Introduction
Primary essential hypertension is second only to diabetic nephropathy as a primary etiology for end-stage renal disease (ESRD)1. Additionally, co-existent/superimposed hypertension plays a major role in the progression of most forms of chronic kidney disease (CKD) including diabetic nephropathy2-5. Nevertheless, the individual risk is very low with less than 1% of the hypertensive population developing ESRD. Such data indicate that there must be mechanisms that normally protect the kidneys from hypertensive injury of a severity sufficient to result in ESRD. The following brief review summarizes the evidence that indicates that the renal autoregulatory response, primarily mediated by the myogenic mechanism, is largely responsible for such protection. Moreover, the differing patterns of renal damage that are observed in clinical and experimental hypertension are best explained when considered in the context of alterations in renal autoregulatory capacity. Recent data also indicate that hypertensive renal damage correlates most strongly with systolic BP6-8. Accordingly, the review additionally emphasizes the kinetic characteristics of the renal myogenic response to oscillating BP signals that render it particularly capable of providing protection against systolic pressures.
Patterns of hypertensive renal damage
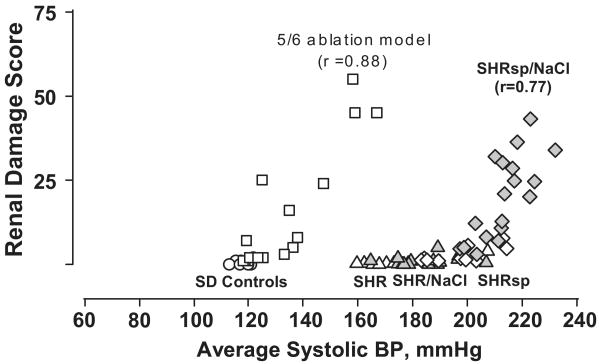
Most individuals with primary hypertension develop the modest vascular pathology of benign nephrosclerosis5. The glomeruli are largely spared and therefore, proteinuria is not a prominent feature. Because it progresses fairly slowly with limited ischemic nephron loss, renal function is not seriously compromised except in some genetically susceptible individuals or groups, such as African-Americans in whom a more accelerated course may be seen2-5. Thus, the slope of the relationship between renal damage and BP through most of the hypertensive range is fairly flat in individuals with benign nephrosclerosis2-4. However, if the hypertension becomes very severe and exceeds a critical threshold, severe acute disruptive injury of malignant nephrosclerosis to the renal arteries and arterioles develops that often extends into the glomeruli5,9. Many glomeruli show evidence of ischemia from more upstream vascular injury, but lesions of focal and segmental glomerulosclerosis (GS) are uncommon. Proteinuria, hematuria and renal failure develop rapidly. By contrast, patients with pre-existent diabetic and non-diabetic proteinuric CKD, exhibit a markedly enhanced susceptibility to renal damage with even moderate BP elevations2-4. Moreover, in contrast to the predominantly vascular pathology in patients with benign or malignant nephrosclerosis, the dominant lesion associated with the progressive proteinuric CKD is that of GS suggesting a somewhat different pathogenesis of hypertensive injury in such patients2-5. Similar patterns of relationships between BP and renal damage and the accompanying differences in renal pathology have been demonstrated in experimental models of benign nephrosclerosis (SHR), malignant nephrosclerosis (salt-supplemented SHRsp) and CKD (5/6 renal ablation) through the use of chronic BP radiotelemetry as illustrated in Fig. 12-5,10-13.
Fig. 1.
Relationship between renal injury and systolic BP in rat models with intact autoregulation (normotensive Sprague-Dawley [SAD; circles]; spontaneously hypertensive rat [SHR, triangles]; stroke-prone SHR [SHRsp, diamonds]; SHR [gray triangles] and SHRsp [gray diamonds] placed on increased dietary salt intake) and in the 5/6 remnant kidney model [squares], with impaired autoregulation. The renal damage score represents a composite of vascular and glomerular damage scores in the SHRsp and % GS in the 5/6 ablation model. Patterns of injury parallel that of renal autoregulation. The remnant kidney exhibits impaired autoregulation, and exhibits a much lower BP threshold for hypertensive injury than SHR and SHRsp kidneys. (Reprinted with permission from Reference #13 with data reproduced with permission from references #10, and #11).
