Abstract
Peptoids that inhibit the group I intron RNA from Candida albicans, an opportunistic pathogen that kills immunocompromised hosts, have been identified using microarrays. The arrayed peptoid library was constructed using submonomers with moieties similar to ones found in small molecules known to bind RNA. Library members that passed quality control analysis were spotted onto a microarray and screened for binding to the C. albicans group I intron ribozyme. Each ligand binder identified from microarray-based screening inhibited self-splicing in the presence of 1 mM nucleotide concentration of bulk yeast tRNA with IC50’s between 150 and 2200 µM. The binding signals and the corresponding IC50’s were used to identify features in the peptoids that predispose them for RNA binding. After statistical analysis of the peptoids’ structures that bind, a second generation of inhibitors was constructed using these important features; all second generation inhibitors have improved potencies with IC50’s <100 µM. The most potent inhibitor is composed of one phenylguanidine and three tryptamine submonomers and has an IC50 of 31 µM. This compound is 6-fold more potent than pentamidine, a clinically used drug that inhibits self-splicing. These results show that: 1.) modulators of RNA function can be identified by designing RNA-focused chemical libraries and screening them via microarray; 2.) statistical analysis of ligand binders can identify features in leads that predispose them for binding to their targets; and 3.) features can then be programmed into second generation inhibitors to design ligands with improved potencies.
INTRODUCTION
RNA plays a variety of roles in biological systems that cement its status as a therapeutic target.(1–3) Therefore, developing compounds that modulate RNA activity is important. Microarrays have proven to be an effective platform to identify small molecules (4–7) that bind protein or RNA. They have also been used to study the interactions between aminoglycosides and mimics of rRNA A-sites and to identify RNA internal loops that bind aminoglycoside derivatives when the loops are presented as members of an RNA motif library.(8–10)
In order to better understand how small molecules interact with RNA targets, we designed and studied a library of compounds that are predisposed for binding RNA. Cues were taken from small molecules that are known to bind RNA and portions of them were displayed on a peptoid scaffold. Peptoids are ideal because chemically diverse compounds can be easily synthesized from amine-containing submonomers that are modularly installed onto polymeric chains.(11–13) Furthermore, a chemical handle for surface immobilization can be installed at the end of each peptoid to enable high throughput screening via microarray.
Many potential RNA drug targets are available to screen for binding to arrayimmobilized small molecules, however few can be directly assayed in vitro for modulation of activity. One such class of RNAs to which an activity assay can be easily applied is group I introns, which are catalytically active in the absence of protein.(14) The group I intron from Candida albicans was selected for microarray-based screening of peptoids because its activity is essential for the assembly of active ribosomes since the intron is embedded in the large subunit rRNA precursor.(15–17) Moreover, C. albicans is a pathogenic fungus that infects and kills AIDS patients and is acquiring resistance to current treatments. (18–20)
Results show that all binders identified from microarray screening inhibit self-splicing. In addition, several features in the peptoids that are important for RNA binding and inhibition of self-splicing were identified, including the presence of N-1-akylated imidazole, indole, and phenylguanidine side chains (Figure 1). Interestingly, these studies afforded six compounds that are as or more potent than a clinically used drug for inhibition of group I intron selfsplicing, pentamidine. These studies should allow for more effective design and screening of ligands that bind to and modulate the activity of RNAs to serve as therapeutics or chemical genetics probes of function. Furthermore, these studies improve our understanding of how the results from microarray screening correlate to modulation of a target’s activity.
Figure 1.
A, structures of the peptoid submonomers that were used to construct the library. Submonomers 2 and 4 were protected during synthesis with t-Butoxycarbonyl (Boc), and 7 with 2,2,5,7,8-pentamethyl-chromane-6-sulfonyl (PMC); protecting groups were removed upon cleavage from the resin. B, the general structure of the peptoid library that was synthesized. C, the Huisgen dipolar cycloaddition reaction that was used to anchor azide-displaying peptoids onto alkyne-functionalized agarose (R5= agarose) or to conjugate to propargylamine (R5 = H) for testing inhibition of self-splicing. Unless indicated, the compounds tested for inhibition of self-splicing contained a triazole.
RESULTS AND DISCUSSION
Many important RNAs are being discovered in genomic sequences. Most of these represent untapped potential as targets for therapeutics or chemical genetic probes. The present study seeks to develop a general method to identify lead ligands that bind to highly structured RNA targets and modulate their activities. Critical to this study were the selection of ligands and the use of microarray-based screening to score members of the ligand library for binding.
