Abstract
High-speed countercurrent chromatography (HSCCC) and preparative liquid chromatography were successively used for separation of epigallocatechin and flavonoids from Hypericum perforatum L. The two-phase solvent system composed of ethyl acetate-methanol -water (10:1:10, v/v) was used for HSCCC. About a 900 mg amount of the crude extract was separated by HSCCC, yielding 7.8 mg of quercetrin at a purity of over 97%, 12.6 mg of quercetin at a purity of over 93%, and 38.9 mg of a mixture of hyperoside, isoquercitrin and miquelianin constituting over 97% of the fraction. A mixture of epigallocatechin and avicularin pooled from three HSCCC runs, a total amount of 54.3 mg, was further separated by Prep-LC yielding 23.4 mg of epigallocatechin and 15.3 mg of avicularin each at a purity of over 97%.
Keywords: High-speed counter-current chromatography, Preparative liquid chromatography, Hypericum perforatum L, flavonoids, epigallocatechin
1. Introduction
Hypericum perforatum L., commonly named St. John's wort in Western Europe, is an herbaceous perennial plant of Hypericeae family widely distributed in Europe, Asia, and North Africa; it is also naturalized in North America [1]. It is well known as a medicinal plant and its extracts are used as an anti-inflammatory and an antidepressant medicine, which has been demonstrated in numerous clinical trials challenging the conventional antidepressant drugs [2]. Its extracts contain a variety of constituents with documented biological activity including naphthodianthrones (hypericin, pseudohypericin, protohypericin, protopseudohypericin), phloroglucinols (hyperforin, adhyperforin), a broad range of flavonoids (quercetin, quercitrin, isoquercitrin, hyperoside, astilbin, miquelianin, I3, II8-biapigenin), and phenolic acids (chlorogenic acid, 3-O-coumaroylquinic acid), epigallocatechin, etc.[3] Flavonoids present in Hypericum perforatum L. extracts have been shown to have antidepressive activities [4].
Isolation and purification of bioactive compounds in Hypericum perforatum L. using HPLC and macroporous resin has been reported [5-7]. High-speed counter-current chromatography (HSCCC), being a support-free liquid–liquid partition method, eliminates irreversible adsorption of sample onto the solid support, and has been widely used in preparative separation of natural products [8-10]. In many cases, Prep-LC is a method needed to satisfy the purity specifications required on a routine basis, and it is also an important industrially applied separation process for the isolation and purification of pharmaceuticals and other valuable products [11].
The aim of the present study was to develop a method using HSCCC and Prep-LC for purifying flavonoids from Hypericum perforatum L.
2. Experimental
2.1 Apparatus
The preparative high-speed counter-current instrument was used with a Model GS10AB multilayer coil planet centrifuge equipped with a PTFE (polytetrafluoroethylene) multilayer coil of 110 m ×1.6 mm I.D. with a total capacity of 230 ml. The beta values of the coil range from 0.5 at the internal terminal and 0.75 at the external terminal. It is designed and constructed in Beijing Institute of New Technology Application (Beijing, China).
The preparative liquid chromatography system equipment used was a Waters 4000 Prep LC controller, a 2487 Dual absorbance detector, an Empower workstation (Waters, USA) and a reversed phase C18 column (19×300 mm, 7 μm, Symmetry Prep TM).
The high performance liquid chromatography (HPLC) equipment used was a Shimadzu LC-20AVP system equipped with two LC-20AT solvent delivery units, an SPD-M20AVP UV-VIS photodiode array detector (DAD), a Model 7725 injection valve with a 20 μl loop and an auto-sampler, an SCL-20AVP system controller, and a Class-VP-LC work station (Shimadzu, Kyoto, Japan).
Identification of CCC peak fractions was carried out by MS (Finnigan MAT711), 1H-NMR and 13C-NMR spectra (av600).
