Abstract
Sustained inhibition of HIV-1, the goal of antiretroviral therapy, is often impeded by the emergence of viral drug resistance. For patients infected with HIV-1 resistant to conventional drugs from the viral reverse transcriptase and protease inhibitor classes, the recently approved entry and integration inhibitors effectively suppress HIV-1 and offer additional therapeutic options. Entry inhibitors are particularly attractive because, unlike conventional antiretrovirals, they target HIV-1 extracellularly, thereby sparing cells from both viral- and drug-induced toxicities. The fusion inhibitor enfuvirtide and the CCR5 antagonist maraviroc are the first entry inhibitors licensed for patients with drug-resistant HIV-1, with maraviroc restricted to those infected with CCR5-tropic HIV-1 (R5 HIV-1) only. Vicriviroc (another CCR5 antagonist) is in Phase III clinical trials, whereas the CCR5 antibodies PRO 140 and HGS 004 are in early stages of clinical development. Potent antiviral synergy between maraviroc and CCR5 antibodies, coupled with distinct patterns of resistance, suggest their combinations might be particularly effective in patients. In addition, given that oral administration of maraviroc achieves high drug levels in cervicovaginal fluid, combinations of maraviroc and other CCR5 inhibitors could be effective in preventing HIV-1 transmission. Moreover, since CCR5 antagonists prevent rejection of transplanted organs, maraviroc could both suppress HIV-1 and prolong organ survival for the growing number of HIV-1 patients with kidney or liver failure necessitating organ transplantation. Thus, maraviroc offers an important treatment option for patients with drug-resistant R5 HIV-1, who presently account for >50% of drug-resistance cases.
Keywords: maraviroc, CCR5 antagonists, CCR5 tropism, HIV resistance, HIV entry inhibitors
Introduction
Highly Active Antiretroviral Therapy (HAART), through the combination of nucleoside analog reverse transcriptase inhibitors (NRTIs), non-NRTIs (NNRTIs) and protease inhibitors (PIs), has significantly changed the progression and outcome of infection with HIV-1.1 However, high pill burdens, inconvenient dosing, and long-term toxicities contribute to poor compliance and emergence of drug-resistant virus in many patients.2,3 For those patients in whom resistant virus develops, treatment options become limited and more complicated regimens are necessary to prevent further disease progression. Fortunately, the elucidation of HIV-1 entry steps has offered new opportunities for therapeutic intervention4 (Fig. 1). Two entry inhibitors are currently licensed, the fusion inhibitor enfuvirtide (T-20)5 and the small-molecule CCR5 antagonist maraviroc.6 Vicriviroc, another CCR5 antagonist, is in advanced clinical development.7 The CCR5 antibodies PRO 1408 and HGS004,9 and the CD4 antibody ibalizumab10 are in early clinical development. Entry inhibitors, as well as integrase inhibitors and newer NNRTIs and PIs, have demonstrated potent HIV-1 inhibition in treatment-experienced patients, with significantly improved suppression when at least two active drugs were used.11-13 Accordingly, current treatment guidelines recommend the use of at least two, and preferably three, fully active agents in a new regimen in patients with evidence of HIV-1 resistance.14 This review will focus on maraviroc (Selzentry, Pfizer Inc), the first licensed CCR5 antagonist for patients with drug-resistant CCR5-tropic HIV-1 infection.
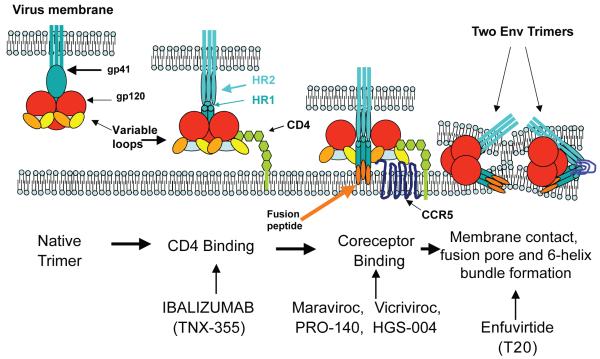
Figure 1. Therapeutic opportunities for inhibition of HIV-1 entry.
HIV-1 entry is mediated by the viral Env protein, which comprises the glycoproteins gp120 and gp41 arranged in trimeric spikes on the viral surface. Entry encompasses three steps: CD4 binding, coreceptor binding and fusion. The viral gp120 first binds to CD4, causing a repositioning of the variable loops V1/V2 and V3 and thereby exposing the bridging sheet and forming a coreceptor binding site. Upon coreceptor binding, conformational changes in gp120 and gp41 lead to the insertion of gp41 fusion peptide into the cell membrane. Subsequent conformational changes result in the formation of a six-helix bundle, with the HR2 domains folding back and packing into grooves on the outside of the triple-stranded HR1 domains. This brings the fusion peptide and transmembrane region of gp41 in close proximity, forming a fusion pore that allows transfer of the viral core into the cell. Each step on HIV-1 entry can be targeted by inhibitors currently approved or in clinical development. CD4 binding is targeted by ibalizumab (formerly TNX-355), a humanized monoclonal antibody that binds to CD4. Coreceptor binding is blocked by small-molecule CCR5 antagonists (maraviroc and vicriviroc) and by CCR5 antibodies (PRO-140 and HGS-004). Finally, the formation of a six-helix bundle, and thereby fusion, is prevented by enfuvirtide.145
Maraviroc: Mechanism of Action and Factors Impacting Antiviral Activity
Maraviroc is a spirodiketopiperazine that targets CCR5, a main coreceptor for HIV-1.15 The identification of CCR5 as a viral coreceptor16-19 was prompted by the discovery of HIV-1 inhibition by the CCR5 ligands β-chemokines (MIP-1α [CCL-1], MIP-1β [CCL-2] and RANTES [CCL-3]).20 CCR5, which belongs to the G-protein-coupled receptor (GPCR) family and is expressed on activated T lymphocytes, macrophages and dendritic cells, regulates cell trafficking to inflamed tissues.21 CCR5-tropic HIV-1 strains (referred to as R5 HIV-1) are the most persistent and predominantly transmitted ones.22,23 HIV-1 strains that use the alternative chemokine receptor CXCR4 (X4 HIV-1) or both CCR5 and CXCR4 (R5X4 dual-tropic HIV-1) emerge in approximately 50% of patients and are generally associated with a more rapid loss of CD4+ lymphocytes and faster progression to AIDS.24,25 However, transition to CXCR4-using HIV-1 strains is not required for the development of AIDS since CD4 depletion and disease progression does occur in patients carrying R5 HIV-1 strains only.26 Several polymorphisms that impact CCR5 expression have been described. A mutation in the CCR5 open reading frame leads to premature truncation and consequently a 32-bp deletion in the CCR5 protein (CCR5Δ32). Individuals homozygous for the Δ32 mutation (~1% in the Caucasian population) are generally resistant to infection with HIV-1, while heterozygous individuals can acquire infection but progress to AIDS more slowly than wild-type individuals.27,28
To infect a host cell, the envelope (Env) proteins on the surface of HIV-1 first bind to cellular CD4 receptors. Env bound to CD4 receptors then interacts with the coreceptor (CCR5 or CXCR4) to trigger the fusion of viral and cellular membranes leading to viral entry into cells.4 In R5 HIV-1 infection, following CD4 engagement, the CCR5 N-terminus binds to the bridging sheet (formed between the C1, C2 and C4 domains) and basal V3 regions of gp120 whereas the CCR5 extracellular loops bind to the tip of V3.29 Small-molecule CCR5 antagonists, such as maraviroc, bind to a hydrophobic cavity formed by the first, second, third and seventh transmembrane domain helices of CCR5 (Fig. 2). Within this cavity, maraviroc interacts with amino acids Trp86, Glu283, Tyr108, Tyr251 and Ile198, inducing conformational changes in the extracellular loops that are not recognized by the HIV-1 V3 region.30-34 Thus, CCR5 antagonists act as allosteric, non-competitive inhibitors. Unlike the natural β-chemokine CCR5 ligands, which induce transduction signaling and coreceptor internalization,35,36 antagonist-bound CCR5 does not signal and remains on the cell surface. Several factors, including densities of CCR5 and CD4 on the cell surface as well as affinity of HIV-1 Env for CCR5 can impact the efficiency of viral binding, entry, and infection.