Small molecule microarrays allow for facile screening for binding of target biomolecules including proteins and RNAs.(5, 6, 21–25) Though screening is relatively easy, it is often difficult to correlate this information to modulation of activity or as a general design tool for other biomolecules. One way microarrays could facilitate the elucidation of general design principles is to screen a diverse chemical space to pinpoint features in small molecules that allow them to bind RNA. These studies are important given the low hit-rates for RNA binders (compared to their protein counterparts) from high throughput screening assays.(26) We designed a peptoid library biased for binding RNA by selecting submonomers that have similarities to known RNAbinding compounds or that have the potential to stack and/or hydrogen bond with RNA. Other methods that could be used to introduce bias include conjugation of small molecules to aminoglycosides and using information about aminoglycoside binding modes to design compounds that display functional groups in a similar manner. (27, 28)
Selection of library members to be synthesized and arrayed
A small peptoid library was synthesized using solid-phase methods and arrayed onto alkyne-functionalized agarose to create small molecule microarrays.(5–7, 10, 25) The submonomers for construction of the library were chosen because they have a high probability of interacting with RNA due to similarities in their structure to known RNA binders or due to their potential to interact with RNA by stacking or hydrogen bonding (Figure 1A). For example, 1 was chosen because pyridines bind to a mimic of the bacterial rRNA A-site.(29) The other submonomers have similar structural features present in other known RNA binders: 2 (4',6-diamidino-2-phenylindole, DAPI);(30) 3 (4-heterocyclic 2-deoxystreptamine derivatives);(31) 4 (DAPI and peptides that bind RNA);(30, 32, 33) 5 (similar to the oxazolidinone ring in linezolid), (34) 6 (xanthinol, which binds TAR RNA);(35) and 7 (pentamidine and arginine-rich peptides that bind RNA).(36, 37) The benzene submonomer (8) was chosen because it may stack with RNA bases and is a common group in known drugs (38) while the benzenesulfonaminde (9) may stack with and hydrogen bond to RNA. The last submonomer installed in each synthesis was 3-azidopropylamine (10) to allow for surface conjugation onto alkyne-agarose via a Huisgen dipolar cycloaddition reaction (HDCR). (39)
A library of 109 peptoids containing three positions of diversity was arrayed; compounds were arrayed only after they passed a mass spectral quality control analysis (40) in order to ensure that each compound was successfully synthesized. This is in contrast to arrays of compounds synthesized from a split-and-mix approach where there is no quality control step. By arraying quality-tested compounds, binding patterns can be used to infer features in ligands that confer or inhibit binding to RNA without having to be concerned if these results are obscured due to inefficient synthesis. Such features can then be further studied or used to design more potent inhibitors.
Serial dilutions of each azide-containing peptoid were conjugated onto alkyne-agarose arrays via a HDCR. (10, 39, 41) Agarose arrays were used because they provide a threedimensional surface for high ligand loading that resists non-specific binding.(42, 43) In addition, the HDCR provides each compound with a triazole group upon immobilization; triazoles are present in many small molecules that bind RNA.(31, 44) Therefore, the immobilization chemistry could also contribute favorably to RNA binding.
Array-based screening identifies peptoids that bind to the C. albicans ribozyme
Once constructed, arrays were hybridized with a 32P-labeled ribozyme construct that is derived from the large subunit rRNA precursor from the fungal pathogen C. albicans. (16) These experiments were completed in the presence of unlabeled bulk yeast tRNA that is in excess over the total amount of ligand delivered to the array surface (Figure 2A). The signal to noise ratio on the array for binding to labeled ribozyme is quite good (>50/1), and 12 of the 109 peptoids bind (Figure 2A); in order to be considered a binder, a ligand had to give at least 20% of the highest signal observed on the array, or from 4-4-3-12 (Table 1). For all peptoids that bind the ribozyme, a dose-response is observed (Figure 2B and C). In general, the binders have similar structural features, such as side chains from the phenylguanidine (2), N-1-alkyated imidazole (3), indole (4), or theophylline (6) submonomers. Interestingly, each of the binders contained either 2 or 3 at the third position. It should be noted that due to synthetic challenges with submonomer 2, it was only installed at the third position (Figure 1B). Attempts to install this submonomer at other positions yielded impure peptoids, which were discarded.
Figure 2.
A, image of a peptoid microarray after hybridization with the C. albicans ribozyme, with the arrows pointing to the spots from the highest loading of the compounds. B and C, plots of radioactive signal for each peptoid binder as a function of the moles of peptoid delivered to the array surface; errors are standard deviations from three measurements. Panel B contains the peptoids without the phenylguanidine in the third position while panel C contains the peptoids with phenylguanidine in the third position. Data were normalized by comparison to the highest loading spot of compound 4-4-3-12, which gave the highest signal. Only compounds that gave at least 20% of the normalized signal of 4-4-3-12 at the highest ligand loading were scored as binders.
Table 1.
Data for inhibition of precursor self-splicing by peptoids that bind to the ribozyme when displayed on a microarray.
| Sequence R1,R2,R3,R4 | IC50 (µM)a | Relative Array Binding Signal b, c |
|---|---|---|
| 4-4-3-12 | 158 ± 14 | 1.00 |
| 4-6-3-12 | 150 ± 40 | 0.35 |
| 4-3-3-12 | 157 ± 40 | 0.66 |
| 6-4-3-12 | 209 ± 90 | 0.71 |
| 3-3-2-12 | 399 ± 14 | 0.56 |
| 7-6-2-12 | 500 ± 110 | 0.42 |
| 8-3-2-12 | 633 ± 153 | 0.48 |
| 3-4-3-12 | 817 ± 76 | 0.24 |
| 3-8-2-12 | 1217 ± 301 | 0.79 |
| 1-3-2-12 | 1320 ± 814 | 0.27 |
| 3-1-2-12 | 1733 ± 681 | 0.34 |
| 3-6-3-12 | 2200 ± 1304 | 0.35 |
| 1-5-8-12d | >5,000 | 0.00 |
| Pentamidine | 200±35 | - |
All reactions were completed in the presence of 1 mM nucleotide concentration of bulk yeast tRNA competitor.
In these assays, peptoids are conjugated to alkyne-functionalized agarose microarrays via HDCRs.
Values are determined by dividing the signal from the compound by the signal from 4-4-3-12 at the highest loading spot from Figure 2.
Compound 1-5-8-12 is a negative control that did not give signal for binding to the ribozyme on the array.