2.2 Reagents
All organic solvents used for sample preparation and HSCCC were of analytical grade and purchased form Beijing Chemical Factory (Beijing, China). Methanol used for HPLC analysis and Prep-LC was of chromatography grade and purchased from Beijing Chemical Factory (Beijing, China). Crude extract of Hypericum perforatum L. was purchased from Shanxi Undersun Biomedtech Co. Ltd (Shanxi, China). The standard compounds of quercetin and hyperoside, etc. were purchased from Chinese Ministry of Health.
2.3 Preparation of two-phase solvent and sample solution for HSCCC
The solvent system utilized in the present study was prepared by mixing n-hexane-ethyl acetate-methanol-water (1:1:1:1, v/v) or ethyl acetate-methanol-water (5:1:5 or 10:1:10 or 50:1:50, v/v), and thoroughly equilibrating the mixture in a separatory funnel at room temperature, the two phases being separated shortly before use. The sample solution for HSCCC was prepared by dissolving the crude extract in the lower phase at suitable concentration according to the preparative purpose. The sample solution for Prep-LC was prepared by dissolving the dried HSCCC peak fraction containing two compounds in the mobile phase of Prep-LC at suitable concentration according to the preparative purpose.
2.4 Separation procedure
HSCCC separation was performed as follows: the multi-player coiled column was first entirely filled with the upper phase. The lower phase was then pumped into the head end of the column at a flow rate of 2.0 ml/min, while the apparatus was run at a revolution speed of 800 rpm. After hydrodynamic equilibrium was established, as indicated by a clear mobile phase eluting at the tail outlet, the sample solution was injected through the sample port. The effluent from the tail end of the column was continuously monitored with a UV detector at 254 nm. Each peak fraction was collected according to the chromatogram. The retention of the stationary phase was computed from the volume of the stationary phase collected from the column after the separation was completed.
The preparative liquid chromatography separation was performed as follows: the column was a reversed phase C18 column (19×300 mm, 7 μm, Symmetry Prep TM), the solvent system consisted of methanol as eluent A and water as eluent B (45:55, v/v), and the eluent was pumped at 5 ml/min for about 30 min, and 2 ml sample solution was injected through the sample injector. The peak fractions were collected according to the chromatogram.
2.5 HPLC analyses and identification of HSCCC peak fractions
HPLC conditions of the crude extract from Hypericum perforatum L. are as follows: YMC-Pack ODS-A, 150×4.6 mm I.D. ODS column; gradient elution was performed using an A eluent (MeOH) and a B eluent (0.08% (v/v) H3PO4 in water) with the following linear gradient combinations: at time 0, 95% B; at time 5 min, 70% B; at time 25 min, 55% B; at time 30 min, 50% B; at time 35 min, 50% B; and at time 45 min, 95% B. Total run time was 45 min. Flow rate was 0.8 ml/min, and 10 μl portion was injected into the column. Flavonoids were detected by absorbance at 254 nm.
3. Results and discussion
As shown in Fig.1, the HPLC analyses of the crude extract of Hypericum perforatum L. indicated that it contained several compounds: hyperoside (quercetin-3-β-D-galactopyranoside), isoquercitrin (quercetin-3-β-D-glucopyranoside), miquelianin (quercetin-3-β-D-glucuropyranoside), quercitrin (quercetin-3-α-L-rhamnopyranoside), avicularin (quercetin-3-α-L-arabinoside), quercetin, etc. Based on the external standard curve, the purities of quercitrin, avicularin and quercetin are 1.06%, 0.98% and 1.63%, respectively.
Fig.1.
