Abstract
Solar ultraviolet radiation (UVR) is the major etiological factor in skin carcinogenesis. However, in vivo studies demonstrate that mice exposed to arsenic and UVR exhibit significantly more tumors and oxidative DNA damage than animals treated with either agent alone. Interactions between arsenite and UVR in the production of reactive oxygen species (ROS) and stress-associated signaling may provide a basis for the enhanced carcinogenicity. In this study keratinocytes were pretreated with arsenite (3μM) then exposed to UVA (10 kJ/m2). We report that exposure to UVA following arsenite pretreatment enhanced ROS production, p38 MAP kinase activation and induction of a redox sensitive gene product, heme oxygenase-1, compared to either stimulus alone. UVR exposure resulted in rapid and transient NADPH oxidase activation whereas the response to arsenite was more pronounced and persistent. Inhibition of NADPH oxidase decreased ROS production in arsenite treated cells but had little impact on UVA exposed cells. Furthermore, arsenite-, but not UVA-, induced p38 activation and HO-1 expression were dependent upon NADPH oxidase activity. These findings indicate differences in mechanisms of ROS production by arsenite and UVA that may provide an underlying basis for the observed enhancement of redox-related cellular responses upon combined UVA and arsenite exposures.
Keywords: ultraviolet radiation, reactive oxygen species, p38 MAP kinase, Heme oxygenase-1, NADPH oxidase, keratinocytes
Introduction
Skin cancer is linked to ultraviolet radiation (UVR) via sun exposure [1]. UVA (320 - 400 nm) and UVB (280 - 320 nm) comprise the wavelengths of primary concern in human skin cancers [2]. A majority of the studies on UVR-induced skin cancers have focused on UVB-induced DNA damage. Pyrimidine photoproducts are induced by UVB, but UVR also causes oxidative DNA lesions due to the production of reactive oxygen species (ROS) [3]. ROS is generated by the reaction of UVR with endogenous photo-sensitizers, blocking of antioxidant activity, and inflammation in the dermis. UVA is primarily associated with elevated levels of ROS, but both UVA and UVB are reported to induce ROS production in keratinocytes [4].
Arsenic is also a skin carcinogen and chronic exposure has been linked with increased incidence of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [5-7]. Arsenic enhances tumor development when combined with other carcinogens [8-10], with chronic overexpression of growth factors [11], and when combined with UVR [12,13]. For example, mice chronically exposed to arsenite in drinking water develop significantly more skin tumors than mice exposed to arsenite or UVR alone [12,13]. Additionally, increased oxidative DNA damage was detected in the skin and tumors of the dually treated animals [14]. Therefore, arsenic may be better characterized as a co-carcinogen capable of potentiating the actions of a carcinogenic partner, such as UVR.
Arsenite exposure elevates ROS levels leading to increased cellular oxidative stress [15,16] resulting in oxidative DNA and protein damage [17-19]. ROS production is, therefore, a shared consequence of UVR and arsenic exposure. However, DNA damage is not the only result of excess ROS production. ROS and arsenite also cause damage to proteins and lipids, and alterations in intracellular signaling pathways [4]. Altered signaling due to ROS can ultimately lead to activation of transcription factor complexes, such as AP-1 or NF-κB. Activation of these transcription factors is crucial in the stimulation of the complex transcriptional alterations required for keratinocyte transformation [20,21] and are likely to be wavelength dependent [22]. Both UVA and UVB are known to activate mitogen-activated protein kinase (MAP kinase) signaling and stimulate gene transcription in human and mouse models of UVR exposed tissues. UVA induces phosphorylation and activation of extracellular signal-regulated kinases (ERKs), c-jun N-terminal kinases (JNKs), and p38 kinases [23,24]. Arsenic, typically as arsenite (AsIII), also stimulates MAP kinase cascades including ERK and the stress-activated kinases JNK and p38 [25-29]. Recently we have shown that arsenite acts through at least two distinct pathways; ERK activation is epidermal growth factor (EGF) receptor dependent, but p38 activation is independent of EGF receptor activation [26]. Further research demonstrated that in human keratinocytes ROS, specifically superoxide, is upstream of p38 activation by arsenite and leads to robust expression of heme oxygenase (HO)-1 with concurrent EGF receptor activation contributing to the persistence and magnitude of the response [30].
There are many potential sources for increased ROS, including mitochondrial activity and activation of enzymes, such as NADPH oxidase [31-33]. Arsenite has been shown to increase the activity of NADPH oxidase via upregulation and phosphorylation of key subunits [34] and NADPH oxidase has been implicated in arsenite-induced oxidative DNA damage [35]. NADPH oxidase has been reported to be a major target involved in the production of ROS following arsenite exposure [36]. However, the mechanism of UVR-induced ROS production remains controversial.
Although mouse models demonstrate co-carcinogenic activity with arsenite and UVR [12,13], the underlying mechanisms remain as yet unknown. The similarities of action between arsenite and UVR in the production of ROS and activation of MAP kinases may provide a basis for understanding the cooperative actions in skin carcinogenesis and are further investigated here. In this study we report that exposure to UVA following arsenite pretreatment results in an additive increase in ROS production. Furthermore, NADPH oxidase was required for arsenite-induced ROS, but was only partially involved in UVA-induced ROS. The combination of arsenite and UVR led to enhanced p38 MAP kinase activation and induction of a redox sensitive gene product, HO-1, compared to either stimulus alone. Together these data demonstrate cooperation between arsenite and UVR in signaling pathways implicated in skin carcinogenesis.
