Abstract
Background
Specificities for carbohydrate IgG antibodies, thought to be predominantly of the IgG2 subclass, have never been broadly examined in healthy human subjects.
Objective
To examine commercial intravenous immunoglobulin (IVIg) preparations for their ability to recognize a wide range of glycans and to determine the contribution of IgG2 to the binding pattern observed.
Methods
We employed a glycan microarray to evaluate IVIg preparations and a control mix of similar proportions of human myeloma IgG1 and IgG2 for binding to 377 glycans courtesy of the Consortium for Functional Glycomics Core H (http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml). Glycans recognized were categorized using public databases for their likely cellular sources. IgG2 was depleted from IVIg using immunoaffinity chromatography and depletion confirmed using nephelometry and surface plasmon resonance.
Results
Nearly half of the glycans bound IgG. Some of the glycans with the greatest antibody binding can be found in structures of human pathogenic bacteria (e.g., Streptococcus pneumoniae, Mycobacterium tuberculosis, Vibrio cholera) and non-pathogenic bacteria, including lipopolysaccharide and lipoteichoic acid, capsular polysaccharides and exopolysaccharides. Surprisingly, depletion of IgG2 had only a modest effect on anti-carbohydrate recognition patterns compared to the starting IVIg preparation. Little to no binding activity was detected to human endogenous glycans including tumor-associated antigens.
Conclusions
This novel, comprehensive analysis provides evidence that IVIg contains a much wider range than previously appreciated of anti-carbohydrate IgG antibodies, including those recognizing both pathogenic and non-pathogen-associated prokaryotic glycans.
Keywords: IgG subclasses, gamma globulin, anti-carbohydrate antibodies, glycans, IgG2, glycan microarray
Introduction
Production of immunoglobulins by plasma cells defines the net effect of an antigen-specific humoral response. This process results in the production of IgG, IgA and IgE antibodies, which themselves are the end-result of immunoglobulin class switching and gene rearrangements.1 Implicit in this paradigm is that the major epitopes recognized by the vast majority of immunoglobulins are those from proteins. However, antibodies can be produced against non-protein antigens. Examples include the generation of carbohydrate-specific antibodies following immunization with carbohydrate vaccines such as Pneumovax; anti-DNA antibodies in systemic lupus erythematosis; antibodies to blood group carbohydrate antigens; and antibody responses to glycolipids including lipopolysaccharide (LPS) and lipoteichoic acid (LTA).2 The vast majority of these antibodies are of the IgG class, and current paradigms suggest that IgG1 and IgG3 subclasses represent the bulk of protective anti-protein antibodies due in part to their ability to more efficiently activate complement. In contrast, the IgG2 subclass is believed to be more commonly associated with anti-carbohydrate immune responses.3-7 A broad and comprehensive analysis of this concept, however, is lacking.
In 2001, the National Institute of General Medical Sciences funded the Consortium for Functional Glycomics (CFG, www.functionalglycomics.org) to facilitate new discoveries in the field of glycobiology. Among the technologies created was the development of a glycan microarray in which now over 300 human and non-human glycans have been immobilized on a testing surface to facilitate high throughput screening of lectins, antibodies and other structures for their carbohydrate ligands. Numerous publications have demonstrated the utility of this technology in facilitating the discovery of candidate ligands for known or unknown glycan binding structures, as well as for screening a variety of existing antibodies and human serum samples for the presence of anti-glycan binding activity.8
In the current paper, the CFG glycan microarray was used to test preparations of human pooled intravenous immunoglobulin, commonly known as IVIg, for its anti-glycan binding activity. The main hypothesis to be tested was whether normal human IgG contains a limited spectrum of anti-carbohydrate antibodies that are predominantly IgG2.
Methods
Reagents
The commercially available IVIg preparations used in this study were Sandoglobulin and Privigen (kindly provided by CSL Behring, Berne, Switzerland) and Gamunex (purchased from Talecris, Research Triangle Park, NC). Sandoglobulin and Privigen (IgG content ≥ 98%) contain 2% and 0.0021% of IgA respectively and only traces of IgM, IgD and IgE (as reported by CSL Behring, Berne, Switzerland). The IgG subclass composition of Sandoglobulin was analyzed by nephelometry (Quest Diagnostics, Baltimore, USA) and contained mainly IgG1 (61%) and IgG2 (32%), with small amounts of IgG3 (5%) and IgG4 (2%). Privigen contained 66% IgG1, 29% IgG2, 3% IgG3 and 3% IgG4 (as reported by CSL Behring, Berne, Switzerland). To remove the stabilizing sucrose component of Sandoglobulin, this IVIg preparation was dialyzed as previously described.9
A control IgG preparation was prepared by mixing two monoclonal human myeloma proteins IgG1, l (67%) and IgG2, k (33%), purchased from Sigma-Aldrich (St. Louis, MO) yielding a k/l ratio of 0.5, well within the range found in normal serum (0.26-1.65).
Glycan array analysis
The glycan microarrays from the CFG (http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh11.shtml) are prepared from amine functionalized glycan structures covalently coupled in microarrays to N-hydroxysuccinimide (NHS)-derivatized microscope slides as previously described.10 IVIg preparations and mixed human myeloma IgG1 and IgG2 were analyzed for binding to version 3.1 of the printed array (377 glycan targets).
IgG2 Depletion of IVIg
Purified murine monoclonal anti-human IgG2 Fd (HP6014, Hybridoma Reagent Laboratory, Baltimore, MD)11 was coupled to cyanogen bromide (CNBr)-activated Sepharose 4B-CL at 1 mg per ml of packed beads and blocked with 1 M ethanolamine. The anti-human IgG2-Sepharose (20 ml of 50% v/v) was packed into a Biorad glass column, washed with 20 bed volumes of PBS and the IVIg (Sandoglobulin) was applied to the column and recycled 5 times. The final eluate was collected and the column was then washed with PBS until no protein was detected in the eluate at OD280 with an online monitor (Pharmacia-LKB Uvicord SD, Uppsala, Sweden). The bound human IgG2 fraction was eluted from the anti-IgG2 affinity column with 10 mM NaOH. The protein peak, once completely eluted based on on-line OD monitoring, was immediately neutralized to pH 7.4.