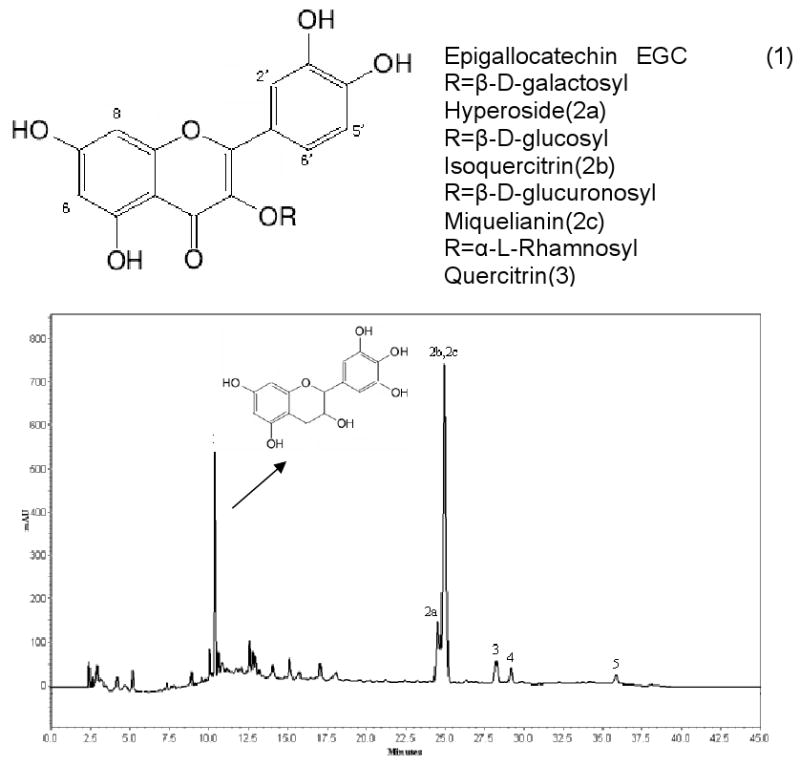
HPLC chromatogram of the crude extract from Hypericum perforatum L. with the chemical structure of epigallocatechin, hyperoside, isoquercitrin, miquelianin, quercitrin, avicularin and quercetin
HPLC conditions: C18 column (150×4.6mm, 5 μm, YMC-Pack), column temperature: 30°C. Mobile phase: MeOH (eluent A) and 0.08% (v/v) H3PO4 in water (eluent B). Gradient conditions: at time 0, 95% B; at time 5 min, 70% B; at time 25 min, 55% B; at time 30 min, 50% B; at time 40 min, 50% B; at time 45 min, 95% B. Flow rate: 0.8 ml/min, monitored at 254 nm. Peak 1: epigallocatechin. Peak 2 (a, b, c): hyperoside, isoquercitrin and miquelianin. Peak 3: quercitrin. Peak 4: avicularin. Peak 5: quercetin.
In order to achieve an efficient resolution of target compounds, the two-phase solvent system of ethyl acetate-methanol-water was examined by varying the volume of methanol. The results showed that the ethyl acetate-methanol-water system with the volume ratio of 10:1:10 was most suitable for the HSCCC run for purification of the flavonoids (Fig. 2). In Fig. 2a, the flavonoids were resolved at a volume ratio (50:1:50), while the elution of all 4 peaks required a long time of 6.7 hours. As shown in Fig.2b, the resolution of all flavonoid peaks was improved at a volume ratio (10:1:10). At the volume ratio of 5:1:5 peak resolution between the flavonoids 3 and 4 became much worse (Fig.2c). The two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (1:1:1:1, v/v) was also examined (Fig.2d). However, only quercetin (peak 2) was obtained with a high purity according to HPLC analysis.
Fig.2.
