Abstract
Klebsiella pneumoniae (Kp) causes extensive lung damage. Toll-like receptor (TLR) signaling involves adaptors TRIF and MyD88. However, the relative contribution of TRIF and MyD88 signaling in host defense against pulmonary Kp infection have not been elucidated. Therefore, we investigated the role of TRIF and MyD88 in Kp pneumonia. TRIF−/− mice infected with Kp showed impaired survival and reduced bacterial clearance, neutrophil influx, histopathologic evidence of inflammation, and TNF-α, IL-6, KC, MIP-2, but not LIX, expression in the lungs. In addition, Kp-induced late NF-κB activation and phosphorylation of MAP kinases was attenuated in the lungs of TRIF−/− mice. However, MyD88−/− mice infected with Kp showed a much more remarkable phenotype, including impaired survival and reduced bacterial clearance, histopathology, and TNF-α, IL-6, KC, MIP-2 and LIX expression with almost no neutrophil influx in the lungs. In MyD88−/− mice, Kp-induced early NF-κB and MAPK activation in the lungs was also reduced. Furthermore, the role of MyD88 is dominant over TRIF because TRIF/MyD88 double-knockout mice displayed a more pronounced phenotype than TRIF−/− mice. Moreover, human alveolar macrophages pretreated with MyD88 blocking peptide showed attenuated TNF-α, IL-6 and IL-8 expression. Also, C57Bl/6 mice pretreated with MyD88 blocking peptide (BP) exhibited attenuation in Kp-induced neutrophil influx and enhanced bacterial burden in the lungs and dissemination. Overall, this investigation provides new insights into the TRIF and MyD88 signaling triggered by pulmonary Kp infection in the lungs and demonstrate the therapeutic potential of MyD88 in reducing excessive neutrophil influx in human disease during Gram-negative bacterial pneumonia.
Keywords: Extracellular pathogen, neutrophils, cytokines/chemokines, host defense, mouse model
INTRODUCTION
Bacterial pneumonia is a serious illness with substantial morbidity and mortality(1–3). Klebsiella pneumoniae (Kp) is a frequent cause of severe pneumonia with extensive lung destruction. Neutrophil recruitment to the lung, the pathological hallmark of bacterial pneumonia (4, 5), is required to augment host defense (1, 4). However, excessive neutrophil accumulation can result in Acute Lung Injury (ALI) or Acute Respiratory Distress Syndrome (ARDS) (6). Therefore, therapeutic strategies to modulate uncontrolled neutrophil influx in bacterial pneumonia and ALI/ARDS are sought to minimize lung damage.
Pathogens can be detected by receptors that recognize common molecular patterns (PAMPs) (7, 8). Toll-like receptors (TLRs) are vital sensors of PAMPs and are transmembrane proteins found on the cell surface or within endocytic vesicles (9, 10). For example, TLR2, 4 and 5 recognize bacterial peptidoglycan, endotoxin (LPS), and flagellin respectively (8–10). Upon ligand binding to TLRs, TIRAP and MyD88 are recruited to the TLR signaling complex, which results in the activation of MAP kinases and NF-κB leading to production of cytokines/chemokines. This cascade is called the MyD88-dependent pathway (11, 12). Activation of TLRs also recruits other adaptor proteins including TRIF and TRAM. This pathway activates NF-κB and a type I interferons and is called the TRIF-dependent (MyD88-independent) pathway (11, 12).
The MyD88-dependent cascade of TLRs involving MyD88 and TIRAP has been the primary focus of previous studies on bacteria-induced lung inflammation. In this context, MyD88 has been shown to be important for pulmonary host defense against Pseudomonas aeruginosa (13–15), non-typeable Haemophilus influenzae (16), E. coli (17), Burkholderia pseudomallei (18) and Legionella pneumophila (19–21), whereas TIRAP plays a critical role in host defense in the lungs against E. coli (17) and Kp (22). Although we have shown previously that MyD88−/− mice had attenuated neutrophil influx in response to Kp infection, the host defense mechanisms associated with MyD88 have not been elucidated against Kp (22). Regarding the TRIF-dependent signaling, TRIF has been shown to be important for host defense against some bacterial pathogens, such as E. coli (23) and P. aeruginosa (24), although it is not essential to host defense against a non-typeable H. influenzae (16) and B. pseudomallei (18). The role of the TRIF-dependent signaling cascade against Kp has not been established.
In the current study, we characterized the role of TRIF and MyD88 in pulmonary host defense against Kp. Although we observed that activation of both TRIF and MyD88 signaling cascades is required for neutrophil-mediated host defense in the lungs against Kp, the MyD88-dependent cascade seems more important. Our results demonstrate that the MyD88-dependent signaling is dominant over the TRIF pathway since TRIF/MyD88−/− mice showed a phenotype identical to MyD88−/− mice. Our findings reveal that MyD88 has a therapeutic potential in humans because 1) MyD88 blocking peptide attenuates chemokine/cytokine expression in human alveolar macrophages (AMs); and 2) C57Bl/6 mice pretreated with MyD88 blocking peptide (BP) showed a reduction in neutrophil recruitment and a higher bacterial burden in the lungs and dissemination. Taken together, our findings support a model in which these two cascades play essential and independent roles in host defense in the lungs against Kp, with the MyD88 signaling being dominant over the TRIF cascade. These findings also support the therapeutic potential of MyD88 in attenuating excessive lung inflammation in human disease.
MATERIALS AND METHODS
Mice
TRIF−/−, MyD88−/− and TRIF/MyD88−/− mice (12, 25) were on a C57Bl/6 background. Therefore, C57Bl/6 mice were used as controls. All animal studies were approved by the Louisiana State University Animal Care and Use Committee. The mice were 8- to 10-wk-old females, ranging from 19 to 25 g in weight.