Renal autoregulatory capacity and hypertensive renal damage
The concept supporting the protective importance of renal autoregulatory capacity is based on the proposition that for a given vascular segment to be injured by hypertension, it has to be exposed to it. Normally, increases in BP, episodic or sustained, result in proportionate increases in renal vascular resistance such that renal blood flow (RBF) is unchanged (Fig. 2)2-4,13-16. Because these resistance changes are confined to the preglomerular resistance vessels, primarily the afferent arteriole, glomerular capillary pressures (PGC) are also maintained relatively constant. Thus, the glomerular capillaries are protected from barotrauma as long as the autoregulatory mechanisms are intact and the BP remains within the autoregulatory range as is the case in the vast majority of patients with primary essential hypertension. As would be expected, the remodeling changes are seen in the resistance vessels exposed to the increased pressures and over time benign nephrosclerosis develops. However, when the BP exceeds the threshold for vascular injury, acute malignant nephrosclerosis ensues and the autoregulatory ability of the preglomerular vasculature to protect the glomerular capillaries is breached.
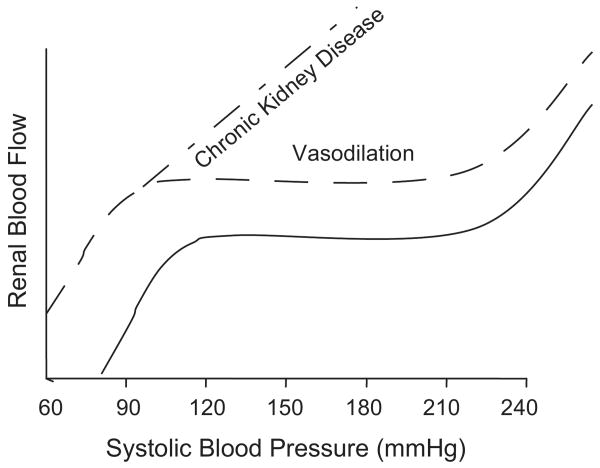
Fig. 2.
Renal autoregulatory response patterns (‘steady-state’ RBF after step changes in blood pressure) in normal rats with intact renal mass, with vasodilation but preserved autoregulation such as after uninephrectomy, and in the 5/6 renal ablation model of chronic kidney disease (vasodilation and impaired autoregulation). (Reprinted with permission from reference #4).
By contrast, if renal autoregulatory ability is impaired, even modest increases in systemic BP are expected to be transmitted to the glomerular capillaries. The increased transmission of pressure manifests as a reduced BP threshold for glomerular injury and a linear relationship between BP and GS, the steepness of which is proportionate to the severity of autoregulatory impairment2-4,10. Thus a marked increase in susceptibility to hypertensive renal injury is seen in the remnant kidney model, in which severe (>3/4) renal mass reduction results in impaired autoregulation, but only a modest increase in susceptibility is seen following uninephrectomy as in the latter case autoregulation is preserved, despite the associated vasodilation (Fig 2).2-4,10,13,14,17,18 Increased susceptibility to hypertensive renal injury is also observed in genetic and other models exhibiting impaired renal autoregulation.13,16,19-22 In the absence of hypertension severe enough to cause necrotizing vascular glomerular injury, the predominant lesion seen in these models is that of GS suggesting that it may be the consequence of more chronic and moderate glomerular capillary hypertension. Further support for the concept of autoregulatory capacity as a major determinant of the glomerular susceptibility to hypertensive injury is provided by the effects of dihydropyridine calcium channel blockers (CCBs) in the 5/6 ablation model of CKD23-25. Given the critical dependence of myogenic responses on calcium entry through voltage-gated calcium channels, these agents not unexpectedly, further impair the already impaired renal autoregulation in the 5/6 ablation model13,23-26. And predictably, CCBs also further reduce the BP threshold and increase the slope of the relationship between GS and BP (percent increase in GS/mmHg in BP) such that greater GS is observed at any given BP elevation as compared with untreated rats, and protection is not achieved without achieving normotension (Fig. 3)2-4,23-25,27. Conversely, if preglomerular vasodilation and autoregulatory impairment are prevented in this model through the substitution of a low-protein diet, GS is also ameliorated despite continued hypertension17,28. However, if CCBs are given to the low-protein diet-fed rats, renal autoregulation is impaired and the protection against GS is also abolished28. Similar adverse effects of dihydropyridine CCBs, and/or protective effects of a low-protein diet on GS, have also been noted in other proteinuric models including the streptozotocin-induced diabetes model29,30. That these adverse effects of CCBs on glomerular capillary injury are due to their effects on renal autoregulation and are not non-specific is indicated by the fact that CCBs are very effective in situations where the target site for hypertensive injury is the larger vessels such as in malignant nephrosclerosis or in clinical cardiovascular end point trials6,31.
