Abstract
Purpose
A publication on behalf of the European Society of Urologic Oncology (ESUO) questioned the need for removing the seminal vesicles (SV) during radical prostatectomy (RP) in patients with PSA < 10 ng/ml, except when biopsy Gleason score is >6 or with > 50% positive biopsy cores. Our objective was to apply the ESUO algorithm to an independent data set to determine its predictive value.
Materials and Methods
Data of 1406 men who underwent RP and SV removal between 1998 and 2004 were analyzed. Patients with and without seminal vesicle invasion (SVI) were classified as positive or negative according to the ESUO algorithm
Results
Of 90 patients (6.4%) with SVI, 81 were ESUO positive for a sensitivity of 90%; 656 of 1,316 patients without SVI were ESUO negative for a specificity of 50%. The negative predictive value was 98.6%. In decision-analytic terms, if the loss in health when SV are invaded and not completely removed is considered at least 75 times greater than removing them unnecessarily, then the algorithm proposed by the ESUO should not be used.
Conclusions
Whether or not to use the ESUO algorithm depends not only on its accuracy but on the relative clinical consequences of a false positive and a false negative. Our threshold of 75 is an intermediate number that is difficult to interpret given uncertainties about the benefit of SV -sparing and harm associated with untreated SVI. We recommend more formal decision analysis to determine the clinical value of the ESUO algorithm.
Keywords: Seminal vesicle preservation, radical prostatectomy, decision analysis
Introduction
A recent communication from the European Society of Urologic Oncology (ESUO)[1] questioned the routine practice of complete resection of the seminal vesicles (SV) during radical prostatectomy (RP). This was based on a on a retrospective analysis of 1283 patients, 137 (10.6%) of whom had seminal vesicle involvement (SVI). The authors first noted that prostate-specific antigen (PSA) level, biopsy Gleason grade and number of biopsy cores positive for cancer were predictive for SVI. They went on to propose a prediction rule: patients with PSA ≥ 10 ng / ml or biopsy Gleason grade > 6 or > 50% biopsy cores positive were at high likelihood of SVI and should have SV removal; patients who with PSA < 10 ng / ml and biopsy Gleason grade < 7 and ≤ 50% biopsy cores positive are unlikely to have SVI and might be spared complete resection of the SV. The recommendation was that this strategy be applied in a prospective study comparing “SV-sparing surgery ... to standard retropubic prostatectomy in selected individuals [i.e. those negative on the algorithm] analyzing potential benefits on erectile function and urinary continence.”
The rationale for SV preservation is that the tip of the SV is very close to both the arterial supply of the bladder base and the proximal neurovascular bundles. Complete dissection of the SV might therefore damage these structures, raising the likelihood of postoperative urinary incontinence and erectile dysfunction. If resection of the tip of the SV is not oncologically necessary, it would therefore seem prudent to preserve this structure.
Our objective in this paper was to apply the ESUO algorithm to data from our institution in order to determine its predictive validity (“external validation”). We also planned to use a decision analytic method to explore the effects of implementing the algorithm in clinical practice. We believe that a prospective trial, as recommended by the ESUO investigators, is likely infeasible. For example, a trial wishing to show that SV-sparing improved function, but did not increase recurrence rates by more than 2%, might well require more than 10,000 patients. Accordingly a decision analysis remains the most feasible method for assessing the clinical impact of a SV-sparing strategy for selected patients.
MATERIALS & METHODS
Study cohort
The study cohort consisted of 2,959 consecutive men with localized prostate cancer who underwent radical prostatectomy between January 1998 and August 2004; we chose January 1998 as the start of the cohort as very few men treated before 1998 had data for number of biopsy cores positive. Men who received treatment prior to surgery (neoadjuvant hormonal therapy, chemotherapy, or radiation therapy) were excluded (n=228). Of the remaining 2,731 patients, 1,406 had complete information regarding preoperative serum PSA, biopsy Gleason score and percentage of biopsies with prostate cancer involvement, defined as the number of cores with any amount of prostate cancer divided by the total number of prostate biopsies. There were no exclusions for type of surgery or pathology.
Data from the cohort were treated according to the U.S. Health Insurance Portability and Accountability Act (HIPAA). The data set was obtained following institutional board approval and was de-identified prior to analysis.