Materials and methods
Reagents and antibodies
Bovine serum albumin (BSA), cell culture reagents, dimethylsulfoxide (DMSO), nitroblue tetrazolium (NBT), dihydroethidium (DHE), diphenyliodium (DPI), lucigenin, tiron, diethyldithiocarbamate, NADPH, apocynin, rotenone and sodium arsenite were purchased from Sigma (St. Louis, MO). Newborn calf serum was acquired from Life Technologies (Gaithersburg, MD). Atpenin and MnTMPyP were purchased from CalBiochem (San Diego, CA) and were dissolved in DMSO. Phospho-specific phospho- p44/42 MAP kinase (Thr202/Tyr204) and phospho-p38 MAP kinase (Thr180/Tyr182) antibodies and pan-ERK and total p38 antibodies were purchased from Cell Signaling, Inc. (Beverly, MA). HO-1, p22phox, p67phox, and β-tubulin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-specific phospho-serine antibodies were purchased from Abcam, Inc. (Cambridge, MA). Peroxidase-conjugated anti-rabbit-IgG and anti-mouse-IgG were purchased from Promega (Madison, WI). SuperSignal chemiluminescent detection system was purchased from Pierce (Rockford, IL). Caution: Inorganic arsenic is toxic and classified as a human carcinogen. It must be handled with appropriate care.
UV Source
UVR exposures were performed using an Oriel 1000 W Watt Solar Ultraviolet Simulator (Oriel Corp., Stratford, CT). This solar simulator produces a high intensity UVR beam in both the UVA (320-400 nm) and UVB (280-320nm) spectrum. Clear glass plates were used to reduce the amount of UVB in the beam. The proportion of UVA was measured using a radiospectrometer (Optronics laboratories, Inc.; Orlando, FL) and exposure times calculated to give the desired doses. Filtration resulted in a final UVR spectrum containing approximately 97% UVA and 3% UVB (data not shown).
Cell Culture and Treatment
HaCaT cells are a spontaneously transformed, non-tumorigenic human keratinocyte cell line [37], and were generously provided by Dr. Mitch Denning (Loyola University Medical Center, Maywood, IL). HaCaT cells were maintained in a 1:1 mixture of Dulbecco's Modified Eagle's Medium F:12 HAM (DMEM:F12) supplemented with 10% newborn calf serum, 4X final concentration of MEM amino acids, and antibiotics (penicillin, 100U/ml and streptomycin, 50μg/ml). Cells were cultured at 37°C in 95% air / 5% CO2 humidified incubators. HaCaT cells were typically grown to 50-70% of confluent density, rinsed in phosphate-buffered saline (PBS) and placed into serum-free medium (DMEM:F12 containing 0.1% bovine serum albumin (BSA)) overnight prior to treatments. For all experiments, cellular viability was assessed by employing the Promega CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay to ensure treatments were not significantly inducing cell death (data not shown). For UVA exposures cells were placed in sterile PBS and kept on ice while exposed to the UVR then original media was replaced and cells returned to the incubator. All cells were treated in an identical manner and conditions were consistently maintained for each treatment group. Cells were treated with arsenite, UVR, or both and collected as indicated in the figure legends. For combined arsenite and UVR treatment, cells were exposed to UVR (10 kJ/m2) following 24h pretreatment with arsenite (3 μM). For inhibitor experiments cells were treated as described above following 30 min. pretreatment with the appropriate inhibitor. NADPH oxidase was inhibited with DPI (10 μM) or apocynin (10 μM), the electron transport chain complexes I and II with Rotenone (200 nM) and Atpenin A5 (50 nM), respectively and MnTMPyP (5 μM) a cell permeable superoxide dismutase mimic was used to inhibit ROS.
ROS Detection
Cells were cultured on glass coverslips in complete medium. When cells reached approximately 40% of confluent density, cultures were placed in serum-free media and treated with arsenite (3 μM), UVA (10 kJ/m2) or both for the times indicated in the figure legends. For the combined treatments, the cells were exposed to UVA following a 24h pretreatment with arsenite. Thirty minutes prior to cell fixation, DHE (5 μM) was added as a fluorescent indicator of ROS generated in response to the described treatment. Coverslips were washed three times with PBS, fixed with paraformaldehyde (3.7%), and mounted to glass slides with Vecta-Shield (Vector Labs. Inc., Burlingame, CA). Images were collected with an Olympus IX70 fluorescence microscope fitted with an Olympus America camera and MagnaFire 2.1 software.
When measuring relative fluorescence, cells were cultured on coverslips in 12 well plates to approximately 50% of confluent density and treated under the same conditions used for image collection. Relative fluorescence intensity was quantified by measuring the intensity of fluorescence emission using a Wallac Victor 2 fluorescence spectrophotometer equipped with 390 nm excitation and 410 nm emission filters. A minimum of 3 independent samples were analyzed per treatment and time point. Values were normalized to total DNA fluorescence as previously described [38]. Briefly, plates previously analyzed for ROS were rinsed with Kreb Ringer buffer (20mM HEPES, 10mM dextrose, 127mM NaCl, 5.5mM KCl, 1mM CaCl2. 2mM MgSO4, pH 7.4) then frozen at -80°C overnight. Plates were thawed for at least 2h at room temperature, stained with Hoechst dye (10 μg/ml bis Benzimide) overnight and fluorescence determined using a Tecan plate reader equipped with 350nm excitation and 460nm emission filters. This method of fluorescence quantification was validated by comparison with data obtained using Metamorph software (version 6.3r6) as previously described [30]. Results were graphed using Microsoft Excel 2003 and statistical significance determined using GraphPad QuickCalcs.
Immunoblot Analyses
Control and treated cells were washed with ice cold PBS and harvested in lysis buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol and 0.1% w/v bromophenol blue). Cell lysates were clarified (10,000 x g, 4oC, 10 minutes), and 10-30 μg of total cell lysate was resolved by electrophoresis through 8-12% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose membranes (Millipore Corp., Bedford, MA) and probed with the indicated antibodies according to the vendor's instructions [26,39]. The membranes were developed using the SuperSignal chemiluminescent detection system (Pierce, Rockford, IL). Quantification of immunoblot results was performed using a Digital Science Image Station on a Kodak 440CF Imager with ID Image Analysis software. A minimum of 3 independent samples were analyzed per treatment and time point. Students t-test was used to determine statistical significance of data obtained from densitometric analysis.
