected for PBMC isolation. Following separation of plasma, cells were isolated by Ficoll gradient separation (lymphocyte separation medium; MP Biomedicals, Aurora, OH) and washed in 4°C 0.9% NaCl solution. Use of phosphate buffered saline was avoided during sample preparation due to its components negative effects on analysis. Isolated cells were washed with an NH4Cl solution to remove contaminating red blood cells as described previously.9 Isolated cell pellets (typically containing approximately 14 million PBMC) were immediately frozen at −70°C until further processing and analysis.
Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) instrumentation
LC methods used an HTS PAL autosampler with cooled sample storage stacks set at 6 °C (Leap Technologies, Carrboro, NC) and an LC-20AD ternary pump system (Shimadzu Scientific Instruments, Columbia, MD). All LC-MS/MS analyses were done in positive ion and multiple reaction monitoring modes using a Sciex API-4000 mass spectrometer (Applied Biosystems, Foster City, CA).
Quantification of Plasma Concentrations of TFV
Plasma samples were subject to protein precipitation by addition of acetonitrile and formic acid to final concentrations of 66 and 0.2%, respectively. The structurally related nucleotide analog adefovir was added as an internal standard. Samples were analyzed by a validated LC-MS/MS method. Analytes were separated using a 4 µm 75 × 2 mm Synergi Hydro column (Phynomenex, Torrance, CA) using 0.2% formic acid and a linear gradient from 1 to 90% acetonitrile at a flow rate of 250 µl/min over 4.5 min. Six point standard curves prepared in blank plasma covered concentrations of 3-orders of magnitude and showed linearity in excess of an r2 value of 0.98. The lower limit of TFV quantification in plasma was 2.6 nM. Separately prepared quality control samples of 10, 80 and 4,000 nM were analyzed at the beginning and end of each sample set to assure accuracy and precision within 15%.
PBMC Sample Preparation
Frozen PBMC pellets had 2.6 pmol of the internal standards GS-9148 (used for TFV), GS-9148-MP (used for TFV-MP) and Cl-ATP (used for TFV-DP, dATP and dGTP) added. Lysis of cell pellets was achieved by vortexing in 1 mL of ice cold 70% methanol. Cellular debris were removed by spinning at 15,000 × g and the pellet kept to evaluate the number of PBMC using a previously described DNA-based biochemical assay using a standard curve generated from manually counted macaque PBMC.10 Supernatants were dried at 37°C under nitrogen flow (Turbovap evaporator, Zymark) and resuspended in 50 µl of 1 mM phosphate buffer. A final spin (15,000 × g, 5 min) was used to remove any remaining particulate matter from extracts before injection on the LC-MS/MS system.
Simultaneous Quantifiaction of PBMC Concentrations of TFV-MP, TFV-DP, dATP and dGTP
Elements of prior LC/MS/MS ion pairing nucleotide detection methods were combined including the use of 1,5-dimethylhexylamine as an ion pairing agent11 and low flow LC coupled with positive ion mode MS/MS detection12. Briefly, analytes were separated using a 1.7 µm 2.5 × 50 mm Acquity BEH column with a VanGaurd pre-column (Waters Corporation, Milford, MA) using an ion pairing buffer containing 10 mM 1,5-dimethylhexylamine and 3 mM ammonium formate and a multistage linear gradient from 5 to 75% acetonitrile at a flow rate of 100 µl/min over 10 min. Six point standard curves prepared in blank PBMC matrices covered concentrations of 3-orders of magnitude and showed linearity for each analyte in excess of an r2 value of 0.98. Quantification of endogenous dATP and dGTP was accomplished using standard curves of stable isotopically labeled nucleotides in cellular matrices as previously described.12,13 The lower limits of quantification were between 38 and 66 fmol/sample for all analytes. Separately prepared quality control samples of 100 to 30,000 fmol/sample were analyzed at the beginning and end of each sample set to assure accuracy and precision within 25%. Results in fmol/sample were then divided by the number of PBMC the extract for each injection was derived from (on average 2 to 3 million cells) to obtain concentrations in fmol/106 PBMC. Intracellular concentrations were then calculated assuming a mean cell volume of 0.2 µl/million PBMC.14
Quantification of PBMC Concentrations of TFV
The presence of chromatographic interference in the method used for PBMC analyses of nucleotides described above did not allow for simultaneous quantification of TFV levels in PBMC. Therefore, all PBMC samples were subject to a second analysis to determine TFV levels using a similar LC-MS/MS methodology as that used for plasma (see above). Five point standard curves prepared in blank PBMC matrices showed linearity in excess of an r2 value of 0.99. The lower limit of quantification was 153 fmol/sample.