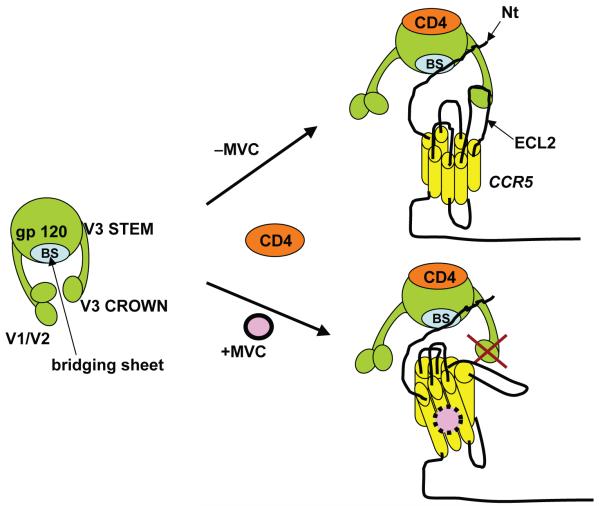
Figure 2. Model for maraviroc mechanism of action.
Binding of HIV-1 gp120 to CD4 exposes the bridging sheet and creates a coreceptor binding site. In the absence of maraviroc, the bridging sheet and the base of V3 interact with the N-terminus of CCR5, while more distal regions of V3 interact with extracellular loops (mainly ECL2). Binding of maraviroc to the transmembrane region of CCR5 locks CCR5 in a conformation that does not recognize the distal regions of V3.
Effect of CCR5 density on antiviral activity of CCR5 antagonists
Early studies with cell lines demonstrated that CCR5 density (molecules/cell) can limit HIV-1 entry, with a threshold below ~2 × 103 CCR5 molecules/CD4+ cell resulting in inefficient infection.37 In addition, coreceptor density on cell lines influences the kinetics of fusion and therefore susceptibility of R5 HIV-1 to the fusion inhibitor enfuvirtide.38-40 However, one caveat of studies using enfuvirtide in cell lines is that they may not accurately reflect the situation in primary CD4+ T cells. In this regard, CCR5 levels among cell lines may vary by several orders of magnitude (from <7 × 102 to >105 molecules/cell), whereas CD4+ T cells from normal individuals vary only by ~5-fold (2–10 × 103 CCR5 molecules/cell).21,37,41,42 In an effort to evaluate the impact of CCR5 expression on HIV-1 entry inhibitor activity, we showed a positive correlation between CCR5 density on primary CD4+ T cells and decreased sensitivity of R5 HIV-1 strains to enfuvirtide.43 In addition, we demonstrated that inhibition of CCR5 expression by the immunomodulatory drug rapamycin synergistically enhanced enfuvirtide activity against R5 HIV-1.44
It is reasonable to think that the activity of CCR5 antagonists will depend on the amount of CCR5 available on the cell surface. Indeed, Reeves et al have shown that lower concentrations of the CCR5 antagonist TAK-779 were required to inhibit the infection of cell lines engineered to express low CCR5 levels compared to cells expressing high CCR5 levels.39 Willey et al have reported similar results using the cell line U87 and several CCR5 inhibitors.45 Moreover, Platt et al demonstrated that TAK-779 was ~15-fold more potent against infection of HeLa-CD4 cells expressing low levels of CCR5 (~2 × 103 CCR5 molecules/cell) than of cells with higher CCR5 levels (~2 × 104 CCR5 molecules/cell).38 However, as indicated above, studies of CCR5 antagonists conducted in cell lines may not reflect the situation in primary CD4+ T cells. In addition to differences in coreceptor expression levels between cell lines and primary cells, it is possible that differences may exist in processing or post-translational modification. For example, differences at the processing/post-translational level could result in altered antagonist affinity. Our results using vicriviroc demonstrated that CCR5 levels on donor lymphocytes correlated with its antiviral activity against R5 HIV-1. Moreover, we showed that reduction of CCR5 expression by rapamycin enhanced the antiviral activities of the CCR5 antagonists TAK-77946 and vicriviroc47 in primary CD4+ T cells.
Interdependence of CCR5 and CD4 levels on HIV-1 entry
Studies on cell lines have demonstrated that densities of CD4 and CCR5 required for R5 HIV-1 infection are interdependent.37 Cell lines with a high CD4 density (4.5 × 105 receptors/cell) require low levels of CCR5 (~2 × 103 receptors/cell) for infection, whereas cells with low CD4 (<104 receptors/cell) require a higher CCR5 level (1–2 × 104 receptors/cell). Primary CD4+ lymphocytes express ~2–10 × 103 CCR5 receptors/cell and ~2 × 104–1 × 105 CD4 receptors/cell.21 Accordingly, small changes on levels of CCR5, but not CD4, on donor lymphocytes may be expected to impact R5 HIV-1 infection. In support of this hypothesis, we have reported that changes on CCR5 density, but not CD4 density, on donor lymphocytes impact infectivity by R5 HIV-1.43
Reciprocal modulation of CCR5 and β-chemokine levels
Expression of the CCR5 receptor on lymphocytes is transcriptionally controlled by cellular activation and requires interleukin-2 signaling for continuous expression.48 Transcription is initiated at multiple sites in exons 1 or 2 and alternative promoter usage gives rise to different transcripts.49 Early studies in transformed cell lines showed that CCR5 transcription is mainly driven by promoter 1 (Pr 1), leading to the assumption that Pr 1 governs the expression of CCR5 on primary cells.49,50 However, a study by Mummidi et al has demonstrated that, unlike in cell lines, CCR5 transcription in primary lymphocytes is mostly driven by Pr 2.51 As pointed out earlier on this review, these findings underscore the importance of using primary lymphocytes in studies of CCR5 expression and HIV-1 entry inhibition. This study also showed that synthesis of the CCR5 mRNA isoforms CCR5A and CCR5B is associated with the level of CCR5 surface expression on lymphocytes. Moreover, the transcription factors Oct-2 was shown to enhance the synthesis of these isoforms and CCR5 surface expression, a finding that extended early observations by Moriuchi et al.52
The levels of extracellular β-chemokines and CCR5 receptors are reciprocally modulated via internalization of chemokine-bound CCR5 receptors. Increased production of CCL3L1 (MIP-1αP) by individuals carrying a duplication in the CCL3L1 gene results in greater internalization of CCR5 and therefore fewer CCR5 receptors on the cell surface.53 Similarly, individuals who are homozygous for the Δ32 deletion in the CCR5 gene and as a result lack CCR5 protein expression, show high levels of β-chemokines in culture supernatants due to their inability to induce internalization.54,55
Impact of HIV-1 diversity on antiviral activity of CCR5 antagonists
Targeting components of the HIV-1 Env proteins (gp120 and gp41) with an entry inhibitor faces the challenge of genetic variation. The Env gene is the most variable HIV-1 gene, with up to 30% diversity among clades, 20% diversity within a clade, and up to 10% diversity within an individual.56 Within gp120, the bridging sheet and V3 regions participate in coreceptor binding.57 Although the exact epitope on gp120 that interacts with the coreceptor is not known, it is likely to differ among HIV-1 strains. However, currently available data indicate that most viruses, from same or different subtypes, are similarly inhibited by maraviroc (IC90 of 2 nM; 95% CI of 1.8 to 2.4 nM).15 In agreement, all patients from a maraviroc phase IIa trial had viral load reductions of >1 log10.58 One exception to the broad antiviral activity of maraviroc is Subtype G HIV-1, which is less sensitive to maraviroc and to vicriviroc, at least in vitro.15,59
Maraviroc Pharmacokinetics
Maraviroc is orally administered, 300 mg twice daily without regard to food. The drug is rapidly absorbed, with peak drug concentrations between 0.5 and 4 h after oral dosing.60 At the licensed dose of 300 mg, maraviroc has a bioavailability of 33% and a terminal t1/2 to steady state of 14–18 h, with steady state reached within 7 days.61 Maraviroc is substrate for CYP3A4 and p-glycoprotein, and thus, doses need to be adjusted when coadministered with inhibitors or inducers of these pathways as follows: 150 mg twice daily in the presence of CYP3A4 inhibitors (PIs, delavirdine, ketoconazole, itraconazole, clarithromycin), and 600 mg twice daily in the presence of CYP3A4 inducers (efavirenz, rifampicin, carbamazepine, phenobarbital and phenytoin).62,63 The standard dose of 300 mg twice daily can be used in the presence of tipranavir/ritonavir, nevirapine, NRTIs and enfuvirtide.