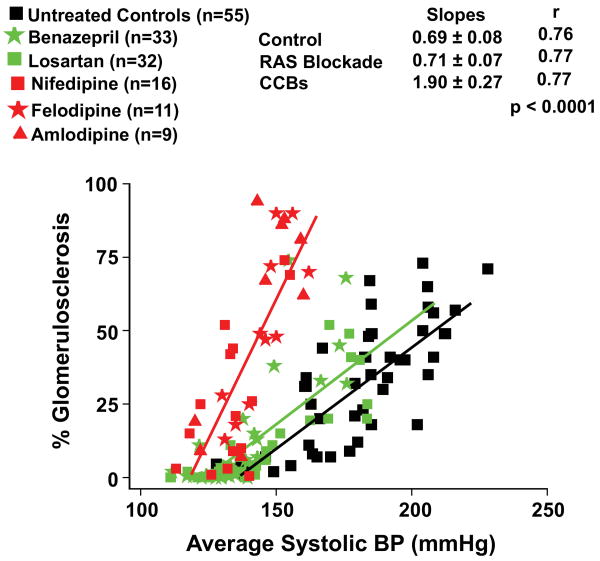
Fig. 3.
Quantitative relationships between BP and GS in rats with 5/6 renal ablation which had been left untreated or had received dihydropyridine calcium channel blockers (DHP CCBs) for 7 weeks (data from references #23-25). For comparison, data are also shown for rats with 5/6 ablation who had been similarly treated with renin-angiotensin systems (RAS) blockade with either the ACE inhibitor, benazepril or the AT1 receptor blocker, losartan. The doses of benazepril used were 25, 50 or 100 mg/L and of losartan 50, 120 or 180 mg/L of drinking water (data from reference #27). Note the significant adverse effects of the DHP CCBs as compared to untreated and RAS blockade treated rats on the slope of the relationship between average systolic BP and % GS (increase in % GS/mmHg increase in systolic BP) (reprinted with permission from reference #4).
It should be noted that autoregulation is not instantaneous and autoregulatory capacity in the studies discussed above was assessed by the steady state RBF responses to “step” changes in BP (Fig. 2). While these data clearly show that an impairment of the steady state magnitude of these responses to somewhat artificial ‘step’ changes in BP is associated with an enhanced susceptibility to hypertensive renal damage, BP in-vivo fluctuates continuously at multiple frequencies13,14,32-34. The mechanics by which the renal autoregulatory mechanisms are able to provide protection against the more rapid fluctuations such as those due to the heart beat is considered in the discussion that follows.
Mechanisms underlying renal autoregulation
The phenomenon of renal autoregulation is believed to be mediated by the combined and interacting contributions of two mechanisms, a faster myogenic and a slower tubuloglomerular feedback (TGF) system13-16,35. Recently additional and even slower mechanisms have been postulated15. Although the myogenic and TGF mechanisms are thought to act in concert in order to both insulate the renal excretion functions from BP fluctuations and to concurrently provide protection against hypertensive injury, several lines of evidence indicate that it is the myogenic response that is primarily responsible for mediating the protective function. This evidence which has been reviewed in detail elsewhere13,32-34, is briefly summarized here.