Statistical analysis of binders identified from array-based screening
Structural features of the binders were then subjected to statistical analysis to quantify their significance relative to the entire peptoid library. Briefly, similarities and trends in the binders were search for, and the percentage of binders with the trend of interest was then calculated. This percentage was compared to the percentage of peptoids in the entire library that display the same trend. If the percentage was greater for the binders, a Z-test was used to determine if the over-representation in the binders was not due to random chance. The generated Z-value was then converted to a confidence level, or a two-tailed p-value, indicative of statistical significance. Please see the Supporting Information for example calculations.
The first set of p-values was generated by simply considering the representation of each submonomer in the binders relative to their representation in the whole library. This analysis yielded p-values of <0.0001 for the imidazole (3), 0.009 for the indole (4), and 0.01 for the theophylline (6) features. The positional dependence of the features was then computed. This analysis showed that phenylguanidine (2) or 3 was preferred at the third position, with p-values of 0.04 and 0.0007, respectively. There was no other statistically significant positional dependence for single submonomers in the analysis. A final analysis was completed by determining if the relative positioning of two or more submonomers is statistically significant. This calculation revealed that peptoids with 3 in the second and 2 in the third position have a pvalue of 0.0026.
Inhibition of C. albicans precursor self-splicing by the arrayed peptoids
Many of the peptoids that bind the ribozyme were studied for their ability to inhibit self-splicing of the C. albicans group I intron precursor (Table 1). In order to mimic the arrayed structure, each peptoid was synthesized to contain a triazole by conjugation to propargylamine (Figure 1C). The IC50’s for inhibition of self-splicing were determined at 2 mM Mg2+ in the presence of competing 1 mM nucleotide concentration of bulk yeast tRNA. Compound 1-5-8-12 was used as a negative control as it did not bind the ribozyme above background when displayed on the microarray surface. As expected, it did not inhibit precursor self-splicing up to the highest concentration tested, 4000 µM.
All peptoids that bind to the ribozyme when displayed on the microarray surface inhibit group I intron self-splicing, with IC50’s ranging from 150 to 2200 µM (Table 1). Compounds 6-4-3-12, 4-6-3-12, 4-3-3-12, and 4-4-3-12 were the most potent inhibitors and also gave some of the highest signals from the microarray screening assay. Compound 3-8-2-12 is an outlier, however, since it has the second highest signal for binding on the array, with 79% of the signal from 4-4-3-12, but is 8-fold less potent at inhibiting precursor self-splicing. Based on its binding signal, the IC50 of 3-8-2-12 was expected to be more in line with compound 6-4-3-12. Therefore, microarray screening identifies inhibitors of self-splicing; however, in order to gain clearer insight into the features in the peptoid that lead to enhanced potency, each peptoid was tested for inhibition of self-splicing.
Design of potent second generation inhibitors using insights from statistical analysis of microarray binding data
Using the data from statistical analysis of binders identified from the array and their corresponding IC50’s, two subsets of second generation inhibitors were designed and tested. The first subset contained submonomer 2 in the third position since statistical analysis identified a preference for 2 or 3 in this position. Compounds 4-4-3-12 and 4-6-3-12 gave excellent binding signal on the array and were the two most potent inhibitors of selfsplicing (Figure 2A and Table 1). The initial library screened by microarray, however, did not contain the corresponding compounds with 2 in the third position. Therefore, 4-4-3-12 and 4-6-3-12 were modified to contain submonomer 2 in the third position, yielding compounds 4-4-2-12 and 4-6-2-12. Both peptoids showed ~1.5-fold improvement in potency compared to their parent compounds, with IC50’s of 97 and 109 µM for 4-4-2-12 and 4-6-2-12, respectively. Evidently, having submonomer 2 in the third position provides more potent inhibitors (Table 2).
Table 2.
Structure-based analyses of 4-4-3-12 and 4-4-2-12 for inhibition of precursor self-splicing. Submonomers were either sequentially deleted or replaced with 11.
| Sequence R1,R2,R3 | IC50 (µM) |
|---|---|
| 4-4-3-12 | 158 ± 14 |
| 4-4-3-10 | 533 ± 58 |
| 4-4-3 | 633 ± 153 |
| 4-4-2-12 | 97 ± 49 |
| 4-4-2-10 | 338 ± 88 |
| 4-4 | 733 ± 115 |
| 3 | >5,000 |
| 11-4-3-12 | >5,000 |
| 4-11-3-12 | 1,333 ± 144 |
| 11-4-2-12 | 1,083 ± 227 |
| 4-11-2-12 | 1,233 ± 252 |
| 4-4-11-12 | 767 ± 153 |
| TBTA | >5,000 |
Structure-based analysis of high potency peptoids
Peptoids 4-4-3-12 and 4-4-2-12 were further analyzed to detemine the contribution of each feature to inhibition potency (Table 2). In the first method, a fragmentation approach was taken in which submonomers in the peptoids were sequentially removed and the the resulting fragment was tested for inhibition. First, the triazole functionality was removed leaving a free azide corresponding to compounds 4-4-3-10 and 4-4-2-10. Both were ~3-fold less potent than the corresponding parent compound. Removal of the azide to afford compounds 4-4-3 and 4-4-2 also decreased potency. When the submonomer in the third position was removed from either peptoid to afford compound 4-4, the IC50 increased to 733 µM (an ~4-fold increase in IC50 compared to 4-4-3-12 and an ~7-fold increase compared to 4-4-2-12). As expected monomeric 3 is a poor inhibitor of self-splicing with no inhibition observed up to the highest concentration tested (5000 µM). Monomer 4 was not soluble in water so accurate measurements of its potency could not be completed.