Infection model
Kp intratracheal (i.t.) inoculation was performed as described in our previous publications (22, 26). Kp serotype 2 (American Type Culture Collection strain 43816) was grown for 16 h at 37°C in tryptic soy broth. Bacteria were harvested by centrifugation, washed twice in sterile isotonic saline, and resuspended in saline at a concentration of 20 × 103 CFU/ml. Mice were anaesthetized with i.p. ketamine/xylazine and the trachea was exposed through a mid-ventral incision followed by i.t. inoculation of 50 μl of bacteria (103 CFU/50 μl/mouse). The neck incision was closed with sterile staples. Control mice were inoculated i.t. in a similar manner with 50 μl of saline. The initial mouse inoculums were confirmed by plating serial 10-fold dilutions on MacConkey and Tryptic Soy Agar (TSA) plates. For enumerating bacterial CFU in the lungs, whole lungs were homogenized in 2 ml sterile saline for 30 s, and 20 μl of the resulting homogenates were plated by serial 10-fold dilutions on MacConkey and TSA plates. Bacterial colonies were counted after incubation at 37°C for 24 h. To demonstrate Kp dissemination, spleens were homogenized for 15 s in 1 ml saline for bacterial culture.
Bronchoalveolar lavage fluid (BALF) collection
BALF was obtained from the whole lung to collect cells in the airspace and to determine cytokine and chemokine levels as described previously (27–30). Approximately 3.0 ml BALF was retrieved from each mouse, and 0.1 ml of BALF was sedimented by centrifugation and stained with Diff-Quik staining (Fisher) to determine leukocyte subtypes. A total of 500 cells were counted in this respect. Leukocytes in BALF were determined using a hemocytometer. For determination of cytokines/chemokines, the remainder (2 ml) of the undiluted cell-free BALF was passed via a 0.22-μm filter and used immediately or stored at −20°C.
MPO assay
Myleoperoxidase (MPO), a marker of neutrophil accumulation in the lungs, was measured as previously described (27–30). Excised whole lungs were weighed, kept frozen at −70°C, and then homogenized. The resulting homogenates were centrifuged and the pellet was resuspended in 50 mM potassium phosphate buffer, pH 6.0 (supplemented with 0.5% hexadecyl trimethyl ammonium bromide) to determine the MPO level. Lungs were homogenized, incubated at 60°C for 2 h, and assayed for activity in a hydrogen peroxide/O-dianisidine buffer at 460 nm at 0 sec and 90 sec. The MPO activity was calculated between these time points using the following formula: MPO activity = the change in absorbance between 0 and 90 s/time (min) × 1.13 × 10−2. Samples were processed within 2 weeks after collection.
NF-κB activation
NF-κB/p65 binding assays (TransAM ELISA kit) were performed according to manufacture’s protocol. A total of 7.5 μg nuclear extract obtained from each lung was collected at 24 and 48 h post-Kp or -saline administration, mixed with binding buffer, added to the precoated plate (with the DNA binding motif of NF-κB) and incubated for 1 h at room temperature. Wells were then washed, and plates were incubated with NF-κB/p65 antibody for 1 h. Plates were then washed three times with wash buffer and HRP-conjugated anti-rabbit IgG was added to each well and incubated for 1 h. Plates were read at 450 nm after adding the developing reagent (27–30).
Cytokine and chemokine measurement
Cytokine and chemokine concentrations were measured in BALF or lung homogenates using a cytokine- or chemokine-specific sandwich ELISA as described in our earlier publications (27–30). The minimum detection limit is 2 pg/ml of cytokine or chemokine protein.
Lung pathology
The lungs were perfused from the right ventricle of heart with 10 ml isotonic saline. Lungs were then removed and fixed in 4% phosphate-buffered formalin for 24 h. Fixed tissues were embedded in paraffin, and 5 μm sections were prepared and stained with hematoxylin and eosin (H&E). These H&E sections were evaluated by a Veterinary Pathologist in a blinded fashion according to the following scoring system for inflammation: Score of 0: No inflammatory cells (macrophages or neutrophils) present in section; score of 1: <5% of section is infiltrated by inflammatory cells; score of 2: 5–10% of section is infiltrated by inflammatory cells; score 3: >10% of section is infiltrated by inflammatory cells. These lung sections were also evaluated for bacterial burden: Score of 0: no bacteria; score of 1: <5 bacteria per 10 high power fields (×40 objective); score of 2: 5–20 bacteria per 10 high power fields; score of 3; >20 bacteria per 10 high power fields.
Immunoblotting
At the designated time points, the lungs were homogenized for 45 s in 1 ml of buffer containing 0.1% Triton X-100 in PBS, complete protease inhibitor cocktail (Thermo Scientific, Waltham, MA 02454), complete phosphatase inhibitor cocktail (Thermo Scientific, Waltham, MA 02454), and 1 mM DTT, followed by centrifugation at maximum speed in a microcentrifuge at 4°C. The resulting supernatants were used for Immunoblotting. To ensure equal amounts of protein onto the gel, a Bradford protein assay was performed (Biorad, Herculus, CA). Equal amounts of protein from lung homogenates were loaded and separated by SDS-PAGE according to the method of Lammelli and electroblotted on to nitrocellulose membrane (Hybond ECL, Amersham Life Science, Birmingham, UK). Membranes were blocked for 1h at 4°C in Tris-buffered saline (TBS containing 0.1% Tween-20) with 5% non-fat dry milk at room temperature for 1h, followed by overnight incubation with primary antibody. The primary Abs to VCAM-1, ICAM-1, phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), phospho-p38 MAPK (Thr180/Tyr182) and phospho-SAPK/JNK (Thr183/Tyr185) were added at a 1:1,000 dilution. The primary Abs to total p38 and GAPDH were added at 1:5,000 dilution. Immunostaining was performed using appropriate secondary Ab at a dilution of 1:2,000 and developed with ECL plus Western blot detection system (Amersham Pharmacia Biotech, Piscataway, NJ). To demonstrate equal protein loading on gels, the blots were stripped and reprobed with Ab specific for total p38 and GAPDH.
