Abstract
Objective
To review the current status of antibiotic prophylaxis for cesarean delivery, newly evolving strategies to enhance the effectiveness of antibiotic prophylaxis in reducing post-cesarean infection, and the implications of the emerging practices.
Data Sources
We conducted a full PubMed (January 1966-July 2008) search using the key words “cesarean section” and “antibiotic prophylaxis.” A total of 277 articles were identified and supplemented by a bibliographic search.
Methods of Study Selection
We selected a total of 15 studies which included all published clinical trials, meta-analyses of clinical trials, and observational studies evaluating either the timing of antibiotics or the use of extended-spectrum prophylaxis. We also reviewed nine reports involving national recommendations or technical reviews supporting current standards for antibiotic prophylaxis.
Tabulation, Integration, and Results
We conducted an analytic review and tabulation of selected studies without further meta-analysis. Although current guidelines for antibiotic prophylaxis recommend the administration of narrow-spectrum antibiotics (cefazolin) after clamping of the umbilical cord, the data suggest that antibiotic administration prior to surgical incision or the use of extended-spectrum regimens (involving azithromycin or metronidazole) after cord clamp may reduce postcesarean maternal infection by up to 50%. However, their impact relative to each other has not been studied. In addition, their impact on neonatal infection or infection with resistant organisms warrants further study.
Conclusion
The use of either cefazolin alone prior to surgical incision or an extended-spectrum regimen after cord clamp appears to be associated with a reduction in postcesarean maternal infection. Confirmatory studies focusing additionally on neonatal outcomes and the impact on resistant organisms, as well as studies comparing both strategies, are needed.
Introduction
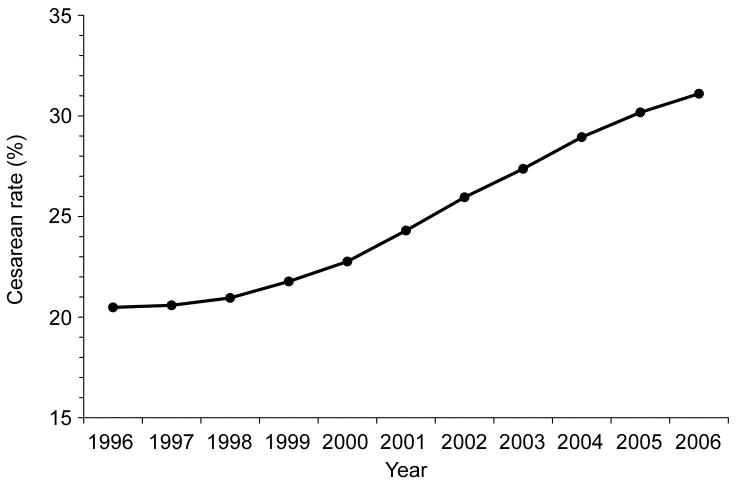
Prophylactic antibiotics reduce surgical site infections1–4 and evidence-based national guidelines recommend their administration prior to surgical incision.2–3,5 An exception to this pre-incision prophylactic approach is cesarean delivery, the most common major surgical procedure in the United States6 and elsewhere. Driven by concerns about the sequelae of fetal antibiotic exposure with pre-incision administration, for nearly 30 years, the standard to prevent post-cesarean infection has been the administration of narrow spectrum antibiotic prophylaxis after delivery of the baby and clamping of the umbilical cord.4,7 Meanwhile, US cesarean delivery rates are increasing - from 20.7% in 1996 to 31.1% in 2006, an absolute increase of 50% over a decade (Figure I).8–9 The overall increase mirrors increases in both primary (laboring and non-laboring) cesareans and repeat cesareans. Recent data also indicate that primary cesareans in the absence of obstetric indications are rapidly rising, reflecting both shifting obstetric practices and maternal preference.10 If these trends continue, cesareans will make up approximately 50% of the more than 4 million annual deliveries by 2020. Therefore, the health and economic burden of post-cesarean infection will likely continue to rise. In this article, we review the current status of antibiotic prophylaxis for cesarean delivery, newly evolving strategies to enhance the effectiveness of antibiotic prophylaxis in reducing post-cesarean infection, and the implications of the emerging practices.2–3,7
Figure 1.
Trends in total cesarean delivery rate, US 1996–2006. Data from Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: Final data for 2005. Natl Vital Stat Rep 2007;56:1–103, and Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2006. Natl Vital Stat Rep. 2007;56:1–18. {AQ: Figure 1 redrawn by editorial office. Please review and indicate approval.}
Data Sources and Study Selection
We searched the entire PubMed computerized database from January 1966 through July, 2008, using the keywords “cesarean delivery” and “antibiotic prophylaxis.” All published studies (without restriction by national origin, language, and presence or absence of risk factors) focusing on national recommendations for antibiotic prophylaxis, timing of prophylaxis and/or extended spectrum regimens for cesarean delivery were selected for abstraction. Our definition of “extended-spectrum” antibiotic prophylaxis specifically refers to the combined use of the standard narrow-spectrum beta-lactam and a 2nd antibiotic of a different class. This is different from the use of broad spectrum penicillins (latter generation cephalosporins, ureidopenicillins or monobactams) or other single agents which have been generally demonstrated to be no more effective than the standard. A bibliographic review of selected studies was also used to supplement eligible studies.
