Abstract
BACKGROUND
Systematic analysis of the influence of diet on the initiation and progression of prostate cancer is often difficult in human populations, for which dietary variables overlap a diversity of genetic backgrounds and social behaviors. Animal models that emulate human prostate cancer allow experimental analysis of the mechanisms of action of nutritional agents that show anti-prostate cancer activity.
METHODS
We have used an orthotopic implant model to characterize the in vivo response of androgen-sensitive LNCaP prostate tumors to three well-characterized soy dietary supplements: isoflavone depleted soy protein, soy phytochemical concentrate (SPC), and genistin.
RESULTS
In male SCID mice orthotopically implanted with the androgen-sensitive human prostate cell line LNCaP, dietary supplements of soy protein, genistin, and SPC reduced primary tumor weight by 42% (P=0.07), 57% (P < 0.05) and 70% (P < 0.005), respectively. All three soy supplements significantly increased tumor apoptosis and decrease microvessel density, with no significant change in tumor proliferation. Each supplement produced a distinct serum androgen response, with genistin producing the greatest decrease in total serum testosterone and dihydrotestosterone (DHT) (P < 0.05) and the greatest increase in testosterone to DHT ratio (P < 0.05) and soy protein the greatest decrease in bioactive androgen (P < 0.05). Only SPC significantly inhibited metastases to lymph nodes and lungs, and only SPC produced a significant increase in tumor p53 expression.
CONCLUSION
Taken together, these data suggest that the anti-prostate cancer activity of dietary soy protein, soy phytochemicals, and genistin use different molecular pathways. In addition, we have demonstrated that this animal model can be used in the design of dietary strategies for prostate cancer prevention and therapy.
Keywords: soy bioactive components, prostate cancer, orthotopic model, biomarker
INTRODUCTION
Prostate cancer is the most frequently diagnosed invasive cancer and the second leading cause of cancer related death in men in the United States. Epidemiologic studies indicate that the rate of clinically indolent prostate cancer is similar worldwide [1], while the rate of clinically significant and metastatic prostate cancer is significantly lower in Asia than in Western populations [2,3]. It has been proposed that these differences are largely due to environmental influences such as diet and nutrition rather than genetic variations [4–6], because Asian men who adopt a Western lifestyle show an increased incidence of clinically significant disease. Among dietary factors, a greater consumption of soybean products in the traditional Asian diet has been suggested to contribute to reduced risks of prostate cancer progression and mortality [3,7].
Several studies have been conducted to investigate the role of soy components on prostate cancer growth [8–11], but none on prostate cancer metastasis. Although no animal model provides all of the characteristics of the human disease, androgen-regulated growth and the potential for metastasis are two clinically important characteristics that are captured in orthotopic implant models using the SCID mouse and the LNCaP human prostate cancer cell line [12]. In addition, this model allows us to use serum prostate specific antigen (PSA) as a surrogate marker of tumor growth in response to defined soy-based dietary interventions. In the present study, we applied an orthotopic SCID-LNCaP model to evaluate the effectiveness of soy supplements as a preventive intervention in animals raised on a conventional low-soy diet. We evaluated the effects of three different dietary soybean components on prostate cancer growth and metastasis: isoflavone depleted soy protein, soy phytochemical concentrate (SPC), and genistin. Our results demonstrate that genistin and SPC supplements significantly inhibit tumor weight of orthotopic LNCaP prostate tumor. However, only SPC significantly inhibits metastasis of these LNCaP tumors to lymph nodes and lungs. Moreover, analysis of a series of serum and tissue tumor biomarkers indicates that these three soy components have mechanistically distinct activities as anti-prostate cancer agents.