Maraviroc has poor penetration into the CNS of rats, suggesting it has limited antiviral activity in the brain.64 In contrast, vicriviroc seems to have better CNS penetration.65 The reason for this discrepancy may be related to vicriviroc not being a substrate for p-glycoprotein and to its greater lipophilic properties compared to maraviroc.64,66,67 Clearly, additional studies are needed to examine and compare the pharmacokinetics of CCR5 antagonists in human CNS.
Clinical Experience with Maraviroc
HIV-1 tropism determination
Because CCR5 antagonists are active against R5 HIV-1 only, assessment of viral tropism is recommended prior to treatment with CCR5 antagonists. In patients failing treatment with maraviroc or vicriviroc, the main pathway of viral escape was the selection of preexisting CXCR4-using HIV-1 variants,68-70 further underscoring the importance of tropism determination. Tropism can be assessed by both phenotypic and genotypic assays. Traditionally, phenotyping was done in the MT-2 cell line,71 which expresses CXCR4 but not CCR5.19,25,72 Because MT-2 culture assays can take several weeks, they may not be suitable for clinical use. Genotyping by population sequencing of the V3 region of Env from plasma virions, followed by tropism inference using an algoritm, such as geno2pheno (g2P) or PSSM, has faster turn-around times.73 However, genotyping has a low sensitivity for detection of X4 variants.74 As a result, clinical trials with CCR5 antagonists have generally used the Trofile assay (Monogram Biosciences, San Francisco, CA), a phenotypic assay that evaluates coreceptor use by Env sequences amplified from plasma by PCR. Following PCR amplification, complete or partial (V1 to V3) Env regions are cloned into an expression vector and used to generate pseudoviruses for infection of indicator cell lines expressing CD4 and CCR5 or CXCR4. The early version of the Trofile assay, which was used in the maraviroc trials, detected X4 variants with a 100% sensitivity but only when such variants represented ≥10% of viral variants in the sample. Despite the superior sensitivity of Trofile compared to genotyping, preliminary data suggest that both the Trofile assay and genotyping are similarly effective at detecting CXCR4-using variants and thereby at predicting viral load reductions, at least in patients with multidrug-resistant virus.75 Yet, the original Trofile assay is not sufficiently sensitive for detection of X4 viruses because patients initially classified as R5 HIV-1 actually contained low-frequency X4 variants, which emerged under treatment with CCR5 antagonists.68-70 There is currently an improved Trofile assay, called “enhanced Trofile”, with a sensitivity of 100% for detecting X4 viruses accounting for ≥0.3% of the virus population (Monogram Biosciences, San Francisco, CA). There are also improved genotyping methodologies using pyrosequencing, with reported detection of X4 variants representing 0.5% of the viral population.68,76 It will be critical to compare enhanced Trofile and pyrosequencing in detection of minor X4 variants in patients considering treatment with CCR5 antagonists.
Maraviroc efficacy
Maraviroc efficacy, first evaluated clinically in a 10-day monotherapy trial in patients with R5 HIV-1, reduced viral loads by ≥1.6 log10.58 These encouraging data led to the phase III clinical trials, MOTIVATE-1 and -2. MOTIVATE-1 was conducted in the United States and Canada, and MOTIVATE-2 in the United States, Australia and Europe. Both trials, with identical designs, evaluated the efficacy and safety of adding maraviroc (either once or twice daily) to Optimized Background Therapy (OBT) in a total of 1049 treatment-experienced patients without detectable X4 HIV-1 (determined by the original Trofile assay). Virologic response, defined as <50 copies HIV-1 RNA/ml plasma at 48 weeks, occurred in 47% of patients in the OBT plus twice-daily maraviroc group and 42% in the OBT plus once-daily maraviroc group versus 16% of those receiving OBT only (both P < 0.001). In addition, CD4+ count recovery was greater with maraviroc once or twice daily than with placebo (both P < 0.001).77,78
In a Phase II study similar to MOTIVATE but in patients with X4- or R5X4- HIV, there was no statistically significant differences in week 48 viral loads between the OBT plus maraviroc regimens versus OBT alone (−0.62 and −1.11 vs. −0.84 log10 HIV-1 RNA copies/ml, respectively) or change in CD4+ count (+65 and +78 vs. +51 cells/μl, respectively).79 Thus, maraviroc offers no significant clinical benefit in patients with X4 or R5X4 HIV-1 viruses.80
The efficacy of maraviroc in treatment-naïve patients was evaluated in the MERIT study, a Phase III trial comparing maraviroc and efavirenz, each in combination with zidovudine and lamivudine.81 Patients with R5-only HIV-1 were randomized to zidovudine/lamivudine with either efavirenz or once- or twice-daily maraviroc. The once-daily maraviroc arm was closed because of inferior efficacy. The twice-daily maraviroc arm demonstrated similar results to the control, with 69.3% vs. 65.3% of patients having <50 HIV-1 RNA copies/ml. Yet, this small difference in response did not meet the predefined 10% criteria for noninferiority. It is likely that failure to demonstrate noninferiority was due to the relatively low sensitivity of the original Trofile assay used for screening of patients. A recent analysis of the data following tropism screening with the more sensitive “Enhanced Trofile assay” (see above), demonstrated that for those patients with a true R5-only phenotype, there were almost identical virological responses in the maraviroc and efavirenz treatments.82
Maraviroc safety
Maraviroc has consistently demonstrated a safety profile similar to that of placebo.58,77,78,80 The most common adverse effects are cough, pyrexia, infections of the upper respiratory tract, rash, musculoskeletal symptoms, lightheadedness, and abdominal pain. Importantly, maraviroc is not associated with cardiovascular events, hepatotoxicity or development of malignancies, all of which raised serious concerns in early clinical studies with other CCR5 antagonists. In this regard, the clinical development of aplaviroc ended because of hepatotoxicity83 and that of Sch-C (a vicriviroc precursor) because of electrocardiographic QTc interval prolongation.59 A Phase II trial suggested an association between vicriviroc and increased risk of malignancy,7 but was not confirmed in a later study.84 Although 11 patients (1.3%) in the maraviroc Phase III studies reported cardiovascular events such as myocardial ischemia, these patients had either heart disease or heart disease factors, precluding a clear association between maraviroc and cardiovascular toxicity. Likewise, out of 1300 patients enrolled in the Phase IIb/III studies, one had severe hepatotoxicity, but again, could not be associated with maraviroc because the patient was taking other medications with potential hepatotoxicity. Finally, the clinical data on maraviroc does not support an association with malignancy development. Overall, maraviroc safety profile is encouraging. However, given the potential for malignancies and cardiovascular and liver toxicities with CCR5 antagonist use, the Food and Drug Administration (FDA) requires long-term follow-up toxicity studies.
HIV-1 Resistance to Maraviroc and Other CCR5 Antagonists
Resistance to CCR5 antagonists may arise from viral use of the alternative coreceptor CXCR4, either by acquiring mutations that allow switch to CXCR4 use or by selection of preexisting CXCR4-using variants. In vitro data indicate that CXCR4 switch under CCR5 antagonist pressure is rare.85 In vivo, some patients failing treatment with maraviroc or vicriviroc were found to harbor X4 variants, but sequencing analysis demonstrated that such variants were most likely selected from minor populations already present prior to treatment.69,70 In MOTIVATE-1 and -2, approximately 5% of patients with R5 HIV-1 only at screening (4–6 weeks prior to the beginning of the trial) had evidence of X4 HIV-1 at trial entry. For this subset of patients, 27% of those receiving OBT plus maraviroc once daily, 18% of those receiving OBT plus maraviroc twice daily and 18% of those receiving OBT alone, had <50 HIV-1 RNA copies/ml at week 24. In contrast, among patients with R5 HIV-1 only at both screening and study entry, 50% in both OBT plus maraviroc groups and 26% in the OBT-alone group had <50 HIV-1 RNA copies/ml at week 24. That ~5% of patients classified as having R5 HIV-1 only at screening turned out to have X4 HIV-1 variants at study entry may reflect the limited sensitivity of the HIV-1 coreceptor tropism assay used (sensitivity of 100% for detection of X4 variants when such variants represented ≥10% of the viral population, see above). These data suggest that selection of preexisting, yet undetected, X4 HIV-1 variants may account for virologic failure in patients taking maraviroc.