In a second method, submonomers were replaced with propylamine (11) to determine the contribution of each heterocycle displayed at the corresponding position (Table 2 and Figure 3). For compound 4-4-3-12, the first position contributes the most to potency as replacement with submonomer 11, affording 11-4-3-12, results in an inactive compound (IC50 >5000 µM). Replacement of the second and third positions with 11 increased the IC50’s by ~8-fold and ~5-fold, respectively. The results for replacing the submonomers of 4-4-2-12 with 11 are similar. Replacement of the first, second, or third position with 11 caused an ~11-fold, ~13-fold, and ~8-fold increase in IC50, respectively. Therefore, each submonomer contributes to the ability of the ligands to inhibit self-splicing.
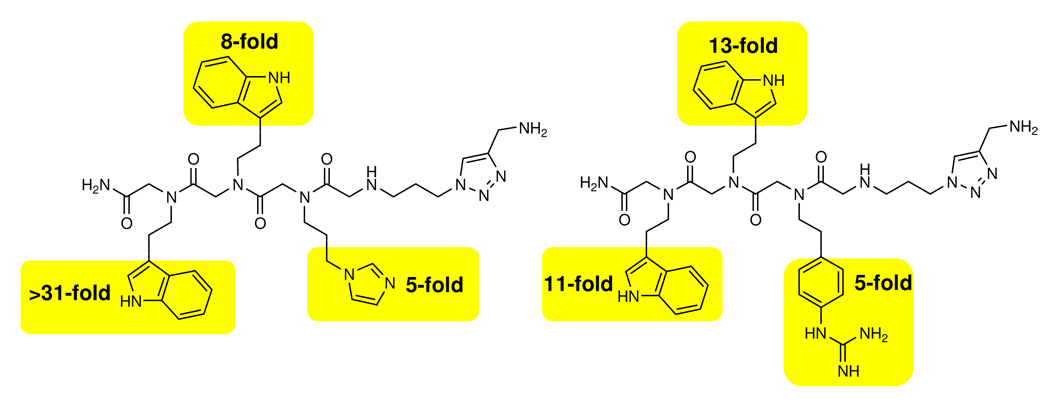
Figure 3.
Studies to determine the contribution of each submonomer to the potency of compounds 4-4-3-12 and 4-4-2-12. The values above each heterocycle are the decrease in IC50 for inhibition of self-splicing when the highlighted position is replaced with a propylamine submonomer (11).
Combining multiple trends from statistical analysis affords the most potent compounds
Another subset of second generation inhibitors incorporated other trends identified by statistical analysis (Table 3). The first trend is the importance of having 3 in the second position and 2 in the third position (two-tailed p-value of 0.0026). A peptoid with four points of diversity similar to 4-4-3-12 and 4-4-2-12, compound 4-4-3-2-12, was synthesized and tested for its ability to inhibit self-splicing. Addition of the 2 side chain improved the IC50 from 158 µM (4-4-3-12) to 88 µM. Another trend is the presence of the tryptamine submonomer (4). Therefore, two statistically significant trends, having 4 submonomers and the 2 submonomer in the last position, were used to design 4-4-4-2-12. This afforded the most potent peptoid with an IC50 of 31 µM. Thus, incorporating multiple trends from statistical analysis can provide inhibitors with increased potency relative to those identified from microarray screening. In fact, 4-4-4-2-12 is a ~5-fold better inhibitor than the three most potent inhibitors identified from the microarray screen.
Table 3.
Data for inhibition of precursor self-splicing by second generation peptoids designed by using statistical analysis. All IC50s are lower than the most potent first generation peptoid.
| Sequence R1,R2,R3,R4 | IC50 (µM) |
|---|---|
| 4-4-2-12 | 97 ± 49 |
| 4-6-2-12 | 109 ± 19 |
| 4-4-3-2-12 | 88 ± 4 |
| 4-4-4-2-12 | 31 ± 6 |
The effect of Mg2+ and bulk yeast tRNA on inhibitor potency
Identification of compounds that are active and specific for the target of interest is essential for rational drug design. Inclusion of competing bulk yeast tRNA in microarray screening yielded compounds that were specific to our target by suppressing signals from non-specific binders. In order to further address the specificity of the most potent inhibitor of self-splicing identified in this study, 4-4-4-2-12, the effect of increasing concentrations of Mg2+ and bulk yeast tRNA on the potency of 4-4-4-2-12 were determined (Table 4).
Table 4.
The effect of bulk yeast tRNA and Mg2+ on the potency of 4-4-4-2-12 to inhibit precursor self-splicing.
| [Mg2+], mM a | IC50, µM |
|---|---|
| 2 | 31 ± 6 |
| 5 | 56 ± 23 |
| 10 | 57 ± 26 |
| [bulk yeast tRNA], mM nucleotides b | |
| 1 | 31 ± 6 |
| 2 | 21 ± 5 |
| 4 | 28 ± 4 |
| 8 | 26 ± 2 |
These assays were completed with 1 mM nucleotide concentration of bulk yeast tRNA.
These assays were completed in H2Mg buffer supplemented with the indicated nucleotide concentration of bulk yeast tRNA.