Surgical treatment and pathology
All patients underwent transrectal ultrasound guided prostate biopsies with a minimum of 6 cores. Pathologic slides of patients with biopsies performed in other institutions were reviewed by a specialized urology pathologist at MSKCC. The Gleason system was scored on all biopsies by the sum of the primary and secondary patterns.
All radical prostatectomies included the complete removal of the seminal vesicles and pelvic lymph nodes. The procedures were performed by experienced surgeons using the standard described technique.[2] All radical prostatectomy specimens were sectioned with whole-mount technique by specialized urologic pathologists. Tumor invasion of the seminal vesicles (SVI) was recorded when malignant cells invaded into the muscular layer of the wall of the seminal vesicle.
Statistical analysis
Patients with PSA ≥ 10 ng / ml or biopsy Gleason grade > 6 or > 50% biopsy cores positive were classed as ESUO positive; the remainder as ESUO negative. Patients were classed as SVI positive or negative irrespective of lymph node status (e.g. a patient with positive lymph nodes but no SVI was classed as disease negative). We calculated the sensitivity, specificity, and positive and negative predictive value of the ESUO prediction rule.
Although no doubt informative, measures of diagnostic accuracy, such as sensitivity, have limited value for clinical decision making. For example, how high would sensitivity and specificity need to be in order to be “high enough” to justify clinical use of the ESUO prediction rule? Our answer to this question depends on the clinical consequences of our results: a false negative – failing to remove completely a cancerous SV – has more serious consequences than that of a false positive – unnecessarily radical resection of SV free of cancer. We therefore used a decision analytic technique, decision curve analysis,[3] to help evaluate the clinical value of the ESUO rule. Decision curve analysis incorporates the clinical consequences of using a prediction rule by applying a different weight to true and false positives. This weighting can be varied to reflect different patient preferences or differences in opinion about the risks of a procedure. These preferences are expressed in terms of a threshold probability for action. A man has a threshold probability of X% if he would choose complete SV removal if his risk of SVI was X% or greater but choose SV preservation if his risk of SVI was less than X%. Decision curve analysis provides a “net benefit” for each treatment strategy at each threshold probability, calculated at true positives – false positives where the latter is weighted by the odds at the threshold probability (i.e. p / [1-p]). The optimal strategy is the one with the highest net benefit. All statistical analyses were conducted using Stata 9.2 (Stata Corp., College Station, Tx).
RESULTS
Preoperative and pathologic patient characteristics are shown in table 1. Seventy-two patients with biopsy Gleason 6 were upgraded on pathological analysis of the RP specimen (69, 2, and 1 to Gleason 7, 8 and 9 respectively); 4 of these patients (all Gleason 7) were classed as ESUO negative but had SVI. Of the 1406 patients, 90 (6.4%) had SVI. ESUO classification is described in table 2. Approximately half of the patients were classified as ESUO positive (n=741, 53%), primarily due to biopsy Gleason grade > 6. The principal results of the study are given in table 3. Of the 90 patients with SVI, 81 were ESUO positive for a sensitivity of 90%; 656 of 1,316 patients without SVI were ESUO negative for a specificity of 50%. Negative predictive value was 98.6%. None of the 9 patients with SVI but negative by the ESUO criteria had positive lymph nodes.
Table 1.
Patient characteristics. Data are median (interquartile range) or frequency (percentage).
| N=1406 | |
|---|---|
| Preoperative Prostate Specific Antigen (ng/ml) | 5.53 (4.32, 7.68) |
| Percentage of positive biopsy cores | 33 (17, 50) |
| Clinical stage ≥ T2 (n=1315) | 518 (39%) |
| Biopsy Gleason grade | |
| ≤ 6 | 887 (63%) |
| 7 | 422 (30%) |
| ≥ 8 | 97 (7%) |
| Pathology Gleason grade (n=1394) | |
| ≤ 6 | 641 (46%) |
| 7 | 665 (47%) |
| ≥ 8 | 88 (6%) |
| Extracapsular extension (n=1399) | 353 (25%) |
| Seminal vesicle invasion | 90 (6%) |
| Positive surgical margins (n=1403) | 220 (16%) |
| Lymph node involvement (n=1264) | 50 (4%) |
Table 2.