Resistance to CCR5 antagonists can also arise from emergence of R5 HIV-1 variants with increased affinity for CCR5 (partial resistance) or from variants capable of infection via antagonist-bound CCR5 (full resistance) (Fig. 3). Partial and full resistance have been observed both in vitro85,86 and in vivo.69 In genotypic assays, resistance is generally associated with mutations in Env, generally in the V3 region,69,87-89 but no signature mutations for resistance to CCR5 antagonist have been identified to date. Some of these mutant Envs are less dependent on interactions with CCR5 extracellular loops (mainly ECL2) but more dependent on interactions with the CCR5 N-terminus than wild-type Envs.90-92 In addition, there is in vitro evidence that full resistance to vicriviroc can be conferred by mutations in the fusion peptide of gp41 without changes in V3.88,93 Thus, resistance to CCR5 antagonists can follow both V3 dependent and V3 independent pathways. It will be important to determine the relative contribution of each resistance pathway in patients. Resistance to CCR5 antagonists is commonly diagnosed using the Phenosense Entry Susceptibility Assay (Monogram Biosciences), a single-cycle, Env-pseudotype assay based on U87 cells expressing high levels of CD4 and CCR5/CXCR4. In this assay, partial resistance is manifested by drug inhibition curves with increased values of EC50 (effective concentration that inhibits virus by 50%), whereas full resistance is manifested by incomplete dose response curves with inhibition plateaus at <100% inhibition.85,86 The height of the inhibition plateau in infection with fully resistant HIV-1 is indicative of the relative efficiencies with which free and antagonist-bound CCR5 are used, with greater inhibition plateaus indicating higher efficiencies in use of free CCR5. Currently, the factors determining the magnitude of inhibition plateaus in resistance phenotypic assays, and therefore the efficiency with which resistant viruses use antagonist-bound CCR5, are not well known. Elucidation of these factors is important because it will help understand resistance to CCR5 antagonists and its manifestation in phenotypic assays currently used in clinical studies.94 We have recently demonstrated that reduced CCR5 density in lymphocytes (either in donors with low CCR5 levels or in donors treated with rapamycin) sensitizes R5 HIV-1 resistant to vicriviroc.47 This impact of CCR5 density on antagonist activity against resistant HIV-1 was confirmed on cell lines with varying levels of CCR5 expression. These results represented the first indication that i) a host factor (CCR5 density) influences the way resistance to a CCR5 antagonist is manifested in a phenotypic assay, and ii) R5 HIV-1 strains that are fully resistant to a CCR5 antagonist recover drug sensitivity when CCR5 density is decreased, suggesting CCR5 reduction as an approach to control resistance.
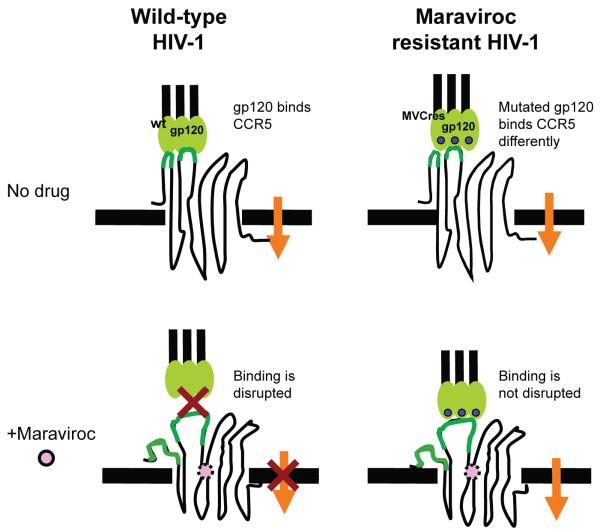
Figure 3. Model for maraviroc mechanism of resistance.
Maraviroc binds to the transmembrane region of CCR5, thereby inducing confomational changes that cannot be recognized by R5 HIV-1 gp120. One mechanism of resistance involves changes in HIV-1 Env that permit recognition of maraviroc-bound CCR5. As such, resistant viruses are not blocked by increasing maraviroc doses.
It is currently unclear whether resistance to maraviroc confers broad drug-class resistance.85,95 In one study, vicriviroc resistant viruses were resistant to other CCR5 antagonists (aplaviroc, maraviroc, AD101 and CMPD-167).95 However, in another study, maraviroc-resistant HIV-1 was inhibited by aplaviroc.85 It is possible that CCR5 antagonists may lock CCR5 in an antagonist-dependent conformation that is recognized by some, but not all, resistant viruses. Alternatively, aplaviroc inhibition of maraviroc-resistant HIV-1 might be explained by the rather unique aplaviroc binding to CCR5. Whereas most small-molecule antagonists have fewer interactions with CCR5 extracellular domains and insert deeply into the transmembrane region,32,33,96,97 aplaviroc binds in an almost horizontal position underneath the extracellular β-hairpin loop. As Phase III trials of vicriviroc advance, it will be critical to determine whether maraviroc-resistant clinical isolates can be inhibited by vicriviroc. In addition, the recently completed mapping of the CCR5 binding pocket could provide critical insights for structure-based design of novel CCR5 antagonists with activity against antagonist-resistant viruses.31,98
Potential Use of Maraviroc in Treatment-Naïve Patients and in Selected Settings
Potential use of maraviroc in treatment-naïve patients
Maraviroc has favorable antiviral interactions (additivity or slight synergy) with NRTIs, NNRTIs, PIs and enfuvirtide,15 and thus, incorporating maraviroc in drug regimens could increase viral suppression in patients. Although maraviroc is currently indicated for treatment-experienced patients carrying R5 HIV-1 strains, anticipated results from a Phase II vicriviroc study (initiated in January, 2008) could recommend vicriviroc, and perhaps maraviroc, for treatment-naïve patients.99 The trial initially included 95 treatment-naïve patients with R5 HIV taking vicriviroc plus ritonavir-boosted atazanavir vs. Truvada (emtricitabine and tenofovir, two NRTIs) plus ritonavir-boosted atazanavir. Following a formal interim analysis and safety data review at week 24, the study was expanded to 105 additional patients. The primary efficacy end-point will be the mean change from baseline in viral loads at week 48. Should vicriviroc demonstrate non-inferiority compared to Truvada, the data will suggest that vicriviroc, and possibly maraviroc, could offer a new first-line therapy option in treatment-naïve patients. Because 80% to 90% of treatment-naïve patients carry R5 HIV-1 strains only,100 first-line therapy with CCR5 antagonists could benefit many patients by preserving other antiretroviral classes for later treatment.
Potential use of maraviroc in prevention of HIV-1 transmission
To date, topical administration of the CCR5 antagonist CMPD-167 or RANTES analogs (PSC-RANTES, 5P12-RANTES and 6P4-RANTES) has demonstrated efficacy in a macaque model of vaginal R5-tropic SHIV-1 transmission.101-103 Vaginal transmission was prevented (8/10 and 5/5 protected animals for CMPD-167 and for each RANTES analog, respectively) but only at high doses (5 mM CMPD-167 and 1 mM RANTES analog), probably due to drug delivery pharmacology.104 Encouraging data demonstrate that antiviral synergy by different inhibitors prevents viral transmission at lower effective doses.105
Oral administration of CMPD-167 has shown efficacy in preventing R5-SHIV-1 transmission, but only at doses higher than those used topically.106 Importantly, however, oral administration of maraviroc gives similar drug concentrations in plasma and cervicovaginal fluid, suggesting that achievable maraviroc doses might prevent viral transmission.107 Thus, the potential use of maraviroc, perhaps in synergistic combinations with other entry inhibitors, in pre- and post-exposure prophylaxis against HIV-1 transmission requires further investigation.