Human alveolar macrophage (AM) isolation and stimulation with Kp
AMs were isolated from lungs of humans who had no history of lung diseases, as described in our previous publication (17). Thereafter, the human AMs in each well (2×106 cells/well in 6 well plate in 2 ml media) were pretreated either with 200 μg of MyD88 blocking peptide (BP) (100 μg/ml), control peptide (CP) or left untreated for 2 h, followed by stimulation with 1 × 104 CFU/ml Kp, for 18 h. Culture media were collected for TNF-α, IL-6 and IL-8 protein measurement by ELISA. Media were centrifuged at 500 × g for 10 min to discard remaining cell debris, and supernatants were stored at −80°C until use. We found that MyD88 BP or CP did not alter the viability of cells or bacterial growth after pretreatment (data not shown).
Statistical analysis
All data are expressed as means ± SE. Data were analyzed with the Student’s t test (between two groups) or with the one-way ANOVA (>2 groups). Survival curves were compared by Wilcoxon rank sign test. Differences in data values were defined significant at a P value of less than 0.05 using Kaleidagraph (Synergy software, Reading, PA).
RESULTS
TRIF is required for pulmonary host defense against Kp
To determine the importance of TRIF in mucosal host immunity in the lung, we used an experimental model of pulmonary Kp infection. We first examined the importance of TRIF in survival from Kp infection. Mice deficient in TRIF (TRIF−/−) and their littermate controls (TRIF+/+) were challenged with i.t. Kp (103 CFU/mouse) and survival was monitored up to 14 d. As demonstrated in Fig. 1A, TRIF−/− mice showed accelerated mortality as compared with their WT counterparts. A high percent (70%) of the TRIF−/−mice died on day 3 whereas all the remaining mice died by the day 5 post infection with Kp as compared with their WT counterparts (Fig. 1A).
Figure 1.
Importance of TRIF in host defense against pulmonary Kp infection: A. Reduced survival in TRIF−/− mice following i.t. Kp infection. TRIF+/+ (WT) and TRIF−/− (KO) mice were i.t. inoculated with 103 CFU/mouse of Kp, and survival was monitored for up to 14 d. n=14 mice in each group from 2 independent experiments. *, P<0.05 determined by Wilcoxon Rank Sign Test. B–C. Impaired bacterial clearance in the lungs and spleens in TRIF−/− mice against i.t. Kp infection (103 CFU/mouse). Infected TRIF+/+ and TRIF−/− mice were evaluated for bacterial CFU in the lungs and spleens. Data represent mean + SE of 4–6 mice at each time point. *, Significant differences between TRIF+/+ and TRIF−/− mice (P<0.05). D–F. Attenuated leukocyte and neutrophil accumulation in the lungs of TRIF−/− mice following Kp inoculation. Both TRIF+/+ and TRIF−/− animals underwent BALF and lung harvest after challenge with Kp. Data shown are the mean ± SE of 4–6 animals in each group at each time point (P<0.05). G. Reduced lung pathology in TRIF−/− mice following Kp inoculation. Lung sections were made 24 h after bacterial or saline challenge and stained with H&E. Inflammation and bacterial burden was calculated as described in Materials and Methods. These are representative sections of 4 mice in each condition with identical results (Magnification ×200).
Having established that TRIF is important for host defense, we sought to investigate the mechanisms associated with enhanced mortality in TRIF−/− mice followed by Kp infection. Mice were infected with Kp (103 CFU) i.t. and sacrificed at 24 and 48 h post-infection. The lungs and spleens were isolated to determine the bacterial CFU. TRIF−/− mice had greater numbers of CFU in the lungs and spleens at 48 h post-infection (Figs. 1B and C).
We then investigated whether TRIF mediates Kp-induced neutrophil influx in the lungs to augment host defense. In TRIF−/− mice, neutrophil influx into the airspaces (BALF) and lung parenchyma (MPO activity) was reduced in response to 103 CFU/mouse Kp at 24 and 48 h post-infection (Figs. 1D–F), demonstrating that TRIF is important for neutrophil-mediated lung defense against Kp. TRIF+/+ mice similarly showed moderate suppurative bronchopneumonia (score of 2.0) with intralesional bacteria (score of 1.0) (Fig. 1G) whereas TRIF−/− mice displayed mild suppurative pneumonia (score of 1.0) with high intralesional bacteria (score of 2.0). No pathological changes were however observed in saline challenged (control) lungs obtained from both TRIF−/− and TRIF+/+ animals (Fig. 1G).
Cytokine production in response to Kp requires TRIF
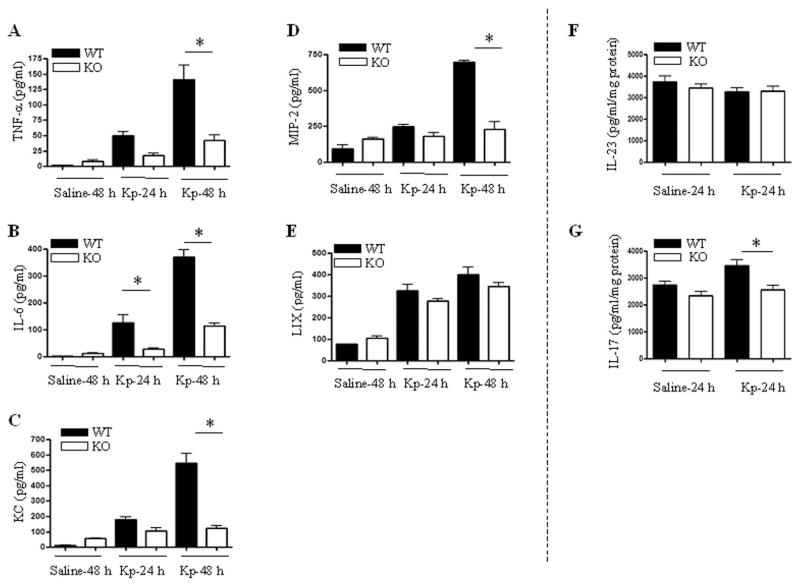
It has been demonstrated that cytokines and ELR+ CXC chemokines contribute to neutrophil influx into the lungs (31–33). In this regard, BALF studies were performed following challenge with Kp to determine cytokine and chemokine levels. Although Kp-induced TNF-α, IL-6, KC, MIP-2 production in BALF was reduced in TRIF−/− mice at 48 h (Figs. 2A–D), LIX expression was not differed between TRIF+/+ and TRIF−/− mice at this time point (Fig. 2E).