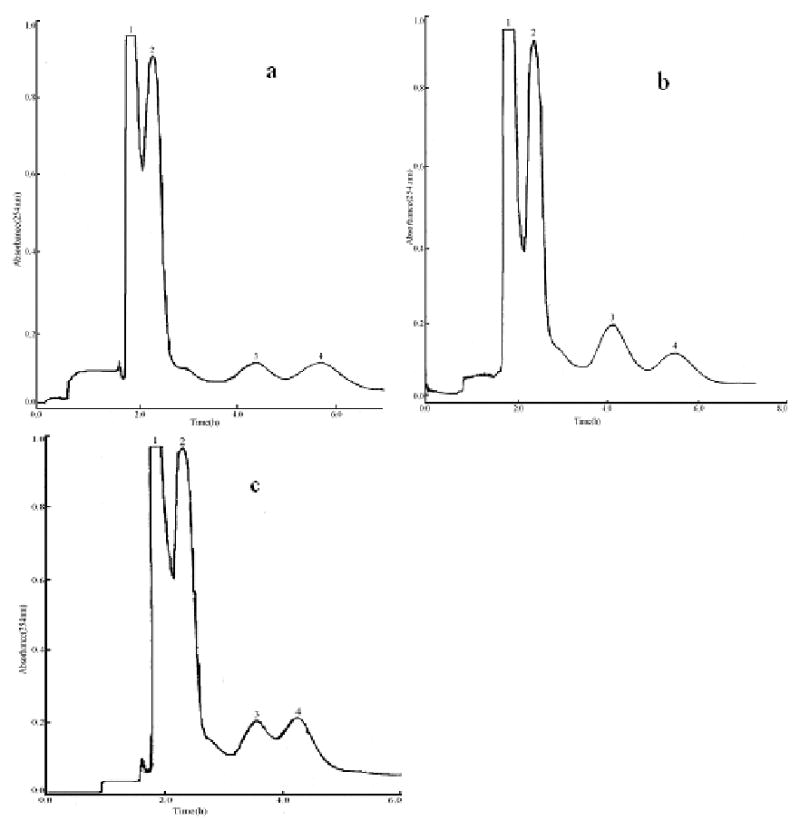
Chromatogram of the crude extract from hypericum perforatum by HSCCC Solvent system: ethyl acetate-methanol-water 50:1:50 (v/v) (a), 10:1:10 (v/v) (b), 5:1:5 (v/v) (c), n-hexane-ethyl acetate-methanol-water (1:1:1:1, v/v) (d); stationary phase: upper organic phase; mobile phase: lower aqueous phase; flow rate: 2.0 ml/min; revolution speed: 800 rpm; sample: 500 mg dissolved in 10 ml lower phase. Peak 2 (in Fig.2a, 2b, 2c): mixture of hyperoside, isoquercitrin and miquelianin. Peak 3: mixture of epigallocatechin and avicularin. Peak 4: quercitrin. Peak 2 (in Fig.2d): quercetin.
Fig.3 shows the HSCCC separation obtained from 900 mg of the crude extract of hypericum perforatum L. The peak 2 was cut and concentrated, yielding 38.9 mg containing a mixture of hyperoside, isoquercitrin and miquelianin at 97% based on the HPLC analysis. The peak 3 was cut and concentrated, yielding 18.2 mg of a mixture of avicularin and epigallocatechin. Then, 54.3 mg of the mixture obtained from 3 HSCCC runs was separated by Prep-LC (Fig.4), yielding 23.4 mg of epigallocatechin and 15.3 mg of avicularin each at 97% purity based on the HPLC analysis. The Prep-LC mobile phase was selected based on the polarity of the two compounds and the HPLC condition. The peak 4 was cut and concentrated, yielding 7.8 mg quercetrin at a high purity of over 97%. At the end of the HSCCC experiment (Fig.3), the retention of the stationary phase was pumped out by N2 and concentrated, yielding 12.6 mg quercetin at 93% purity based on the HPLC analysis.
Fig.3.