Potential use of maraviroc in solid-organ transplantation in HIV-1 infection
Since the introduction of HAART, HIV-1 patients are living longer and comorbidities such as heart, liver and kidney disease are becoming serious medical problems.108,109 Among HIV-1 patients living in New York City, 22% of African American and 11.4% of Whites have either chronic kidney disease or end-stage renal disease.110 In France, the proportion of HIV-1 patients dying of end-stage liver disease has increased from 2% in 1995 to 17% in 2005.111 As a result of increased morbidity and mortality rates in HIV-1 patients with end-stage organ disease,112,113 many transplant centers have begun to transplant organs into selected patients.114-116 Transplant patients receive both antiretrovirals and immunosuppressants (commonly cyclosporine, tacrolimus, mycophenolic acid and rapamycin), but there is no consensus on drug combinations.115,117,118 The combinations of mycophenolic acid and zidovudine (AZT) or stavudine (D4T) are generally avoided118 due to antiviral drug antagonism.119 Because many immunosuppressants are metabolized by the cytochrome P450 (CYP450) enzyme system (mainly through the CYP3A4 isoform),120 which is inhibited by PIs and induced by NNRTIs,121 co-administration of immunosuppressants and PIs/NNRTIs often results in significant drug interactions. These drug interactions can perturb drug levels and thereby lead to toxicity, insufficient immunosuppression and reduced HIV-1 control.118 For these reasons, close monitoring and adjustment of drug levels in patients treated with immunosuppressants and HAART are of critical importance.118 Since maraviroc and other CCR5 antagonists are neither inhibitors nor inducers of CYP3A4, their use in transplant patients may prevent potentially harmful drug interactions resulting from altered immunosuppressant blood levels. In addition, combinations of immunosuppressants and CCR5 antagonists will have lesser effects on blood levels of CYP3A4-substrate non-HIV-1 medications (many macrolide antibiotics, statins and psychotropic drugs) for the treatment of comorbidities especially frequent in older patients. In this regard, a retrospective study of HIV-1 patients older than 55 in New York City found that 89% had comorbidities and 81% were taking non-HIV medications.122
A recent analysis of 100 HIV-1 patients receiving kidney transplants demonstrated 1-year patient survival was similar between HIV-1-infected and uninfected groups (95.4% and 96.2%, for infected and uninfected, respectively; P = 0.32).123 However, 1-year organ survival was significantly lower for HIV-1 patients (87.9% vs. 94.6%; P = 0.03). After subsequent subgroup analyses, the different outcomes were explained by several risk factors, especially older donor age and delayed graft function (DGF) in HIV-infected recipients. As in previous studies,114,124 the increased susceptibility of HIV-1 transplant patients to the detrimental effects of DGF was related to nephrotoxicity by combinations of calcineurin inhibitors and PIs. Because CCR5 antagonists, unlike PIs and NNRTIs, do not alter calcineurin inhibitor concentrations,125 their use could provide a safer antiretroviral option. In addition, lower transplant rejection rates in individuals lacking CCR5 expression (CCR5Δ32 homozygous)126 or following CCR5 blockade,127,128 further supports the idea that CCR5 antagonists will prolong survival of the transplanted organ in HIV-1 patients.
Potential use of maraviroc in other settings
As recently reviewed by Soriano et al,129 maraviroc may provide a convenient antiretroviral option for HIV-1 patients with an increased risk for heart disease. Commonly used PIs and NRTIs (most notably abacavir and didanosine) are associated with increased risk of cardiovascular events,130,131 and thus, switching to a maraviroc-containing regimen could be beneficial for those patients with both R5 HIV-1 and increased risk for heart disease. Likewise, since HIV-1 entry inhibitors are associated with a recovery of CD4+ counts even in the absence of complete viral suppression, maraviroc could serve as a strategy for immunological restoration in selected patients.132,133 It is also possible that maraviroc will improve treatment options for HIV-1 patients with tuberculosis and for those coinfected with hepatitis viruses.129
Summary
The recently developed antiretroviral classes of entry and integrase inhibitors offer much-needed treatment options for patients with drug-resistant HIV-1 infection. The fusion inhibitor enfuvirtide and the small-molecule CCR5 antagonist maraviroc are the first two licensed entry inhibitors, while vicriviroc and the CCR5 antibodies PRO 140 and HGS004 are in clinical trials.7,8,134 Entry inhibitors are currently recommended for patients with drug-resistant HIV-1. Favorable pharmacokinetic and antiviral interactions between entry inhibitors and drugs from the NRTI, NNRTI, PI and integrase inhibitor classes suggest that their combinations will be conveniently administered.15,59 Importantly, combinations of small-molecule CCR5 antagonists and CCR5 antibodies exhibit potent antiviral synergies, probably explained by their binding to different regions of CCR5 and thereby disruption of sequential steps on viral entry.135-138 Due to coreceptor specificity, the use of CCR5 inhibitors is limited to patients lacking X4 viruses. However, since 50% to 62% of patients with drug-resistant HIV-1 carry R5 HIV-1 only,139 maraviroc and other CCR5 inhibitors will benefit a majority of the treatment-experienced HIV-1 infected population. Moreover, with expanded access of antiretroviral therapy to other countries, CCR5 inhibitors could be particularly effective against Subtype C HIV-1, which rarely switches to CXCR4 coreceptor use, accounts for >50% infections worldwide, and is the most prevalent subtype in sub-Saharan Africa and parts of Asia.56,140
In addition, maraviroc (and perhaps other CCR5 inhibitors) could improve treatment for the increasing numbers of older HIV-1 patients with organ failure receiving organ transplants.115,141 Because maraviroc does not inhibit or induce CYP3A4, combinations of maraviroc and transplantation immunosuppressants have low potential for organ rejection secondary to insufficient immunosuppression or for toxicity secondary to overdosing. Of note, our results demonstrate that selected immunosuppressants reduce CCR5 expression and have synergistic antiviral activities with both enfuvirtide and CCR5 antagonists.44,46,47,142 Moreover, CCR5 antagonists prevent acute and chronic rejection of transplanted organs,127,128 providing an additional potential benefit for HIV-1 infected transplant recipients. Prevention of vaginal SHIV transmission in animal models by CCR5 inhibitors,101-103,105,106 coupled with achievement of high levels of maraviroc in cervicovaginal fluid following oral administration,107 suggest that maraviroc could curtail sexual transmission of HIV-1, which is virtually always driven by R5 strains.143
Finally, results from a vicriviroc trial in treatment-naïve HIV-1 patients, could lead to the use of CCR5 antagonists as a new first-line therapy, preserving drugs from other classes for later treatment.99
Acknowledgements
We thank Dr. Robert R. Redfield and Dr. Robert C. Gallo for their continual mentoring and support. This project was supported in part by NIH grant AI084417 to A.H.
The authors grant exclusive rights to all commercial reproduction and distribution to Libertas Academica. Commercial reproduction and distribution rights are reserved by Libertas Academica. No unauthorised commercial use permitted without express consent of Libertas Academica. Contact tom.hill@la-press.com for further information.
Footnotes
This is an open access article distributed under the terms of the Creative Commons Attribution License (http://www.creativecommons.org/licenses/by/2.0) which permits unrestricted use, distribution and reproduction provided the original work is properly cited.