Data Analyses
Non-compartmental pharmacokinetic parameters were calculated using WinNonLin 5.01 (Pharsight Corporation, Mountain View, CA). Inter and intra arm statistical comparisons were made using the non-parametric Mann-Whitney U-test. Statistical calculations were done using GraphPad Prism 4 (GraphPad Prism, Inc., La Jolla, CA). P-values less than or equal to 0.05 were considered significant.
Results
Determination of doses resulting in equivalent plasma TFV exposures
Initial pharmacokinetic studies were done at oral TDF doses of 20 and 30 mg/kg (9.1 and 13.6 mg TFV equivalents per kg, respectively) and subcutaneous TFV doses of 2.5 and 10 mg/kg to allow for the estimation of doses resulting in equivalent plasma exposures to TFV (2 to 3 monkeys per dose). Based on the observation of mg/kg dose normalized exposures approximately 8-fold higher following subcutaneous TFV relative to oral TDF administration (as determined by the area under the concentration versus time curve (AUC); data not shown), subsequent studies were done at doses of 30 mg/kg oral TDF and 4 mg/kg subcutaneous TFV. The 30 mg/kg oral TDF dose is approximately 2-fold higher than the surface area adjusted human dose of 300 mg used clinically but allowed for accurate detection of PBMC levels of TFV and its phosphorylated metabolites at the corresponding subcutaneous dose.
Plasma pharmacokinetics following oral TDF and subcutaneous TFV
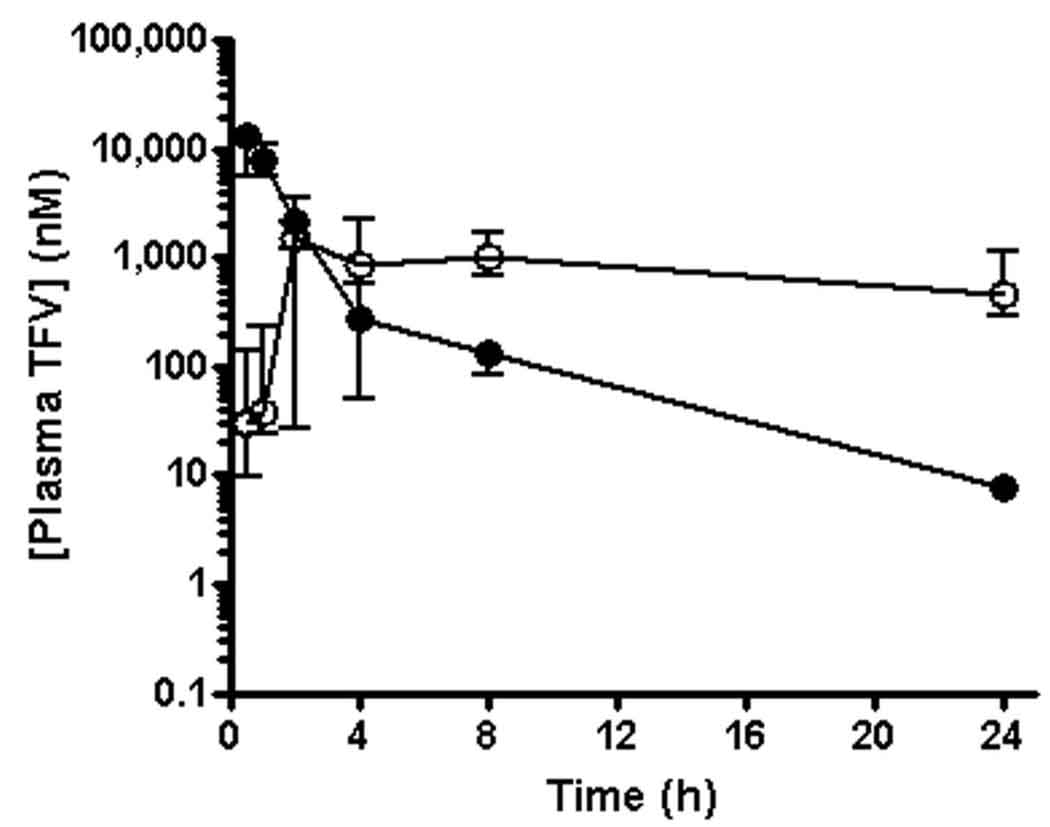
A sequential study design with a 6 week wash out period and 5 macaques per arm was chosen to compare the plasma and PBMC pharmacokinetics following a single administration of 30 mg/kg oral TDF or 4 mg/kg subcutaneous TFV. The non-compartmental plasma pharmacokinetic parameters are summarized in Table 1. As estimated from the initial studies summarized above, TFV plasma exposures over 24 h (AUC0–24) and exposures extrapolated to infinity (AUC0-∞) did not significantly differ between the oral TDF and subcutaneous TFV study arms. Dose proportional changes in plasma