Immunochemical Characterization of the Original and IgG2-Depleted IVIg
The IgG2-adsorbed IVIg that passed through the anti-IgG2 affinity column was initially analyzed for IgG1, 2, 3 and 4 by nephelometry (CSL Behring, Berne, Switzerland) using Binding Site Reagents (San Diego, CA) and the CRM470 serums standard. The levels of IgG subclass protein measured by nephelometry in the IgG2 depleted fraction were: IgG1: 14 mg/dl, IgG2: <17 mg/dl, IgG3: 2 mg/dl and IgG4: 0.4 mg/dl. Since the analytical sensitivity of the IgG2 nephelometric assay was insufficient to demonstrate effective removal of IgG2, we performed an alternative assay using surface plasmon resonance on the Biacore 3000 instrument (General Electric, Piscataway, NJ). The following purified monoclonal antibodies were coupled to separate flow cells on a carboxymethyl cellulose (CM5) chip using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC)-NHS chemistry: HP6019 (murine anti-human IgG1, 3 and 4 Fc exclusion antibody that does not bind human IgG2), HP6043 (murine anti-human IgG1, 2, 3 and 4 Fc that is pan reactive with all four human IgG subclasses), and HP6014 (anti-human IgG2 Fd). The levels of the IgG subclass protein in the original Sandoglobulin and IgG2-depleted IVIg eluate were analyzed by measuring the level of binding to each of the antibodies in each of the flow cells in separate runs. The level of resonance units measured were analyzed with BIAsimulation software to compute the relative quantity of IgG 2 or IgG1,3,4 protein binding to the anti-IgG2 and anti-IgG1,3,4, respectively.
Glycan analysis using the Bacterial Carbohydrate Structure Data Base
For an analysis of glycans recognized by IVIg, the online Bacterial Carbohydrate Structure Database (BCSDB) was consulted (http://www.glyco.ac.ru/bcsdb/start.shtml). For the top 100 glycans binding to IVIg glycan names were entered as terms to search for expression of these glycans in bacteria. The top 20 glycans for which registries were found were grouped according to expression by pathogenic versus non-pathogenic, gram positive versus gram negative bacteria, and according to the presence in LPS, lipooligosaccharides (LOS) and LTA, capsular polysaccharide (CPS) and endopolysaccharide (EPS).
Results
In cooperation with the CFG, IVIg was analyzed for the presence of anti-carbohydrate antibodies using a glycan microarray containing 377 glycan structures. To broaden the analysis, preparations of IVIg tested included Sandoglobulin (donors from the United States and Europe), Privigen (donors from Germany) and Gamunex preparation (donors from the USA). These IVIg preparations contained all four IgG subclasses but were mainly composed of the antibody subclasses IgG1 and IgG2 as measured by their respective manufacturers. Thus, a 2:1 mix of human myeloma-derived IgG1 and IgG2 protein (“IgG control mix”) was also concurrently analyzed to determine levels of background nonspecific binding.
Based on results of pilot experiments that involved testing a range of dilutions of IVIg (data not shown), a concentration of 180 μg/ml of IVIg was determined to be optimal for screening in the CFG glycan microarray. At this concentration, reproducible glycan-binding patterns were achieved with minimal background (see Fig. 1). Sandoglobulin and Privigen each bound to 181 (48%) and Gamunex to 172 (46%) of the 377 glycans of the microarray at a level above background, defined as the level of the highest binding signal obtained using the IgG myeloma control mix (Fig. 1 and 2a). The maximal values of relative fluorescence (RFUmax) were similar for all three IVIg preparations (RFUmax for Sandoglobulin = 49379; for Privigen = 49710; for Gamunex = 48348). Among the various specifically recognized glycans, the levels of IVIg binding varied considerably. In contrast, binding of the IgG myeloma control was low for all 377 glycans on the microarray (Fig.1), suggesting that the anti-carbohydrate binding activity of IVIg is due to the presence of specific anti-glycan antibodies and not due to nonspecific binding.
Figure 1. IVIg contains many different anti-carbohydrate IgG antibodies.