BP lability and the requirements of protection against hypertensive injury
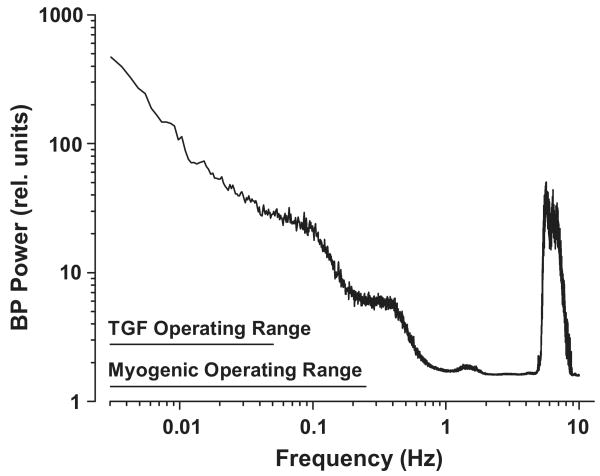
If hypertensive injury is considered to be a consequence of an excess energy delivered to the target organ vasculature from continuously oscillating pressures, an examination of the BP power (energy/unit time) and its frequency distribution provides clues to the requirements for effective protection against hypertensive injury (Fig. 4)13,14,32-34. Although a 1/frequency relationship is observed for the slower BP fluctuations below the heart beat frequency, there is very substantial BP power at the heart beat frequency itself (6 Hz in the rat). This is consistent with the recent findings that suggest that the systolic (peak) blood pressure is the most damaging component of the blood pressure load, as elevations in the systolic blood pressure have been found to exhibit the closest correlation with hypertensive target organ injury including renal damage6-8,13,32-34. Fig. 4 also shows the frequency range over which the myogenic and TGF mechanisms can attenuate pressure induced changes in RBF13-16,26,32-35. TGF is relatively slow and can contribute to stabilization of RBF over frequencies that are less than 0.05 Hz or events occurring over intervals of 20s or longer. The faster myogenic mechanisms can elicit compensatory responses that stabilize RBF when pressure oscillations present at frequencies below ∼0.3 Hz (events lasting more than 3 s). However, based on such transfer function analysis of simultaneously recorded BP and RBF, it had been believed that the vasculature behaved passively with BP fluctuations faster than 0.3 Hz, given that such fluctuations appear to be accompanied by parallel and proportional changes in RBF13,14,26,32-35. In regard to insulation of renal function including RBF and GFR, this potential limitation is inconsequential. As shown in Fig. 4, the operational range of these mechanisms is sufficient to accommodate the larger-amplitude blood pressure variations seen at low frequencies and achieve autoregulation of RBF and GFR over this range. The very rapid events (>1 Hz) would have minimal impact on mean RBF or GFR. For effective renal protection, however, the vascular response to pressure must extend over the entire range of frequencies and, most importantly, it must include a response to the systolic blood pressure, which is presented at the heart beat frequency13,14,32-34.
Fig. 4.
Blood pressure (BP) power spectrum in the conscious rat (mean data, n=10). The BP signal is a complex wave form derived from various fluctuations occurring at different frequencies. BP power is proportional to the square of the amplitude of these fluctuations (from the mean BP) and is plotted as a function of oscillation frequency (f). Note the 1/f relationship seen at frequencies below 1 Hz and the natural frequencies of TGF and the myogenic response. A major BP power peak is produced at the heart rate frequency (6 Hz in the rat). By current interpretations, this signal is beyond the myogenic operating range and, accordingly, is handled passively by the renal vasculature. (Reprinted with permission from Reference #13).