Figure 2.
Impaired cytokine responses in the airspaces of TRIF−/− mice in response to infection with Kp. A–E. Cytokine and chemokine levels in BALF were measured by sandwich ELISA after infection with Kp. Protein levels expressed as mean ± SE with 4–6 six animals used at each time point. Significant differences between TRIF+/+ and TRIF−/− mice are indicated by asterisks (P<0.05). F–G. Levels of IL-23 and IL-17 in lung homogenates from TRIF+/+ and TRIF−/− mice. Data are expressed as mean ± SE with 4–6 six animals used at each group (P<0.05).
Since IL-23 and IL-17 can regulate ELR+ CXC chemokines, such as KC and MIP-2, in response to Kp infection (34, 35), we have determined the levels of IL-23 and IL-17 in our model. Our data show less IL-17 in TRIF−/− mice, although IL-23 levels were not different between TRIF−/− and TRIF+/+ mice (Fig. 2F–G). We measured these cytokines in lung homogenates and BALF, however, their levels were not detectable in BALF (data not shown).
TRIF deficiency impairs NF-κB activation, ICAM-1 and VCAM-1 upregulation and MAPK activation in the lung against Kp
To investigate further mechanisms underlying attenuated neutrophil recruitment to the lungs in TRIF−/− mice, we investigated NF-κB activation, ICAM-1 and VCAM-1 expression, and MAPK activation in the lungs following Kp infection. Although substantial NF-κB activation was observed in the lungs of TRIF+/+ mice, a modest reduction in NF-κB activation was observed in the lungs of TRIF−/− mice against Kp at 48 h (Fig. 3A). In addition, ICAM-1, but not VCAM-1, expression was consistently reduced in TRIF−/− mice at 24 and 48 h following Kp challenge (Fig. 3B–C). Furthermore, TRIF−/− mice infected with Kp showed reduced activation of JNK and p38 kinases at 24 h whereas ERK kinase was substantially attenuated only at 48 h (Fig. 3B–C).
Figure 3.
Activation of NF-κB, upregulation of ICAM-1 and VCAM-1, and activation of MAPK against infection with Kp. A. Reduced NF-κB activation in TRIF−/− mice following Kp infection. Nuclear translocation of the p65 subunit of NF-κB as detected by p65 ELISA of nuclear extracts of mouse lungs at 24 and 48 h following Kp infection. N=3–5 per group at a time point. Values that are significantly different between TRIF+/+ and TRIF−/− are indicated by asterisks (P < 0.05). B. Attenuated upregulation of ICAM-1 and activation of MAPK in TRIF−/− mice. Total protein in the lungs was prepared from TRIF+/+ and TRIF–/–mice at 24 and 48 h following infection with Kp, run on SDS-PAGE gel and the membrane was blotted with the appropriate Ab as described in Materials and Methods. This is a representative of 3 separate experiments with identical results. C. Densitometric analysis of western blots from these experiments (n=3) were performed to quantify the protein levels of adhesion molecules (ICAM-1 and VCAM-1) and phospho-MAP kinases following Kp infection. The results obtained were normalized against GAPDH and expressed as mean ± SE.
MyD88-dependent cascade regulates host defense against Kp
We next examined the importance of MyD88-dependent signaling cascade in host defense against Kp infection since 1) TRIF-independent (MyD88-dependent) and -dependent cascades use different signaling mechanisms to boost antibacterial defense against Kp; and 2) to test whether these two cascades use the same mechanism(s) to augment host defense against Kp. As revealed in Fig. 4A, MyD88−/− mice showed early mortality (85% animals died on day 2 post-infection) compared to control mice (no death till day 2) and therefore, we performed experiments only at 24 h post-infection in MyD88−/− mice. In addition, MyD88−/− mice showed higher CFUs in the lungs and spleens compared to controls (Fig. 4B). Furthermore, MyD88−/− mice had minimal neutrophil accumulation in airspaces and showed reduced neutrophil recruitment to lung parenchyma (4C–E). Moreover, MyD88+/+ mice showed moderate suppurative bronchopheumonia (score of 2.0) with intralesional bacteria (score of 1.0) (Fig. 4F) whereas MyD88−/− mice showed no detectable histopathological changes (score of 0) with high intralesional bacteria (score of 3.0). Importantly, no significant histopathological changes were observed in the lungs of either MyD88−/− or MyD88+/+ mice in response to saline challenge (data not shown).
Figure 4.