MATERIALSANDMETHODS
Soy Protein and Soy Phytochemical Extracts
Three different soybean components were used as dietary supplements, and all supplements were provided by Archer Daniels Midland Co. (Decatur, IL). Isoflavone-depleted soy protein isolate (soy protein supplement) contains more than 90% protein, less than 4% fat, less than 5% ash, less than 6.5% moisture, and 0.01% soy isoflavones as aglycone equivalents. SPC contains 51.9% soy isoflavones by weight (50.8% genistein aglycone equivalents, 40.5% daidzein aglycone equivalents, and 8.7% glycitein aglycone equivalents, other phytochemicals not quantified) and genistin (the glycoside form of soy isoflavone genistein, the proposed major bioactive ingredient in soy). Genistin was purified from SPC and contains 100% soy isoflavones by weight (90.1% genistein aglycone equivalents, 9.1% daidzein aglycone equivalents, and 0.8% glycitein aglycone equivalents). In all three soy components, glycoside forms of isoflavones represented over 95% of total isoflavones. All isoflavone levels were determined by high performance liquid chromatography (HPLC) by the Archer Daniels Midland Co.
Diet Formulations and Treatment Groups
The following are the four experimental diets by Research Diets, Inc. (New Brunswick, NJ): (1) AIN-76 as the control diet; (2) AIN-76 with isoflavone-depleted soy protein in place of casein, 20% by weight, providing 20 mg of total isoflavone aglycone equivalents/kg diet; (3) AIN-76 with SPC at 0.5% of the diet, providing 1,700 mg of total isoflavone aglycone equivalents (812 mg of genistein aglycone equivalents, 648 mg of daidzein aglycone equivalents, and 139 mg of glycitein aglycone equivalents)/kg; and (4) AIN-76 with genistin at 0.14% of the diet, providing 812 mg of genistein aglycone equivalents/kg, the same amount of genistein equivalents as that in the diet 3.
Orthotopic Implantation of LNCaP Cells
Immediately before implantation, exponentially growing LNCaP cells were trypsinized and resuspended in DMEM with 10% fetal bovine serum, cell viability was determined by trypan blue exclusion, and a single cell suspension with >90% viability was used for implantation. A transverse incision was made in the lower abdomen, and the bladder and seminal vesicles were delivered through the incision to expose the dorsal prostate. LNCaP cells (2×106 cells/50 µl medium) were carefully injected under the prostatic capsule by means of a 30-gauge needle. Proper inoculation of cell suspension was indicated by blebbing under the prostatic capsule. The incision was closed by using a running suture of 5-0 silk.
Animal Study
Thirty-two 8-week-old male SCID-beige mice were purchased from Taconic (Germantown, NY) and housed in a pathogen-free environment. After 1 week of acclimation and adaptation to the AIN-76A diet, mice were randomized into four groups (in each group, n = 8) and fed one of the experimental diets for 2 weeks. Mice were then inoculated intraprostatically with LNCaP human prostate cancer cells and continued on experimental diets. Body weight and food intake were measured twice weekly. At 4 weeks and 8 weeks after tumor cell inoculation, phlebotomy was performed by accessing the retro-orbital venous plexus to obtain 100 µl of blood from each animal. Serum PSA level was measured by enzyme-linked immunosorbent assay (ELISA) assay to estimate the tumor-take rate and tumor size. The experiment was finished when the average tumor weight in the control animals reached 2–5% of the body weight (10 weeks after tumor cell inoculation). The mice were killed; primary tumors were excised and weighed. A tumor slice from each primary tumor tissue was carefully dissected and fixed in 10% buffer-neutralized formalin, paraffin-embedded, and sectioned at 4-µm thickness for histology and immunohistochemistry. Lymph nodes and lungs were harvested, fixed, paraffin-embedded, and hematoxylin and eosin (H&E) stained for metastases quantification. Blood samples were collected. All procedures with animals were reviewed and approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center according to National Institutes of Health guidelines.
Both lymph nodes and lungs developed microscopic metastases. Lymph node metastases were determined by the number of lymph nodes with tumor cells in the H&E-stained section. Lung metastases were determined by the number of micrometastases in lungs of each mouse by averaging numbers of metastases in five histologic sections that were collected equally across lungs.