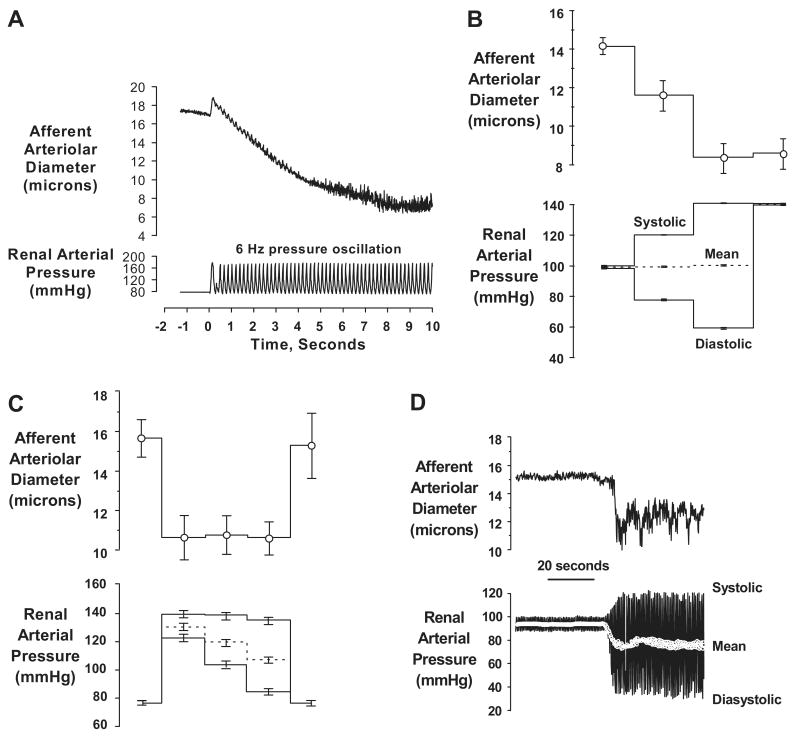
Recent observations obtained using the in-vitro perfused hydronephrotic rat kidney preparation have provided an explanation by showing that when exposed to pressure oscillation presented at the heart rate (6 Hz), the afferent arteriole does not behave passively, but rather responds with a sustained vasoconstriction13,32-34 (Fig. 5a). Moreover, as also shown in Fig. 5, when the peak and nadir pressures are varied independently, only the peak signal corresponding to the systolic pressure determined the response tone. Thus, the afferent arteriole constricts when the systolic signal is increased even if mean pressure is unaltered (Fig. 5b). Moreover, when a sub-maximal level of myogenic tone is established by an elevated peak pressure, reductions in the diastolic and mean pressures have no effect on the level of myogenic tone (Fig. 5c). Essentially, an identical response is seen when an oscillating BP signal rather than step changes are used for the input (Fig. 5d). Such vasoconstrictive increases in prevalent tone in response to increases in systolic (peak) pressure in-vivo would be expected to limit the downstream transmission of not only systolic pressure but also of pressure fluctuations at all other slower frequencies13,14,32-34.
Fig. 5.
Data illustrating the afferent arteriolar responses in the rat hydronephrotic rat kidney preparation to pressure inputs using high speed video analysis. Note, all pressures are measured within the renal artery. (a) A tracing illustrating the sustained afferent arteriolar vasoconstriction elicited by pressure oscillations presented at the rat heart rate (6 Hz). (b) afferent arteriole responds to increase in peak pressure signal (systolic) even when mean perfusion pressure is maintained constant (n=10). (c) myogenic tone established by submaximal increase in systolic (peak) pressure signal is not altered when mean pressure is reduced by marked reductions in the nadir (diastolic) pressure (n=7). (d) tracing illustrating the afferent arteriolar response to changes in the oscillating pressure signal. Note that modest increase in systolic BP evokes vasoconstriction even though mean pressure is reduced. (Reprinted with permission from reference #13 and #32).