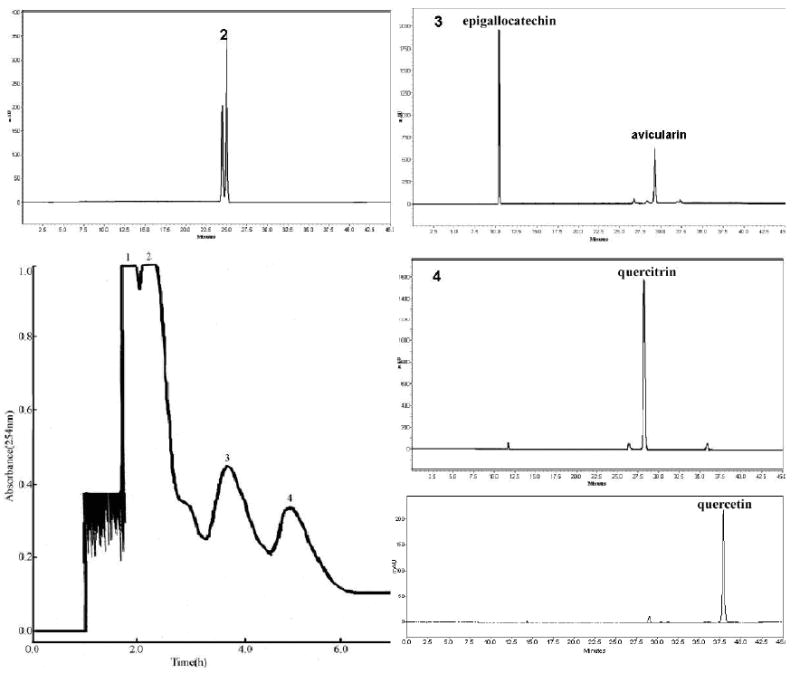
Chromatogram of the crude extract from hypericum perforatum by high-speed countercurrent chromatography (HSCCC)
Solvent system: ethyl acetate-methanol-water (10:1:10); stationary phase: upper organic phase; mobile phase: lower aqueous phase; flow rate: 2.0 ml/min; revolution speed: 800rpm; sample: 900 mg dissolved in 10ml of lower phase. HPLC conditions: the same as Fig 1. Peak 2: mixture of hyperoside, isoquercitrin and miquelianin. Peak 3: mixture of epigallocatechin and avicularin. Peak 4: quercitrin. Pumped out peak by N2: quercetin
Fig.4.
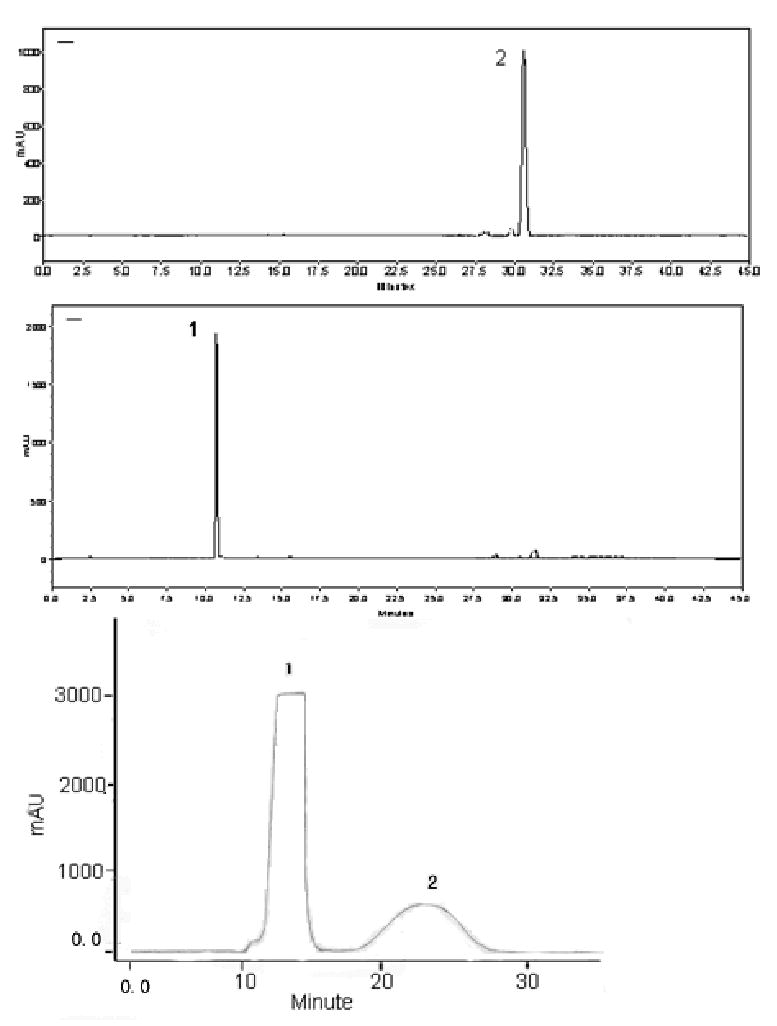
Pre-LC chromatogram of peak 3 in HSCCC
Prep-LC conditions: reversed phase C18 column (19×300 mm, 7 μm, Symmetry Prep TM). Mobile phase: MeOH/H2O (45:55, v/v). Flow rate: 5 ml/min. Peak 1: epigallocatechin, Peak 2: avicularin. HPLC conditions: the same as Fig.1.