Determinations of Serum Levels of PSA, Testosterone, and Dihydrotestosterone
Serum levels of total PSA were determined by ELISA assay following the procedures provided by the manufacturer (Diagnostic Systems Laboratory, Inc., Webster, TX). Serum levels of testosterone and dihydrotestosterone (DHT) were measured by enzyme immunoassay, following the procedures provided by the manufacturer (Diagnostic Systems Laboratory, Inc.)
Determinations of Serum Levels of Bioactive AR Ligand by Yeast-Based AR Transactivation Assay
The recombinant Saccharomyces cerevisiae strain used in the microbioassay, yAR-1, is constructed by transforming JC2LZ host yeast [13] with the pG1-hAR expression plasmid [14]. JC2LZ (a ade8 leu2 lys2 his3 trp1 ura3) is constructed by chromosomal integration of a single ARE-lacZ reporter gene at the ura3–52 locus. This reporter cassette contains a promoter with three copies of a 15-bp sequence (ggtacaaaatgttct) that functions as an androgen-dependent transcriptional enhancer, and this androgen response element controls transcription of a β-galactosidase coding sequence [15]. Transformation of JC2LZ with the AR expression plasmid pG1-hAR results in constitutive expression of full-length human AR (hAR) protein from a glyceraldehyde-3-phosphate dehydrogenase gene promoter. In the presence of AR ligand, AR transactivation results in ARE-lacZ transcription, which is detected and measured as β-galactosidase activity.
For each yeast AR bioassay, 1.2×105 yAR-1 yeast cells in 10 µl of medium were incubated with 10 µl of serum or standard for 5 hr, with vigorous shaking, at room temperature. All assays were performed in triplicate. The incubation was terminated by freezing at −80°C and β-galactosidase activity was measured with a 1,2-dioxetane chemiluminescent β-galactosidase substrate (Galacton-Star in Gal-Screen assay system, Tropix, Inc., Bedford MA). Yeast AR bioassay activity was defined relative to reference sera. The relative AR-ligand bioactivities were expressed as the percentage of the control group.
Determinations of Serum Soy Isoflavones and Metabolites
Extraction and analysis of isoflavonoids as aglycones from serum samples after enzymatic hydrolysis of conjugates followed a previously applied HPLC protocol [16]. Liquid chromatography photo diode array mass spectrometry (LC/PDA/MS) was carried out with a Spectra-Physics, Inc., designed quaternary solvent delivery liquid chromatography system with multiple channel diode-array detection and a quadrupole ion trap mass spectrometer model “LCQ” (Thermo Finnigan Corp., San Jose, CA). Mean interassay variability for genistein, daidzein, glycitein, equol, and O-desmethylangolensin (DMA) in serum was determined to be 8, 5, 17, 9, and 15%, respectively, for a serum concentration range between 5 and 500 nM.
In Situ Detection of Apoptotic Index
Apoptotic cells were determined by a terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay using the ApopTag in situ Apoptosis Detection System (Oncor, Inc., Gaithersburg, MD) according to our previous procedures [11,17]. The apoptotic index was expressed as the percentage of positive apoptotic tumor cells to total tumor cells.
Immunohistochemical Detection of Microvessel Density
Microvessel density (MVD) was used as a marker for tumor angiogenesis and quantified by immunohistochemical staining of Factor VIII following a previous described method [11,17].
Immunohistochemical Determination of Proliferation Index
Proliferating cell nuclear antigen (PCNA) was determined by immunohistochemical staining to quantify proliferation index, as described previously [11,17]. The proliferation index was calculated as the percentage of PCNA-positive tumor cells to total tumor cells.