exposures were observed relative to the initial studies completed to determine doses resulting in equivalent plasma exposures in both the oral TDF and subcutaneous TFV study arms (data not shown). However, marked differences in the plasma pharmacokinetic profile of TFV administered by the two routes were observed (Figure 2). Most notably, the terminal plasma half-life (t1/2) of TFV was significantly longer following oral TDF relative to subcutaneous TFV administration. The increased persistence of plasma TFV in the oral TDF arm resulted in a 57-fold higher median TFV concentration measured at 24 h (C24) despite an 8.2-fold lower median maximal plasma TFV concentration (Cmax) relative to the subcutaneous TFV arm. Assuming complete bioavailability of the subcutaneous TFV dose, the relative AUC0-∞ values led to the calculation of an oral bioavailability of approximately 31% for TFV following oral administration of TDF. Due to plasma instability, a validated method for TFV disoproxil could not be obtained and TFV disoproxil was not detected in plasma taken at any time. Consistent with previously reported findings in dogs,15 low levels of the mono-substituted, intermediate metabolite of TFV disoproxil were observed in plasma from the oral treatment arm (AUC0–24 less than 0.3% that of plasma TFV).
Table 1.
Plasma TFV pharmacokinetic parameters following a single administration of 4 mg/kg subcutaneous TFV or 30 mg/kg oral TDF to macaques
| Parameter | 4 mg/kg subcutaneous TFV |
30 mg/kg oral TDF |
Ratioa |
|---|---|---|---|
| AUC0–24 (nM•h) | 24,200 (16,100–28,300)b |
19,900 (14,400–32,900) |
0.8 |
| AUC0-∞ (nM•h) | 24,200 (16,200–28,400) |
25,700 (21,900–65,900)d |
1.1 |
| Cmax (nM) | 13,000 (10,900–16,300) |
1,580 (1,280–2,300) |
0.12**,c |
| C24 (nM) | 7.92 (7.50–8.49) |
451 (292–1,110) |
57** |
| t1/2 (h) | 3.9 (3.4–4.3) |
15.3 (11.7–20.6)d |
3.9** |
Value for oral TDF divided by subcutaneous TFV.
Values represent the median (minimum-maximum) of n = 5 animals in each dosing arm unless noted otherwise.