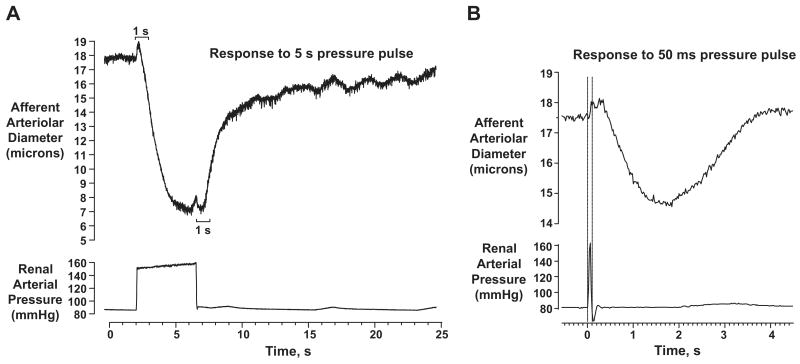
Unfortunately, technical limitations have precluded a direct demonstration of similar characteristics of the autoregulatory responses in-vivo thus far. However, mathematical modeling combined with the observations in the hydronephrotic kidney preparation have provided insights into the characteristics of the afferent arteriolar myogenic response which allow it to respond exclusively to the peak pressure36. These features are dependent on the differences in the kinetics of the pressure induced vasoconstriction and vasodilatation response and are illustrated in Fig. 6. Critical to this response are the unusually short delay in the onset of the vasoconstriction of 200-300 msec and the much longer delay in the onset of relaxation (∼1 sec) after a pressure change. Moreover, once initiated, both vasoconstriction and/or vasorelaxation events proceed during these delay periods (Fig. 6). Although recent data obtained by Just and Arendhorst indicates that the difference in delays between constriction and relaxation in-vivo are much smaller than in the hydronephrotic kidney preparation (∼140 rather than ∼700 msec), primarily due to a shorter delay in relaxation37, they are nevertheless still consistent with the systolic BP acting as the primary determinant of the myogenic response in-vivo36. Such modeling considerations however also indicate that pathophysiologic processes that may alter the kinetics of the myogenic response, even in the absence of a clear impairment of steady-state autoregulatory responses, could result in an increased transmission of the systolic pressure transients to glomerular capillaries and contribute to an enhanced susceptibility to hypertension induced renal damage. However, such has also not yet been experimentally validated.
Fig. 6.
(a) Illustration of the kinetic features of the afferent arteriolar myogenic response in the hydronephrotic kidney preparation to a step change in pressure. Note, the very short “delay” in onset of vasoconstriction (200-300 ms) and a much longer “delay” in onset of relaxation (∼1 sec). (b) The afferent arteriolar vasoconstriction response to a 50 msec pressure pulse. Note that once initiated, events proceed during these “delay” periods.
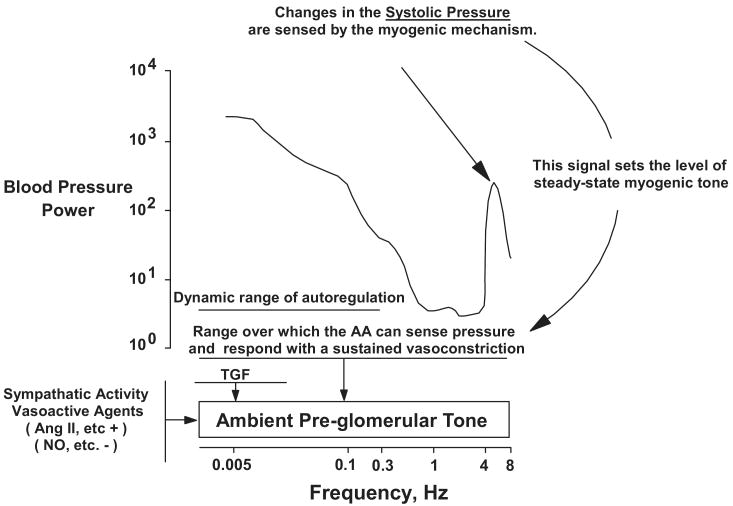
Fig. 7 summarizes these concepts in a working model of the interactions between the various mechanisms that serve to integrate the protective and regulatory functions of the renal vasculature13. It proposes that effective protection is achieved because the afferent arteriolar myogenic response is able to sense and respond to changes in systolic BP by setting the ambient preglomerular tone. This limits the downstream transmission of the oscillating pressures at all frequencies including the systolic BP and provides an explanation as to how a myogenic mechanism operating at 0.3 Hz can nevertheless protect the renal microcirculation from more rapidly oscillating systolic BP. Since under most circumstances changes in systolic BP are paralleled by changes in mean BP, concurrent autoregulation of RBF and GFR also occurs. Additionally, the absolute ambient preglomerular tone may need further modulation to achieve a regulation of RBF, GFR and volume status that is appropriate to the needs of the animal. This likely occurs through an alteration of TGF, sympathetic activity and vasoactive mediators as indicated. Given that renal autoregulatory impairment both clinically and experimentally, primarily manifests itself as an enhanced susceptibility to hypertensive renal injury and not in volume dysregulation, there are probably additional redundant and as yet incompletely defined compensatory mechanisms for regulating renal function and volume status in states of impaired autoregulation.