Immunohistochemical Determinations of AR, p53, p21/wafl, bcl-2,Vascular Endothelial Growth Factor, and Basic Fibroblast Growth Factor
Automated immunohistochemistry followed by image analysis was applied to quantify the effects of dietary soy treatments on the expression of AR, p53, p21/wafl, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF). In brief, after deparaffinization, rehydration, and washing, the sections was soaked in 10mM citrate buffer, heated for 10 min in a microwave oven and then stained by using an automated staining machine (ES, Ventana Medical Systems, Inc., Tucson, AZ). The section was incubated with the primary antibody for 32 min and incubated with a biotinylated universal anti-mouse/rabbit IgG (Vectastain, 1:100 dilution). The section was then stained with 3-3′-diaminobenzidine, and counterstained with hematoxylin and a bluing agent by using 3-3′-diaminobenzidine Detection Kit (Ventana Medical Systems, Inc.). Digital images of five fields in each tissue section were acquired at 400× magnification with a digital camera (CoolSNAP, RS Photometrics, Tucson, AZ) mounted on a light microscope (Leica DMLS, Leica Microsystems Wetzlar GmbH, Ernst-Leitz-Strasse, Germany). True-color image analysis was performed by using IPLab 3.5 image analysis software (Scanalytics, Inc., Fairfax, VA) to quantify the percentages of positive p53, bcl-2, or p21/wafl tumor cells to total tumor cells and to measure the staining intensities of p53, bcl-2, p21/wafl, AR, bFGF, and VEGF. The staining intensities in the treatment groups were expressed as the percentages of that in the control group. Digital image capture and image analysis were performed by an experienced investigator who was not involved in the experimental design and animal experiments, thus without knowledge of the treatment group. Both positive and negative control slides were used to confirm the sensitivity and specificity of staining. The antibodies and dilutions were as follows: a mouse anti-human AR monoclonal antibody (1:50, DAKO), a mouse anti-human p53 monoclonal antibody (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), a mouse anti-human p21/wafl monoclonal antibody (1:100, Calbiochem, San Diego, CA), a mouse anti-human bcl-2 monoclonal antibody (1:50, DAKO), a rabbit anti-human bFGF polyclonal antibody (1:500, Sigma Chemical Co., St. Louis, MO), and a rabbit antihuman VEGF polyclonal antibody (1:100, Santa Cruz Biotechnology, Inc.).
Statistical Analysis
Tumor weight, average numbers of metastases in lymph nodes and lungs, and measured levels of serum and tumor biomarkers were expressed as group means ± SEM. They were initially analyzed by analysis of variance followed by Fisher’s protected least-significant difference [18] to calculate two-sided comparisons among experimental groups by use of Statview 5.0 program (SAS Institute, Inc., Cary, NC). A P value of less than 0.05 was considered as statistically significant. Linear regression was performed to test the association between changes in the biomarker levels and differences in tumor growth rate as a function of each dietary treatment. Chi-square test or Fisher’s exact test [18] was applied to test statistical significance of the effect of treatment on the incidence of metastasis to lymph nodes or lungs.
RESULTS
Effects of Dietary Soy Components on Orthotopic Growth and Metastasis of Human LNCaP Tumors
In this study, an animal model that produces orthotopic growth and metastasis of an androgen-dependent, PSA-producing LNCaP human prostate tumor was used to evaluate the effects of dietary soy products on prostate cancer growth and metastasis. In animals fed the control diet, intraprostatic implantation of LNCaP cells resulted in a 91% tumor-take rate. In addition to primary tumor development, this animal model also resulted in a 75% rate of lymph node metastasis and a 50% rate of lung metastasis. These results were consistent with intraprostatic implantation resulting in 89% tumor-take rate, 100% retroperitoneal lymph node metastases, and 40% lung metastases, reported by Sato and coworkers [12].