European Society of Urologic Oncology (ESUO) classification with Prostate Specific Antigen (PSA), Biopsy Gleason grade and % Biopsy cores positive
| ESUO classification | |
| Negative | 665 (47%) |
| Positive | 741 (53%) |
| Criteria met for classification of ESUO positive | |
| Only Preoperative PSA >= 10ng/ml | 66 (5%) |
| Only Biopsy Gleason grade > 6 | 287 (20%) |
| Only >50% Biopsy cores positive | 133 (9%) |
| Preoperative PSA >= 10ng/ml & Biopsy Gleason grade >6 | 54 (4%) |
| Preoperative PSA >= 10ng/ml & >50% Biopsy Cores Positive | 23 (2%) |
| Biopsy Gleason grade >6 & >50% Biopsy Cores Positive | 146 (10%) |
| Preoperative PSA >=10ng/ml & Biopsy Gleason grade >6 & >50% Biopsy Cores Positive | 32 (2%) |
Table 3.
Diagnostic results: patients with true seminal vesicle invasion (SVI) by European Society of Urologic Oncology (ESUO) algorithm results.
| SVI in our series |
||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| ESUO criteria for SVI | Positive | 81 | 660 | 741 |
| Negative |
9 |
656 |
665 |
|
| Total | 90 | 1316 | 1406 | |
Sensitivity = 81 / 90 = 90.0%
Specificity = 656 / 1316 = 49.9%
Positive predictive value = 81 / 741 = 10.9%
Negative predictive value = 656/665 = 98.7%
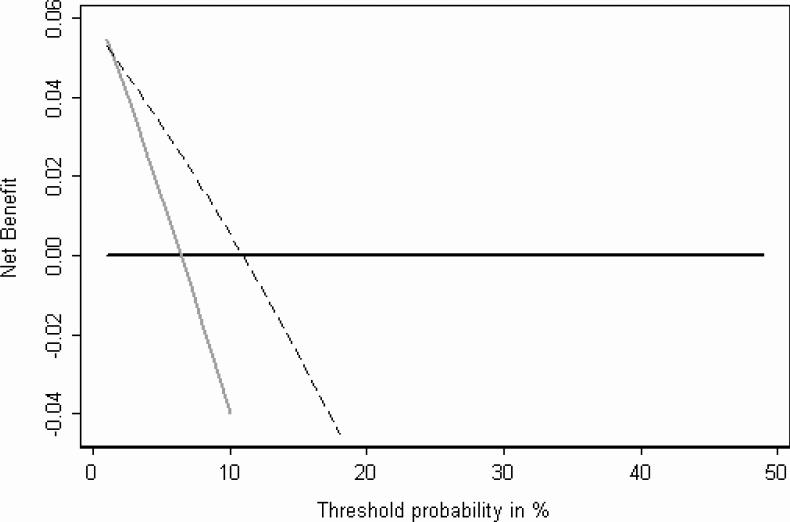
The decision curve is shown in figure 1. The x axis is the threshold for action, that is, the probability of SVI at which a man would opt for complete SV resection. The y axis gives the clinical “net benefit” in terms of the probability of a true positive (i.e. removing cancerous SV), minus the probability of a false positive (i.e. unnecessarily resecting SV without cancer) where the latter is weighted by the odds at the threshold.
Figure 1.