The search produced 277 PubMed articles for preliminary abstract review. Case reports, descriptive studies, and letters to the editor as well as irrelevant studies were excluded. A total of 15 studies including all relevant clinical trials, metaanalyses or observational studies evaluating the timing of antibiotics or extended-spectrum prophylaxis for cesarean11–25 and 9 publications of national recommendations or metaanalyses supporting current standards for antibiotic prophylaxis were selected and fully reviewed.2–5,7,26–29 Key outcome measures were any post-cesarean maternal infection (primarily endometritis and wound infection based on standard definitions regardless of duration of follow-up), neonatal sepsis and sepsis work-ups. Descriptive data including study design, sample size and results for relevant studies were abstracted. Because of the varying study designs and the inclusion of metaanalyses, we conducted an analytic as opposed to a synthetic or metaanalytic review i.e. focused on analyzing study results without further metaanalysis. We present descriptive data for each study and comment on the relative risks of total maternal post-cesarean infection comprising endometritis and wound infection with or without other infections (e.g., urinary tract infection). We also present data for endometritis, wound infection; neonatal infection and/or hospital stay where available. Whenever possible we compute the relevant relative risk using data presented in the study reports. Finally we provide a review of the data supporting the rationale for each strategy (based primarily on bibliographic review of the selected articles) and comment on clinical and research implications for the use of the interventions.
Results
Current Standards for Cesarean Antibiotic Prophylaxis
Infections remain among the top 5 causes of pregnancy-related mortality and account for a disproportionate contribution to maternal morbidity, both in the US and around the world.30 Cesarean delivery is the single most important risk factor for postpartum infection. Compared to women delivered vaginally, those delivered by cesarean classically face a 5 to 20-fold increase in risk.31 The most common post-cesarean infections are surgical site infections (endomyometritis and wound infection) and infection of the urinary tract. Pelvic abscess, septic pelvic phlebitis, pneumonia and sepsis, although rare, are also increased. These infections are associated with considerable health and economic burdens.32 The incidence of post-cesarean infection varies widely by population profile depending on several risk factors. Low socioeconomic status, unscheduled delivery (e.g., due to labor arrest, failed induction, and fetal emergencies), and obesity feature among these risk factors for post-cesarean infection.31,33–34
Routine use of prophylactic antibiotics reduces the risk of post-cesarean fever and infections by over 50% from baseline rates as high as 20–50%.4 Based on systematic reviews of over eighty clinical trials, this benefit applies to both non-elective and elective (scheduled) procedures.4,26 Because antibiotic prophylaxis shortens overall length of hospitalization and reduces treatment costs associated with cesarean, it is highly cost-effective.27–28 Consequently, antibiotic prophylaxis is recommended for all women undergoing cesarean section.7 The American College of Obstetricians and Gynecologists (ACOG) specifically recommends a narrow-spectrum 1st generation cephalosporin (cefazolin) over ampicillin as the regimen of choice because of increasing microbial resistance to the latter.7
Despite currently recommended antibiotic prophylaxis protocols, at least 10% of cesareans overall are complicated by infection, and over 15% by fever.4 Fifteen to 80% of post-cesarean infections, particularly those involving wounds, may actually occur after initial discharge from the hospital.2,35 Therefore, underestimation of the incidence of post-cesarean infection is pervasive, particularly when based solely on in-patient data and/or follow-up duration of less than 4–6 weeks. Given the rising rates of cesarean delivery (Figure I), prevention of post-cesarean infection remains a public health priority.36 Evolving concepts affecting both the timing of antibiotic administration and the selection of antimicrobial agents have implications for addressing this priority.
Pre-Incision Antibiotic Prophylaxis
Rationale
Both animal models and clinical studies in non-pregnant patients suggest that prophylactic antibiotics are more effective if administered just prior to surgery (as opposed to long before, during, or after the procedure).1,37 Administration within 30–60 minutes of surgery is optimal; this maximizes tissue and blood antibiotic concentrations at surgical sites.2–3,5,29 Antibiotics commonly used for cesarean prophylaxis are rapidly transferred to the fetal compartment (within 2 hours for cefazolin) raising concerns that fetal exposure to antibiotics might mask infection in the neonate and promote the selection of resistant organisms.7 Thus, historically, pediatricians were inclined to perform invasive and costly sepsis work-ups on neonates who were exposed to antibiotics immediately prior to delivery.12 Moreover, 2 non-randomized studies of timing of antibiotic prophylaxis for cesarean (Table I) suggested that while pre-incision administration did not reduce post-cesarean infection,11–12 it did increase invasive neonatal sepsis evaluations and costs.10
Table 1.