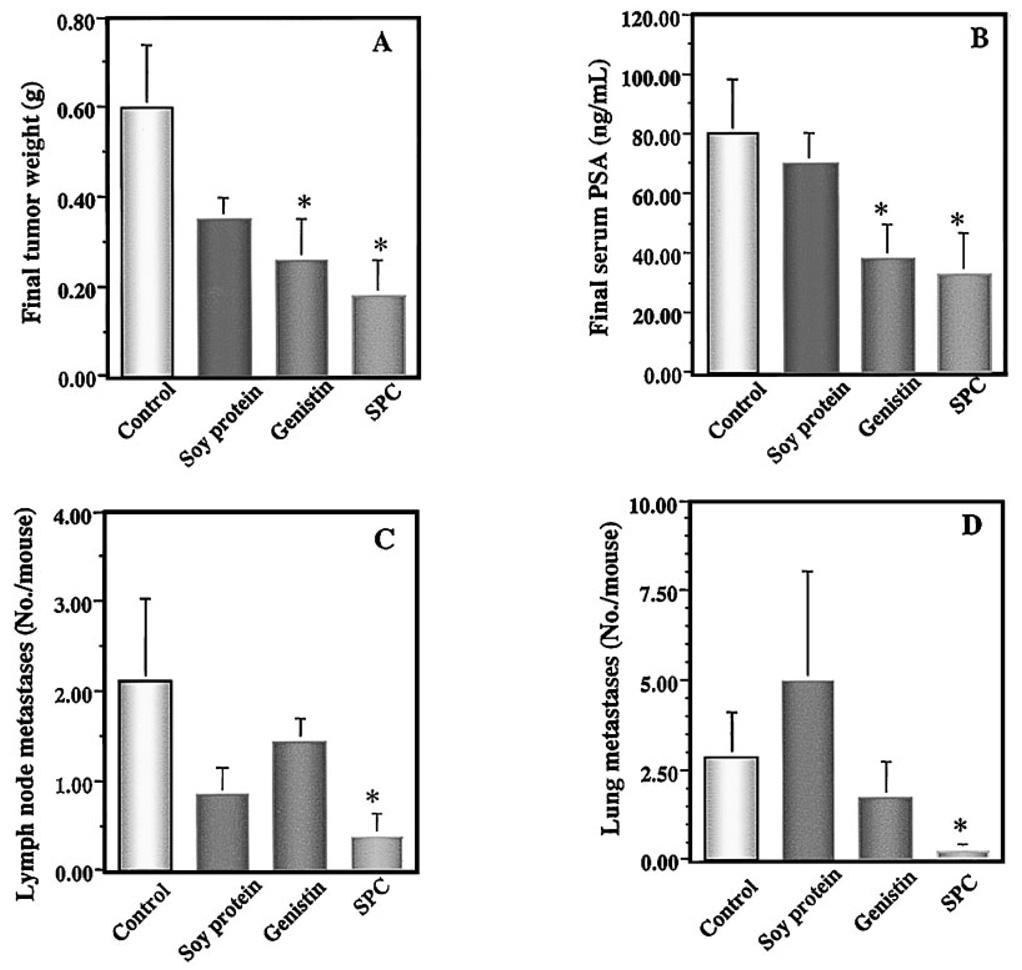
The animal experiments were terminated when mean primary tumor weight in the control group, which was estimated by serum PSA values, reached 2–5% of the body weight. The experimental dietary treatments did not significantly alter food intake or final body weight (data not shown). Final tumor weights in mice treated with soy protein, genistin, and SPC were reduced by 42% (P=0.07), 57% (P < 0.05), and 70% (P < 0.005), respectively, compared with that of the control mice treated with AIN-76 control diet (Fig. 1A). In parallel, serum levels of PSA in mice treated with soy protein, genistin, and SPC were reduced by 14% (P=0.59), 53% (P < 0.05), and 59% (P < 0.005), respectively, compared with that of the control group (Fig. 1B). Linear regression analysis indicated that there was a statistically significant positive correlation between primary tumor weight and serum PSA (R2=0.76; P < 0.0001).
Fig 1.
Effects of soy components on final tumor weight (A), serum levels of prostate specific antigen (PSA;B), lymph node metastases (C), and lung micrometastases (D) in SCID mice. Mice were treated with soy components containing experimental diets for 2 weeks, implanted intraprostatically with LNCaP cells, and continued experimental diets for10 more weeks. Dietary supplements of soy protein, genistin, and soy phytochemical concentrate (SPC) all reduced final tumor weights and final serum PSA. SPC was the only soy supplement SPC that significantly inhibited metastases to the lymph nodes and lungs. Values represent mean ± SEM. Asterisks indicate P at least <0.05 compared with the control.