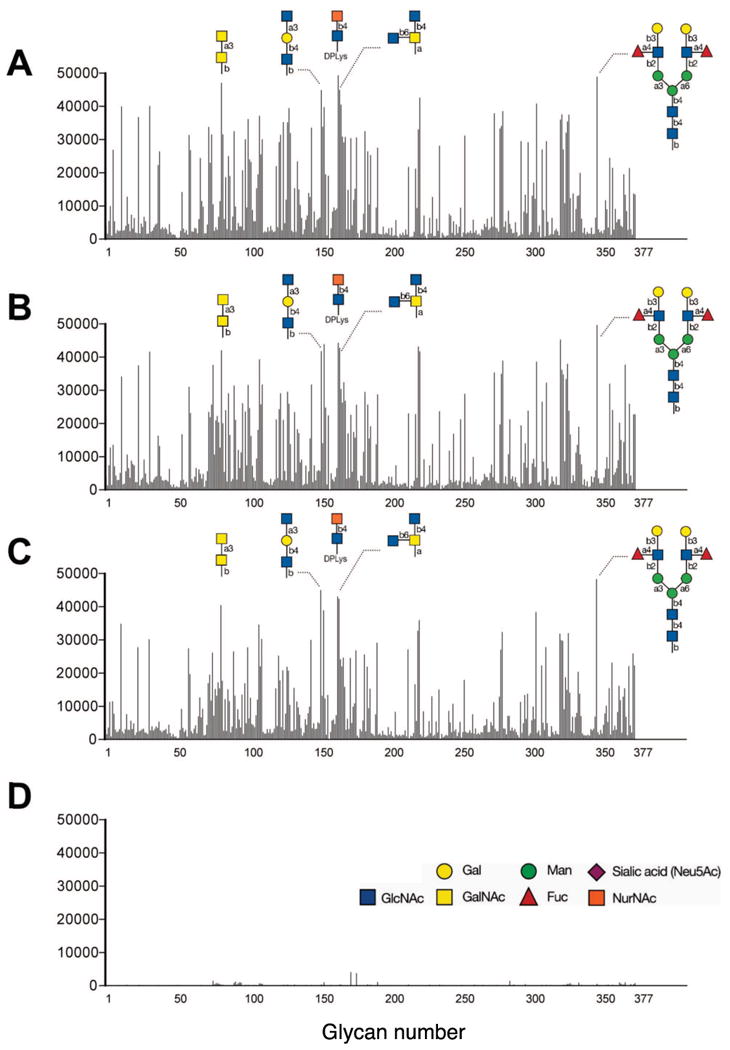
A total of 377 glycans were screened for binding of IgG using commercial IVIg brands Sandoglobulin (A), Privigen (B), and Gamunex (C) or a control mix of human myeloma (D; containing IgG1 and IgG2 in a 2:1 proportion, similar to IVIg). Glycan binding for each was tested at 180 μg/ml. As examples the carbohydrate structures of several of the top IVIg bound glycans are shown.
Figure 2. Different commercial IVIg preparations derived from different donor populations recognize similar patterns of glycans.
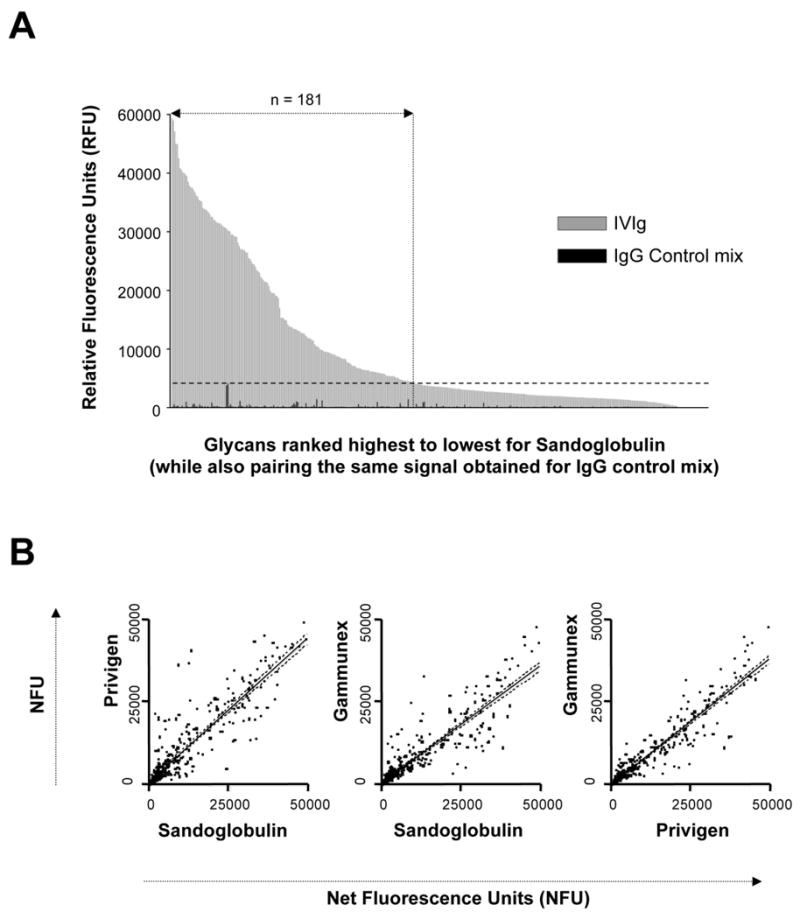
A) Glycan-binding patterns of the Sandoglobulin IVIg as measured by relative fluorescence units (RFU) and ranked in descending order of signal (grey bars) paired with the results of the control IgG mix (black bars). Binding of IVIg to 181 glycans (48%) was above background, as defined by the highest signal of the control IgG mix (dotted horizontal line). B) Comparison of glycan binding activities of Sandoglobulin, Privigen and Gamunex. Values are indicated as net fluorescence units (NFU) as calculated by subtraction of the corresponding glycan binding value for the IgG control mix. The majority of glycans were recognized with comparable binding intensity by the three IVIg preparations (r2 from left to right: 0.82, 0.81 and 0.88).
Since all three IVIg preparations produced the same relative level of binding to the glycan microarray (46-48% of glycans recognized; see above), it was important to determine whether the glycan recognition patterns were similar. As shown in Fig. 1 and Fig. 2B the glycan recognition patterns of Sandoglobulin, Privigen and Gamunex were similar and for the majority of glycans, there was only a minor variation in signal intensity produced by the three IVIg preparations. Table 1 shows results for the top twenty glycans bound by IVIg. A comprehensive list showing binding intensities of all three IVIg preparations to each of the 377 glycans is provided in supplementary materialM to this manuscript (Online Supplement Table 1). All raw data are also presented on the CFG website at http://www.functionalglycomics.org/glycomics/publicdata/selectedScreens.jsp.
Table 1. The Top 20 Glycans Bound by Antibodies Contained in IVIg.