Statistical significance assessed by the non-parametric Mann-Whitney U-test (**, P < 0.01).
Values represent the median (minimum-maximum) of n = 4 animals. One animal was excluded from analyses due to a poorly defined terminal phase.
Figure 2.
Pharmacokinetic profiles of TFV in plasma following a single administration of either 4 mg/kg subcutaneous TFV (filled circles) or 30 mg/kg oral TDF (open circles) to macaques. Values represent the median (minimum-maximum) of 5 monkeys dosed with each regimen in a sequential study design with a 6 week washout period.
PBMC Pharmacokinetics
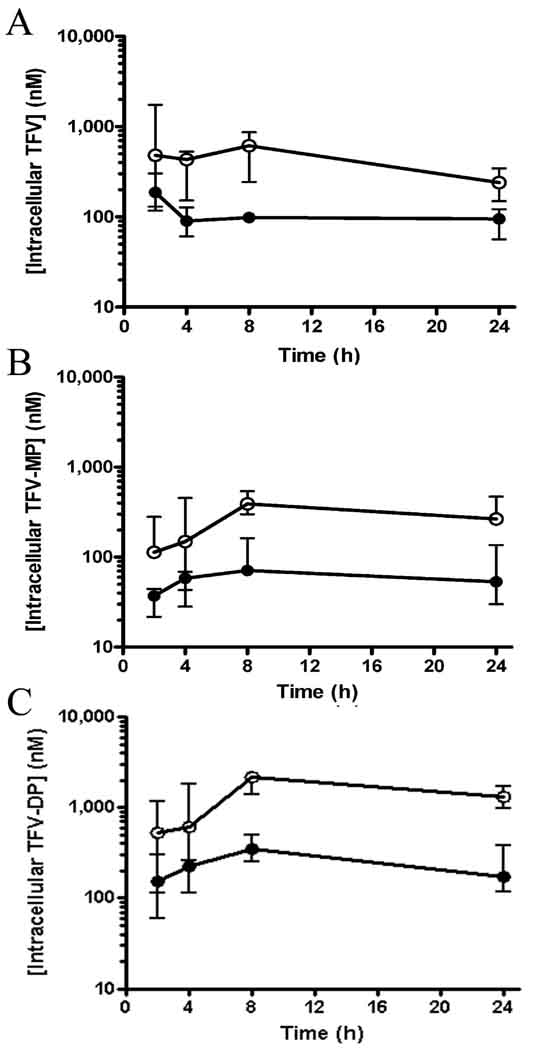
In contrast to the equivalent plasma TFV exposures observed, intracellular TFV, TFV-MP and TFV-DP AUC0–24 in PBMC were significantly higher following oral TDF relative to subcutaneous TFV administration (Table 2). Intracellular TFV, TFV-MP and TFV-DP levels were consistently greater at all time-points in the oral TDF arm (Figure 3). For example, the median Cmax of TFV-DP in PBMC were 2,200 and 323 nM in the oral TDF and subcutaneous TFV arms, respectively (P = 0.0079). TFV-DP rapidly accumulated in PBMC and was found to persist with a t1/2 of >24 h in both arms. Dose proportional changes in PBMC exposures to TFV and its phosphorylated metabolites were observed relative to the initial studies completed to determine doses resulting in equivalent plasma exposures in both the oral TDF and subcutaneous TFV study arms (data not shown).
Table 2.
PBMC AUC0–24 to TFV, TFV-MP and TFV-DP following a single administration of 4 mg/kg subcutaneous TFV or 30 mg/kg oral TDF to macaques
| Nucleotide | Intracellular AUC0–24 (nM•h) |
Ratioa | |
|---|---|---|---|
| 4 mg/kg subcutaneous TFV |
30 mg/kg oral TDF |
||
| TFV | 2,800 (1,110–3,200)b |
10,200 (5,420–13,700) |
4.9**,c |
| TFV-MP | 1,220 (384–2,990) |
6,630 (5,540–11,100) |
5.4** |
| TFV-DP | 4,650 (1,910–9,150) |
36,600 (24,500–44,400) |
7.9** |
Value for oral TDF divided by subcutaneous TFV.