In addition to its inhibitory effect on primary tumor size, the dietary supplement SPC inhibited tumor metastasis both to the lymph nodes and to the lungs. Dietary SPC significantly reduced the multiplicity of lymph node metastases by 82% (P < 0.05; Fig. 1C) and the multiplicity of lung metastases by 91% (P < 0.05; Fig. 1D) when compared with the control. Dietary SPC also reduced the incidences of LNCaP cell metastasis to lymph nodes by 67% (P < 0.05) and to lungs by 50% (P > 0.05) compared with the control. In contrast, soy protein and genistin treatments did not significantly (P > 0.05) reduce either the incidence or the multiplicity of lymph node or lung metastases (data not shown). Because primary tumor was inhibited by soy treatment, it is possible that inhibition of metastases to lymph nodes and to lungs are in part due to inhibition of primary tumor.
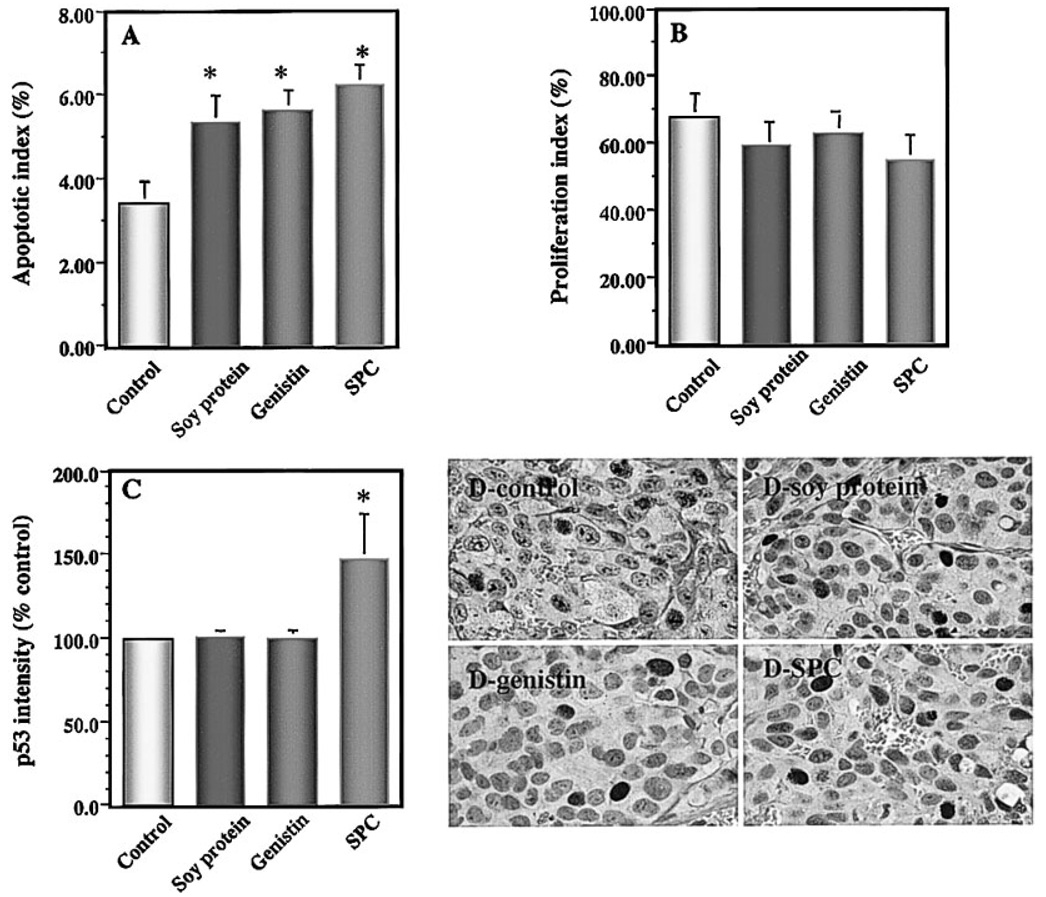
Effects of Dietary Soy Components on Apoptosis and Proliferation, and the Expression of p53 and p21/wafl of Primary Tumor
Apoptosis rates of primary tumors in mice treated with soy protein, genistin, and SPC were significantly increased by 56% (P < 0.05), 64% (P < 0.01), and 83% (P< 0.005), respectively, compared with that of the control (Fig. 2A). In contrast, proliferation rates of primary tumors in mice treated with the above diets were not significantly reduced by 13% (P=0.31), 7% (P=0.56), and 19% (P=0.17), respectively, compared with that of the control (Fig. 2B).