Fig. 7.
Proposed model of pressure-induced activation of the renal vasculature. Changes in the oscillating systolic pressure are sensed by the myogenic mechanism and it is this signal that sets the level of steady-state myogenic tone. This response provides protection over the full range of BP frequencies by limiting the transmission of pressure transients to the glomerular capillaries. Dynamic autoregulation of RBF and GFR occurs at frequencies below the myogenic operating range as a consequence of this myogenic response and, at lower frequencies, as mediated by TGF. AA, afferent arteriole. (Reprinted with permission from reference #13).
Acknowledgments
Source of Funding: This research was supported by the NIH grants DK-40426 (Anil Bidani), DK-61653 (Karen A. Griffin) and a VA Merit Review grant (Karen A. Griffin) and the Canadian Institutes of Health Research and Alberta Heritage Foundation for Medical Research (Rodger Loutzenhiser).
Footnotes
Conflict(s) of Interest/Disclosure(s): None
References
- 1.US Renal Data System: USRDS 2005 Annual Data Report, Atlas of End Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2005. [Google Scholar]
- 2.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens. 2002;11:73–80. doi: 10.1097/00041552-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertens. 2004;44:1–7. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 4.Griffin KA, Bidani AK. Progression of renal disease: the renoprotective specificity of renin angiotensin system blockade. Clin J Am Soc Nephrol. 2006;1:1054–1065. doi: 10.2215/CJN.02231205. invited review. [DOI] [PubMed] [Google Scholar]
- 5.Olson JL. Renal Disease caused by hypertension. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall's Pathology of the Kidney. sixth. II. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 937–990. [Google Scholar]
- 6.Joint National Committee on PreventionDetectionEvaluation and Treatment of High Blood Pressure. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood pressure. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 8.Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP) J Am Soc Nephrol. 2002;13:2776–2782. doi: 10.1097/01.asn.0000031805.09178.37. [DOI] [PubMed] [Google Scholar]
- 9.Bidani AK, Griffin KA, Plott W, Schwartz MM. Renal ablation acutely transforms “benign” hypertension to “malignant” nephrosclerosis in hypertensive rats. Hypertens. 1994;24:309–316. doi: 10.1161/01.hyp.24.3.309. [DOI] [PubMed] [Google Scholar]
- 10.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric BP monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 11.Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens. 2001;14:311–320. doi: 10.1016/s0895-7061(00)01282-6. [DOI] [PubMed] [Google Scholar]
- 12.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure dependent. Hypertens. 2003;41:201–206. doi: 10.1161/01.hyp.0000049881.25304.73. [DOI] [PubMed] [Google Scholar]
- 13.Loutzenhiser, Griffin KA, Williamson G, Bidani AK. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. ‘Step’ vs ‘Dynamic’ autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol. 2003;285:F113–F120. doi: 10.1152/ajprenal.00012.2003. [DOI] [PubMed] [Google Scholar]
- 15.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol. 2007;292:R1–R17. doi: 10.1152/ajpregu.00332.2006. [DOI] [PubMed] [Google Scholar]
- 16.Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol. 2007;292:F1105–F1123. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:F1003–F1010. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 18.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical determinant of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 19.Hill GS, Heptinstall RH. Steroid-induced hypertension in the rat: a microangiographic and histologic study on the pathogenesis of hypertensive vascular and glomerular lesions. Am J Pathol. 1968;52:1–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Van Dokkum RP, Alonso-Galicia M, Provoost AP, Jacob HJ, Roman RJ. Impaired autoregulation of renal blood flow in the fawn-hooded rat. Am J Physiol. 1999;276:R189–R196. doi: 10.1152/ajpregu.1999.276.1.R189. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Ajikobi DO, Salevsky FC, Cupples WA. Impaired myogenic autoregulation in kidneys of Brown Norway rats. Am J Physiol. 2000;278:F962–F969. doi: 10.1152/ajprenal.2000.278.6.F962. [DOI] [PubMed] [Google Scholar]