Disclosures
The authors report no conflicts of interest.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14(3):309–18. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- 3.Yerly S, Vora S, Rizzardi P, et al. Acute HIV infection: impact on the spread of HIV and transmission of drug resistance. Aids. 2001;15(17):2287–92. doi: 10.1097/00002030-200111230-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kuhmann SE, Hartley O. Targeting chemokine receptors in HIV: a status report. Annu Rev Pharmacol Toxicol. 2008;48:425–61. doi: 10.1146/annurev.pharmtox.48.113006.094847. [DOI] [PubMed] [Google Scholar]
- 5.Robertson D. US FDA approves new class of HIV therapeutics. Nat Biotechnol. 2003;21(5):470–1. doi: 10.1038/nbt0503-470. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez E. Maraviroc—new HIV drug. Emerging options need to be used wisely. Posit Aware. 2007;18(4):18–9. [PubMed] [Google Scholar]
- 7.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196(2):304–12. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson JM, Saag MS, Thompson MA, et al. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis. 2008;198(9):1345–52. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- 9.Lalezari J, Yadavalli GK, Para M, et al. Safety, pharmacokinetics, and antiviral activity of HGS004, a novel fully human IgG4 monoclonal antibody against CCR5, in HIV-1-infected patients. J Infect Dis. 2008;197(5):721–7. doi: 10.1086/527327. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson JM, Kuritzkes DR, Godofsky E, et al. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53(2):450–7. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavirritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369(9568):1169–78. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 12.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369(9569):1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 13.Lalezari JP, Henry K, O’Hearn M, et al. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348(22):2175–85. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 14.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 15.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–32. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 17.Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85(7):1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 18.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381(6584):661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 21.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96(9):5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael NL, Chang G, Louie LG, et al. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997a;3(3):338–40. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 23.Michael NL, Louie LG, Rohrbaugh AL, et al. The role of CCR5 and CCR2 polymorphisms in HIV-1 transmission and disease progression. Nat Med. 1997b;3(10):1160–2. doi: 10.1038/nm1097-1160. [DOI] [PubMed] [Google Scholar]
- 24.Brumme ZL, Goodrich J, Mayer HB, et al. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. J Infect Dis. 2005;192(3):466–74. doi: 10.1086/431519. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272(5263):872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 26.Moyle GJ, Wildfire A, Mandalia S, et al. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J Infect Dis. 2005;191(6):866–72. doi: 10.1086/428096. [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86(3):367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 28.Smith MW, Dean M, Carrington M, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277(5328):959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 29.Huang CC, Tang M, Zhang MY, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310(5750):1025–8. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dragic T, Trkola A, Thompson DA, et al. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci U S A. 2000;97(10):5639–44. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondru R, Zhang J, Ji C, et al. Molecular interactions of CCR5 with major classes of small-molecule anti-HIV CCR5 antagonists. Mol Pharmacol. 2008;73(3):789–800. doi: 10.1124/mol.107.042101. [DOI] [PubMed] [Google Scholar]
- 32.Maeda K, Das D, Ogata-Aoki H, et al. Structural and molecular interactions of CCR5 inhibitors with CCR5. J Biol Chem. 2006;281(18):12688–98. doi: 10.1074/jbc.M512688200. [DOI] [PubMed] [Google Scholar]
- 33.Seibert C, Ying W, Gavrilov S, et al. Interaction of small molecule inhibitors of HIV-1 entry with CCR5. Virology. 2006;349(1):41–54. doi: 10.1016/j.virol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Tsamis F, Gavrilov S, Kajumo F, et al. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J Virol. 2003;77(9):5201–8. doi: 10.1128/JVI.77.9.5201-5208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amara A, Gall SL, Schwartz O, et al. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186(1):139–46. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mack M, Luckow B, Nelson PJ, et al. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187(8):1215–24. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72(4):2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt EJ, Durnin JP, Kabat D. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J Virol. 2005;79(7):4347–56. doi: 10.1128/JVI.79.7.4347-4356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A. 2002;99(25):16249–54. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves JD, Miamidian JL, Biscone MJ, et al. Impact of mutations in the coreceptor binding site on human immunodeficiency virus type 1 fusion, infection, and entry inhibitor sensitivity. J Virol. 2004;78(10):5476–85. doi: 10.1128/JVI.78.10.5476-5485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol. 1999;163(4):2306–13. [PubMed] [Google Scholar]
- 42.Reynes J, Portales P, Segondy M, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181(3):927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 43.Heredia A, Gilliam B, Devico A, et al. CCR5 density levels on primary CD4 T cells impact the replication and Enfuvirtide susceptibility of R5 HIV-1. Aids. 2007a;21(10):1317–22. doi: 10.1097/QAD.0b013e32815278ea. [DOI] [PubMed] [Google Scholar]
- 44.Heredia A, Gilliam B, Latinovic O, et al. Rapamycin reduces CCR5 density levels on CD4 T cells and this effect results in potentiation of Enfuvirtide (T-20) against R5 HIV-1 in vitro. Antimicrob Agents Chemother. 2007b;51:2482–96. doi: 10.1128/AAC.01602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willey S, Peters PJ, Sullivan WM, Dorr P, Perros M, Clapham PR. Inhibition of CCR5-mediated infection by diverse R5 and R5X4 HIV and SIV isolates using novel small molecule inhibitors of CCR5: effects of viral diversity, target cell and receptor density. Antiviral Res. 2005;68(2):96–108. doi: 10.1016/j.antiviral.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Heredia A, Amoroso A, Davis C, et al. Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV beta-chemokines: an approach to suppress R5 strains of HIV-1. Proc Natl Acad Sci U S A. 2003;100(18):10411–6. doi: 10.1073/pnas.1834278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heredia A, Latinovic O, Gallo RC, Melikyan GB, Reitz MNL, Redfield R. Reduction of CCR5 with low-dose Rapamycin enhances the antiviral activity of Vicriviroc against both sensitive and drug-resistant HIV-1. Proc Natl Acad Sci U S A. 2008;105(51):20476–81. doi: 10.1073/pnas.0810843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94(5):1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mummidi S, Ahuja SS, McDaniel BL, Ahuja SK. The human CC chemokine receptor 5 (CCR5) gene. Multiple transcripts with 5′-end heterogeneity, dual promoter usage, and evidence for polymorphisms within the regulatory regions and noncoding exons. J Biol Chem. 1997;272(49):30662–71. doi: 10.1074/jbc.272.49.30662. [DOI] [PubMed] [Google Scholar]
- 50.Liu R, Zhao X, Gurney TA, Landau NR. Functional analysis of the proximal CCR5 promoter. AIDS Res Hum Retroviruses. 1998;14(17):1509–19. doi: 10.1089/aid.1998.14.1509. [DOI] [PubMed] [Google Scholar]
- 51.Mummidi S, Adams LM, VanCompernolle SE, et al. Production of specific mRNA transcripts, usage of an alternate promoter, and octamer-binding transcription factors influence the surface expression levels of the HIV coreceptor CCR5 on primary T cells. J Immunol. 2007;178(9):5668–81. doi: 10.4049/jimmunol.178.9.5668. [DOI] [PubMed] [Google Scholar]
- 52.Moriuchi M, Moriuchi H. Octamer transcription factors up-regulate the expression of CCR5, a coreceptor for HIV-1 entry. J Biol Chem. 2001;276(12):8639–42. doi: 10.1074/jbc.M008391200. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez E, Kulkarni H, Bolivar H, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307(5714):1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 54.Paxton WA, Liu R, Kang S, et al. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of beta-chemokines. Virology. 1998;244(1):66–73. doi: 10.1006/viro.1998.9082. [DOI] [PubMed] [Google Scholar]
- 55.Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2(4):412–7. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 56.Geretti AM. HIV-1 subtypes: epidemiology and significance for HIV management. Curr Opin Infect Dis. 2006;19(1):1–7. doi: 10.1097/01.qco.0000200293.45532.68. [DOI] [PubMed] [Google Scholar]