Results for each of the 377 glycans on the array and for three different IVIg preparations (Sandoglobulin, Privigen, Gammunex) are shown. The complete list is provided as an Online Supplement.
| Chart # |
Masterlist Name | Relative Fluorescence Units (RFUs) | ||||
|---|---|---|---|---|---|---|
| Sandoglobuli n (mean) |
Privige n (mean) |
Gammune x (mean) |
Averag e |
S.E.M . |
||
| 156 | GlcNAcβ1-2Galβ1-3GalNAcα | 38808 | 42989 | 37997 | 39931 | 2187 |
| 154 | GlcNAcα1-3Galβ1-4GlcNAcβ | 44652 | 41549 | 44768 | 43656 | 1491 |
| 24 | GlcNAcβ1-3(GlcNAcβ1-4)(GlcNAcβ1-6)GlcNAc | 36572 | 37350 | 27618 | 33846 | 4416 |
| 166 | 2GlcNAcβ1-4MDPLys | 49248 | 44222 | 42953 | 45475 | 2718 |
| 167 | GlcNAcβ1-4(GlcNAcβ1-6)GalNAcα | 44791 | 42589 | 42158 | 43179 | 1153 |
| 168 | GlcNAcβ1-4Galβ1-4GlcNAcβ | 40247 | 30117 | 23867 | 31410 | 6749 |
| 102 | Galα1-3Galβ1-3GlcNAcβ | 36030 | 31401 | 27631 | 31687 | 3435 |
| 110 | Galα1-4GlcNAcβ | 36518 | 38687 | 33955 | 36387 | 1934 |
| 147 | Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ | 33402 | 31560 | 29834 | 31599 | 1457 |
| 83 | GalNAcα1-3GalNAcβ | 46913 | 41898 | 40319 | 43043 | 2812 |
| 324 | NeuAc(9Ac)α2-3Galβ1-3GlcNAcβ | 35989 | 45272 | 31884 | 37715 | 5600 |
| 223 | 1Neu5Acα2-3Galβ1-3GlcNAcβ | 33050 | 43183 | 32744 | 36326 | 4851 |
| 224 | 1Neu5Acα2-3Galβ1-3GlcNAcβ | 42560 | 41737 | 35903 | 40067 | 2963 |
| 283 | Neu5Acα2-3Galβ1-4GlcNAcβ1-3Galβ1-3GlcNAcβ | 38558 | 38927 | 32376 | 36620 | 3005 |
| 282 | Neu5Acα2-3Galβ1-3GlcNAcβ1-3Galβ1-3GlcNAcβ | 34023 | 34962 | 27041 | 32008 | 3534 |
| 325 | NeuAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-3GlcNAcβ | 37472 | 36108 | 29887 | 34489 | 3301 |
| 12 | α-L-Rha | 39964 | 34151 | 34807 | 36308 | 2599 |
| 32 | [su]3Galβ1-3GlcNAcβ | 40124 | 41628 | 30224 | 37326 | 5059 |
| 307 | 3HOOC(CH3)CH-3-O-GlcNAcβ1-4GlcNAcβ | 40792 | 38600 | 38355 | 39249 | 1096 |
| 350 | Galβ1-3(Fucα1-4)GlcNAcβ1-2Manα1-3[Galβ1-3(Fucα1-4)GlcNAcβ1-2Manα1-6]Manβ1-4GlcNAcβ1-4GlcNAcβ | 48478 | 49208 | 47846 | 48511 | 557 |
Glycans 223 and 224 differ only by a single carbon in the linkage to the array
A fragment of peptido glycan containing GlcNAcβ1-4MurAc coupled to the array through a lysine
MurAcβ1-4GlcNAcβ (disaccharide of peptidoglycan)
Longstanding doctrine suggests that among human IgG subclasses, IgG2 most commonly binds to carbohydrate epitopes.12, 13 To globally assess the contribution of IgG2 to glycan binding, a preparation of Sandoglobulin was depleted of IgG2 by affinity chromatography for testing on the glycan microarray. The depletion efficacy was analyzed by nephelometry and surface plasmon resonance to determine the IgG2 content of the various fractions including the starting IVIg material, IgG2-depleted IVIg and eluates from the anti-IgG2 affinity column. These fractions were tested for binding to anti-human IgG2 mAb antibody clone HP6014 and anti-human IgG1/IgG3/IgG4 clone HP6019, two well-characterized antibodies covalently immobilized on a carboxymethyl cellulose coated Biacore chip. In addition, monoclonal human IgG1 and IgG2 antibodies were used as positive and negative specificity controls, respectively.
Figure 3A depicts the surface plasmon resonance association curves obtained with each of the fractions at 100 μg/ml to anti-IgG2 mAb immobilized on the chip flow cell. Total IVIg and affinity-purified IgG2 fractions demonstrated a high level of binding that was similar to the IgG2 myeloma isotype control. In contrast, IgG2–depleted IVIg and the IgG1 myeloma control demonstrated no evidence of binding with the anti-IgG2 antibody. These data indicated that the IgG2 protein had been removed from this IVIg fraction. Analogous reciprocal binding patterns were obtained on the flow cell containing the anti-IgG1/IgG3/IgG4 antibody. From these data for binding levels, the percentage of IgG2 and IgG1/IgG3/IgG4 was computed in each of the fractions (Fig. 3B) and demonstrate that >99% of the IgG2 had been removed from the anti-IgG2 affinity column adsorbed IVIg fraction.
Figure 3. Analysis of IgG2-depletion from IVIg: Evaluation of IVIg Fractions Pre and Post IgG2 Adsorption by Surface Plasmon Resonance.