- 22.Churchill PC, Churchill MC, Bidani AK, Griffin KA, Picken M, Pravenec M, Kren V, St Lezin E, Wang JM, Wang N, Kurtz TW. Genetic susceptibility to hypertension-induced renal damage in the rat: evidence based on kidney specific genome transfer. J Clin Invest. 1997;100:1373–1382. doi: 10.1172/JCI119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium channel blockers in the rat remnant kidney model. Kidney Int. 1999;55:1849–1860. doi: 10.1046/j.1523-1755.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 25.Griffin KA, Picken MM, Bakris GL, Bidani AK. Comparative effects of T- and L- type calcium channel blockade in the remnant kidney model. Hypertens. 2001;37:1268–1272. doi: 10.1161/01.hyp.37.5.1268. [DOI] [PubMed] [Google Scholar]
- 26.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol. 2004;286:F1136–F1143. doi: 10.1152/ajprenal.00401.2003. [DOI] [PubMed] [Google Scholar]
- 27.Bidani AK, Picken MM, Bakris G, Griffin KA. Lack of evidence of BP independent protection by renin-angiotensin system blockade after renal ablation. Kidney Int. 2000;57:1651–1661. doi: 10.1046/j.1523-1755.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 28.Griffin KA, Picken M, Giobbie-Hurder A, Bidani AK. Low protein diet mediated renoprotection in remnant kidneys: renal autoregulatory vs hypertrophic mechanisms. Kidney Int. 2003;63:607–616. doi: 10.1046/j.1523-1755.2003.00759.x. [DOI] [PubMed] [Google Scholar]
- 29.Schnermann J, Gokel M, Weber PC, Schubert G, Briggs JP. Tubuloglomerular feedback and glomerular morphology in Goldblatt hypertensive rats on varying protein diets. Kidney Int. 1986;29:520–529. doi: 10.1038/ki.1986.30. [DOI] [PubMed] [Google Scholar]
- 30.Kloke HJ, Braten AJ, Hyysmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal disease: risks or benefits of calcium channel blockers? Kidney Int. 1998;53:1559–1573. doi: 10.1046/j.1523-1755.1998.00912.x. [DOI] [PubMed] [Google Scholar]
- 31.Griffin KA, Picken M, Litbarg N, Bidani AK. All major antihypertensive classes provide comparable and BP-dependent renoprotection in the model of malignant nephrosclerosis in the stroke prone SHR. JASN. 2005;16:186A. [Google Scholar]
- 32.Loutzenhiser R, Bidani A, Chilton L. The renal myogenic response: kinetic attributes and physiologic role. Circ Res. 2002;90:1316–1324. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 33.Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand. 2004;181:1–7. doi: 10.1111/j.1365-201X.2004.01312.x. review. [DOI] [PubMed] [Google Scholar]
- 34.Loutzenhiser R, Griffin KA, Bidani AK. Systolic blood pressure as the trigger for the renal myogenic response: Protective or autoregulatory? Curr Opin Nephrol and Hypertens. 2006;15(1):41–49. doi: 10.1097/01.mnh.0000199011.41552.de. [DOI] [PubMed] [Google Scholar]
- 35.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertens. 1997;30:975–983. doi: 10.1161/01.hyp.30.4.975. [DOI] [PubMed] [Google Scholar]
- 36.Williamson GA, Loutzenhiser R, Wang X, Griffin K, Bidani AK. Systolic and mean blood pressures and afferent arteriolar myogenic response dynamics: a modeling approach. Am J Physiol. 2008;295:R1502–R1511. doi: 10.1152/ajpregu.00490.2007. [DOI] [PubMed] [Google Scholar]
- 37.Just A, Arendshorst WJ. Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol. 2005;569:959–974. doi: 10.1113/jphysiol.2005.094888. [DOI] [PMC free article] [PubMed] [Google Scholar]