Decision curve for the ESUO algorithm. The dashed line gives the net benefit for seminal vesicle dissection only to men classified as ESUO positive; the gray line gives net benefit for seminal vesicle dissection in all men; the black line is for seminal vesicle preservation for all men
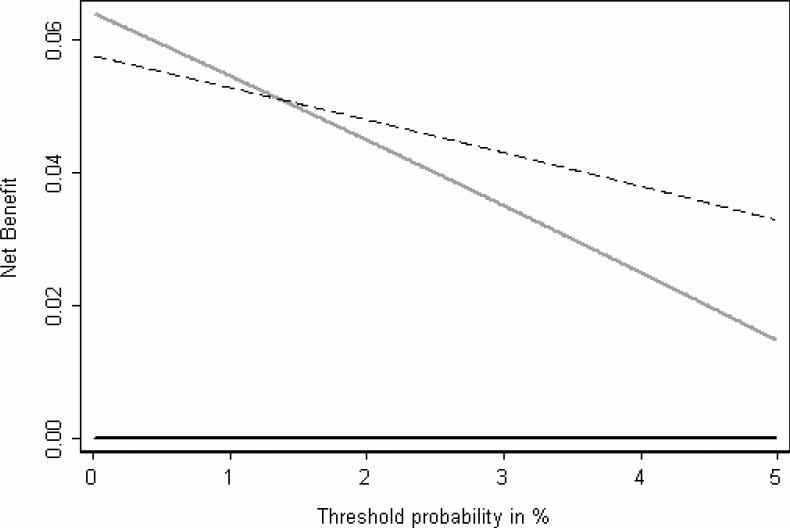
To interpret this figure, we considered what a reasonable range of thresholds might be in the community. Clearly any man told that he has a 50% chance of SVI would opt for SV removal; similarly, a man told that he had only a one in a million chance of SVI would opt for SV preservation. We thought that few if any men would ever have a threshold much above 10%; however, given that the benefits of SV preservation are unclear, some men might chose complete SV resection even if their probability of SVI was 1% or less. It can be seen from figure 1 that although the net benefit of the ESUO prediction rule is higher than the curve for the strategy of SV resection in all men for thresholds of 2% or higher, it is actually lower at thresholds of approximately 1% and lower. This is shown more clearly in figure 2.
Figure 2.
Decision curve for the ESUO algorithm showing the range 0 − 5%. The dashed line gives the net benefit for seminal vesicle dissection only to men classified as ESUO positive; the gray line gives net benefit for seminal vesicle dissection in all men; the black line is for seminal vesicle preservation for all men
The net benefit of the prediction rule is lower than that of treating all men when the threshold probability for resection is 1.35% or less (this is simply 1 minus the negative predictive value). As the odds at 1.35% probability is close to 1 in 75 (0.0135 / [1−0.0135]), this result can be phrased in the following terms: if the loss in health when SV are invaded and not completely removed is considered at least 75 times greater than an unnecessarily radical resection, then the algorithm proposed by the ESUO should not be used. We consider 75 to be an intermediate number that is difficult to interpret given uncertainties about both the value of SV -sparing and harm associated with untreated SVI.
DISCUSSION
SV-sparing surgery might be just one of a number of technical options that aims to reduce the surgical trauma of RP. But whether the original nerve-sparing technique of Walsh [4] or the fascia sparing techniques piloted at the Vattikuti Urology Institute [5], any technique must weigh any benefits in terms of functional preservation against possible risks in terms of decreased oncologic control. SV involvement has historically been associated with poor prognosis in patients undergoing RP [6]; however, the cumulative probability of freedom from biochemical recurrence (BCR) in patients with SVI and no nodal metastases treated with RP alone was 36% and 32% at 10 and 15 years in our contemporary series of 4441 men.[7] The cumulative 10- and 15-year cancer specific survival probabilities for those patients were 89% and 81%, respectively, although men remain at risk for many more years. Other studies have shown similar findings.[8]
There are no compelling data to estimate recurrence and survival for men with SVI who did not undergo complete SV removal. It may be that surgery may still be adequate as the tumor usually extends only into the proximal portions of the SV and rarely involves the tip; hence a man with SVI undergoing tip preservation may still have cancer-appropriate surgery. But in about 20% of patients with SVI, the pattern of spread is diffuse, similar to satellite metastases, and often involves the tip of the SV. [9] However, patients who have cancer in preserved tissue may not experience nadir of their PSA postoperatively and will undoubtedly require adjuvant therapy that will worsen their quality of life.[10] In comparison, adjuvant therapy for SVI in a patient with completely resected SV is a matter of surgeon choice and is by no means mandated in all cases.