The results show that increasing the Mg2+ concentration from 2 mM to 10 mM in the presence of 1 mM nucleotide bulk yeast tRNA resulted in an increase in the IC50 from 31 µM to 57 µM. Previous studies on oligonucleotide inhibition of C. albicans intron self-splicing have also observed a decrease in potency as the concentration of Mg2+ is increased.(45) The effect of increasing the amount of tRNA was also studied at 2 mM Mg2+. Bulk yeast tRNA was added to splicing reactions at 1, 2, 4, and 8 mM nucleotide concentrations. Even when 8 mM nucleotide concentration of competitor is added, the IC50 is unaffected (Table 4). If this compound was a non-specific inhibitor, then a significant decrease in potency would be expected. Thus, 4-4-4-2-12 is specific for the C. albicans group I intron over bulk yeast tRNA.
Testing 4-4-4-2-12 for inhibition of the group I introns precursors from Tetrahymena thermophilla (46) and Pneumocystis carinii (47)
In order to determine if 4-4-4-2-12 is selective for the C. albicans precursor relative to other group I introns, it was tested for inhibiting the self-splicing of the T. thermophila and P. carinii group I intron precursors which both reside in the large subunit pre-rRNA. Experiments were completed in 10 mM Mg2+ and 1 mM nucleotide concentration of bulk yeast tRNA. The IC50’s were >200 µM and >500 µM for the T. thermophila and the P. carinii precursors, respectively. Thus, 4-4-4-2-12 is specific for the C. albicans group I intron.
Comparison of the most potent compound to other inhibitors of self-splicing
Compound 4-4-4-2-12 is similar in structure to several compounds that have been found to inhibit group I intron self-splicing, including similarities between the indole side chain and the benzimidazole in Hoechst 33258.(48) It is also similar to pentamidine in that both compounds contain a phenyl ring capped by a positively charged group, a benzenecarboximidamide (pentamidine) or phenylguanidine (submonomer 2). The array structure-activity relationship (SAR) data identified that all inhibitors had either a phenylguanidine or imidazole group at the third position and each likely provides critical interactions to stabilize the ligand-target complex.
Summary and Conclusions
Using cues from small molecules that target RNA, a peptoid library was designed and screened for binding to the C. albicans group I ribozyme. Of the 109 arrayed peptoids, 12 bound the RNA, and a subset was tested for their abilities to inhibit self-splicing. We combined the data from microarray screening, statistical analysis of ligand binders, and compound potency, and identified modules 2 and 4 as important determinants in the molecular recognition of the C. albicans group I intron. These analyses guided the rational design of 4-4-4-2-12, which is the most potent inhibitor identified from this study. This compound is a 5-fold more potent inhibitor of self-splicing compared to the best ligand identified from the microarray screen, 4-6-3-12 (Table 2 and Table 3). It is also a 6-fold more potent inhibitor than pentamidine, a clinically used drug to treat Pneumocystis carinii infections that has also been shown to inhibit self-splicing of the C. albicans intron in vitro and in vivo. (17, 37, 49) In fact, all second generation inhibitors were more potent than the original library and a known drug that targets self-splicing RNAs.
Many features that can predispose ligands for specific recognition of RNA may lie outside of presently known scaffolds that bind RNA. By expanding the results disclosed herein and probing diverse chemical space for binding RNA via microarray, these scaffolds are likely to be identified. Future developments using microarray-based screening of peptoids or other small molecules would benefit from using chemoinformatics approaches to compute chemical diversity to rationally design libraries with broad chemical landscapes. By using statistical analysis of binders, general ligand features for binding RNA can be elucidated quickly.
METHODS
General Methods
All chemicals used were from Acros, Sigma-Aldrich, or MP Biochemicals and used without purification unless noted otherwise. Please see the Supporting Information for detailed procedures for the syntheses of peptoid submonomers 2 and 6 and all peptoids.
Diethyl pyrocarbonate (DEPC)-treated nanopure water was used for all buffers and in splicing assays. HXMg buffer is 50 mM Na+ HEPES, pH 7.5, 135 mM KCl, and X mM MgCl2. Plasmids encoding the C. albicans group I intron precursor and ribozyme (16), the T. thermophila precursor (46), and the P. carinii precursor (47) were transcribed as described. RNAs were internally labeled using an Ambion MEGAscript or a Stratagene RiboMaxx transcription kit and [ α-32P]ATP and purified as described.(16) RNAs were resuspended in DEPC-treated water and stored at −20 °C.
Microarray construction
Glass slides (4″ × 5″) were cleaned in piranha solution (70:30 H2SO4 : 30% (v/v) aqueous H2O2) overnight at room temperature. Slides were functionalized with amines by submersion in a 3% (v/v) solution of 3-aminopropyltriethoxysilane in 95% ethanol for 1 h at room temperature.(6) This solution was prepared prior to use by stirring for 10 min at room temperature. Arrays were then washed with 95% ethanol and cured by heating at 110 °C for 1 h. After cleaning (sonicated for 1 h in water), the amine-functionalized arrays were coated with ~15 mL of a 2% (w/v) agarose solution, and the agarose was allowed to dry to a thin film at room temperature. The agarose was then oxidized by submersion in 20 mM NaIO4 for 3 h at room temperature. Alkyne-functionalized arrays were afforded by submerging the slides in an aqueous solution of 10 mM propargylamine and 10 mM NaHCO3 overnight. Arrays were quenched in a 200 mL solution (70 mL ethanol and 130 mL phosphate buffered saline) containing 200 mg NaBH3CN for 5 min. Arrays were washed and submerged in a 20% (v/v) aqueous ethylene glycol solution for 1 h, and then washed with water (6 × 100 mL for 15 min each) and dried to a clear film on the bench top.