Overview of Published Studies of Timing of Antibiotic Prophylaxis for Cesarean
| Study Description | Study Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Sample Size |
Antibiotic Prophylaxis (dose) |
Total Infection* |
Endometriti s* |
Wounds* | Neonatal Sepsis*‡ |
| Gordon, 197910 | RCT (Un-blinded) | 78 | Ampicillin (1g) | 1.40 (0.3, 5.9) | N/A | N/A | 0 vs. 2.7% |
| Cunningha m, 198211 | 20 analysis of 2 clinical trials | 305 (642 neonates) | Cefamandale or Penicillin G & Gentamycin | N/A | 1.09 (0.6, 1.9) | N/A | 9.2 vs. 6.7% |
| Fejgin, 199312 | Cohort (Historical comparison) | 435 | A cephalosporin (Cefonicid, ceftriaxone or cefamezine) | 0.40 (0.20, 0.81) | 0.40 (0.12, 1.3) | 0.11 (0.01, 0.9) | N/A |
| Wax, 199713 | RCT | 90 | Cefazolin (1g) | 0.28 (0.03, 2.6) | 0.84 (0.05, 13.0) | 0.42 (0.04, 4.5) | 3.35 (0.8, 14.9) |
| Thigpen, 200514 | RCT | 303 | Cefazolin (2g) | 0.58 (0.34, 1.0)† | 0.67 (0.42, 1.1) | 0.84 (0.45, 1.6) | 0.96 (0.68, 1.3) |
| Sullivan, 200715 | RCT | 357 | Cefazolin (1g) | 0.40 (0.18, 0.87) | 0.20 (0.2, 0.94) | 0.52 (0.18, 1.5) | 1.0 (0.67, 1.55) |
| Costantine, 200816 | Metaanalysis (the 3 preceding RCTs) | 749 (771 neonates) | Cefazolin | 0.50 (0.33, 0.78) | 0.47 (0.26, 0.85) | 0.60 (0.30, 1.21) | 0.93 (0.45, 1.96) |
| Kaimal, 200817 | Cohort (Historical) | 1316 | Cefalozin | 0.33 (0.14, 0.76) | 0.34 (0.13, 0.92) | N/A | N/A |
RR (95% CI) for “Before Incision” vs. “After Cord Clamping” extracted from published reports or calculated using the reported data (Referent is the “After cord clamping” group)
Calculated assuming that no patient had both wound infection and endometritis;
Neonatal sepsis: either proven or suspected
RCT= Randomized clinical trial; N/a = Relevant data “Not available”;
Therefore, to prevent fetal exposure, the traditional practice has been to administer antibiotics only after delivery of the infant and clamping of the umbilical cord,4,7 and federal and national organizations including the CDC, the Center for Medicaid and Medicare Services and the American Society of Hospital Pharmacists all identify cesarean as the exception to general recommendations to administer antibiotic prophylaxis prior to surgical incision.2–3,5,29 Although ACOG currently does not make an explicit recommendation regarding timing, it recognizes that prophylactic antibiotics for cesarean delivery are generally administered after cord clamping.7 The most recent Cochrane systematic review, involving over 80 clinical trials, recommended administration after cord clamp pending further studies of pre-incision administration.4
Study Findings
Compared with the older studies,11–12 4 more recent studies of antibiotic prophylaxis for cesarean delivery suggest that prophylactic antibiotics administered prior to incision are more effective in preventing post-cesarean infection than administration after umbilical cord clamping (Table I).13–18 These studies do have sample size limitations and were not all randomized trials. The largest and most recent randomized trial reported by Sullivan involved 357 patients from a single center.16 Significant reductions in the pre-incision group were observed in endometritis and total infection (endometritis, wound infections, urinary infections and pneumonia) but not in wound infections alone.16 A meta-analysis of all 3 randomized trials found a 50% reduction in post-cesarean infection associated with pre-incision antibiotic administration.17 Although antibiotic exposure did not appear to influence neonatal sepsis in any single trial or in the metaanalysis (n=759), none of these studies was sufficiently powered to determine a clinically significant difference in this rare outcome (as many as 4,800 cesareans would be needed to ascertain a 33% difference in neonatal sepsis with 80% power assuming a baseline incidence of about 5%). No differences in frequency of neonatal sepsis work-ups or proven sepsis were noted under the blinded conditions of these clinical trials. One retrospective cohort study has also associated pre-incision antibiotic prophylaxis with greater than a 50% reduction in maternal infection compared to prophylaxis after cord clamp.18