The structural identification of the flavonoids was carried out by MS, 1H NMR and 13C-NMR spectra as follows.
Mixture of hyperoside, isoquercitrin and miquelianin: ESI-MS: m/z 463, 477 (M-H)-. 1H-NMR (600MHz, CD3COCD3): δppm 12.37, 8.00, 7.60, 6.96, 6.29, 5.21, 5.10, 5.03. The results were similar to those in reference [3]. Prep-LC was also used to separate these three compounds, but the pure compound has not been obtained. The experiments have indicated that it is difficult to separate hyperoside, isoquercitrin and miquelianin by Prep-LC in this condition which should be selected by further study.
Quercitrin (quercetin-3-α-L-rhamnopyranoside): ESI-MS: m/z 447 (M-H)-. 1H-NMR(600MHz, CD3COCD3+D2O): 7.60(1H, H-2′), 7.45(1H, H-5′), 7.00(1H, H-6′), 6.50(1H, H-8), 6.29(1H, H-6), 5.38(1H, brs, H-1″), 4.10(1H, H-2″), 3.58(1H, H-3″), 3.32(1H, H-4″), 3.21(1H, H-5″), 0.89(3H, CH3). 13C-NMR(600MHz, CD3COCD3+D2O): 177.5(C-4), 164.2(C-7), 161.3(C-5), 156.4(C-9), 156.2(C-2), 148.5(C-4′), 144.8(C-3′), 133.4(C-3), 121.7(C-1′), 121.2(C-6′), 116.3(C-5′), 115.3(C-2′), 104.0(C-10), 100.9(C-1″), 98.7(C-6), 93.6(C-8), 77.6(C-5″), 76.5(C-3″), 74.1(C-2″), 70.0(C-4″), 61.0(C-6″). The results were similar to those in reference [3,12].
Quercetin: ESI-MS: m/z 302 (M-H)-. 1H-NMR(600MHz, CD3COCD3+D2O): 7.62(1H, H-2′), 7.51(1H, H-6′), 6.86(1H, H-5′), 6.39(1H, H-8), 6.18(1H, H-6). 13C-NMR(600MHz, CD3COCD3+D2O): 176.8(C-4), 164.2(C-7,C-5), 160.8(C-9), 156.2(C-2), 148.5(C-4′), 144.8(C-3′), 136.4(C-3), 122.7(C-1′), 121.0(C-6′), 116.3(C-2′), 115.3(C-5″), 103.6(C-10), 98.7(C-6), 93.8(C-8). The results were similar to those in reference [3,12].
Avicularin (quercetin-3-α-L-arabinoside): ESI-MS: m/z 433 (M-H)-. 1H-NMR(600MHz, CD3COCD3+D2O): 7.47(1H, H-2′), 7.35(1H, H-5′), 6.99(1H, H-6′), 6.46(1H, H-8), 6.25(1H, H-6), 5.44(1H, brs, H-1″), 4.24-3.46(5H, glu-H). 13C-NMR(600MHz, CD3COCD3+D2O): 179.4(C-4), 165.0(C-7), 169.2(C-5), 162.6(C-9), 157.9(C-2), 149.1(C-4′), 145.7(C-3′), 134.4(C-3), 122.6(C-6′), 122.3(C-1′), 116.2(C-2′, 5′), 109.0(C-1″), 105.2(C-10), 99.4(C-6), 94.5(C-8), 89.2(C-4″), 82.1(C-2″), 78.6(C-3″), 62.5(C-5″). The results were similar to those in reference [12].