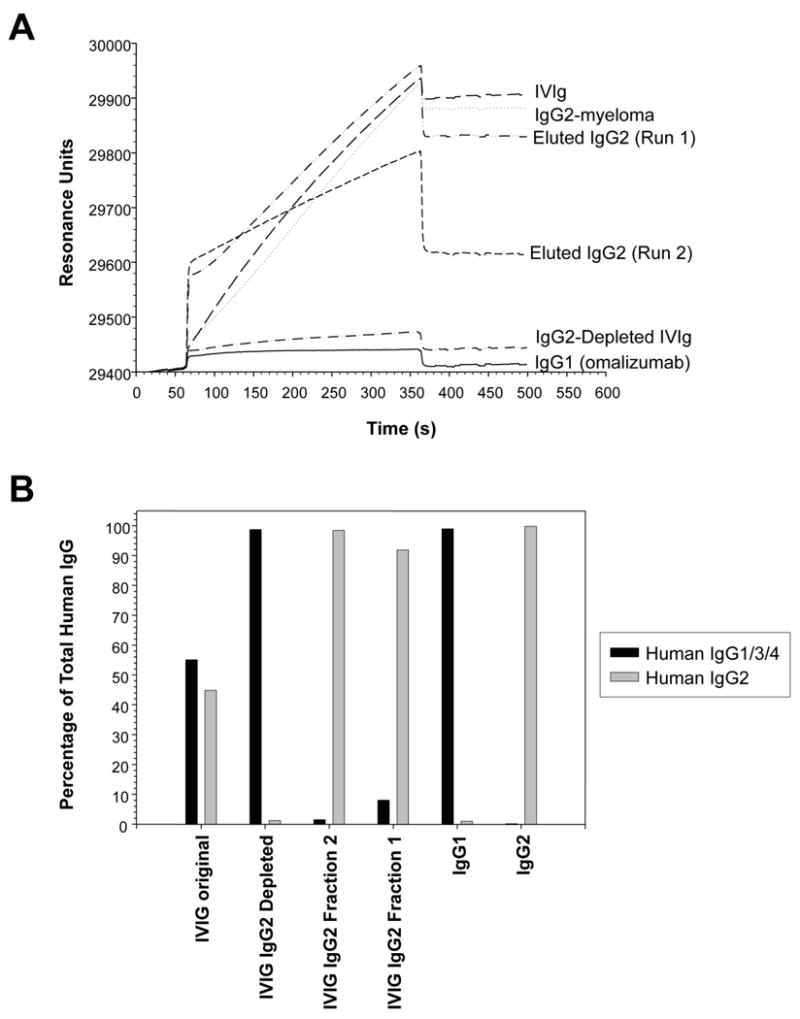
A) Binding of IVIg fractions after immunoaffinity IgG2-depletion. B) The proportion of IgG2 versus IgG1/IgG3/IgG4 for the various fractions is shown.
The glycan binding properties of total and IgG2-depleted-IVIg were next evaluated using the glycan microarray (Fig. 4). For this analysis, we arbitrarily defined IgG2 subclass predominance as IgG2-depleted-IVIg signal intensities <33.3% of total IVIg signals and non-IgG2-predominance as response levels >66.67%. While a broad range of glycans were still recognized by IgG2-depleted-IVIg, binding was completely lost for some glycans, suggesting that these structures are exclusively recognized by IgG2 antibodies. For example, among 45 of the top 100 glycans recognized, binding of IgG2-depleted-IVIg was below 33.3% of the signal intensity of non-depleted IVIg. This indicated that for these glycans, recognition was mainly due to anti-glycan antibodies of the IgG2 subclass. In contrast, 22 of the top 100 glycans bound by the IgG2-depleted-IVIg were still above 66.67% of the signal strength as compared to unadsorbed IVIg, revealing that non-IgG2 antibodies play a major role in their recognition. A summary of the results for all 377 glycans is presented as supplementary material (Online Supplement Table 2). These data strongly suggest that human immune responses towards glycans are not exclusively a function of IgG2 and that the IgG subclass antibodies generated from anti-carbohydrate responses may be dependent on the type of glycan.
Figure 4. IgG2 Dependence of Glycan Recognition by IVIg.
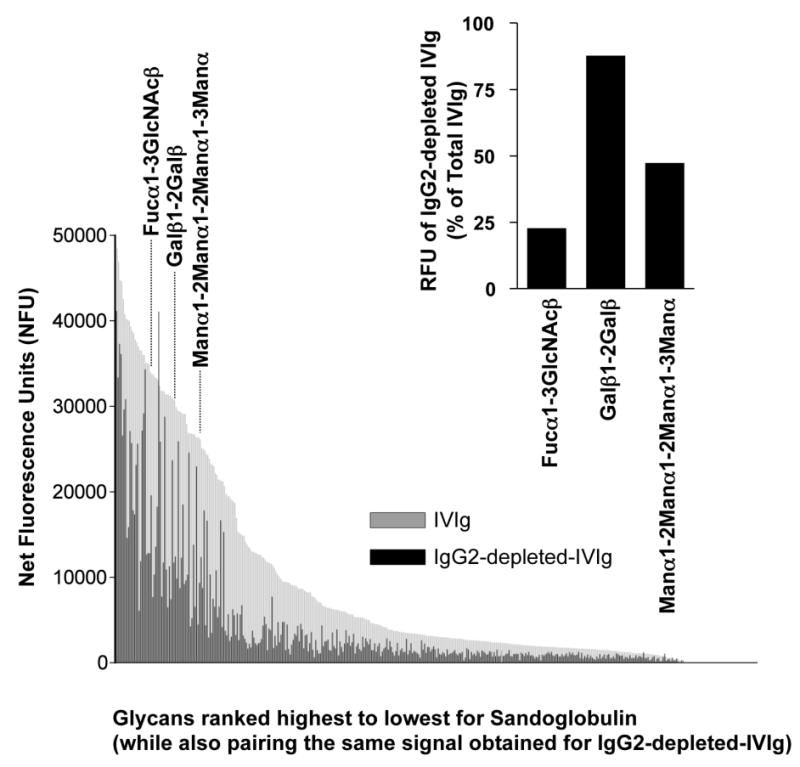
Glycans bound by IgG antibodies in the original and IgG2 depeted Sandoglobulin are ranked from highest to lowest binding. Signal intensities for IVIg binding to the 377 glycans were expressed as net fluorescence units after subtraction of the IgG control mix signal for each respective glycan. Depending on the carbohydrate structure, IgG2-depletion had different effects on glycan recognition by IVIg. For instance, binding to the three bacterial glycans Fucα1-3GlcNAcβ, Galβ1-2Galβ and Manα1-2Manα1-2Manα1-3Manα were largely IgG2-dependent, independent or mixed, respectively (see inset).