Extended Spectrum Antibiotic Prophylaxis
Study findings
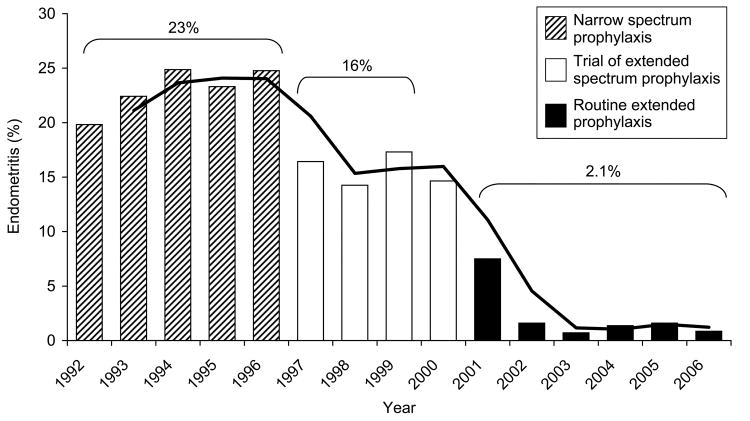
First generation cephalosporins (primarily cefalozin) are recommended over broader spectrum antibiotics, as they are equally effective and less costly than the latter.7,19 However, the broad-spectrum antibiotics that have been evaluated are mainly single-agent extended-spectrum penicillins, or 2nd or 3rd generation cephalosporins (i.e. beta lactams).19 In one small trial, ampicillin alone (compared to ampicillin plus gentamycin) was associated with significantly higher risk of endometritis, febrile morbidity and longer hospitalization.20 Indeed, accumulating evidence from the preceding and other randomized clinical trials (Table II) suggest that such extended-spectrum regimens (i.e., a regimen involving the use of both the standard narrow-spectrum antibiotic in addition to a second antibiotic of a different class e.g. azithromycin, gentamycin or metronidazole) are significantly more effective in reducing post-cesarean infections (by 30–60%) and shortening hospital stay (and costs) than narrow-spectrum agents alone.20–23 Furthermore, a recent cohort study confirmed a corresponding drop in rates of post-cesarean endometritis with increasing use of azithromycin-based extended spectrum prophylaxis (Figure II) at one US center over a period of 14 years.24 The incidence of wound infection also decreased from 3.2 to 1.3% over the same time period.25 These findings are limited by the possibility, albeit unlikely, that they are entirely due to other concurrent changes during the time periods.
Table 2.
Overview of Published Trials of Extended-Spectrum Antibiotic Prophylaxis for Cesarean
| Study Description | Study Outcomes | ||||||
|---|---|---|---|---|---|---|---|
| Study | Design | Sample size | Antibiotics | Total Infection* | Endometritis* | Wounds* | Hospital stay** |
| O’Leary 198619 | RCT | 123 | Gentamycin (+ Ampicilin) | 0.42 (0.17, 1.03) † | 0.38 (0.14, 0.99)* | 0.98 (0.06, 15.0)* | 1.4 days shorter |
| Pitt, 200120 | RCT | 224 | Vaginal metronidazole (+ Cefazolin) | N/A | 0.42 (0.19, 0.92) | 1.67 (0.41, 6.81) | No difference |
| Meyer, 200321 | RCT | 160 | Metronidazole (+ Cefotetan) | N/A | 0.43 (0.23, 0.82) | N/A | 1.4 days shorter |
| Andrews, 200322 | RCT | 597 | Azithromycin/Doxycycline (+ Cefotetan) | 0.68 (0.50, 0.94) | 0.68 (0.49, 0.94) | 0.22 (0.05, 0.99) | ½ day shorter |
RR (95% CI) for “Extended spectrum” vs. “standard narrow spectrum” prophylaxis from published reports or calculated based on reported data (Referent is the narrow-spectrum only group corresponding to the antibiotic in parentheses);
Calculated assuming that no patient had both wound infection and endometritis
Shorter hospital stay with extended-spectrum compared to narrow spectrum prophylaxis;
RCT= Randomized clinical trial; N/a = Relevant data “Not available”
Figure 2.
Annual incidence of post-cesarean endometritis for three time periods, categorized according to type of prophylactic antibiotics at University of Alabama at Birmingham. The line is the moving average trend line for successive years within each period of antibiotic prophylaxis. Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183–9.