NADPH Oxidase Activity
HaCaT cells were placed in serum-free medium overnight and treated as described for ROS experiments. NADPH oxidase activity was detected using the Lucigenin Illumination method. Briefly, following treatment and incubation time cells were rinsed thoroughly with PBS, removed by scraping, resuspended in 500 μl PBS and placed in eppendorf tubes. Cells were frozen at -80°C overnight to lyse cells. Samples were aliquoted (100 μl) in quadruplicate, placed in luminometer tubes and incubated with diethyldithiocarbamate (DTC, 1M) for 20 min at 37°C to inhibit superoxide dismutase activity. Immediately before measuring, 1 μl lucigenin (0.5 mM) was added to the sample followed by 1 μl NADPH (10mM) and mixed. Lumeniscence was measured in a TD20/20 Luminometer (Turner Designs, Sunnyvale, CA) with ten 30 sec. counts. Sample values were integrated and average units calculated. Parallel samples were analyzed with the addition of Tiron (1M) to confirm measurement of NADPH oxidase activity.
Results
Activation of MAP kinases by arsenite and UVA
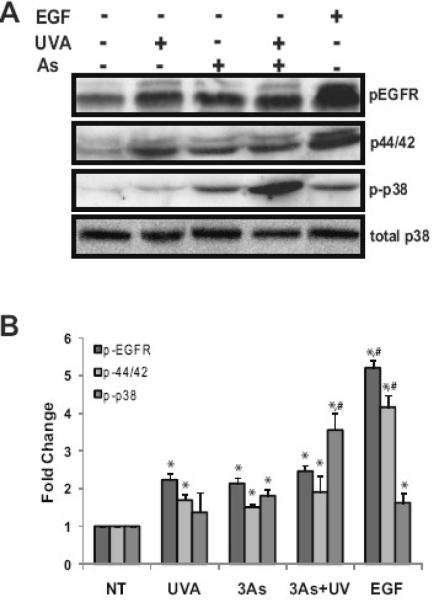
Both arsenite and UVR activate MAPK cascades [4,26,28,30,41,42] however, the effect of the combination has not been investigated. HaCaT cells were pretreated with 3 μM arsenite for 24 hours, a concentration shown previously to elicit sub-maximal MAP kinase activity [26], then exposed to submaximal UVA (10 kJ/m2 ) and incubated for an additional 4 hours. Activation of the EGF receptor and MAP kinases was detected by immunoblot analysis. As expected, UVA or arsenite alone activated the EGF receptor, p38 and ERK (Fig. 1). Arsenite pretreatment followed by UVA exposure led to activation of ERK (1.9 fold) and EGF receptor (2.5 fold) which were not appreciable higher than levels observed with arsenite or UVA alone. However, activation of p38 was increased by 1.37 fold with UVA alone, 1.81 fold with arsenite alone while the combined treatment led to a 3.6 fold increase in activation (Fig. 1A&B). These data demonstrate that arsenite and UVA both stimulate MAP kinase signaling and that arsenite in combination with UVA further activates p38 suggesting that the impact of combined exposures exceeds that of single agents.
Fig. 1.
Stimulation of MAP kinase signaling by arsenite and UVA. HaCaT cells were treated with 3 μM arsenite (As) or 10kJ/m2 UVA. Dually treated samples were pre-incubated with 3 μM arsenite (As) for 24 hrs and then exposed to UVA. EGF was used as a positive control for MAP kinase activation. Total protein was collected 2 hrs post exposure and 20 μg resolved via a 8-12% SDS-PAGE gradient gel and the indicated proteins were analyzed, transferred to nitrocellulose membrane, immunoreacted with the appropriate antibodies and detected by ECL (A) Blots shown are representative of at least 3 independent experiments. Densitometric analysis of immunoblotting was performed (B). Blots were quantified with a Digital Science Image Station on a Kodak 440CF Imager equipped with ID Image Analysis software. Each bar is the mean of at least 3 independent experiments and the error bars indicate ± S.D. * = significantly different from untreated controls (NT), p<0.05; # = significantly different from singly treated samples, p<0.05.
ROS production and HO-1 expression in response to arsenite and UVA
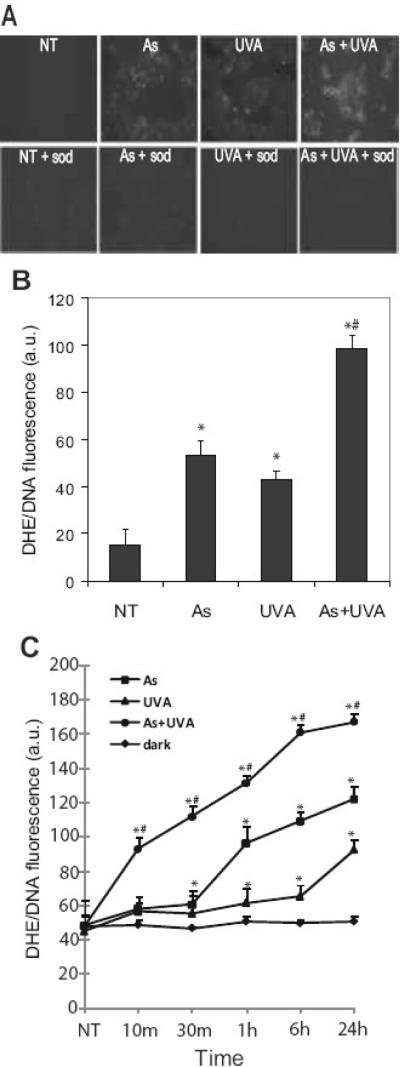
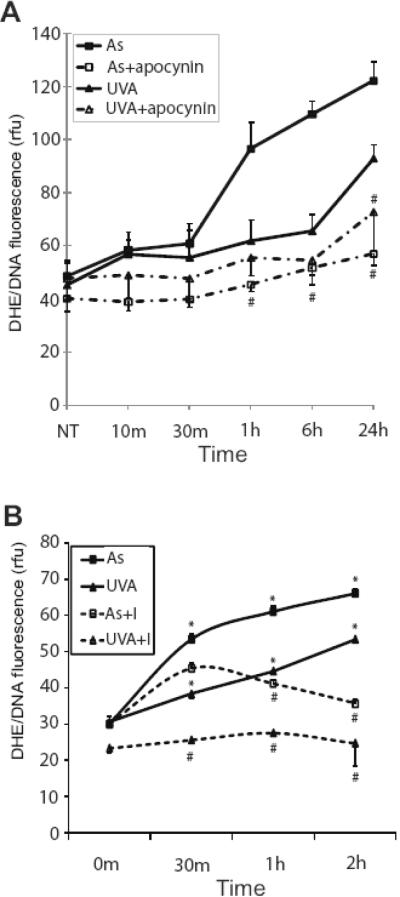
Several researchers have shown that UVR and arsenite induce production of intracellular ROS [4,17,43] and we have shown that ROS is upstream of p38 activation [30]. However, little is known about the action of combined arsenite and UVR on ROS production. Dihydroethidium (DHE) is a molecular probe specific to superoxide that stains the nucleus a bright fluorescent red when oxidized by intracellular superoxide. DHE was used to detect ROS production in order to more specifically investigate the contributions of superoxide as this species has been identified as the “parent species” of intracellular ROS [44]. Immunofluorescence quantification showed arsenite (3 mM) and UVA (10 kJ/m2) increased the production of ROS (2.43 and 1.78 fold, respectively), and the response is further increased (5.34 fold) when cells are treated with arsenite 24h prior to the UVA challenge (Fig. 2A&B). A dose dependent increase in the UVA-stimulated ROS production was detected in both UVR alone and arsenite pretreated samples at 4h post exposure (data not shown). ROS generation by both arsenite and (3 μM) and UVA (10 kJ/m2) is maximal approximately 4h after UVA exposure and is maintained up to 24 h (Fig. 2C). ROS production was significantly elevated in the arsenite and UVA co-treated samples at each time point and maximal ROS production was evident at 2h after exposure (Fig. 2C). The cell permeable superoxide dismutase mimic, MnTMPyP, was employed to confirm that observed and measured fluorescence was indeed from superoxide. In all treatments, ROS was decreased to near control levels when MnTMPyP was included (Fig. 2A).