Given the pattern of glycans bound by IVIg, the identity of these glycans, their biological source and function were explored. The top ranked IVIg binding glycans were elucidated for their occurrence in bacteria using the BCSDB (http://www.glyco.ac.ru/bcsdb/start.shtml). Among the top 100 glycans recognized by IVIg, 39 had entries in the BCSDB. Results of the BCSDB analysis for the top 20 of these sugars are presented in Table 2. Most of the highly recognized glycans represent determinants found in bacteria. They are not limited to one single class of bacteria but instead represent a broad cross-section of species. Besides bacterial commensals, such as Bacteroides spp. and Lactobacillus, these included glycans found in important pathogens such as Bacillus anthracis, Mycobacteria, Vibrio cholerae, Neisseria meningitides, Haemophilus influenzae, Helicobacter pylori, Pseudomonas aeruginosa, Streptococcus pneumoniae, Escherichia coli, Shigella and Salmonella species. Bacterial glycans recognized by IVIg were not restricted to one species, but rather were a part of a variety of structural and secreted molecules such as bacterial cell wall components like LPS, LOS and LTA, or in CPS and even in secreted EPS.
Table 2. The top 20 Glycans Bound by Antibodies in IVIg Recognizing Bacterial Carbohydrate Structures that are Listed in the Bacterial Carbohydrate Structure Data Base (BCSDB; http://www.glyco.ac.ru/bcsdb/start.shtml).
IgG2 subclass distribution of binding antibody is based on data of IgG2-depletion experiments as described. IgG2 predominance was defined as IgG2-depleted-IVIg signal intensity of total IVIg < 33.3 %, and > 66.6 % for non-IgG2 predominance. Abbreviations: CPS = Capsular Polysaccharides, EPS = Exopolysaccharides, LPS = Lipospolysaccharides, LOS = Lipooligosaccharides, LTA = Lipoteichoic acid, Comm. = Commensal, Environ. = Environmental, Ab = Antibody
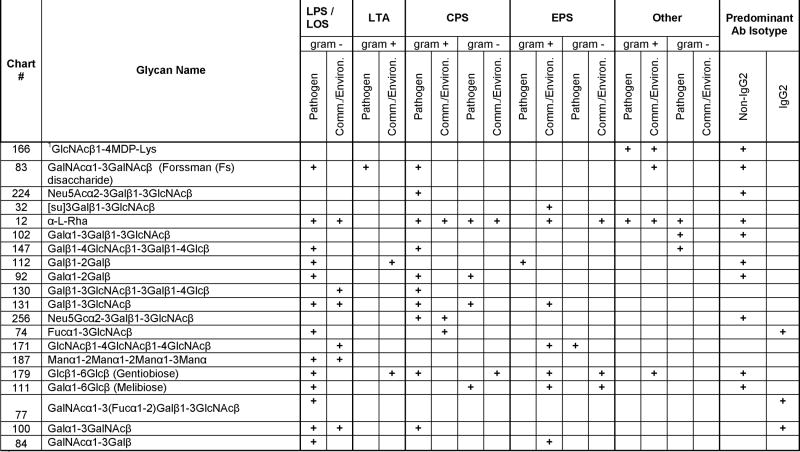 |
A fragment of peptidoglycan containing GlcNAcβ1-4MurAc coupled to the array through a lysine
Comparing data from the untreated and IgG2-depleted IVIgs, it becomes apparent that the IgG2 subclass contribution to the top 20 binding bacterial glycan antibodies was quite variable (see Table 2). Using an arbitrarily defined IgG2 subclass predominance as IgG2-depleted-IVIg signal intensities <33.3 % of total IVIg signals, only 3 of the top 20 IVIg bound glycans were recognized predominantly by IgG2 antibodies. Ten of the top 20 glycans were recognized predominantly by non-IgG2 antibodies and 7 glycans were recognized by both IgG2 and non-IgG2 antibodies. Bacterial capsular structures are thought to be recognized principally by IgG2 antibodies,7 but our BCSDB analysis revealed that many of the top 20 glycans bound by non-IgG2 antibodies are present in capsular polysaccharides. These results indicate that more than a third of the glycans on the microarray bound by IgG antibodies in the IVIg are present in a variety of bacterial components and products of both commensals and pathogens. Furthermore, the bacterial glycan recognition in IVIg is not restricted to the IgG2 subclass, but involves an even higher proportion of non-IgG2 anti-glycan antibodies.
Endogenous human glycans (e.g., blood group antigens, selectin ligands) have important biological functions in health and disease, and binding by IVIg to these glycans may have important modulatory effects. Certain biologically important glycans to which IVIg binding has been assessed using the glycan microarray are depicted in Figure 5. As expected, IVIg contained antibodies to blood group antigens H (core), A and B, but not to the P/E-selectin ligand sialyl Lewis x (sLex), the Siglec-8 ligand 6(Gal)-su-sLex, the L-selectin ligand 6(GlcNAc)-su-sLex, or GD3. Cancer cells frequently display altered glycosylation patterns. Glycans that have been associated with cancer and were represented on the microarray but were not recognized by IVIg included sialyl Lewis a (sLea), sialyl Tn (sTn), Lewis y (Ley), polysialic acid (PSA) and the gangliosides GD2, GD3, fucosyl GM1, and GM2 (see Fig. 5). Taken together, these data demonstrate that IVIg contained antibodies to blood group antigens but not to endogenous human cancer or inflammation-associated glycans, which therefore appear to be relatively non-immunogenic or at least are not represented often enough to be detected among normal healthy donors.