Importance of MyD88 in host defense against pulmonary Kp infection. A. Increased mortality in MyD88−/− mice following i.t. infection with Kp. MyD88−/− and control mice were i.t. inoculated with 103/mouse of Kp, and survival was monitored for up to 14 d. n=14 mice in each group from 2 independent experiments. *, P<0.05 determined by Wilcoxon Rank Sign Test between groups. B. Reduced bacterial clearance in the lungs and spleens in MyD88−/− mice post i.t. Kp infection. Bacterial burden in the lungs and spleens of infected MyD88+/+ and MyD88−/− mice was determined at 24 h post-Kp infection. Data represent mean ± SE of 4–6 mice at each time point. *, Significant differences between MyD88+/+ and MyD88−/− mice (P<0.05). C–E. Attenuated leukocyte and neutrophil influx in the lungs of TRIF−/− mice following infection with Kp. The MyD88+/+ and MyD88−/− animals underwent BALF and lung collection after bacterial inoculation. Data represent as mean ± SE of 3–5 animals in each group at each time point (P<0.05). F. Attenuated lung inflammation and higher bacterial burden in MyD88−/− mice following Kp inoculation. Lung sections were made at 24 h post-bacterial infection and stained with H&E. These are representative sections of 4 mice in each condition with identical results (Magnification ×200).
Cytokine and chemokine expression in response to Kp requires MyD88
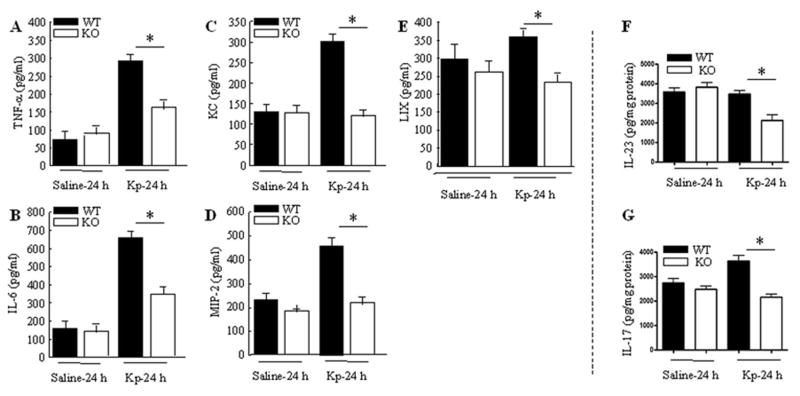
BALF studies were then conducted in mice following challenge with Kp. In MyD88−/− mice, TNF-α, and IL-6, KC, MIP-2 and LIX levels in the BALF in response to i.t. Kp infection were decreased compared to those of WT mice (Fig. 5A–E). We also observed that both IL-23 and IL-17 proteins were reduced in MyD88−/− mice in response to infection with Kp (Fig. 5F–G).
Figure 5.
Cytokine and chemokine expression in airspaces in response to infection with Kp. A–E. Attenuated TNF-α, IL-6, KC and MIP-2 and LIX in MyD88−/− mice following Kp infection. BALF protein levels were measured by sandwich ELISA and are expressed as mean ± SE and 3–5 animals were used at each time point. Significant differences between MyD88+/+ and MyD88−/− mice are indicated by asterisks (P<0.05). F–G. IL-23 and IL-17 levels in lung homogenates of MyD88−/− mice. Reduced IL-23 and IL-17 levels were observed in lung homogenates obtained from MyD88−/− mice after Kp infection. Data are expressed as mean ± SE with 4–6 animals used in each group. Statistical significance between MyD88+/+ and MyD88−/− is indicated by P<0.05.
MyD88 regulates NF-κB activation, ICAM-1 and VCAM-1 upregulation and MAPK activation in the lung against Kp
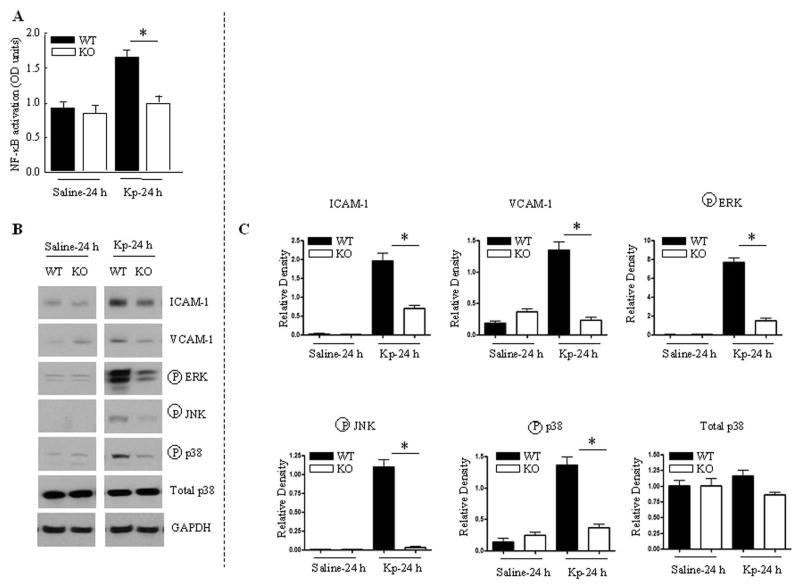
We further delineated the mechanisms associated with less neutrophil influx in MyD88−/− mice following Kp infection. In this context, we determined the role of NF-κB and cell adhesion molecules. NF-κB was activated in the lungs of MyD88+/+ mice against Kp infection, a substantial reduction in NF-κB activation was observed in the lungs of MyD88−/− mice at 24 h (Fig. 6A). Furthermore, both ICAM-1 and VCAM-1 expression was reduced in MyD88−/− mice at 24 h post-Kp infection (Fig. 6B-C). When MyD88−/− mice were infected with Kp, activation of JNK, ERK and p38 kinases was abrogated (Fig. 6B–C).
Figure 6.
NF-κB activation, ICAM-1 and VCAM-1 upregulation, and MAPK activation following Kp infection. A. Reduced NF-κB activation in MyD88−/− mice in response to Kp challenge. Nuclear translocation of the RelA/p65 subunit of NF-κB as detected by ELISA of nuclear extracts of mouse lungs at 24 h post-Kp instillation. N=3–5 per group at a time point. (P < 0.05). B. Attenuated upregulation of ICAM-1 and VCAM-1 and MAPK activation in MyD88−/− mice. Lung homogenates were prepared from MyD88+/+ and MyD88−/− mice at 24 h following infection with Kp. The results are representative of 3 different experiments with identical results. C. Quantification of non-phospho and phospho protein levels normalized against GAPDH in MyD88+/+ and MyD88−/− mice following Kp infection. Data represent mean ± SE (n=3 for each group).