Fig. 2.
Arsenite and UVA induce cellular ROS. HaCaT cells grown on glass coverslips to 50% confluence were treated with 3 μM arsenite (As), 10kJ/m2 UVA (UVA) or left untreated (NT). Dually treated samples were pre-incubated with 3 μM arsenite (As) for 24 hrs and then exposed to UVA. A superoxide-specific dye (5 μM DHE) was added 30 min. prior to fixation. Samples were rinsed with PBS, fixed using 3.7% para-formaldehyde, mounted on glass slide and observed with fluorescence microscopy 4 hours post UV exposure. Lower panels were treated as described above with the addition of the cell permeable SOD mimic MnTMPyP (sod,5 μM) 30 min. prior to exposures to demonstrate fluorescence is derived from superoxide (A). ROS quantification was carried out as described in “Experimental Procedures.” Each image is representative of multiple observations, and error bars indicate ± S.D. (B). ROS generation was monitored over time and quantified as described in “Experimental Procedures” for untreated or dark (◆), arsenite (■), UVA (▲) and dually treated (●) samples (C). All p values were <0.05 versus the untreated control. * = significantly different from untreated controls (NT), p<0.05; # = significantly different from singly treated samples, p<0.05. DHE, dihydroethidium.
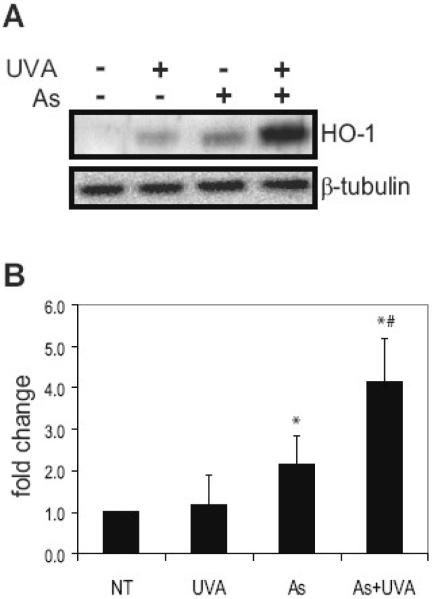
A sensitive indicator of arsenite exposure is heme oxygenase-1 (HO-1), a redox sensitive gene [19,45-47] We have shown previously that induction of HO-1 by arsenite is dependent on ROS and p38 activity. Inhibition of p38 activation or ROS production inhibited arsenite activation of p38 and subsequent induction of HO-1 [30]. HO-1 is upregulated in response to arsenite (Fig. 3) and HO-1 is significantly elevated with the combined exposure (Fig. 3A). Densitometry shows that HO-1 was increased by 1.16 fold with UVA, 2.15 fold with arsenite and combined treatments resulted in a 4.15 fold increase (Fig. 3B). These results suggest that the increased MAPK signaling observed following combined UVA and arsenite exposure leads to significant changes in downstream HO-1 expression.
Fig. 3.
Arsenite and UVA cooperate in HO-1 upregulation. HaCaT cells were incubated with 3 μM arsenite (As), 10kJ/m2 UVA (UVA) or left untreated (NT). Dually treated samples were pre-incubated with 3 μM arsenite (As) for 24 hrs and then exposed to UVA. Total protein was collected 4 hrs post exposure and 60 μg resolved via a 12% SDS-PAGE gel. HO-1 was analyzed using anti-HO-1 antibodies and detected by ECL. Equal protein loading was confirmed by reprobing the original membrane with β-Tubulin (A). Blots shown are representative of at least 3 independent experiments. Densitometric analysis of immunoblotting was performed (B). Each bar is the mean of at least 3 independent experiments and the error bars indicate ± S.D. * = significantly different from untreated controls (NT), p<0.05; # = significantly different from singly treated samples, p<0.05.