Spotting the library onto the microarray surface
Serial dilutions of each peptoid were placed into the wells of a 384-well plate at concentrations of 5 mM, 500 µM, and 50 µM in 1X spotting solution (10 mM Tris•HCl, pH 8.5, 1 mM CuSO4, 100 µM ascorbic acid, 100 µM TBTA,(41) and 10% (v/v) glycerol). Approximately 500 nL of each peptoid solution was delivered to the surface of an alkyne-functionalized slide using a 384-pin replicator (Boekel Scientific). The arrays were then incubated at room temperature for ~3 h. The arrays were washed in buffer (8 mM Na2HPO4, pH 7.0, 1 mM EDTA, and 180 mM NaCl; 3 × 15 min each) to remove uncoupled peptoid and coupling reagents including Cu2+, rinsed with nanopure water, and left to dry at room temperature.
Array hybridization
Peptoid arrays were pre-hybridized with 3 mL of H2Mg buffer for 30 min to hydrate the surface. A 3 mL solution of 32P-labeled ribozyme (~10 nM) was annealed in 1X H2Mg buffer at 60 °C for 5 min and allowed to slow cool to room temperature. BSA and bulk yeast tRNA were then added to final concentrations of 0.162 mg mL−1, and 0.1 mg mL−1, respectively. Bulk yeast tRNA was added as a competitor and is in 10-fold nucleotide excess over the moles of ligand that were delivered to the array surface and in 250-fold nucleotide excess over the ribozyme. The comparison of the moles of tRNA and the moles of ligand are based on earlier experiments to determine the loading of ligands onto alkyne-arrays via a HDCR.(8, 50) As such, ~10% of the ligand delivered to the array surface was assumed to be immobilized. It should be noted that the concentration of MgCl2 does not significantly affect binding signals. The arrays were washed in 1X H2Mg buffer for 10 min, rinsed with water, and air dried. After drying, they were exposed to a phosphor screen and imaged using a BioRad FX imager. Data were quantified using BioRad’s QuantityOne software.
Splicing assays
Splicing assays to determine the ability of the peptoids to inhibit precursor self-splicing were completed as described except that reactions were completed in the presence of 100 µM GMP and various concentrations of bulk yeast tRNA.(16, 45) All data were quantified using BioRad’s QuantityOne software. The resulting curves were fit to SigmaPlot’s four parameter logistic curve to determine IC50’s.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Cech, A. Zaug, and S. Testa for generously providing plasmids. This work was supported by the New York State Center of Excellence in Bioinformatics and Life Sciences, and the National Institutes of Health (R01-GM079235).
Abbreviations
- Boc
t-Butoxycarbonyl
- BSA
bovine serum albumin
- DEPC
diethyl pyrocarbonate
- EDTA
ethylenediaminetetraacetic acid
- GMP
guanosine monophosphate
- HDCR
Huisgen dipolar cycloaddition reaction
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PMC
2,2,5,7,8-pentamethyl-chromane-6-sulfonyl
- rRNA
ribosomal RNA
- SAR
structure-activity relationship
- TBTA
tris(benzyltriazolylmethyl)amine
- Tris
tris(hydroxymethyl)aminomethane
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
REFERENCES
- 1.Gallego J, Varani G. Targeting RNA with small-molecule drugs: therapeutic promise and chemical challenges. Acc. Chem. Res. 2001;34:836–843. doi: 10.1021/ar000118k. [DOI] [PubMed] [Google Scholar]
- 2.Magnet S, Blanchard JS. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 2005;105:477–498. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- 3.Blount KF, Wang JX, Lim J, Sudarsan N, Breaker RR. Antibacterial lysine analogs that target lysine riboswitches. Nat. Chem. Biol. 2007;3:44–49. doi: 10.1038/nchembio842. [DOI] [PubMed] [Google Scholar]
- 4.Barnes-Seeman D, Park SB, Koehler AN, Schreiber SL. Expanding the Functional Group Compatibility of Small-Molecule Microarrays: Discovery of Novel Calmodulin Ligands. Angew. Chem. Int. Ed. Engl. 2003;42:2376–2379. doi: 10.1002/anie.200351043. [DOI] [PubMed] [Google Scholar]
- 5.Hergenrother PJ, Depew KM, Schreiber SL. Small-molecule microarrays: Covalent attachment and screening of alcohol-containing small molecules on glass slides. J. Am. Chem. Soc. 2000;122:7849–7850. [Google Scholar]
- 6.MacBeath G, Koehler AN, Schreiber SL. Printing small molecules as microarrays and detecting protein-ligand interactions en masse. J. Am. Chem. Soc. 1999;121:7967–7968. [Google Scholar]
- 7.Duffner JL, Clemons PA, Koehler AN. A pipeline for ligand discovery using small-molecule microarrays. Curr. Opin. Chem. Biol. 2007;11:74–82. doi: 10.1016/j.cbpa.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Disney MD, Labuda LP, Paul DJ, Poplawski SG, Pushechnikov A, Tran T, Velagapudi SP, Wu M, Childs-Disney JL. Two-dimensional combinatorial screening identifies specific aminoglycoside-RNA internal loop partners. J. Am. Chem. Soc. 2008;130:11185–11194. doi: 10.1021/ja803234t. [DOI] [PubMed] [Google Scholar]