The ESUO investigators[1] argue that the benefit of SV preservation is to lower the probability of damage to the pelvic plexus and the blood supply to the cavernous bodies. There are isolated reports from small non-randomized series of patients undergoing SV -sparing, and authors have typically reported better than expected outcomes for urinary and erectile function.[11-14] More recently, however, Albers et al. have reported the results of a trial of a SV-sparing strategy [15]. Patients were deemed eligible for SV-sparing using more liberal criteria than the ESUO: PSA of 10 ng / ml or less; biopsy Gleason score of 7 or less and total prostate volume ≤ 50 ml. Urinary function was superior in the 146 patients randomized to SV-sparing RP, compared to the 171 patients undergoing total SV resection (96% vs. 86% continence at one-year, p=0.005); potency rates were similar. Unfortunately, however, oncologic control is poorly reported: the authors give recurrence proportions without stating a follow-up time, showing a survival curve or giving a confidence interval for the difference between groups. Moreover, all patients in the main comparison received perineal prostatectomy, which is less common than the retropubic approach.
In short, both the benefits and the risks of SV tip preservation are subject to considerable uncertainty: we have no good estimates as to the degree to which SV preservation improves function; similarly we have no good estimates as to the degree to which SV preservation in a man with SVI increases the risk of recurrence. The latter estimate is likely to remain uncertain. The key number in our analysis is that SV preservation is harmful below a probability threshold for SV removal of 1.35%, equivalent to the loss of health from preserving affected SV being considered 75 times worse than that of SV removal. Had this number been either much lower, say, 0.25%, or higher, such as 10%, our conclusions would be clear: use or avoid the ESUO algorithm respectively. However, the actual number we derived is indeterminate. As such, we recommend a more formal decision analysis to explore the value of the ESUO algorithm. Such an analysis involves entering a range of specific values for the risk of SV preservation (a hazard ratio for recurrence) and harm of SV resection (a relative risk for poor erectile or urinary function) and then exploring the conditions under which use of the ESUO algorithm gives a better expected outcome than the current strategy of resection in all patients.
Regardless of the results of this planned decision analysis, the algorithm proposed by the ESUO could be helpful in patients undergoing external beam radiation therapy. This is because the threshold probability of SVI at which a radiotherapist would choose to irradiate the SV likely differs from the threshold probability of SVI at which a surgeon would completely excise the SV. The volume of normal surrounding tissue irradiated is larger when the seminal vesicle are included in the radiation field.[16] Thus, reduction of target volume may reduce normal tissue reactions (particularly bladder and rectum), facilitate dose escalation and increase local control [17-18] . Accordingly, the balance between the benefit of avoiding unnecessary treatment and the harm of undertreatment differs between radiotherapy and surgery. We believe that a reasonable range of threshold probabilities of SVI that would indicate a wider field irradiation would be 2.5% - 15%. Even for the conservative clinician who would irradiate the SV if the risk of SVI was 2.5% or more, but not if the risk was less than 2.5%, the ESUO algorithm should be used to select patients for SV irradiation because the net benefit for ESUO is superior to the strategy of irradiating all men for all values within this range (figure 1). Hence, decision curve analysis supports the application of the ESUO algorithm for radiotherapy planning.
In summary, whether or not to use the ESUO algorithm depends not only on its accuracy but on the cost of a false positive and a false negative. We recommend further decision analysis to characterize the uncertainty associated with the health effects of SV resection or preservation.
ACKNOWLEDGMENTS
The authors would like to thank Jason Stasi for assistance with data retrieval.
Acknowledgments of research support: This work was supported primarily by the P50-CA92629 SPORE in Prostate Cancer grant from the National Cancer Institute. Additional support came from a gift from the Koch Foundation, the Prostate Cancer Foundation, and the Leon Lowenstein Foundation. F.J.B. is supported in part by the American Foundation for Urologic Disease and a training grant (T32-82088) from the National Institutes of Health.