Mechanisms of ROS production by arsenite and UVA
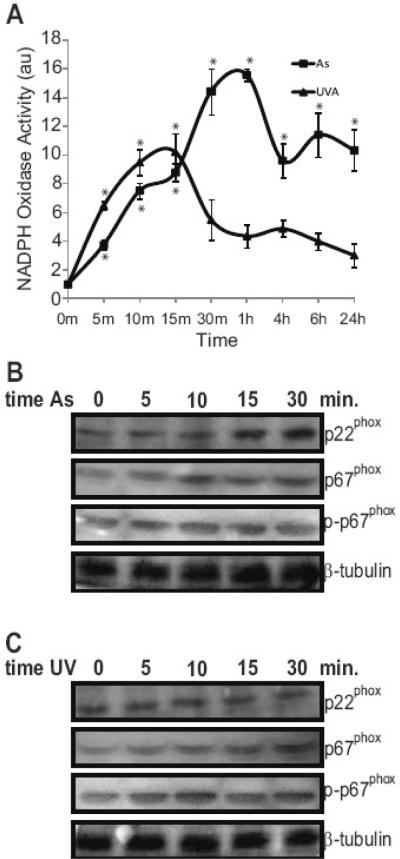
Because arsenite and UVR induce ROS production (Fig. 2) and ROS is upstream of p38 and HO-1 induction [30], we investigated mechanisms of ROS production by arsenite and UVR. There are several potential sources for the production of ROS, with NADPH oxidase and mitochondria being prime candidates [31-33,35,36]. Arsenite and UVR have been reported to induce or increase phosphorylation of the subunits of NADPH oxidase [34,35], thereby increasing the overall activity of the enzyme and ROS production [34,35,48-51]. We assessed NADPH oxidase activity following arsenite and UVA exposure and found that both agents increase activity of the enzyme, but with different kinetics (Fig. 4A). UVA exposure resulted in rapid and transient NADPH oxidase activation whereas the response to arsenite was slightly delayed, but more pronounced and persistent. Immunoblotting showed that arsenite, but not UVA, exposure led to upregulation of the p22phox subunit while both treatments increased phosphorylation of the p67phox subunit of NADPH oxidase (Fig. 4B&C). Inhibiting NADPH oxidase activity with diphenylene iodinium (DPI) or apocynin significantly decreased enzyme activity for both arsenite and UVA (data not shown). However, inhibition of NADPH oxidase only affected ROS production in cells treated with arsenite (Fig. 5A). Since NADPH oxidase is not the only potential source of ROS, the mitochondrial contribution to increased ROS was also investigated. Rotenone and Atpenin A5 were concomitantly added to inhibit complexes I and II of the electron transport chain, respectively. This addition did not affect cellular viability during the short exposures employed (data not shown). Mitochondrial inhibition resulted in significant decreases in UVA- induced ROS at all time points. Arsenite-induced ROS was also reduced, but was not significantly affected at the 30 min. time point (Fig. 5B). These results show that both arsenite and UVA induce ROS and are sensitive to mitochondrial inhibition.
Fig. 4.
Arsenite and UVA activate NADPH oxidase. HaCaT cells were incubated with 3 μM arsenite (■) or 10kJ/m2 UVA (▲) for the indicated times. NADPH oxidase activity was assessed over time as described in “Experimental Procedures” (A). Total protein was collected at the indicated times and 20 μg resolved via a 8-12% SDS-PAGE gradient gel and the indicated proteins were analyzed, transferred to nitrocellulose membrane, immunoreacted with the appropriate antibodies and detected by ECL. Panel (B) shows results following arsenite treatment and UVA results are shown in (C). Equal protein loading was confirmed by reprobing original membranes with β-tubulin. Each point and blot is representative of at least 3 independent experiments and error bars indicate ± S.D. All p values were <0.05 versus the untreated control. * = significantly different from untreated controls.
Fig. 5.
Contribution of NADPH oxidase and the mitochondria to arsenite- and UVA- induced ROS. HaCaT cells were treated with 3 μM arsenite (■), 10kJ/m2 UVA (▲). DHE was added 30 min. prior to fixation. Samples were rinsed with PBS, fixed using 3.7% para-formaldehyde, mounted on glass slide and observed with fluorescence microscopy. ROS quantification was carried out as described in “Experimental Procedures.” Samples were incubated with apocynin (10 μM) (arsenite + inhibitor (□) or UVA + inhibitor (Δ) 30 min prior to any other treatment for inhibition of NADPH oxidase (A) and rotenone and atpenin A5 (arsenite + inhibitors (□) or UVA + inhibitors (Δ) were added for mitochondrial inhibition (B). Each point is representative of multiple observations, and error bars indicate ± S.D. (A) # = significantly different from uninhibited treatment matched samples, p<0.05. (B) * =significantly different from untreated control; # = significantly different from arsenite or UVA treatment. Comparable results were obtained when employing DPI to inhibit NADPH oxidase. Apocy. = apocynin; DPI = diphenyliodium
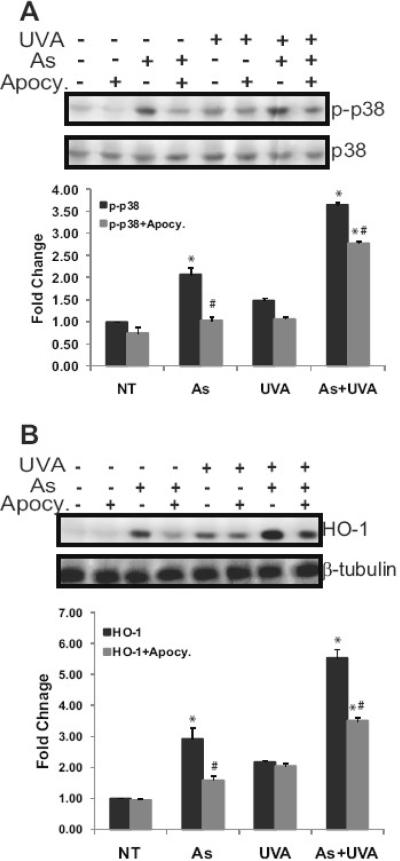
Inhibition of NADPH oxidase decreased the arsenite-induced activation of p38 MAP kinase alone as did arsenite in combination with UVA (Fig. 6A). HO-1 induction by arsenite was abolished with the addition of the NADPH oxidase inhibitor apocynin (Fig. 6B). In contrast, UVA induction of HO-1 was not disrupted by apocynin. With combined exposures, the response was partially inhibited by apocynin, likely reflecting inhibition of arsenite-dependent ROS production (Fig. 6B). These results highlight that there are differences in the mechanisms underlying ROS production by arsenite and UVA and may provide some explanation for enhanced responses observed with combined exposures.