Figure 5. Recognition of known autologous glycans by IVIg.
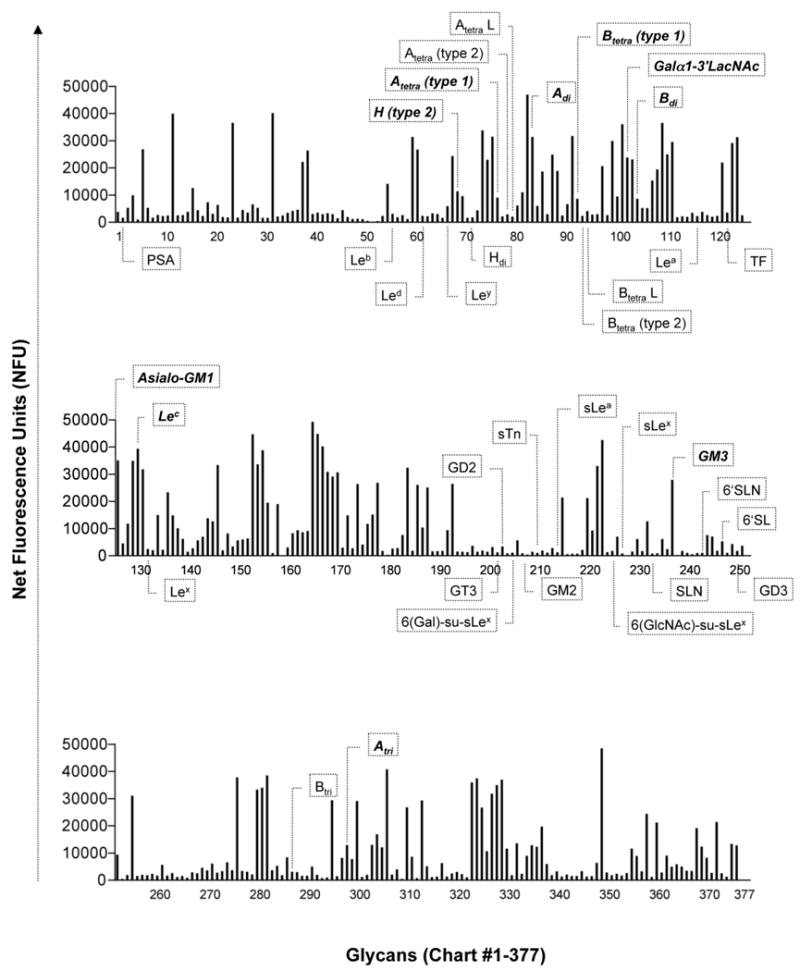
IVIg contained antibodies to blood group antigens H, A and B. Notably absent, however, were antibodies to selectin ligands, Siglec ligands and cancer-associated glycans, such as sialyl Lewis x (sLex), sialyl Lewis a (sLea), 6(Gal)-su-sLex, 6(GlcNAc)-su-sLex, polysialic acid (PSA), sialyl Tn (sTn), Lewis y (Ley) polysialic acid (PSA) and the gangliosides GD2, GD3, fucosyl GM1 and GM2.
Discussion
IVIg preparations contain mostly IgG from thousands of donors with only trace amounts of IgM or IgA. They represent the immunological antibody repertoire of the donor population that has evolved predominantly after T cell-dependent Ig isotype class switching. Most carbohydrate structures are considered to be T cell-independent antigens.2, 14 The present data show that IVIg contains abundant anti-glycan antibodies that are capable of binding to a broad range of carbohydrate structures. Furthermore, a greater than expected proportion of non-IgG2 antibodies in IVIg displayed anti-glycan binding activity because depletion of IgG2 from the IVIg reduced or eliminated binding to only about a half of glycans (45% of the top 100 recognized glycans), suggesting that many carbohydrate-specific IgG antibody responses can be of IgG2, non-IgG2 or mixed subclass distribution. Why certain glycans do not elicit a preferential IgG2 response cannot be determined from the present experiments. However, from an immunogenicity standpoint, their IgG subclass suggests that these anti-glycan antibodies occurred as a result of isotype class switching with the help of T cells. Conversion of glycans into T cell-dependent antigens may occur if the specific glycan is coupled to a carrier (glycoconjugates) and in this form presented by antigen-presenting cells to T cells. Also, glycolipids are presented to T cells by CD1 molecules (CD1a, CD1b, CD1c, CD1d).
The immense structural diversity of bacterial glycans, their variable levels of expression, and the presence in both bacterial pathogens and commensals at various host sites likely all contribute to both short-term and long-term host-bacterial interactions with stimulation of the host's immune system and subsequent antibody production. The present analysis identified a number of carbohydrates that are immunogenic, and many are found in both pathogenic as well as on non-pathogenic bacteria. The bacterial structures containing these glycans included structural molecules such as the cell wall components LPS, LOS and LTA or CPS, and secreted bacterial products such as EPS. Although in the present study we focused on the identification of IVIg binding to bacterial glycans, the results contain information on glycans that are expressed in other non-bacterial microorganisms, such as glycan #196, which represents a mannose structure often found in fungi.
Why certain glycans are more immunogenic than others cannot be ascertained by the present analysis, and ultimately the eliciting source of all of the observed IgG2 and non-IgG2 anti-carbohydrate responses cannot be determined. Nevertheless, these data might help to identify T cell-dependent glycan antigens that could be of major importance in carbohydrate vaccine development. Indeed, at the September 2007 meeting held at the National Institutes of Health entitled ‘Carbohydrate Moieties as Vaccine Candidates’ for vaccine development the “need to understand the unique differences in the degree of immunogenicity among and between types of carbohydrates including the immune responses to glycolipids” was postulated.2 Thus, the results of the present study regarding the recognition of glycans by IgG in IVIg may be of future aid in the development of carbohydrate-based vaccines.