Neutrophil accumulation in the lungs in response to Kp requires both TRIF and MyD88, but MyD88 has a predominant role
From our results, it appears that TRIF-dependent cascade induces a late phase activation of NF-κB and expression of cytokines/chemokines, but not LIX, and VCAM-1 whereas MyD88-dependent pathway induces an early phase activation of NF-κB and expression of cytokines/chemokines, including LIX and VCAM-1 in response to Kp. Based on these findings, we hypothesized that the MyD88-dependent cascades are dominant over TRIF cascade. To test the hypothesis, we generated mice lacking both TRIF and MyD88 (Double knockout mice [DKO]; TRIF/MyD88−/−). In TRIF/MyD88−/− mice, Kp-induced neutrophil influx was almost abolished whereas neutrophil accumulation was attenuated in TRIF−/− mice (Fig. 7A–B). Furthermore, cytokine/chemokine expression, including LIX was reduced in TRIF/MyD88−/− mice at 24 h (Fig. 7C–G). These results show a more pronounced phenotype in TRIF/MyD88−/− mice than in TRIF−/− mice.
Figure 7.
Cellular accumulation and cytokine and chemokine expression in the lungs of double knockout (DKO; TRIF/MyD88−/−) mice following Kp challenge. A–B. Attenuated leukocyte and neutrophil migration in the lungs of TRIF−/− and DKO mice after Kp challenge. Data represent mean ± SE (n=3–4/group/time point. *Significance p<0.05). C–G. Reduced cytokine/chemokine levels in BALF. Protein levels were determined by sandwich ELISA and are expressed as mean ± SE and 3–4 animals were used at each time point. Significant differences between control, TRIF−/− and double knockout mice are indicated by asterisks (P<0.05).
Effect of MyD88 blocking in human AMs in response to Kp
Because AMs play critical roles in the induction of host response against bacteria, we examined the importance of MyD88-dependent signaling cascades in cytokine/chemokine responses using primary human AMs (2 × 106/well) in response to 2 × 104 Kp. Human AMs were stimulated with Kp, in the presence of MyD88 BP or CP, and cytokine/chemokine expression was measured in culture media. Live Kp stimulation of AMs resulted in expression of TNF-α, IL-6 and IL-8 (Fig. 8), and these responses were attenuated by the BP (Fig. 8). On the other hand, CP had no influence on chemokine and cytokine gene expression in response to Kp stimulation (data not shown). In addition, BP or CP alone did not induce cytokine/chemokine expression in AMs (data not shown). These observations demonstrate that MyD88 is a central regulator in the expression of cytokines and neutrophil chemoattractant in response to Kp challenge.
Figure 8.
MyD88 blocking peptide attenuates Kp induced expression of TNF-α, IL-6 and IL-8 in human alveolar macrophages. AMs were pretreated either with control or blocking peptide for 2 h before Kp challenge. Supernatants collected at 18 h post-Kp challenge were used to determine the release of TNF-α (A), IL-6 (B) and IL-8 (C). Data shown as mean ± SE of three experiments each performed in duplicate.
Effect of MyD88 blocking in the lung in response to Kp
To exhibit the importance of MyD88 in pathological settings, control (C57Bl/6) mice were pretreated with 500 μg of MyD88 BP or CP 2 h before Kp administration. When these mice were pretreated with MyD88 blocking peptide prior to Kp infection, neutrophil influx was almost abrogated in the lungs of these mice compared to mice treated with control peptide (CP) at 48 h (Figs 9A–B). We have also observed enhanced bacterial burden in the lungs and bacterial dissemination in the spleens (Fig. 9C–D).
Figure 9.
MyD88 blocking peptide reduces neutrophil influx. A–B. MyD88 blocking peptide (BP) attenuates Kp induced cellular and neutrophil influx in control (C57Bl/6) mice. Mice were pretreated with blocking peptide (BP), control peptide (CP) or untreated for 2 h prior to Kp challenge. BALF was collected from mice at 48 h following challenge with Kp (N=3–5/group). *p<0.05 between CP and BP treated mice after Kp challenge). C–D. Impaired bacterial clearance in the lungs and spleens of BP treated mice following i.t. Kp infection (103 CFU/mouse). Peptide treated and subsequently infected TRIF−/− mice were evaluated for bacterial CFUs in the lungs and spleens. Data indicate mean ± SE of 4–6 mice at each time point. *, Significant differences between BP and CP treated animals (P<0.05).
DISCUSSION
Kp can cause life-threatening pneumonia with extensive lung damage. TLRs are well-characterized family of pattern recognition receptors (PRRs) that provide host defense against pathogens. Ligand binding to TLRs initiates a series of downstream signaling cascades via the interaction of TLRs with the TIR domains of adaptors, which ultimately results in the synthesis and secretion of cytokines and chemokines. Although TLR4 (36) and TLR9 (37) have been shown to play roles in Kp-induced pneumonia, the roles of adaptor molecules in TLR signaling cascades are not well established. Unlike TLRs, TLR adaptors can be used as therapeutic targets because several TLRs can use a single adaptor to induce downstream signaling. Therefore, we elucidated the roles of TRIF and MyD88 signaling cascades in host defense against pulmonary Kp.