Fig. 6.
NADPH oxidase activity is required for arsenite-induced signaling and HO-1 upregulation. HaCaT cells were treated with 3 μM arsenite (As) or 10kJ/m2 UVA (UV). Dually treated samples were incubated with 3 μM arsenite for 24 hrs and then exposed to UVA (As+UVA). Samples were incubated with apocynin (10 μM) 30 min prior to any other treatment for inhibition of NADPH oxidase. Total protein was collected 4 hrs post exposure and 20 μg resolved via 8-12% SDS-PAGE gradient gel and the indicated proteins were analyzed, transferred to nitrocellulose membrane, immunoreacted with the appropriate antibodies and detected by ECL. The effect of NADPH oxidase inhibition on activation of p38 (A). HO-1 upregulation with and without NADPH oxidase inhibition (B). Blots shown are representative of at least 3 independent experiments. Equal protein loading was confirmed by reprobing original membranes with total p38 or β-tubulin as indicated on the figures. Blots were quantified with a Digital Science Image Station on a Kodak 440CF Imager equipped with ID Image Analysis software. Each bar is the mean of at least 3 independent experiments and the error bars indicate ± S.D. * = significantly different from untreated control (NT); # = significantly different from treatment without inhibitor p<0.05. Comparable results were obtained when employing DPI to inhibit NADPH oxidase. Apocy. = apocynin; DPI = diphenyliodium
Discussion
Skin cancer accounts for approximately 40% of all newly diagnosed cancers each year [52] and nonmelanoma tumors represent greater than 95% of these cases. Experimental and epidemiological evidence implicates solar UVR as the most important etiological factor in development of skin tumors [5, 53-55]. Because the skin is exposed to many environmental carcinogens, for example polycyclic aromatic hydrocarbons and various metals, interactions between UVR and other carcinogens in the genesis of skin cancer are receiving increasing attention [5]. Of particular interest are interactions between UVR and arsenic, a known skin carcinogen. UVR-induced skin carcinogenesis is greatly increased in mice that receive arsenic in drinking water with i) nearly 5-fold increase in tumor number per mouse, ii) accelerated time to tumor development, and iii) increase in tumor size and invasiveness [12,13]. The observed decrease in tumor yield with the addition of either vitamin E or an organoselenium compound (p-XSC) to food suggests a role for reactive oxygen species (ROS) in the observed cooperation between arsenite and UVR in the development of skin cancer [14].
Reactive oxygen species are implicated in carcinogenesis through epigenetic mechanisms (i.e. modulation of signal transduction pathways and gene expression) and genetic damage. UVR in sunlight, particularly at the UVA waveband, is able to produce a variety of ROS in the skin [4] and increasing evidence indicates that exposure to arsenic results in the generation of ROS, including superoxide [17,26,30,44]. In this study we demonstrate that arsenite and UVA in combination generate more ROS than either treatment alone (Fig. 2A-C). Interestingly, we find differences in the mechanisms leading to ROS generation by arsenic or UVA in keratinocytes. NADPH oxidase is an important enzyme for ROS generation by arsenite [35,36,56] and UVR [48-51,57]. We find that both arsenite and UVA increase NADPH oxidase activity (Fig. 4A) and induce phosphorylation of p67phox (Fig. 4B&C); however, only arsenite increased expression of p22phox (Fig. 4B) and caused extended NADPH oxidase activation (Fig. 4A). Mitochondria represent another source of intracellular ROS and selective inhibitors of NADPH oxidase or the mitochondrial respiratory chain revealed different relative contributions of these two mechanisms to arsenite or UVA induced ROS production. Arsenite-induced ROS was sensitive to NADPH oxidase inhibition by apocynin at all time points (Fig. 5A) while UVA-induced ROS displayed greater dependence on mitochondrial contribution throughout the time course examined (Fig. 5B). Recently Valencia and Kochevar reported that NADPH oxidase is the primary contributor to UVA-induced ROS [57]; however their studies detected hydrogen peroxide using the indicator DCFDA. We detected ROS using the indicator DHE (selective for O2•-) and when we used DCFDA (more H2O2 and •OH specific) we obtained results comparable to those of Valencia and Kochevar [57, data not shown]. These findings highlight that ROS consist of several unique and distinctive species, including •OH, O2•-, NO, 1O2, H2O2, and ONOO-.[44] and these specific species differ in reactivity, kinetics and sources so studies that focus on specific reactive oxygen species, not “ROS” in general may reveal important distinctions.
Both UVR and arsenite stimulate numerous signal transduction cascades including MAP kinases [23-26,29,58,59]. ROS are signaling molecules and we reported previously that arsenite-induced ROS is upstream of and required for p38 activation [30]. In this study we find that combined arsenite and UVA exposures selectively increase p38 activation compared to either agent alone (Fig. 1). Similarly, expression of a redox sensitive gene (HO-1) was substantially increased by the combined exposure relative to arsenite or UVA alone (Fig. 3). NADPH oxidase is an upstream activator of p38 [31,32]. Both p38 activation (Fig. 1) and HO-1 expression (Fig. 3) were stimulated by arsenite, but not UVA, and were highly sensitive to NADPH oxidase inhibition (Fig. 6 A&B). This suggests that the persistent NADPH oxidase activation by arsenite is an important contributor to arsenite-stimulated ROS production, p38 activation and HO-1 induction.
The observed elevation of ROS and ROS-dependent signaling observed with arsenite and UVA co-exposure may represent a mechanism underlying the synergistic interactions of these agents in producing skin cancer in mouse models [7,10,11]. In vivo data shows increased oxidative DNA damage with combined arsenite and UVR exposures [14] which is likely the result of the convergence of stimuli inducing persistently elevated ROS in the skin. The increased ROS also stimulates signaling cascades leading to expression of tumor relevant genes may further contribute to tumor promotion and progression. Since arsenite appears to be a potent co-carcinogen it is imperative that the mechanisms leading to synergistic interactions be thoroughly investigated.
Acknowledgements
Thanks to Dr. T. Thompson and Dr. G. Timmins for their assistance in experimental design and data analysis. This work was supported by NIH Grant R01 ES012938 and in part by NIH AR 42989, NIEHS P30-ES-012-72, and EPA STAR Fellowship FP-91650201-1.