Epigallocatechin: ESI-MS: m/z 304 (M-H)-. 1H-NMR(600MHz, CD3COCD3+D2O): 2.873(1H, H-4), 4.161(1H, H-3), 4.469(1H, H-2), 5.981(1H, H-8), 5.962(1H, H-6), 6.575(1H, H-2′), 6.575(1H,H-6′). 13C-NMR(600MHz, CD3COCD3+D2O): 78.67(C-2), 67.42(C-3), 29.24(C-4), 157.42(C-5), 96.43(C-6), 157.15(C-7), 95.76(C-8), 156.07(C-9), 99.91(C-10), 131.23(C-1′), 106.81(C-2′, 6′), 146.32(C-3′, 5′), 133.21(C-4′). The results were similar to those in reference [13].
4. Conclusions
In the present study, about a 900 mg amount of the crude extract was separated, yielding 7.8 mg of quercetrin at a purity of over 97%, 12.6 mg of quercetin at a purity of over 93% in addition to 38.9 mg of hyperoside, isoquercitrin and miquelianin mixture consisting of over 97% of the fraction. A 54 mg amount of this epigallocatechin and avicularin mixture obtained by three HSCCC runs was further separated by Prep-LC, yielding 23.4 mg of epigallocatechin and 15.3 mg of avicularin each at a high purity of over 97%. The results of this study demonstrate that HSCCC is a very efficient method for the preparative separation of flavonoids from Hypericum perforatum L. HSCCC combined with Prep-LC has become a new and superior separation mode for natural compounds over other chromatographic methods due to complementary action of these two methods.
Acknowledgments
Financial support from Ministry of Agriculture of the people's republic of China (Project 200803022) is gratefully acknowledged.
References
- 1.Di Carlo G, Borrelli F, Ernst E, Izzo AA. Trends Pharmacol Sci. 2001;22:292. doi: 10.1016/s0165-6147(00)01716-8. [DOI] [PubMed] [Google Scholar]
- 2.Greeson JM, Sanford B, Monti DA. Psychopharmacology. 2001;153:402. doi: 10.1007/s002130000625. [DOI] [PubMed] [Google Scholar]
- 3.Tatsis EC, Boeren S, Exarchou V, et al. Phytochemistry. 2007;68:383. doi: 10.1016/j.phytochem.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Butterweck V, Jurgemlienk G, Nahrstedt A, Winterhorff H. Planta Med. 2000;66:3. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JS, Wang XL, Luo Q. Chinese traditional patent medicine. 2006;28:709. [Google Scholar]
- 6.Wang XL, Zhang JS, Luo Q. Chinese traditional medicine. 2006;29:1047. [Google Scholar]
- 7.Yu L, Li JM, Li YR. Applied Chemical Industry. 2006;35:755. [Google Scholar]
- 8.Wei Y, Zhang TY, Xu GQ, Ito Y. J Chromatogr A. 2001;929:169. doi: 10.1016/s0021-9673(01)01177-3. [DOI] [PubMed] [Google Scholar]
- 9.Yao S, Luo JG, Huang XF. J Chromatogr B. 2008;864:69. doi: 10.1016/j.jchromb.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Cao XL, Huang DF, Dong YM, Zhao H, Ito Y. Journal of Liquid Chromatography &Related Technologies. 2007;30:1657. [Google Scholar]
- 11.Shan Y, Morgenstern AS. J Chromatogr A. 2004;1041:53–62. doi: 10.1016/j.chroma.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Hu YJ. Journal of Guangzhou university of traditional medicine. 2006;23:416. [Google Scholar]
- 13.Kumar NS, Rajapaksha M. Journal of Chromatography A. 2005;1083:223–228. doi: 10.1016/j.chroma.2005.06.013. [DOI] [PubMed] [Google Scholar]