The clearance of bacteria from the lower respiratory tract can be mediated by both resident cells, such as alveolar epithelial cells, and bone marrow derived cells, such as neutrophils and macrophages, which are recruited from the bloodstream into the lungs. Previous investigations have unequivocally demonstrated that neutrophils recruited from the bloodstream play a more important role than resident cells in the initial antibacterial host defense in the lungs (4, 5, 38). Although TRIF is important for neutrophil recruitment against E. coli (23) and P. aeruginosa (24), it does not seem to be important for neutrophil migration to the lungs against non-typeable H. influenzae (16) and B. pseudomallei (18). These findings could demonstrate the pathogen specific role of TRIF in neutrophil-mediated pulmonary host defense. Furthermore, we revealed that MyD88 is also important for antibacterial host defense against a pulmonary pathogen (Kp) and these data are in line with other investigations showing the crucial role of MyD88 in bacterial clearance during infection with both gram-positive and gram-negative bacteria (13–16, 18, 22). It is important to note that the TRIF signaling cascade activated through TLR4 is MyD88-independent and that TRAM is critical for the TLR4-TRIF cascade. TRAM-TRIF signaling occurs from an endosomal compartment after internalization of TLR4-TRAM complex and results in IFN-γ production (39). The role of endocytosis in TRIF signaling in the lungs against Kp infection should be a subject of future investigations.
Neutrophil sequestration within capillaries and migration into lung parenchyma during lung infection is a multistep process that involves neutrophil stiffening, retention in capillaries, adhesion to endothelium, and extravasation to the alveolus (40, 41). Neutrophils bind to various adhesion molecules, such as ICAM-1, E-selectin, and VCAM-1 expressed on endothelial cells. Most importantly, VCAM-1 and ICAM-1 are up regulated by TNF-α during infection/inflammation (42, 43). The data presented herein constitute a strong argument that Kp-induced TRIF signaling leads to the expression of TNF-α and subsequent up-regulation of ICAM-1 on endothelial cells (Fig. 3). It is also possible that Kp induces direct upregulation of these cell adhesion molecules. In addition to TRIF, MyD88 mediates upregulation of LIX and VCAM-1 expression in the lungs against Kp (Fig. 6) and this may involve both direct (Kp-induced) and indirect (TNF-α– mediated) mechanisms.
Leukocyte migration into tissues and subsequent activation is regulated by NF-κB activation and the production of cytokines and chemokines. In particular, critical roles for ELR+ CXC chemokines have been demonstrated in murine models of bacterial pneumonia (44–46). It has been established that TLR signaling can activate NF-κB (47, 48). Our results suggest that TRIF-dependent late NF-κB activation is a critical mediator of TNF-α and IL-6 expression in the lungs in response to Kp. Although similar findings have been reported in investigations using E. coli (23) and P. aeruginosa (24), investigations using H. influenzae (16) and B. pseudomallei (18) have revealed that TRIF is not required for cytokine/chemokine expression in the lungs. The discrepancy between these findings may be explained by the nature of the pathogens and time points used to measure chemokines/cytokines in the lungs. We also provide evidence that MyD88 is an important mediator of Kp-induced early NF-κB activation and cytokine/chemokine production in the lungs. Unlike TRIF, MyD88 is important for the production LIX and VCAM-1 probably via IL-23 in response to Kp. These results demonstrate that MyD88, as compared with TRIF, has additional and essential mechanisms to induce neutrophil influx to the lungs in Kp infection. Since we observed more dramatic attenuation of early NF-κB in MyD88−/− mice as compared with TRIF−/− mice against Kp infection, it appears that early phase of NF-κB activation is required for the induction of LIX and VCAM-1.
The current study also shows that TRIF and MyD88 are important for Kp-induced MAPK activation in the lungs. It is important to mention that we have performed our studies in lung homogenates and therefore, reported data reflect the net effect as a representation of various cell types in the lungs. However, numerous investigations have shown the activation of MAPK in isolated cells (48, 49), rather than in the whole lungs. Nevertheless, our results are consistent with previous reports using isolated macrophages (48, 49). Given the fact that MAPK activation contributes to cytokine/chemokine expression (49), our data suggest that TRIF- and MyD88-mediated MAPK signaling contributes to Kp-induced cytokine/chemokine production and cell adhesion molecule upregulation in the lungs.
From the therapeutic point of view, due to complex adhesion cascades leading to neutrophil accumulation in the lung by Kp, blocking an individual adhesion molecule may not be a feasible strategy to attenuate excessive neutrophil migration during Kp-mediated infection/inflammation. However, blocking the initial signaling steps possibly at the level of adaptor molecules could plausibly attenuate subsequent signaling pathways leading to neutrophil influx. In this context, our results, using TRIF−/− or MyD88−/− mice in response to Kp reveal that targeting upstream signaling, such as TRIF- or MyD88-dependent cascades, using cell-permeable compounds could minimize uncontrolled neutrophil influx into the lungs and subsequent lung damage during Kp infection (Fig. 10). Thus, the therapeutic potential of MyD88 is supported by our findings in human AMs and in a murine model using MyD88 blocking peptide.
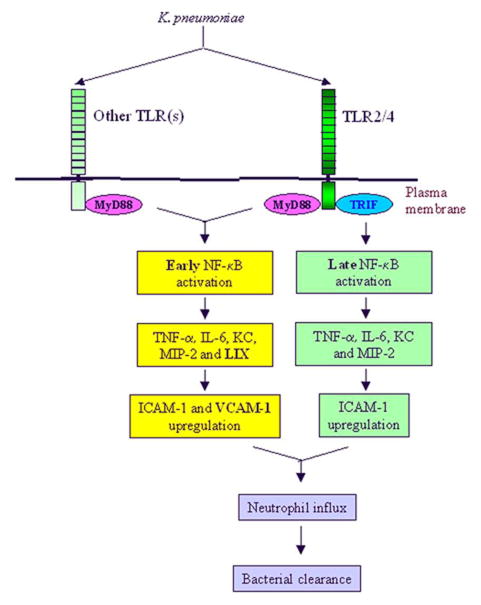
Figure 10.