List of abbreviations
- UVR
ultraviolet radiation
- ROS
reactive oxygen species
- DHE
dihydroethidium
- DCFDA
2',7'-dichlorofluorescein diacetate
- DPI
diphenyleneiodonium
- ERK
extracellular signal-regulated kinase
- MAP kinase
mitogen-activated protein kinase
- JNK
c-jun N-terminal kinases
- PBS
phosphate-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boukamp P. UV-induced skin cancer: similarities—variations. J Dtsch Dermatol Ges. 2005;3(7):493–503. doi: 10.1111/j.1610-0387.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishigori C. Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci. 2006;5(2):208–214. doi: 10.1039/b507471a. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed NU, Ueda M, Nikaido O, Osawa T, Ichihashi M. High levels of 8-hydroxy-2'-deoxyguanosine appear in normal human epidermis after a single dose of ultraviolet radiation. Br J Dermatol. 1999;140(2):226–231. doi: 10.1111/j.1365-2133.1999.02653.x. [DOI] [PubMed] [Google Scholar]
- 4.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6(3):561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 5.Baudouin C, Charveron M, Tarroux R, Gall Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002;18:341–348. doi: 10.1023/a:1019540316060. [DOI] [PubMed] [Google Scholar]
- 6.Morton W, Dunnette D. Health effects of environmental arsenic. In: Nriagy JO, editor. Arsenic in the Environment, Part II: Human Health and Ecosystem Effects. John Wiley and Sons, Inc.; 1994. [Google Scholar]
- 7.Shannon RL, Strayer DS. Arsenic-induced skin toxicity. Human Toxicol. 1989;8:99–104. doi: 10.1177/096032718900800203. [DOI] [PubMed] [Google Scholar]
- 8.Evans CD, LaDow K, Schumann BL, Savage RE, Jr., Caruso J, Vonderheide A, Succop P, Talaska G. Effect of arsenic on benzo[a]pyrene DNA adduct levels in mouse skin and lung. Carcinogenesis. 2004;25(4):493–497. doi: 10.1093/carcin/bgg199. [DOI] [PubMed] [Google Scholar]
- 9.Fischer JM, Robbins SB, Al-Zoughool M, Kannamkumarath SS, Stringer SL, Larson JS, Caruso JA, Talaska G, Stambrook PJ, Stringer JR. Comutagenic activity of arsenic and benzo[a]pyrene in mouse skin. Mutat Res. 2005;588(1):35–46. doi: 10.1016/j.mrgentox.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Waalkes MP, Liu J, Ward JM, Diwan BA. Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology. 2004;198(13):31–38. doi: 10.1016/j.tox.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Germolec DR, Spalding J, Yu HS, Chen GS, Simeonova PP, Humble MC, Bruccoleri A, Boorman GA, Foley JF, Yoshida T, Luster MI. Arsenic enhancement of skin neoplasis by chronic stimulation of growth factors. Am. J. Pathol. 1998;153(6):1775–1785. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol. 2004;198(3):394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol. 2001;176(1):64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- 14.Uddin AN, Burns FJ, Rossman TG. Vitamin E and organoselenium prevent the cocarcinogenic activity of arsenite with solar UVR in mouse skin. Carcinogenesis. 2005;26(12):2179–2186. doi: 10.1093/carcin/bgi180. [DOI] [PubMed] [Google Scholar]
- 15.Hamadeh HK, Trouba KJ, Amin RP, Afshari CA, Germolec D. Coordination of altered DNA repair and damage pathways in arsenite-exposed keratinocytes. Toxicol. Sci. 2002;69:306–316. doi: 10.1093/toxsci/69.2.306. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, L.G. H, Ding W, Wang S, Cooper KL, Chen Y, Shi X, Liu KJ. Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem. Research Toxicol. 2004;17(7):871–878. doi: 10.1021/tx049939e. [DOI] [PubMed] [Google Scholar]
- 17.Ding W, Hudson LG, Liu KJ. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem. 2005;279(12):105–112. doi: 10.1007/s11010-005-8227-y. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Ke Q, Costa M, Shi X. Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem. 2004;255(12):57–66. doi: 10.1023/b:mcbi.0000007261.04684.78. [DOI] [PubMed] [Google Scholar]
- 19.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533(12):37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Dong Z, Crawford HC, Lavrovsky V, Taub D, Watts R, Matrisian LM, Colburn NH. A dominant negative mutant of jun blocking 12-O-tetradecanoylphorbol-13-acetate-induced invasion in mouse keratinocytes. Mol Carcinog. 1997;19(3):204–212. doi: 10.1002/(sici)1098-2744(199707)19:3<204::aid-mc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermis cells. Proc Natl Acad Sci U S A. 1994;91(2):609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE. 2003;167:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 23.Yanase H, Ando H, Horikawa M, Watanabe M, Mori T, Matsuda N. Possible involvement of ERK1/2 in UVA-induced melanogenesis in cultured human epidermal keratinocytes. Pigment Cell Res. 2001;14:103–109. doi: 10.1034/j.1600-0749.2001.140205.x. [DOI] [PubMed] [Google Scholar]
- 24.Englaro W, Derijard B, Ortonne R, Ballotti R. Solar ultraviolet light activates extracellular signal-regulated kinases and the trenary complex factor in human normal keratinocytes. Oncogene. 1998;16:661–664. doi: 10.1038/sj.onc.1201536. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Nartindale KL, Holbrook NJ, Liu Y. Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc. Mol. Cell. Biol. 1998;18:5178–5188. doi: 10.1128/mcb.18.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper KL, Myers TA, Rosenberg M, Chavez M, Hudson LG. Roles of mitogen-activated protein kinases and EGF receptor in arsenite-stimulated matrix metalloproteinase-9 production. Toxicol. Appl. Pharmacol. 2004;200:177–185. doi: 10.1016/j.taap.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig S, Hoffmeyer A, Goebeler M, Kilian K, Hafner H, Neufeld B, Han J, Rapp UR. The stress inducer arsenite activates mitogen-activated protein kinases extracellular signal-regulated kinases 1 and 2 via a MAPK kinase 6/p38-dependent pathway. J. Biol. Chem. 1998;273(4):1917–1922. doi: 10.1074/jbc.273.4.1917. [DOI] [PubMed] [Google Scholar]