Rationale
The association of extended-spectrum antibiotic prophylaxis with reduced rates of post-cesarean infection conforms to the principle that the selected prophylactic antibiotic regimen should have activity against microbial agents commonly involved in surgical site contamination and actual infections.2–3,7 Post-cesarean infections are polymicrobial, involving aerobes, anaerobes and Ureaplasma (or Mycoplasmas). The most frequent microbes isolated from endometrial cultures of women with post-cesarean endometritis include Ureaplasmas/Mycoplasmas, aerobic gram-negative rods, enterococci, Gardnerella and anaerobes.38–41 The most common organisms isolated from wound infections also include Ureaplasma as well as staphylococci and enterococci. 42–43 Furthermore, when specifically identified, Ureaplasma (or Mycoplasma) is the most common organism isolated from the amniotic fluid and chorioamnion at cesarean delivery, and is associated with a 3 to 8-fold increased risk of post-cesarean endometritis or wound infection.44–48 Bacterial vaginosis is also associated with as much as a 6-fold increased risk of post-cesarean endometritis.49 Therefore, the recommended narrow-spectrum regimen of cefazolin alone does not cover frequent isolates or risk factors such as Ureaplasma and anaerobic bacteria. Indeed, narrow-spectrum antibiotic prophylaxis modifies flora towards the increased presence of resistant organisms such as anaerobes.38,50 While azithromycin or metronidazol appropriately suppresses ureaplasma or anaerobes respectively, it is likely that the origin of the observed benefits extends to the suppression of other susceptible organisms. The use of an extended-spectrum regimen involving a 2nd antibiotic is not an entirely new concept in surgical antibiotic prophylaxis. The addition of vancomycin to cefazolin is recommended for other surgeries (e.g. cardiothoracic surgery) when MRSA is a frequent cause of infection.2 Also, extended-spectrum prophylaxis is well established for non-surgical obstetric antibiotic prophylaxis: ampicillin + erythromycin (or azithromycin) for preterm premature rupture of membranes to reduce maternal and fetal infectious morbidity, and to prolong the time from membrane rupture to delivery.7,51
Antibiotic selection
Azithromycin appears to be the leading option for the 2nd antibiotic for extended-spectrum regimens for cesarean delivery. It has a longer half-life (68 hours), higher tissue concentration and lower potential for fetal transfer than the other antibiotics in published studies.52–53 In addition, azithromycin has both aerobic and some anaerobic coverage, uniquely covers Ureaplasma, and is the only choice associated with significantly reduced incidence in both endometritis and wound infection.20–25 The main advantage of metronidazole over azithromycin is lower antibiotic cost. Furthermore, in light of evidence suggesting that greater than 20% of preterm neonates may have Ureaplasma bacteremia,54 and suggestions that bronchopulmonary dysplasia (BPD) is associated with neonatal Ureaplasma infection,55 pre-incisional use of azithromycin-based extended spectrum prophylaxis may theoretically prevent neonatal sepsis syndrome and BPD. However, this hypothesis needs to be tested.
The rationale and evidence for azithromycin-based extended-spectrum antibiotic prophylaxis for cesarean delivery are demonstrated in a series of studies conducted at one center. 23–25,46 First in a cohort study of 575 women undergoing cesarean with intact membranes and without any clinical evidence of infection, those with positive chorioamnion cultures for Ureaplasma (with and without other bacteria) were three times more likely to develop post-cesarean endometritis than those with negative Ureaplasma cultures.46 In a subsequent clinical trial involving 597 cesareans, azithromycin-based extended-spectrum prophylaxis significantly reduced incidence of post-cesarean endometritis and wound infection, and shortened hospital stay compared to the use of a cephalosporin only.23 Finally, institutional surveillance studies demonstrated significant reductions in endometritis and wound infection.24–25
Conclusion
A recent joint publication by ACOG and the American Academy of Pediatrics included new wording that “… an antibiotic before the procedure [cesarean] has been demonstrated to be more effective than administration immediately after umbilical cord clamping.”56 Still, none of the major national guidelines on antibiotic prophylaxis, including ACOG’s, explicitly recommends pre-incision administration of antibiotic prophylaxis (or use of an extended-spectrum regimen) for cesarean. Nevertheless, after some 25–30 years of narrow-spectrum prophylaxis after cord clamping, interventions to improve on this clinical standard are emerging.
We acknowledge that our review findings are inherently limited by the likelihood of publication bias; studies with negative findings are less likely to be published. The inclusion of observational studies further raises the possibility of bias.