Proposed scheme for TRIF and MyD88 signaling cascades leading to neutrophil influx into the lungs (in vivo) against Kp. Kp activates single or multiple TLRs to induce downstream signaling. TRIF signaling induces late NF-κB activation and subsequent TNF-α, IL-6, KC and MIP-2 production and ICAM-1 upregulation whereas MyD88 signaling induces early NF-κB activation and subsequent TNF-α, IL-6, KC MIP-2 and LIX production and ICAM-1 and VCAM-1 upregulation.
Acknowledgments
Supported by a Research Grant from the American Lung Association (RG-22442-N), a Scientist Award from the Flight Attendant Medical Research Institute (YCSA-062466); and grants from the NIH (R01 HL-091958 and R01 HL-091958S1 via ARRA) to SJ
The authors thank Robert Mason at National Jewish Health for providing human AMs. We also thank to Lung Biology (Jey) lab members, including Gayathriy Balamayooran, Kohila Mahadevan, and Theivanthiran Balamayooran for critical reading of the manuscript. The authors thank Rachel Zemans, Mike Fessler and Ken Malcolm for helpful discussions and critical reading of the manuscript.
Abbreviations in this paper
- KC
Keratinocyte cell-derived chemokine
- MIP-2
macrophage inflammatory protein-2
- TIRAP
Toll-IL-1R domain-containing adapter protein
- TRAM
TRIF-related adaptor molecule
- TRIF
Toll/IL-1 Receptor Domain-Containing Adaptor Inducing IFN- {beta}
- MAPK
Mitogen Activated Protein Kinase
- ICAM-1
Intracellular Cell Adhesion Molecule
- VCAM-1
Vascular Cell Adhesion Molecule
- PAMPs
Pathogen Associated Molecular Patterns
- CFU
Colony Forming Unit
- AM
Alveolar macrophages
References
- 1.Lynch JP, 3rd, Martinez FJ. Community-acquired pneumonia. Curr Opin Pulm Med. 1998;4:162–172. [PubMed] [Google Scholar]
- 2.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fein AM. Pneumonia in the elderly: overview of diagnostic and therapeutic approaches. Clin Infect Dis. 1999;28:726–729. doi: 10.1086/515218. [DOI] [PubMed] [Google Scholar]
- 4.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861–868. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 13.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292:L312–322. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 14.Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–3934. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- 15.Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J Biol Chem. 2004;279:49315–49322. doi: 10.1074/jbc.M402111200. [DOI] [PubMed] [Google Scholar]
- 16.Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, Akira S, van der Poll T. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol. 2005;175:6042–6049. doi: 10.4049/jimmunol.175.9.6042. [DOI] [PubMed] [Google Scholar]
- 17.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Toll-IL-1 receptor domain-containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J Immunol. 2005;175:7484–7495. doi: 10.4049/jimmunol.175.11.7484. [DOI] [PubMed] [Google Scholar]
- 18.Wiersinga WJ, Wieland CW, Roelofs JJ, van der Poll T. MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS One. 2008;3:e3494. doi: 10.1371/journal.pone.0003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires’ disease. Infect Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 21.Sporri R, Joller N, Albers U, Hilbi H, Oxenius A. MyD88-dependent IFN-gamma production by NK cells is key for control of Legionella pneumophila infection. J Immunol. 2006;176:6162–6171. doi: 10.4049/jimmunol.176.10.6162. [DOI] [PubMed] [Google Scholar]
- 22.Jeyaseelan S, Young SK, Yamamoto M, Arndt PG, Akira S, Kolls JK, Worthen GS. Toll/IL-1R domain-containing adaptor protein (TIRAP) is a critical mediator of antibacterial defense in the lung against Klebsiella pneumoniae but not Pseudomonas aeruginosa. J Immunol. 2006;177:538–547. doi: 10.4049/jimmunol.177.1.538. [DOI] [PubMed] [Google Scholar]
- 23.Jeyaseelan S, Young SK, Fessler MB, Liu Y, Malcolm KC, Yamamoto M, Akira S, Worthen GS. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 24.Power MR, Li B, Yamamoto M, Akira S, Lin TJ. A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2007;178:3170–3176. doi: 10.4049/jimmunol.178.5.3170. [DOI] [PubMed] [Google Scholar]
- 25.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 26.Deng JC, Zeng X, Newstead M, Moore TA, Tsai WC, Thannickal VJ, Standiford TJ. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J Immunol. 2004;173:4075–4083. doi: 10.4049/jimmunol.173.6.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun. 2005;73:1754–1763. doi: 10.1128/IAI.73.3.1754-1763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72:7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32:531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai S, Zemans RL, Young SK, Worthen GS, Jeyaseelan S. Myeloid differentiation protein-2-dependent and -independent neutrophil accumulation during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2009;40:701–709. doi: 10.1165/rcmb.2008-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- 32.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol. 1995;58:359–364. doi: 10.1002/jlb.58.3.359. [DOI] [PubMed] [Google Scholar]
- 34.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun. 2005;73:532–545. doi: 10.1128/IAI.73.1.532-545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, Zeng X, Moore TA, McMillan TR, Krieg AM, Akira S, Standiford TJ. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 38.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol. 2002;14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 39.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazan-Socha S, Bukiej A, Marcinkiewicz C, Musial J. Integrins in pulmonary inflammatory diseases. Curr Pharm Des. 2005;11:893–901. doi: 10.2174/1381612053381710. [DOI] [PubMed] [Google Scholar]
- 41.Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 42.Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels. J Immunol. 2002;169:2111–2119. doi: 10.4049/jimmunol.169.4.2111. [DOI] [PubMed] [Google Scholar]
- 43.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 44.Mizgerd JP, Lupa MM, Spieker MS. NF-kappaB p50 facilitates neutrophil accumulation during LPS-induced pulmonary inflammation. BMC Immunol. 2004;5:10. doi: 10.1186/1471-2172-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178:1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 49.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]