- 28.Patterson TJ, Reznikova TV, Phillips MA, Rice RH. Arsenite maintains germinative state in cultured human epidermal cells. Toxicol Appl Pharmacol. 2005;207(1):69–77. doi: 10.1016/j.taap.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Souza K, Maddock DA, Zhang Q, chen J, Chiu C, Mehta S, Wan Y. Arsenite activation of PI3K/AKT cell survival pathway is mediated by p38 in cultured human keratinocytes. Mol. Med. 2001;7(11):767–772. [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper KL, Liu KJ, Hudson LG. Contributions of reactive oxygen species and mitogen-activated protein kinase signaling in arsenite-stimulated hemoxygenase-1 production. Toxicol Appl Pharmacol. 2007;218(2):119–127. doi: 10.1016/j.taap.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 32.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43(3):332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orient A, Donko A, Szabo A, Leto TL, Geiszt M. Novel sources of reactive oxygen species in the human body. Nephrol Dial Transplant. 2007;22(5):1281–88. doi: 10.1093/ndt/gfm077. [DOI] [PubMed] [Google Scholar]
- 34.Qian Y, Liu KJ, Chen Y, Flynn DC, Castranova V, Shi X. Cdc42 regulates arsenic-induced NADPH oxidase activation and cell migration through actin filament reorganization. J Biol Chem. 2005;280(5):3875–3884. doi: 10.1074/jbc.M403788200. [DOI] [PubMed] [Google Scholar]
- 35.Lynn S, Gurr JR, Lai HT, Jan KY. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ Res. 2000;86(5):514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- 36.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A. 2004;101(13):4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rago R, Mitchen J, Wilding G. DNA fluorometric assay in 96-well tissue culture plates using Hoechst 33258 after cell lysis by freezing in distilled water. Anal Biochem. 1990;191(1):31–34. doi: 10.1016/0003-2697(90)90382-j. [DOI] [PubMed] [Google Scholar]
- 39.McCawley LJ, Li S, Wattenberg EV, Hudson LG. Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine kinase specificity for matrix metalloproteinase-9 induction and cell migration. J Biol Chem. 1999;274(7):4347–4353. doi: 10.1074/jbc.274.7.4347. [DOI] [PubMed] [Google Scholar]
- 40.Dib K, Melander F, Axelsson L, Dagher M, Aspenstrom P, Andersson T. Down-regulation of Rac activity during beta 2 integrin-mediated adhesion of human neutrophils. J Biol Chem. 2003;278(26):24181–24188. doi: 10.1074/jbc.M302300200. [DOI] [PubMed] [Google Scholar]
- 41.Cooper S, Ranger-Moore J, Bowden TG. Differential inhibition of UVB-induced AP-1 and NF-kappaB transactivation by components of the jun bZIP domain. Mol Carcinog. 2005;43(2):108–116. doi: 10.1002/mc.20101. [DOI] [PubMed] [Google Scholar]
- 42.Finch S, Joseloff E, Bowden T. JunB negatively regulates AP-1 activity and cell proliferation of malignant keratinocytes. J Cancer Res Clin Oncol. 2002;128(1):3–10. doi: 10.1007/s00432-001-0298-x. [DOI] [PubMed] [Google Scholar]
- 43.Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004;43(5):326–335. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 44.Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255(12):67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 45.Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH. Global alterations of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis. 2003;24:747–756. doi: 10.1093/carcin/bgg010. [DOI] [PubMed] [Google Scholar]
- 46.Maines MD, Gibbs PE. 30 some years of heme oxygenase: from a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338(1):568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 47.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005;7(12):80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- 48.Cho HJ, Jeong HG, Lee JS, Woo ER, Hyun JW, Chung MH, You HJ. Oncogenic H-Ras enhances DNA repair through the Ras phosphatidylinositol 3-kinase/Rac1 pathway in NIH3T3 cells. J Biol Chem. 2002;277(22):19358–19366. doi: 10.1074/jbc.M200933200. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Q, Zhou C, Healey S, Chu W, Kouttab N, Bi Z, Wan Y. UV radiation down-regulates Dsg-2 via Rac/NADPH oxidase-mediated generation of ROS in human lens epithelial cells. Int J Mol Med. 2006;18(2):381–387. [PubMed] [Google Scholar]
- 50.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nirtric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007;75(2):349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Wan Y, Wang Z, Shao Y, Xu Y, Voorhees J, Fisher G. UV-induced expression of GADD45 is mediated by an oxidant sensitive pathway in cultured human keratinocytes and in human skin in vivo. Int J Mol Med. 2000;6(6):683–688. doi: 10.3892/ijmm.6.6.683. [DOI] [PubMed] [Google Scholar]
- 52.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signaling. Nat Rev Cancer. 2004;4(1):23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 53.Campbell FA, Gupta G. The management of non-melanoma skin cancer. Hosp Med. 2005;66(5):288–293. doi: 10.12968/hmed.2005.66.5.18423. [DOI] [PubMed] [Google Scholar]
- 54.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(13):8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 55.de Gruijl FR, Van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B. 2001;63(13):19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 56.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177(2):324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 57.Valencia A, Kochevar IE. Nox-1 based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J Invest Dermatol. 2008;128(1):214–222. doi: 10.1038/sj.jid.5700960. [DOI] [PubMed] [Google Scholar]
- 58.Dong J, Ramachandiran S, Tikoo K, Jia Z, Lau SS, Monks TJ. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol. 2004;287(5):F1049–1058. doi: 10.1152/ajprenal.00132.2004. [DOI] [PubMed] [Google Scholar]
- 59.Patterson TJ, Ngo M, Aronov PA, Reznikova TV, Green PG, Rice RH. Biological activity of inorganic arsenic and antimony reflects oxidation state in cultured human keratinocytes. Chem Res Toxicol. 2003;16(12):1624–1631. doi: 10.1021/tx034146y. [DOI] [PubMed] [Google Scholar]