Amongst the published studies of timing of antibiotic prophylaxis for cesarean, one well-designed and implemented randomized trial provides evidence that pre-incision administration is superior to administration after cord clamp in preventing post-cesarean endometritis and total infectious morbidity.16 The study’s power to delineate the impact on wound infections alone, or on neonatal outcomes, was limited. Universal use of pre-incision administration will expose all neonates delivered by cesarean (1.3 million annually in the US for example) to prenatal antibiotics. The already widespread prenatal use of antibiotics for preterm membrane rupture and to prevent GBS sepsis (both of which are without demonstrable neonatal harm), and commitments from pediatricians to avoid invasive neonatal sepsis evaluations solely because of antibiotic exposure, may serve to diminish concerns regarding safety. However, conclusive information regarding the safety of pre-incision prophylactic antibiotics, and their potential impact on the rate of neonatal infections, and emergence of anti-microbial resistance or selection of known resistant organisms is not available. Specifically, given reports suggesting an increase in E. coli neonatal sepsis with GBS prophylaxis, attention should be paid to the possibility of resistant infections.57–58 Obstetric and neonatal antibiotic practices, by inducing early abnormal gut colonization, have also been implicated in the significant rise in childhood allergy and asthma.59–60 Consequently, adoption of these emerging strategies for antibiotic prophylaxis during cesarean should be tempered by caution. Follow-up studies addressing these concerns including additional well-designed trials to confirm the effectiveness and neonatal safety of pre-incision timing of antibiotic prophylaxis for cesarean delivery are prudent. It is important to verify that neonates are not subjected to invasive and costly sepsis work-ups solely because of antibiotic exposure under the non-blind conditions of routine clinical practice.
The available experimental evidence supporting extended-spectrum prophylaxis for cesarean comes from single centers using varying antibiotic regimens.20–23 Given the marked inter and intra-regional variation in populations and rates of post-cesarean infection, additional trials are needed to assess the generalizability of the benefit observed with extended regimens administered after cord clamping, and to compare the strategy to pre-incision administration of cefazolin alone. Furthermore, considering the potential for a shift in practices to pre-incision antibiotic prophylaxis for cesarean delivery, studies assessing whether pre-incision administration of extended-spectrum antibiotics provides additional benefits over pre-incision administration of cefazolin only may also be indicated. Such studies should assess the impact on maternal and neonatal infection, and monitor for disparities in microbial resistance and pseudomembraneous colitis. Because the overall incidence of post-cesarean infections will vary immensely by socio-demographic profile and other risk factors within populations, the urgency with which to adopt and/or further evaluate these emerging strategies may vary by setting. Nonetheless, efforts to optimize the impact of antibiotic prophylaxis should obviously be part of a comprehensive strategy to reduce surgical site infections including improved patient preparation and surgical technique.2
The use of either cefazolin alone prior to surgical incision or an extended-spectrum regimen after cord clamp appears to be associated with a reduction in postcesarean maternal infection. Confirmatory studies focusing additionally on neonatal outcomes and the impact on resistant organisms, as well as studies comparing both strategies, are needed.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–6. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–132. quiz 133–4; discussion 96. [PubMed] [Google Scholar]
- 3.American Society of Health System Pharmacists. ASHP Therapeutic Guidelines on Antimicrobial Prophylaxis in Surgery. American Society of Health-System Pharmacists. Am J Health Syst Pharm. 1999;56(18):1839–88. doi: 10.1093/ajhp/56.18.1839. Review. [DOI] [PubMed] [Google Scholar]
- 4.Smaill F, Hofmeyr GJ. Antibiotic prophylaxis for cesarean section. Cochrane Database Syst Rev. 2002;(3):CD000933. doi: 10.1002/14651858.CD000933. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services. [Accessed June 18, 2008];Physician Quality Reporting Initiative (PQRI) Quality Measures Specifications. 2008 http://www.cms.hhs.gov/PQRI/downloads/2008PQRIMeasureSpecifications123107.pdf.