- 57.Kwong PD, Doyle ML, Casper DJ, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420(6916):678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 58.Fatkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11(11):1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 59.Strizki JM, Tremblay C, Xu S, et al. Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2005;49(12):4911–9. doi: 10.1128/AAC.49.12.4911-4919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abel S, van der Ryst E, Muirhead G. Pharmacokinetics of single and multiple oral doses of UK-427,857—a novel CCR5 antagonist in healthy volunteers; Abstracts of the Tenth Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2003; Alexandria, VA, USA: Foundation for Retrovirology and Human Health. 2003;Abstract 547. [Google Scholar]
- 61.Abel S, Russell D, Whitlock LA, Ridgway CE, Nedderman AN, Walker DK. Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br J Clin Pharmacol. 2008c;65(Suppl 1):60–7. doi: 10.1111/j.1365-2125.2008.03137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abel S, Jenkins TM, Whitlock LA, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inducers with and without CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008a;65(Suppl 1):38–46. doi: 10.1111/j.1365-2125.2008.03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abel S, Russell D, Taylor-Worth RJ, Ridgway CE, Muirhead GJ. Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008b;65(Suppl 1):27–37. doi: 10.1111/j.1365-2125.2008.03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker DK, Bowers SJ, Mitchell RJ, Potchoiba MJ, Schroeder CM, Small HF. Preclinical assessment of the distribution of maraviroc to potential human immunodeficiency virus (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT) Xenobiotica. 2008;38(10):1330–9. doi: 10.1080/00498250802447409. [DOI] [PubMed] [Google Scholar]
- 65.Tagat JR, McCombie SW, Nazareno D, et al. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-[2-methoxy-1(R)-4-(trifluoromethyl)phenyl] ethyl-3(S)-methyl-1-piperaz inyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J Med Chem. 2004;47(10):2405–8. doi: 10.1021/jm0304515. [DOI] [PubMed] [Google Scholar]
- 66.Schurmann D, Fatkenheuer G, Reynes J, et al. Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS. 2007;21(10):1293–9. doi: 10.1097/QAD.0b013e3280f00f9f. [DOI] [PubMed] [Google Scholar]
- 67.Walker DK, Abel S, Comby P, Muirhead GJ, Nedderman AN, Smith DA. Species differences in the disposition of the CCR5 antagonist, UK-427,857, a new potential treatment for HIV. Drug Metab Dispos. 2005;33(4):587–95. doi: 10.1124/dmd.104.002626. [DOI] [PubMed] [Google Scholar]
- 68.Tsibris AM, Korber B, Arnaout R, et al. Quantitative deep sequencing reveals dynamic HIV-1 escape and large population shifts during CCR5 antagonist therapy in vivo. PLoS One. 2009;4(5):e5683. doi: 10.1371/journal.pone.0005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsibris AM, Sagar M, Gulick RM, et al. In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject. J Virol. 2008;82(16):8210–4. doi: 10.1128/JVI.00444-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Westby M, Lewis M, Whitcomb J, et al. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol. 2006;80(10):4909–20. doi: 10.1128/JVI.80.10.4909-4920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bozzette SA, McCutchan JA, Spector SA, Wright B, Richman DD. A cross-sectional comparison of persons with syncytium- and non-syncytium-inducing human immunodeficiency virus. J Infect Dis. 1993;168(6):1374–9. doi: 10.1093/infdis/168.6.1374. [DOI] [PubMed] [Google Scholar]
- 72.Alkhatib G, Broder CC, Berger EA. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70(8):5487–94. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poveda E, Briz V, Quinones-Mateu M, Soriano V. HIV tropism: diagnostic tools and implications for disease progression and treatment with entry inhibitors. AIDS. 2006;20(10):1359–67. doi: 10.1097/01.aids.0000233569.74769.69. [DOI] [PubMed] [Google Scholar]
- 74.Low AJ, Dong W, Chan D, et al. Current V3 genotyping algorithms are inadequate for predicting X4 co-receptor usage in clinical isolates. AIDS. 2007;21(14):F17–24. doi: 10.1097/QAD.0b013e3282ef81ea. [DOI] [PubMed] [Google Scholar]
- 75.Harrigan PR, McGovern R, Dong W, et al. Screening for HIV Tropism Using Population-Based V3 Genotypic Analysis: A Retrospective Virological Outcome Analysis Using Stored Plasma Screening Samples From MOTIVATE 1; 18th HIV Drug Resistance Workshop; Ft. Myers Florida. 2009 June 9–12.2009. [Google Scholar]
- 76.Archer J, Braverman MS, Taillon BE, et al. Detection of low-frequency pretherapy chemokine (CXC motif) receptor 4 (CXCR4)-using HIV-1 with ultra-deep pyrosequencing. AIDS. 2009;23(10):1209–18. doi: 10.1097/QAD.0b013e32832b4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med. 2008;359(14):1442–55. doi: 10.1056/NEJMoa0803154. [DOI] [PubMed] [Google Scholar]
- 78.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359(14):1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goodrich JM, Saag M, van der Ryst E. 48-week safety and efficacy of maraviroc, a novel CCR5 antagonist, in combination with optimized background therapy (OBT) for the treatment of antiretroviral-experienced patients infected with dual/mixed-tropic HIV-1; Program and abstracts of the 45th Annual Meeting of the Infectious Disease Society of America; San Diego, CA. 2007 October 4–7; Abstract LB-2. [Google Scholar]
- 80.Saag M, Goodrich J, Fatkenheuer G, et al. A Double-Blind, Placebo-Controlled Trial of Maraviroc in Treatment-Experienced Patients Infected with Non-R5 HIV-1. J Infect Dis. 2009;199(11):1638–47. doi: 10.1086/598965. [DOI] [PubMed] [Google Scholar]
- 81.Saag M, Prudence I, Heera J. A multicenter, randomized, double-blind, comparative trial of a novel CCR5 antagonist, maraviroc versus efavirenz, both in combination with Combivir (zidovudine [ZDV]/lamivudine [3TC]), for the treatment of antiretroviral naive patients infected with R5 HIV 1: week 48 results of the MERIT study; Abstracts of the Fourth International AIDS Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. 2007; Abstract WESS 104. [Google Scholar]
- 82.Vandekerckhove L, Verhofstede C, Vogelaers D. Maraviroc: perspectives for use in antiretroviral-naive HIV-1-infected patients. J Antimicrob Chemother. 2009;63(6):1087–96. doi: 10.1093/jac/dkp113. [DOI] [PubMed] [Google Scholar]
- 83.Nichols WG, Steel HM, Bonny T, et al. Hepatotoxicity observed in clinical trials of aplaviroc ( GW873140) Antimicrob Agents Chemother. 2008;52(3):858–65. doi: 10.1128/AAC.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Landovitz RJ, Angel JB, Hoffmann C, et al. Phase II study of vicriviroc versus efavirenz (both with zidovudine/lamivudine) in treatment-naive subjects with HIV-1 infection. J Infect Dis. 2008;198(8):1113–22. doi: 10.1086/592052. [DOI] [PubMed] [Google Scholar]
- 85.Westby M, Smith-Burchnell C, Mori J, et al. Reduced maximal inhibition in phenotypic susceptibility assays indicates that viral strains resistant to the CCR5 antagonist maraviroc utilize inhibitor-bound receptor for entry. J Virol. 2007;81(5):2359–71. doi: 10.1128/JVI.02006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pugach P, Marozsan AJ, Ketas TJ, Landes EL, Moore JP, Kuhmann SE. HIV-1 clones resistant to a small molecule CCR5 inhibitor use the inhibitor-bound form of CCR5 for entry. Virology. 2007;361(1):212–28. doi: 10.1016/j.virol.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuhmann SE, Pugach P, Kunstman KJ, et al. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J Virol. 2004;78(6):2790–807. doi: 10.1128/JVI.78.6.2790-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marozsan AJ, Kuhmann SE, Morgan T, et al. Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D) Virology. 2005;338(1):182–99. doi: 10.1016/j.virol.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 89.Ogert RA, Wojcik L, Buontempo C, et al. Mapping resistance to the CCR5 co-receptor antagonist vicriviroc using heterologous chimeric HIV-1 envelope genes reveals key determinants in the C2-V5 domain of gp120. Virology. 2008;373(2):387–99. doi: 10.1016/j.virol.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Berro R, Sanders RW, Lu M, Klasse PJ, Moore JP. Two HIV-1 variants resistant to small molecule CCR5 inhibitors differ in how they use CCR5 for entry. PLoS Pathog. 2009;5(8):e1000548. doi: 10.1371/journal.ppat.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin G, Bertolotti-Ciarlet A, Haggarty B, et al. Replication-competent variants of human immunodeficiency virus type 2 lacking the V3 loop exhibit resistance to chemokine receptor antagonists. J Virol. 2007;81(18):9956–66. doi: 10.1128/JVI.00385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nolan KM, Del Prete GQ, Jordan AP, et al. Characterization of a human immunodeficiency virus type 1 V3 deletion mutation that confers resistance to CCR5 inhibitors and the ability to use aplaviroc-bound receptor. J Virol. 2009;83(8):3798–809. doi: 10.1128/JVI.01751-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anastassopoulou CG, Ketas TJ, Klasse PJ, Moore JP. Resistance to CCR5 inhibitors caused by sequence changes in the fusion peptide of HIV-1 gp41. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mascolini M, Richman D, Larder B, Mellors J, Boucher CA. Clinical implications of resistance to antiretrovirals: new resistance technologies and interpretations. Antivir Ther. 2008;13(2):319–34. [PubMed] [Google Scholar]
- 95.Pugach P, Ketas TJ, Michael E, Moore JP. Neutralizing antibody and antiretroviral drug sensitivities of HIV-1 isolates resistant to small molecule CCR5 inhibitors. Virology. 2008;377(2):401–7. doi: 10.1016/j.virol.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maeda K, Nakata H, Koh Y, et al. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J Virol. 2004;78(16):8654–62. doi: 10.1128/JVI.78.16.8654-8662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watson C, Jenkinson S, Kazmierski W, Kenakin T. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric noncompetitive HIV entry inhibitor. Mol Pharmacol. 2005;67(4):1268–82. doi: 10.1124/mol.104.008565. [DOI] [PubMed] [Google Scholar]