ABBREVIATIONS
- ESUO
European Society of Urologic Oncology
- PSA
prostate specific antigen
- RP
radical prostatectomy
- SV
Seminal vesicles
- SVI
Seminal vesicle invasion
References
- 1.Zlotta AR, Roumeguere T, Ravery V, Hoffmann P, Montorsi F, Turkeri L, et al. Is seminal vesicle ablation mandatory for all patients undergoing radical prostatectomy? A multivariate analysis on 1283 patients. Eur Urol. 2004;46(1):42–9. doi: 10.1016/j.eururo.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Ohori M, Scardino PT. Localized prostate cancer. Curr Probl Surg. 2002;39(9):833–957. doi: 10.1067/msg.2002.126335. [DOI] [PubMed] [Google Scholar]
- 3.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–74. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol. 1984;56(6):694–7. doi: 10.1111/j.1464-410x.1984.tb06149.x. [DOI] [PubMed] [Google Scholar]
- 5.Savera AT, Kaul S, Badani K, Stark AT, Shah NL, Menon M. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49(6):1065–73. doi: 10.1016/j.eururo.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Jewett HJ, Bridge RW, Gray GF, Jr., Shelley WM. The palpable nodule of prostatic cancer. Results 15 years after radical excision. Jama. 1968;203(6):403–6. [PubMed] [Google Scholar]
- 7.Secin FP, Bianco FJ, Jr., Vickers AJ, Reuter V, Wheeler T, Fearn PA, et al. Cancer-specific survival and predictors of prostate-specific antigen recurrence and survival in patients with seminal vesicle invasion after radical prostatectomy. Cancer. 2006;106(11):2369–75. doi: 10.1002/cncr.21895. [DOI] [PubMed] [Google Scholar]
- 8.Baccala A, Reuther A, Bianco F, Scardino P, Klein E. Complete resection of the seminal vesicles at radical prostatectomy results in substantial long-term disease-free survival: a multi-institutional study in 6740 patients. J Urol. 2005;173(4):A692. doi: 10.1016/j.urology.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Ohori M, Scardino PT, Lapin SL, Seale-Hawkins C, Link J, Wheeler TM. The mechanisms and prognostic significance of seminal vesicle involvement by prostate cancer. Am J Surg Pathol. 1993;17(12):1252–61. doi: 10.1097/00000478-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Miller D, Sanda M, Hollingsworth I, JH., Dunn RM, JE., et al. Salvage therapy adversely effects long-term hrqol among localized prostate cancer treatment survivorsrs. J Urol. 2005;173(4):A827. [Google Scholar]
- 11.Shidham VB, Lindholm PF, Kajdacsy-Balla A, Basir Z, George V, Garcia FU. Prostate-specific antigen expression and lipochrome pigment granules in the differential diagnosis of prostatic adenocarcinoma versus seminal vesicle-ejaculatory duct epithelium. Arch Pathol Lab Med. 1999;123(11):1093–7. doi: 10.5858/1999-123-1093-PSAEAL. [DOI] [PubMed] [Google Scholar]
- 12.Bellina M, Mari M, Ambu A, Guercio S, Rolle L, Tampellini M. Seminal monolateral nerve-sparing radical prostatectomy in selected patients. Urol Int. 2005;75(2):175–80. doi: 10.1159/000087174. [DOI] [PubMed] [Google Scholar]
- 13.John H, Hauri D. Seminal vesicle-sparing radical prostatectomy: a novel concept to restore early urinary continence. Urology. 2000;55(6):820–4. doi: 10.1016/s0090-4295(00)00547-1. [DOI] [PubMed] [Google Scholar]
- 14.Sanda M, Dunn R, Wei J. Seminal vesicle sparing technique is associated with improved sexual HRQOL outcome after radical prostatectomy. J Urol. 2002;167:A606. [Google Scholar]
- 15.Albers P, Schäfers S, Löhmer H, de Geeter P. Seminal vesicle-sparing perineal radical prostatectomy improves early functional results in patients with low-risk prostate cancer. BJU Int. 2007;100(5):1050–4. doi: 10.1111/j.1464-410X.2007.07123.x. [DOI] [PubMed] [Google Scholar]
- 16.Jacob R, Hanlon AL, Horwitz EM, Movsas B, Uzzo RG, Pollack A. Role of prostate dose escalation in patients with greater than 15% risk of pelvic lymph node involvement. Int J Radiat Oncol Biol Phys. 2005;61(3):695–701. doi: 10.1016/j.ijrobp.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J Urol. 2004;171(3):1132–6. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 18.Peeters ST, Hoogeman MS, Heemsbergen WD, Slot A, Tabak H, Koper PC, et al. Volume and hormonal effects for acute side effects of rectum and bladder during conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1142–52. doi: 10.1016/j.ijrobp.2005.03.060. [DOI] [PubMed] [Google Scholar]