- 6.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13(165):1–209. [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists. ACOG practice bulletin number 47, October 2003: Prophylactic Antibiotics in Labor and Delivery. Obstet Gynecol. 2003;102(4):875–82. doi: 10.1016/s0029-7844(03)00984-0. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Sutton PD, et al. Births: Final data for 2005. Natl Vital Stat Rep. 2007;56 (6):1–103. [PubMed] [Google Scholar]
- 9.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2006. Natl Vital Stat Rep. 2007;56 (7):1–18. [PubMed] [Google Scholar]
- 10.MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008;35(2):293–307. doi: 10.1016/j.clp.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstet Gynecol. 1979;53(2):151–6. [PubMed] [Google Scholar]
- 12.Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping? Obstet Gynecol. 1983;62(2):151–4. [PubMed] [Google Scholar]
- 13.Fejgin MD, Markov S, Goshen S, Segal J, Arbel Y, Lang R. Antibiotic for cesarean section: the case for ‘true’ prophylaxis. Int J Gynaecol Obstet. 1993;43(3):257–61. doi: 10.1016/0020-7292(93)90513-v. [DOI] [PubMed] [Google Scholar]
- 14.Wax JR, Hersey K, Philput C, et al. Single dose cefazolin prophylaxis for postcesarean infections: before vs. after cord clamping. J Matern Fetal Med. 1997;6(1):61–5. doi: 10.1002/(SICI)1520-6661(199701/02)6:1<61::AID-MFM13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005;192(6):1864–8. doi: 10.1016/j.ajog.2004.12.063. discussion 1868–71. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan SA, Smith T, Chang E, Hulsey T, Vandorsten JP, Soper D. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity: a randomized, controlled trial. Am J Obstet Gynecol. 2007;196(5):455, e1–5. doi: 10.1016/j.ajog.2007.03.022. Erratum in: Am J Obstet Gynecol. 2007;197(3):333. [DOI] [PubMed] [Google Scholar]
- 17.Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. Am J Obstet Gynecol. 2008;199(3):301, e1–6. doi: 10.1016/j.ajog.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 18.Kaimal AJ, Zlatnik MG, Cheng YW, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical-site infections. Am J Obstet Gynecol. 2008;199(3):310, e1–5. doi: 10.1016/j.ajog.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins L, Smaill F. Antibiotic prophylaxis regimens and drugs for cesarean section. Cochrane Database Syst Rev. 2000;(2):CD001136. doi: 10.1002/14651858.CD001136. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary JA, Mullins JH, Jr, Andrinopoulos GC. Ampicillin vs. ampicillin-gentamicin prophylaxis in high-risk primary cesarean section. J Reprod Med. 1986;31(1):27–30. [PubMed] [Google Scholar]
- 21.Pitt C, Sanchez-Ramos L, Kaunitz AM. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstet Gynecol. 2001;98(5 Pt 1):745–50. doi: 10.1016/s0029-7844(01)01517-4. [DOI] [PubMed] [Google Scholar]
- 22.Meyer NL, Hosier KV, Scott K, Lipscomb GH. Cefazolin versus cefazolin plus metronidazole for antibiotic prophylaxis at cesarean section. South Med J. 2003;96(10):992–5. doi: 10.1097/01.SMJ.0000060570.51934.14. [DOI] [PubMed] [Google Scholar]
- 23.Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101(6):1183–9. doi: 10.1016/s0029-7844(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 24.Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, Andrews WW. Decreasing incidence of post-cesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111(1):51–6. doi: 10.1097/01.AOG.0000295868.43851.39. [DOI] [PubMed] [Google Scholar]
- 25.Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, Andrews WW. Impact of Extended-Spectrum Antibiotic Prophylaxis on Incidence of Post-Cesarean Surgical Wound Infection. Am J Obstet Gynecol. 2008;199(3):303, e1–3. doi: 10.1016/j.ajog.2008.06.068. [DOI] [PubMed] [Google Scholar]
- 26.Chelmow D, Ruehli MS, Huang E. Prophylactic use of antibiotics for non-laboring patients undergoing cesarean delivery with intact membranes: a meta-analysis. Am J Obstet Gynecol. 2001;184(4):656–61. doi: 10.1067/mob.2001.111303. [DOI] [PubMed] [Google Scholar]
- 27.Mugford M, Kingston J, Chalmers I. Reducing the incidence of infection after caesarean section: implications of prophylaxis with antibiotics for hospital resources. BMJ. 1989;299(6706):1003–6. doi: 10.1136/bmj.299.6706.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chelmow D, Hennesy M, Evantash EG. Prophylactic antibiotics for non-laboring patients with intact membranes undergoing cesarean delivery: an economic analysis. Am J Obstet Gynecol. 2004;191(5):1661–5. doi: 10.1016/j.ajog.2004.03.079. [DOI] [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality. Prevention of Surgical Site Infections (chapter 20) in Evidence Report/Technology Assessment No. 43. [Accessed June 18, 2008];Making Health Care Safer: A Critical Analysis of Patient Safety Practices. 2001 AHRQ Publication No. 01-E058. http://www.ahrq.gov/clinic/ptsafety/
- 30.Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991–1997. Obstet Gynecol. 2003;101(2):289–96. doi: 10.1016/s0029-7844(02)02587-5. [DOI] [PubMed] [Google Scholar]
- 31.Gibbs RS. Clinical risk factors for puerperal infection. Obstet Gynecol. 1980;55(5 Suppl):178S–184S. doi: 10.1097/00006250-198003001-00045. [DOI] [PubMed] [Google Scholar]