Values represent the median (minimum-maximum) of n = 5 animals in each dosing arm.
Statistical significance assessed by the non-parametric Mann-Whitney U-test (**, P < 0.01).
Figure 3.
Pharmacokinetic profiles of TFV (A), TFV-MP (B) and TFV-DP (C) in PBMC following a single administration of either 4 mg/kg subcutaneous TFV (filled circles) or 30 mg/kg oral TDF (open circles) to macaques. Values represent the median (minimum-maximum) of 5 monkeys dosed with each regimen in a sequential study design with a 6 week washout period.
Levels of Endogenous 2’-deoxynucleotides
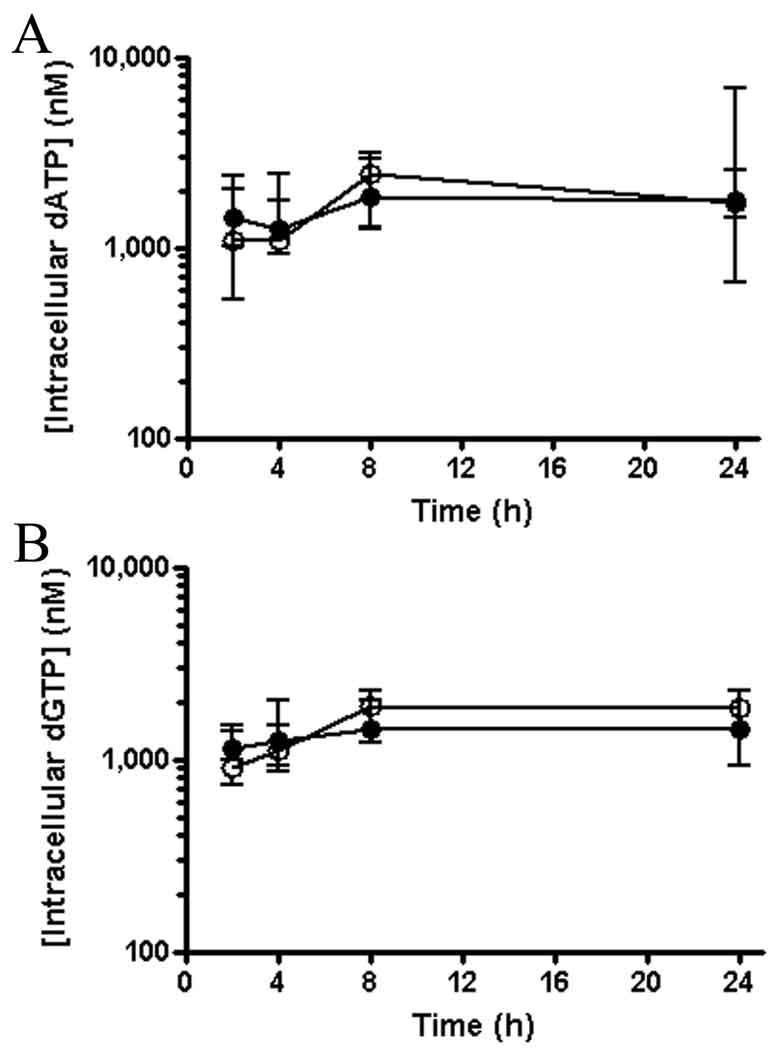
Levels of dATP and dGTP in PBMC were determined to further validate cell collection techniques, the consistency of PBMC collection between the two arms of the study, the effectiveness of the cell lyses procedure and to assure that no dephosphorylation was occurring during sample preparation. Similar levels of dATP and dGTP were observed between arms and over time in the same arm (Figure 3). Determined concentrations of dATP and dGTP (in the 1 to 5 µM range) were similar to those measured previously in lymphoid cells by different methodologies.16,17
Discussion
Development of the acyclic nucleoside phosphonate TFV as an oral antiviral agent was inextricably linked to identification of the disoproxil prodrug required to increase this charged nucleotide analog’s oral bioavailability.18 In the present study, oral administration of TDF resulted in effective delivery of TFV into the systemic circulation in macaques. The oral bioavailability observed in macaques here (31%) is similar to that reported in humans (25%)3. Consistent with a higher surface area adjusted dose relative to the human clinical dose, the 30 mg/kg oral TDF dose in macaques resulted in plasma TFV AUC0-∞ approximately 3-fold higher than the steady state AUC0–24 observed in humans following repeat dosing at 300 mg once a day (29,000 and 9,510 nM•h, respectively)3.