- 9.Disney MD, Barrett OJ. An aminoglycoside microarray platform for directly monitoring and studying antibiotic resistance. Biochemistry. 2007;46:11223–11230. doi: 10.1021/bi701071h. [DOI] [PubMed] [Google Scholar]
- 10.Childs-Disney JL, Wu M, Pushechnikov A, Aminova O, Disney MD. A small molecule microarray platform to select RNA internal loop-ligand interactions. ACS Chem. Biol. 2007;2:745–754. doi: 10.1021/cb700174r. [DOI] [PubMed] [Google Scholar]
- 11.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient Method For The Preparation Of Peptoids [Oligo(N-Substituted Glycines)] By Submonomer Solid-Phase Synthesis. J. Am. Chem. Soc. 1992;114:10646–10647. [Google Scholar]
- 12.Uno T, Beausoleil E, Goldsmith RA, Levine BH, Zuckermann RN. New submonomers for poly N-substituted glycines (peptoids) Tetrahedron Lett. 1999;40:1475–1478. [Google Scholar]
- 13.Burkoth TS, Fafarman AT, Charych DH, Connolly MD, Zuckermann RN. Incorporation of unprotected heterocyclic side chains into peptoid oligomers via solid-phase submonomer synthesis. J. Am. Chem. Soc. 2003;125:8841–8845. doi: 10.1021/ja0352101. [DOI] [PubMed] [Google Scholar]
- 14.Zaug AJ, Cech TR. The intervening sequence RNA of Tetrahymena is an enzyme. Science. 1986;231:470–475. doi: 10.1126/science.3941911. [DOI] [PubMed] [Google Scholar]
- 15.Nikolcheva T, Woodson SA. Association of a group I intron with its splice junction in 50S ribosomes: implications for intron toxicity. RNA. 1997;3:1016–1027. [PMC free article] [PubMed] [Google Scholar]
- 16.Disney MD, Haidaris CG, Turner DH. Recognition elements for 5' exon substrate binding to the Candida albicans group I intron. Biochemistry. 2001;40:6507–6519. doi: 10.1021/bi002008r. [DOI] [PubMed] [Google Scholar]
- 17.Miletti KE, Leibowitz MJ. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob. Agents Chemother. 2000;44:958–966. doi: 10.1128/aac.44.4.958-966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grigull L, Beier R, Schrauder A, Kirschner P, Loening L, Jack T, Welte K, Sykora KW, Schrappe M. Invasive fungal infections are responsible for one-fifth of the infectious deaths in children with ALL. Mycoses. 2003;46:441–446. doi: 10.1046/j.0933-7407.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 19.Mishra NN, Prasad T, Sharma N, Payasi A, Prasad R, Gupta DK, Singh R. Pathogenicity and drug resistance in Candida albicans and other yeast species. A review. Acta Microbiol. Immunol. Hung. 2007;54:201–235. doi: 10.1556/AMicr.54.2007.3.1. [DOI] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff A. Mechanisms of resistance to antifungal agents: yeasts and filamentous fungi. Rev. Iberoam. Micol. 2008;25:101–106. doi: 10.1016/s1130-1406(08)70027-5. [DOI] [PubMed] [Google Scholar]
- 21.Disney MD, Magnet S, Blanchard JS, Seeberger PH. Aminoglycoside Microarrays To Study Antibiotic Resistance. Angew. Chem. Int. Ed. Engl. 2004;43:1591–1594. doi: 10.1002/anie.200353236. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Bowerman D, Marthandan N, Klyza S, Luebke KJ, Garner HR, Kodadek T. Photolithographic synthesis of peptoids. J. Am. Chem. Soc. 2004;126:4088–4089. doi: 10.1021/ja039565w. [DOI] [PubMed] [Google Scholar]
- 23.Kuruvilla FG, Shamji AF, Sternson SM, Hergenrother PJ, Schreiber SL. Dissecting glucose signalling with diversity-oriented synthesis and small-molecule microarrays. Nature. 2002;416:653–657. doi: 10.1038/416653a. [DOI] [PubMed] [Google Scholar]
- 24.Koehler AN, Shamji AF, Schreiber SL. Discovery of an inhibitor of a transcription factor using small molecule microarrays and diversity-oriented synthesis. J. Am. Chem. Soc. 2003;125:8420–8421. doi: 10.1021/ja0352698. [DOI] [PubMed] [Google Scholar]
- 25.Vegas AJ, Fuller JH, Koehler AN. Small-molecule microarrays as tools in ligand discovery. Chem. Soc. Rev. 2008;37:1385–1394. doi: 10.1039/b703568n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swayze EE, Jefferson EA, Sannes-Lowery KA, Blyn LB, Risen LM, Arakawa S, Osgood SA, Hofstadler SA, Griffey RH. SAR by MS: a ligand based technique for drug lead discovery against structured RNA targets. J. Med. Chem. 2002;45:3816–3819. doi: 10.1021/jm0255466. [DOI] [PubMed] [Google Scholar]
- 27.Wong CH, Hendrix M, Manning DD, Rosenbohm C, Greenberg WA. A library approach to the discovery of small molecules that recognize RNA: Use of a 1,3-hydroxyamine motif as core. J. Am. Chem. Soc. 1998;120:8319–8327. [Google Scholar]