Finally, among three different IVIg preparations, the glycan binding patterns were similar in their recognition patterns of many presumed exogenous glycans, yet little IVIg binding was detected to endogenous human cancer or inflammation-associated glycans (e.g., ligands of lectins, Siglecs). The absence of naturally occurring antibodies to sialosides and other glycan structures of potential human origin was expected and suggests that IVIg should not interfere with receptor-ligand interactions of biologically important human lectins. For instance, our data suggest that IVIg treatment would not inhibit sLex-selectin mediated leukocyte emigration into inflamed tissue or tumor-cell migration to distant tissues.15 Altered glycosylation patterns of cancer cells might give rise to anti-carbohydrate antibodies that could be used as serological tumor markers.15, 16 Our glycan microarray included many well-known tumor-associated glycans including gangliosides. The healthy blood donors who contributed to the pooled IVIg do not appear to produce significant levels of antibodies against these gangliosides, suggesting that the appearance and detection of such antibodies in a patient might be diagnostically useful. Therefore, the data presented here set the stage for future investigations into the diagnostic utility of measuring anti-glycan antibodies in various disease states, and point to the potential use of novel carbohydrate-based vaccines to enhance pathogen and cancer immunity.
Supplementary Material
Abbreviations
- BCSDB
Bacterial Carbohydrate Structure Database
- CFG
Consortium for Functional Glycomics
- CNBr
cyanogen bromide
- CPS
capsular polysaccharide
- EPS
endopolysaccharide
- Gal
galactose
- GD2
the ganglioside GalNAcβ(1-4)[NeuAc(α2-8)NeuAc(α2-3)]Galβ(1-4)Glcβ(1-1′)-ceramide
- GD3
the ganglioside NeuAc(α2-8)NeuAc(α2-3)Galβ(1-4)Glcβ(1-1′)-ceramide
- GM1
the ganglioside Galβ(1-4)GalNAcβ(1-4)[NeuAc(α2-3)]Galβ(1-4)Glcβ(1-1′)-ceramide
- GM2
the ganglioside GalNAcβ(1-4)[NeuAc(α2-3)]Galβ(1-4)Glcβ(1-1′)-ceramide
- IVIg
intravenous immunoglobulin
- Ley
Lewis Y
- LOS
lipooligosaccharides
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- NFU
net fluorescence units
- NHS
N-hydroxysuccinimide
- PBS
phosphate-buffered saline
- PSA
polysialic acid
- RFU
relative fluorescence units
- sLea
sialyl Lewis A
- sLex
sialyl Lewis X
- sTn
sialyl Tn (Thomsen-Friedenreich precursor) antigen: Neu5Ac(α2-6)GalNAcα-O-Ser/Thr)
- su
sulfated
Footnotes
Clinical implications: IVIg, and presumably sera from normal individuals, contains many types of IgG antibodies (both IgG2 and others) recognizing carbohydrate structures. This suggests normal immunity involves making IgG responses to many different naturally occurring foreign glycans.
Capsule summary: Humans naturally make a broad range of anti-carbohydrate IgG antibodies, both from the standpoint of making more than just IgG2 subclass responses and in making IgG antibodies against both pathogenic and non-pathogen-associated prokaryotic glycans.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–92. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas AH, Rittenhouse-Olson K, Kronenberg M, Apicella MA, Wang D, Schreiber JR, et al. Carbohydrate moieties as vaccine candidates: Meeting summary. Vaccine. 2008 Jun 9; doi: 10.1016/j.vaccine.2008.05.055. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Siber GR, Schur PH, Aisenberg AC, Weitzman SA, Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303:178–82. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 4.Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986;63:127–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Hammarstrom L, Smith CI. IgG2 deficiency in a healthy blood donor. Concomitant lack of IgG2, IgA and IgE immunoglobulins and specific anti-carbohydrate antibodies. Clin Exp Immunol. 1983;51:600–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Schauer U, Stemberg F, Rieger CH, Buttner W, Borte M, Schubert S, et al. Levels of antibodies specific to tetanus toxoid, Haemophilus influenzae type b, and pneumococcal capsular polysaccharide in healthy children and adults. Clin Diagn Lab Immunol. 2003;10:202–7. doi: 10.1128/CDLI.10.2.202-207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikolajczyk MG, Concepcion NF, Wang T, Frazier D, Golding B, Frasch CE, et al. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin Diagn Lab Immunol. 2004;11:1158–64. doi: 10.1128/CDLI.11.6.1158-1164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulson JC, Blixt O, Collins BE. Sweet spots in functional glycomics. Nat Chem Biol. 2006;2:238–48. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 9.von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–11. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jefferis R, Reimer CB, Skvaril F, de Lange GG, Goodall DM, Bentley TL, et al. Evaluation of monoclonal antibodies having specificity for human IgG subclasses: results of the 2nd IUIS/WHO collaborative study. Immunol Lett. 1992;31:143–68. doi: 10.1016/0165-2478(92)90141-a. [DOI] [PubMed] [Google Scholar]
- 12.Yount W, Dorner M, Kunkel H, Kabat E. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968;127:633–46. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riesen W, Skvaril F, Braun D. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5:383–90. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 14.Snapper CM. Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae. Curr Protein Pept Sci. 2006;7:295–305. doi: 10.2174/138920306778017972. [DOI] [PubMed] [Google Scholar]
- 15.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–88. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Liu S, Trummer BJ, Deng C, Wang A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat Biotechnol. 2002;20:275–81. doi: 10.1038/nbt0302-275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