Consistent with the higher plasma exposures observed following oral administration of 30 mg/kg TDF to macaques relative to administration of 300 mg to patients, approximately 4-fold higher intracellular TFV-DP levels were observed here (2,200 nM) relative to those observed in PBMC from HIV infected patients treated with TDF (approximately 500 nM)11,19. Despite the lack of a significant difference in plasma TFV exposures between arms, intracellular levels of TFV, TFV-MP and TFV-DP were markedly increased following oral TDF relative to subcutaneous TFV. This contrasted the endogenous nucleotides dATP and dGTP, both found to have similar levels between the two arms. In contrast to the oral TDF arm, in the subcutaneous TFV arm the ratio of PBMC TFV-DP to plasma TFV was lower than what has been observed in patients orally administered TDF. Combined, these results suggest that oral administration of TDF to humans causes similar increases in PBMC loading as what has been observed in this study in macaques. Enhanced TFV delivery to PBMC after oral TDF administration has also been observed in beagle dogs.18 As PBMC are circulating lymphocytes and represent a compartment infected by HIV, the increased level of the active metabolite, TFV-DP, in these cells following oral TDF administration has important implication for clinical efficacy. Indeed, a cross-study comparison of short-term clinical efficacy studies comparing intravenous TFV and oral TDF suggests higher efficacy following oral TDF administration.2,20 While the concentrations of intracellular TFV and its phosphorylated metabolites differed significantly, levels persisted over the 24 h sampling period in both arms. Similarly, long TFV-DP half-lives have been observed in various cell types in vitro1,21 and in PBMC taken from patients in vivo11,19.
Following subcutaneous administration, TFV was observed to have a relative short t1/2 (3.9 h) and a limited steady-state volume of distribution (Vss; 0.75 L/kg). This calculated Vss likely represents a slight over estimate, as it assumes immediate and complete bioavailability of the subcutaneous dose. Nevertheless, the Vss estimated here leads to a similar conclusion as that made based on intravenous administration in humans3 and suggests limited distribution outside of total body water (total body water is approximately 0.7 L/kg in monkeys)22. TFV is not catabolized and is cleared from plasma exclusively through renal elimination by the combined action of glomerular filtration and active tubular secretion.23 Consistent with studies in other species, in macaques the clearance of TFV (0.60 L/hr/kg) exceeded the glomerular filtration rate and approached renal blood flow (in monkeys, approximately 0.13 and 1.66 L/h/kg, respectively)22. In addition to enabling effective oral delivery of TFV into plasma and PBMC, the plasma pharmacokinetic profile for TFV following oral administration of TDF suggests that TDF administration also resulted in a wider general distribution of TFV outside of the central plasma compartment. Since plasma t1/2 is a function of volume and clearance and the clearance of TFV is unlikely route dependent, the marked increase in the plasma t1/2 of TFV observed after oral TDF administration likely reflects the prodrug more widely distributing TFV and its phosphorylated metabolites to cells and tissues and slow release of TFV back into the central plasma compartment where it is subject to renal elimination.