- 28.Zhou Y, Gregor VE, Sun Z, Ayida BK, Winters GC, Murphy D, Simonsen KB, Vourloumis D, Fish S, Froelich JM, Wall D, Hermann T. Structureguided discovery of novel aminoglycoside mimetics as antibacterial translation inhibitors. Antimicrob. Agents Chemother. 2005;49:4942–4949. doi: 10.1128/AAC.49.12.4942-4949.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Oost TK, Schkeryantz JM, Yang J, Janowick D, Fesik SW. Discovery of aminoglycoside mimetics by NMR-based screening of Escherichia coli A-site RNA. J. Am. Chem. Soc. 2003;125:4444–4450. doi: 10.1021/ja021354o. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Pilch DS, Srinivasan AR, Olson WK, Geacintov NE, Breslauer KJ. Modulation of nucleic acid structure by ligand binding: induction of a DNA.RNA.DNA hybrid triplex by DAPI intercalation. Bioorg. Med. Chem. 1997;5:1137–1147. doi: 10.1016/s0968-0896(97)00050-3. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y, Hofstadler SA, Swayze EE, Griffey RH. An efficient synthesis of mimetics of neamine for RNA recognition. Org. Lett. 2001;3:1621–1623. doi: 10.1021/ol015794g. [DOI] [PubMed] [Google Scholar]
- 32.Davis B, Afshar M, Varani G, Murchie AI, Karn J, Lentzen G, Drysdale M, Bower J, Potter AJ, Starkey ID, Swarbrick T, Aboul-ela F. Rational design of inhibitors of HIV-1 TAR RNA through the stabilisation of electrostatic "hot spots". J. Mol. Biol. 2004;336:343–356. doi: 10.1016/j.jmb.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 33.Murchie AI, Davis B, Isel C, Afshar M, Drysdale MJ, Bower J, Potter AJ, Starkey ID, Swarbrick TM, Mirza S, Prescott CD, Vaglio P, Aboul-ela F, Karn J. Structure-based drug design targeting an inactive RNA conformation: exploiting the flexibility of HIV-1 TAR RNA. J. Mol. Biol. 2004;336:625–638. doi: 10.1016/j.jmb.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, Duffy EM. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 2008;51:3353–3356. doi: 10.1021/jm800379d. [DOI] [PubMed] [Google Scholar]
- 35.Lind KE, Du Z, Fujinaga K, Peterlin BM, James TL. Structure-based computational database screening, in vitro assay, and NMR assessment of compounds that target TAR RNA. Chem. Biol. 2002;9:185–193. doi: 10.1016/s1074-5521(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 36.Harada K, Martin SS, Frankel AD. Selection of RNA-binding peptides in vivo. Nature. 1996;380:175–179. doi: 10.1038/380175a0. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Leibowitz MJ. Variation and in vitro splicing of group I introns in rRNA genes of Pneumocystis carinii. Nucleic Acids Res. 1993;21:2415–2421. doi: 10.1093/nar/21.10.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999;1:55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 39.Disney MD, Childs-Disney JL. Using Selection to Identify and Chemical Microarray to Study the RNA Internal Loops Recognized by 6'-N-Acylated Kanamycin A. Chembiochem. 2007;8:649–656. doi: 10.1002/cbic.200600569. [DOI] [PubMed] [Google Scholar]
- 40.See the Supporting Information for spectra
- 41.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 42.Barrett OJ, Childs JL, Disney MD. Chemical microarrays to identify ligands that bind pathogenic cells. Chembiochem. 2006;7:1882–1885. doi: 10.1002/cbic.200600260. [DOI] [PubMed] [Google Scholar]
- 43.Afanassiev V, Hanemann V, Wolfl S. Preparation of DNA and protein micro arrays on glass slides coated with an agarose film. Nucleic Acids Res. 2000;28:E66. doi: 10.1093/nar/28.12.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas JR, Liu X, Hergenrother PJ. Size-specific ligands for RNA hairpin loops. J. Am. Chem. Soc. 2005;127:12434–12435. doi: 10.1021/ja051685b. [DOI] [PubMed] [Google Scholar]
- 45.Disney MD, Matray T, Gryaznov SM, Turner DH. Binding enhancement by tertiary interactions and suicide inhibition of a Candida albicans group I intron by phosphoramidate and 2'-O-methyl hexanucleotides. Biochemistry. 2001;40:6520–6526. doi: 10.1021/bi002009j. [DOI] [PubMed] [Google Scholar]
- 46.Been MD, Cech TR. One binding site determines sequence specificity of Tetrahymena pre-rRNA self-splicing, trans-splicing, and RNA enzyme activity. Cell. 1986;47:207–216. doi: 10.1016/0092-8674(86)90443-5. [DOI] [PubMed] [Google Scholar]
- 47.Testa SM, Haidaris CG, Gigliotti F, Turner DH. A Pneumocystis carinii group I intron ribozyme that does not require 2' OH groups on its 5' exon mimic for binding to the catalytic core. Biochemistry. 1997;36:15303–15314. doi: 10.1021/bi9713097. [DOI] [PubMed] [Google Scholar]
- 48.Disney MD, Childs JL, Turner DH. Hoechst 33258 selectively inhibits group I intron self-splicing by affecting RNA folding. Chembiochem. 2004;5:1647–1652. doi: 10.1002/cbic.200400159. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Tidwell RR, Leibowitz MJ. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J. Eukaryot. Microbiol. 1994;41:31–38. doi: 10.1111/j.1550-7408.1994.tb05931.x. [DOI] [PubMed] [Google Scholar]
- 50.Barrett OJ, Pushechnikov A, Wu M, Disney MD. Studying aminoglycoside modification by the acetyltransferase class of resistance-causing enzymes via microarray. Carbohydr. Res. 2008;343:2924–2931. doi: 10.1016/j.carres.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.