- 32.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maharaj D. Puerperal pyrexia: a review. Part I. Obstet Gynecol Surv. 2007;62(6):393–9. doi: 10.1097/01.ogx.0000265998.40912.5e. Review. [DOI] [PubMed] [Google Scholar]
- 34.Henderson E, Love EJ. Incidence of hospital-acquired infections associated with caesarean section. J Hosp Infect. 1995;29(4):245–55. doi: 10.1016/0195-6701(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 35.Opoien HK, Valbo A, Grinde-Andersen A, Walberg M. Post-cesarean surgical site infections according to CDC standards: rates and risk factors. A prospective cohort study. Acta Obstet Gynecol Scand. 2007;86(9):1097–102. doi: 10.1080/00016340701515225. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119(2):345–60. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 37.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–8. [PubMed] [Google Scholar]
- 38.Newton ER, Wallace PA. Effects of prophylactic antibiotics on endometrial flora in women with postcesarean endometritis. Obstet Gynecol. 1998;92(2):262–8. doi: 10.1016/s0029-7844(98)00164-1. [DOI] [PubMed] [Google Scholar]
- 39.Sherman D, Lurie S, Betzer M, Pinhasi Y, Arieli S, Boldur I. Uterine flora at cesarean and its relationship to postpartum endometritis. Obstet Gynecol. 1999;94(5 Pt 1):787–91. doi: 10.1016/s0029-7844(99)00421-4. [DOI] [PubMed] [Google Scholar]
- 40.Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and Chlamydia trachomatis. Obstet Gynecol. 1989;73(1):52–60. [PubMed] [Google Scholar]
- 41.Hoyme UB, Kiviat N, Eschenbach DA. Microbiology and treatment of late postpartum endometritis. Obstet Gynecol. 1986;68(2):226–32. [PubMed] [Google Scholar]
- 42.Emmons SL, Krohn M, Jackson M, Eschenbach DA. Development of wound infections among women undergoing cesarean section. Obstet Gynecol. 1988;72(4):559–64. [PubMed] [Google Scholar]
- 43.Roberts S, Maccato M, Faro S, Pinell P. The microbiology of post-cesarean wound morbidity. Obstet Gynecol. 1993;81(3):383–6. [PubMed] [Google Scholar]
- 44.Rosene K, Eschenbach DA, Tompkins LS, Kenny GE, Watkins H. Polymicrobial early postpartum endometritis with facultative and anaerobic bacteria, genital mycoplasmas, and Chlamydia trachomatis: treatment with piperacillin or cefoxitin. J Infect Dis. 1986;153(6):1028–37. doi: 10.1093/infdis/153.6.1028. [DOI] [PubMed] [Google Scholar]
- 45.Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and Chlamydia trachomatis. Obstet Gynecol. 1989;73(1):52–60. [PubMed] [Google Scholar]
- 46.Andrews WW, Shah SR, Goldenberg RL, Cliver SP, Hauth JC, Cassell GH. Association of post-cesarean delivery endometritis with colonization of the chorioamnion by Ureaplasma urealyticum. Obstet Gynecol. 1995;85(4):509–14. doi: 10.1016/0029-7844(94)00436-H. [DOI] [PubMed] [Google Scholar]
- 47.Keski-Nisula L, Kirkinen P, Katila ML, Ollikainen M, Suonio S, Saarikoski S. Amniotic fluid U. urealyticum colonization: significance for maternal peripartal infections at term. Am J Perinatol. 1997;14(3):151–6. doi: 10.1055/s-2007-994117. [DOI] [PubMed] [Google Scholar]
- 48.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179(5):1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 49.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990;75(1):52–8. [PubMed] [Google Scholar]
- 50.Archer GL. Alteration of cutaneous staphylococcal flora as a consequence of antimicrobial prophylaxis. Rev Infect Dis. 1991;13(Suppl 10):S805–9. doi: 10.1093/clinids/13.supplement_10.s805. Review. [DOI] [PubMed] [Google Scholar]
- 51.Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of the membranes: a systematic review. Obstet Gynecol. 2004;104(5 Pt 1):1051–7. doi: 10.1097/01.AOG.0000143268.36682.21. Review. [DOI] [PubMed] [Google Scholar]
- 52.Whitman MS, Tunkel AR. Azithromycin and clarithromycin: overview and comparison with erythromycin. Infect Control Hosp Epidemiol. 1992;13(6):357–68. doi: 10.1086/646545. Review. [DOI] [PubMed] [Google Scholar]
- 53.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol. 2003;188(3):714–8. doi: 10.1067/mob.2003.141. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, Hauth JC. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):43, e1–5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schelonka RL, Waites KB. Ureaplasma infection and neonatal lung disease. Semin Perinatol. 2007;31(1):2–9. doi: 10.1053/j.semperi.2007.01.001. Review. [DOI] [PubMed] [Google Scholar]
- 56.American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 6. AAP/ACOG; 2007. [Google Scholar]
- 57.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–7. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 58.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121(4):689–96. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 59.Tan S, Holliman R, Russell AR. Hazards of widespread use of erythromycin for preterm prelabour rupture of membranes. Lancet. 2003;361(9355):437. doi: 10.1016/s0140-6736(03)12420-8. [DOI] [PubMed] [Google Scholar]
- 60.Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007;131(6):1753–9. doi: 10.1378/chest.06-3008. [DOI] [PubMed] [Google Scholar]