Taken together, the plasma and PBMC profiles suggest that two factors contribute to the more efficient PBMC loading observed in the oral TDF arm. First, TDF directly loads PBMC immediately following administration while it is still present in plasma. Direct PBMC loading by TDF is illustrated by the higher TFV and phosphorylated metabolite levels observed in PBMC during the first 2 h in the oral TDF arm despite plasma exposures to TFV greater than 10-fold lower than the subcutaneous TFV arm (median AUC0–2 of 842 and 18,900 nM•h, respectively). Second, the wider distribution of TFV resulting from administration of TDF indirectly facilitates continued PBMC loading due to the longer duration of plasma exposures to TFV. Plasma levels of TFV in the oral TDF arm exceeded the subcutaneous TFV arm following the 2 h sampling. Combined, these two processes serve to explain the consistently higher intracellular TFV and phosphorylated metabolite levels over the entire dosing interval following oral TDF administration.
In summary, our study quantitatively shows that the addition of the disoproxil pro-moiety not only allows for effective oral delivery of TFV into the systemic circulation but also enhances loading of TFV-DP into PBMC. In addition to adding valuable insight into the pharmacology of TDF, understanding the effect of pro-moieties on target tissue loading will help in the development of agents with improved efficacy. We’ve studied next generation phosphonoamidate prodrugs that exhibit improved stability, measurable prodrug levels in the systemic circulation and further enhanced target cell and tissue loading4,24–26 and others have applied phosphate prodrugs to nucleoside analogs to improve pharmacologic activity.27–29
Figure 4.
Intracellular levels of the endogenous nucleotides dATP (A) and dGTP (B) in PBMC following administration of either 4 mg/kg subcutaneous TFV (filled circles) or 30 mg/kg oral TDF (open circles) to macaques. Values represent the median (minimum-maximum) of 5 monkeys dosed once with each regimen in a sequential study design with a 6 week washout period.
Acknowledgement
We thank Darius Babusis for contributing to analytical method development. This research was supported by Gilead Sciences, Inc. and grant RR-00169 from the National Center for Research Resources (NCRR; a component of the National Institute of Health (NIH)) to the California National Primate Research Center. The content of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. L.D.G., J.E.V., I.N.H, T.C., H.R., W.A.L, G.R.R and A.S.R. are employed by Gilead Sciences, Inc. the marketer of TDF (Viread).
REFERENCES
- 1.Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, Kearney BP, Coleman RL, Lamy PD, Kahn JO, McGowan I, Lietman PS. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 2001;45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 4.Lee WA, He GX, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, Cundy KC. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005;49:1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Gelder J, Deferme S, Naesens L, De Clercq E, van den Mooter G, Kinget R, Augustijns P. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab. Dispos. 2002;30:924–930. doi: 10.1124/dmd.30.8.924. [DOI] [PubMed] [Google Scholar]
- 6.Tong L, Phan TK, Robinson KL, Babusis D, Strab R, Bhoopathy S, Hidalgo IJ, Rhodes GR, Ray AS. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob. Agents Chemother. 2007;51:3498–3504. doi: 10.1128/AAC.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NationalResearchCouncil. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 8.Van Rompay KK, Blackwood EJ, Landucci G, Forthal D, Marthas ML. Role of CD8+ cells in controlling replication of nonpathogenic Simian Immunodeficiency Virus SIVmac1A11. Virol. J. 2006;3:22. doi: 10.1186/1743-422X-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand-Gasselin L, Da Silva D, Benech H, Pruvost A, Grassi J. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob. Agents Chemother. 2007;51:2105–2111. doi: 10.1128/AAC.00831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benech H, Theodoro F, Herbet A, Page N, Schlemmer D, Pruvost A, Grassi J, Deverre JR. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 2004;330:172–174. doi: 10.1016/j.ab.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Pruvost A, Negredo E, Benech H, Theodoro F, Puig J, Grau E, Garcia E, Molto J, Grassi J, Clotet B. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 2005;49:1907–1914. doi: 10.1128/AAC.49.5.1907-1914.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vela JE, Olson LY, Huang A, Fridland A, Ray AS. Simultaneous quantitation of the nucleotide analog adefovir, its phosphorylated anabolites and 2'-deoxyadenosine triphosphate by ion-pairing LC/MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;848:335–343. doi: 10.1016/j.jchromb.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Vela JE, Miller MD, Rhodes GR, Ray AS. Effect of nucleoside and nucleotide reverse transcriptase inhibitors of HIV on endogenous nucleotide pools. Antivir. Ther. 2008;13:789–797. [PubMed] [Google Scholar]
- 14.Chapman EH, Kurec AS, Davey FR. Cell volumes of normal and malignant mononuclear cells. J. Clin. Pathol. 1981;34:1083–1090. doi: 10.1136/jcp.34.10.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw JP, Sueoko CM, Oliyai R, Lee WA, Arimilli MN, Kim CU, Cundy KC. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res. 1997;14:1824–1829. doi: 10.1023/a:1012108719462. [DOI] [PubMed] [Google Scholar]
- 16.Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoggard PG, Kewn S, Maherbe A, Wood R, Almond LM, Sales SD, Gould J, Lou Y, De Vries C, Back DJ, Khoo SH. Time-dependent changes in HIV nucleoside analogue phosphorylation and the effect of hydroxyurea. AIDS. 2002;16:2439–2446. doi: 10.1097/00002030-200212060-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lee WA, Martin JC. Perspectives on the development of acyclic nucleotide analogs as antiviral drugs. Antiviral Res. 2006;71:254–259. doi: 10.1016/j.antiviral.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins T, Veikley W, St Claire RL, 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 2005;39:406–411. doi: 10.1097/01.qai.0000167155.44980.e8. [DOI] [PubMed] [Google Scholar]
- 20.Deeks SG, Barditch-Crovo P, Lietman PS, Hwang F, Cundy KC, Rooney JF, Hellmann NS, Safrin S, Kahn JO. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(Phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaney WEt, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 2006;50:2471–2477. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 23.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, Wieman LM, Eisenberg EJ, Rhodes GR. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 2006;50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cihlar T, Ray AS, Boojamra CG, Zhang L, Hui H, Laflamme G, Vela JE, Grant D, Chen J, Myrick F, White KL, Gao Y, Lin KY, Douglas JL, Parkin NT, Carey A, Pakdaman R, Mackman RL. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 2008;52:655–665. doi: 10.1128/AAC.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray AS, Vela JE, Boojamra CG, Zhang L, Hui H, Callebaut C, Stray K, Lin KY, Gao Y, Mackman RL, Cihlar T. Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent. Antimicrob. Agents Chemother. 2008;52:648–654. doi: 10.1128/AAC.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiser H, Wang J, Chong L, Watkins WJ, Ray AS, Shibata R, Birkus G, Cihlar T, Wu S, Li B, Liu X, Henne IN, Wolfgang GH, Desai M, Rhodes GR, Fridland A, Lee WA, Plunkett W, Vail D, Thamm DH, Jeraj R, Tumas DB. GS-9219--a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin's lymphoma. Clin Cancer Res. 2008;14:2824–2832. doi: 10.1158/1078-0432.CCR-07-2061. [DOI] [PubMed] [Google Scholar]
- 27.Peyrottes S, Egron D, Lefebvre I, Gosselin G, Imbach JL, Perigaud C. SATE pronucleotide approaches: an overview. Mini Rev. Med. Chem. 2004;4:395–408. doi: 10.2174/1389557043404007. [DOI] [PubMed] [Google Scholar]
- 28.Cahard D, McGuigan C, Balzarini J. Aryloxy phosphoramidate triesters as pro-tides. Mini Rev. Med. Chem. 2004;4:371–381. doi: 10.2174/1389557043403936. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Maag H, Alfredson T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J. Pharm. Sci. 2008;97:1109–1134. doi: 10.1002/jps.21047. [DOI] [PubMed] [Google Scholar]