- 98.Wang T, Duan Y. Binding modes of CCR5-targetting HIV entry inhibitors: partial and full antagonists. J Mol Graph Model. 2008;26(8):1287–95. doi: 10.1016/j.jmgm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schering-Plough . Shering-Plough expands vicriviroc Phase II study in treatment-naive patients with HIV. Press Release; Jul 17, 2009. [Google Scholar]
- 100.Hoffmann C. The epidemiology of HIV coreceptor tropism. Eur J Med Res. 2007;12(9):385–90. [PubMed] [Google Scholar]
- 101.Lederman MM, Veazey RS, Offord R, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306(5695):485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 102.Veazey RS, Klasse PJ, Ketas TJ, et al. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198(10):1551–62. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Veazey RS, Ling B, Green LC, et al. Topically Applied Recombinant Chemokine Analogues Fully Protect Macaques from Vaginal Simian-Human Immunodeficiency Virus Challenge. J Infect Dis. 2009;199(10):1525–7. doi: 10.1086/598685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klasse PJ, Shattock RJ, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3(9):e351. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005a;438(7064):99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 106.Veazey RS, Springer MS, Marx PA, Dufour J, Klasse PJ, Moore JP. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005b;11(12):1293–4. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 107.Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc Concentrates in the Cervicovaginal Fluid and Vaginal Tissue of HIV-Negative Women. J Acquir Immune Defic Syndr. 2009:546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20(16):2051–64. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 109.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 1):S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 110.Wyatt CM, Winston JA, Malvestutto CD, et al. Chronic kidney disease in HIV infection: an urban epidemic. AIDS. 2007;21(15):2101–3. doi: 10.1097/QAD.0b013e3282ef1bb4. [DOI] [PubMed] [Google Scholar]
- 111.Rosenthal E, Salmon-Ceron D, Lewden C, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 Study in collaboration with the Mortalite 2005 survey, ANRS EN19) HIV Med. 2009;10(5):282–9. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 112.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretrans-plant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver Transpl. 2005;11(11):1425–30. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 113.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 114.Kumar MS, Sierka DR, Damask AM, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67(4):1622–9. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 115.Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8(2):355–65. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 116.Stock PG, Roland ME, Carlson L, et al. Kidney and liver transplantation in human immunodeficiency virus-infected patients: a pilot safety and efficacy study. Transplantation. 2003;76(2):370–5. doi: 10.1097/01.TP.0000075973.73064.A6. [DOI] [PubMed] [Google Scholar]
- 117.Carlson L. Clinical management of the HIV-positive kidney transplant recipient. Nephrol Nurs J. 2008;35(6):559–67. quiz 568. [PubMed] [Google Scholar]
- 118.Frassetto LA, Browne M, Cheng A, et al. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant. 2007;7(12):2816–20. doi: 10.1111/j.1600-6143.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 119.Margolis D, Heredia A, Gaywee J, Oldach D, Drusano G, Redfield R. Abacavir and mycophenolic acid, an inhibitor of inosine monophosphate dehydrogenase, have profound and synergistic anti-HIV activity. J Acquir Immune Defic Syndr. 1999;21(5):362–70. [PubMed] [Google Scholar]
- 120.Dooley KE, Flexner C, Andrade AS. Drug interactions involving combination antiretroviral therapy and other anti-infective agents: repercussions for resource-limited countries. J Infect Dis. 2008;198(7):948–61. doi: 10.1086/591459. [DOI] [PubMed] [Google Scholar]
- 121.Long MC, King JR, Acosta EP. Pharmacologic aspects of new antiretroviral drugs. Curr HIV/AIDS Rep. 2009;6(1):43–50. doi: 10.1007/s11904-009-0007-y. [DOI] [PubMed] [Google Scholar]
- 122.Shah SS, McGowan JP, Smith C, Blum S, Klein RS. Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clin Infect Dis. 2002;35(10):1238–43. doi: 10.1086/343048. [DOI] [PubMed] [Google Scholar]
- 123.Locke JE, Montgomery RA, Warren DS, Subramanian A, Segev DL. Renal transplant in HIV-positive patients: long-term outcomes and risk factors for graft loss. Arch Surg. 2009;144(1):83–6. doi: 10.1001/archsurg.2008.508. [DOI] [PubMed] [Google Scholar]
- 124.Granville DJ, Tashakkor B, Takeuchi C, et al. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci U S A. 2004;101(5):1321–6. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hughes A, Barber T, Nelson M. New treatment options for HIV salvage patients: an overview of second generation PIs, NNRTIs, integrase inhibitors and CCR5 antagonists. J Infect. 2008;57(1):1–10. doi: 10.1016/j.jinf.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 126.Fischereder M, Luckow B, Hocher B, et al. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357(9270):1758–61. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- 127.Akashi S, Sho M, Kashizuka H, et al. A novel small-molecule compound targeting CCR5 and CXCR3 prevents acute and chronic allograft rejection. Transplantation. 2005;80(3):378–84. doi: 10.1097/01.tp.0000166338.99933.e1. [DOI] [PubMed] [Google Scholar]
- 128.Schroder C, Pierson RN, 3rd, Nguyen BN, et al. CCR5 blockade modulates inflammation and alloimmunity in primates. J Immunol. 2007;179(4):2289–99. doi: 10.4049/jimmunol.179.4.2289. [DOI] [PubMed] [Google Scholar]
- 129.Soriano V, Geretti AM, Perno CF, et al. Optimal use of maraviroc in clinical practice. AIDS. 2008;22(17):2231–40. doi: 10.1097/QAD.0b013e3283136d95. [DOI] [PubMed] [Google Scholar]
- 130.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003a;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 131.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS. 2003b;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 132.Aquaro S, D’Arrigo R, Svicher V, et al. Specific mutations in HIV-1 gp41 are associated with immunological success in HIV-1-infected patients receiving enfuvirtide treatment. J Antimicrob Chemother. 2006;58(4):714–22. doi: 10.1093/jac/dkl306. [DOI] [PubMed] [Google Scholar]
- 133.Svicher V, Aquaro S, D’Arrigo R, et al. Specific enfuvirtide-associated mutational pathways in HIV-1 Gp41 are significantly correlated with an increase in CD4(+) cell count, despite virological failure. J Infect Dis. 2008;197(10):1408–18. doi: 10.1086/587693. [DOI] [PubMed] [Google Scholar]
- 134.Lalezari J, Thompson M, Kumar P, et al. Antiviral activity and safety of 873140, a novel CCR5 antagonist, during short-term monotherapy in HIV-infected adults. AIDS. 2005;19(14):1443–8. doi: 10.1097/01.aids.0000183633.06580.8a. [DOI] [PubMed] [Google Scholar]
- 135.Ji C, Zhang J, Dioszegi M, et al. CCR5 small-molecule antagonists and monoclonal antibodies exert potent synergistic antiviral effects by cobinding to the receptor. Mol Pharmacol. 2007;72(1):18–28. doi: 10.1124/mol.107.035055. [DOI] [PubMed] [Google Scholar]
- 136.Murga JD, Franti M, Pevear DC, Maddon PJ, Olson WC. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2006;50(10):3289–96. doi: 10.1128/AAC.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olson WC, Rabut GE, Nagashima KA, et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73(5):4145–55. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Safarian D, Carnec X, Tsamis F, Kajumo F, Dragic T. An anti-CCR5 monoclonal antibody and small molecule CCR5 antagonists synergize by inhibiting different stages of human immunodeficiency virus type 1 entry. Virology. 2006;352(2):477–84. doi: 10.1016/j.virol.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 139.Melby T, Despirito M, Demasi R, Heilek-Snyder G, Greenberg ML, Graham N. HIV-1 coreceptor use in triple-class treatment-experienced patients: baseline prevalence, correlates, and relationship to enfuvirtide response. J Infect Dis. 2006;194(2):238–46. doi: 10.1086/504693. [DOI] [PubMed] [Google Scholar]
- 140.Pollakis G, Kang S, Kliphuis A, Chalaby MI, Goudsmit J, Paxton WA. N-linked glycosylation of the HIV type-1 gp120 envelope glycoprotein as a major determinant of CCR5 and CXCR4 coreceptor utilization. J Biol Chem. 2001;276(16):13433–41. doi: 10.1074/jbc.M009779200. [DOI] [PubMed] [Google Scholar]
- 141.Roland ME, Stock PG. Solid organ transplantation is a reality for patients with HIV infection. Curr HIV/AIDS Rep. 2006;3(3):132–8. doi: 10.1007/BF02696657. [DOI] [PubMed] [Google Scholar]
- 142.Latinovic O, Heredia A, Gallo RC, Reitz M, Le N, Redfield RR. Rapamycin enhances aplaviroc anti-HIV activity: Implications for the clinical development of novel CCR5 antagonists. Antiviral Res. 2009;83(1):86–9. doi: 10.1016/j.antiviral.2009.02.199. [DOI] [PubMed] [Google Scholar]
- 143.Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9(7):847–52. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 144.Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci U S A. 2003;100(19):10598–602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melikyan GB. Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm. Retrovirology. 2008;5:111. doi: 10.1186/1742-4690-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Westby M. Resistance to CCR5 antagonists. Curr Opin HIV AIDS. 2007;2(2):137–44. doi: 10.1097/COH.0b013e3280142007. [DOI] [PubMed] [Google Scholar]