Fig 2.
Effects of soy components on apoptosis index (A), proliferation index (B), and the expression of p53 (C,D) in primary prostate tumors in mice. Primary tumors were determined for tumor cell apoptosis by the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay and proliferation by proliferating cell nuclear antigen staining. The expression ofp53 was determined by immunohistochemistry and quantified by image analysis. Dietary supplements of soy protein, genistin, and soy phytochemical concentrate (SPC) significantly increased tumor cell apoptosis but nonsignificantly reduced tumor cell proliferation index. Dietary supplement of SPC significantly up-regulated the expression of p53.In SPC-treated mice, p53-positive tumor cells had increased staining intensity in the nuclei. Values represent mean ± SEM. Asterisks indicate P at least <0.05 compared with the control.
The expressions of the apoptosis inducers p53 and p21/wafl and the apoptosis inhibitor bcl-2 in primary tumors were also detected by immunohistochemistry and quantified by imaging analysis, to further elucidate the possible molecular mechanisms by which dietary soy products induce tumor cell apoptosis in vivo. The soy dietary treatments did not significantly alter the percentage of p53-positive tumor cells in vivo (data not shown). In contrast, dietary SPC significantly increased the intensity of p53 protein expression by 47% (P < 0.05; Fig. 2C) compared with that of the control. No significant changes in p53 intensity were observed with the soy protein and genistin supplements. None of the dietary treatments significantly altered either the percentage of p21/wafl-positive tumor cells or the intensity of p21/wafl expression in tumor cells in vivo (data not shown). The expression of bcl-2 in the primary LNCaP tumors was very weak; therefore, the quantification could not be performed.
Effects of Dietary Soy Components on Angiogenesis and on Expression of bFGF and VEGF in the Primary Tumor
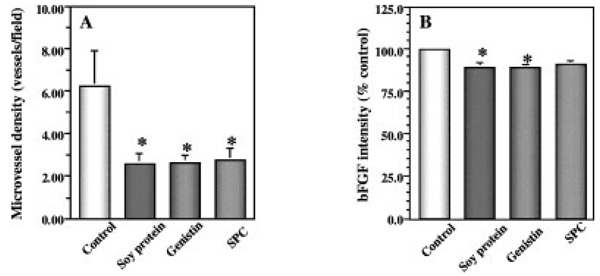
The MVD of primary tumors derived from mice treated with soy protein, genistin, or SPC diets were reduced by 58% (P < 0.01), 58% (P < 0.05), and 56% (P < 0.05), respectively, compared with that of the control (Fig. 3A). All tumor cells expressed both bFGF and VEGF, two major angiogenic factors. bFGF staining intensities in primary tumors from mice treated with soy protein, genistin, and SPC were reduced by 10.3% (P < 0.05), 10.6% (P < 0.05), and 8.6% (P=0.08), respectively, compared with that of the control (Fig. 3B). The above dietary treatments did not significantly reduce the VEGF intensity of the primary tumors (data not shown).
Fig 3.
Effects of soy components on tumor microvessel density (MVD) (A) and the expression of angiogenic factor basic fibroblast growth factor; (bFGF; B) in tumor cells in vivo. Primary tumors were determined for MVD as a parameter of angiogenesis by immunohistochemical staining for factor VIII. Dietary supplements of soy protein, genistin, and soy phytochemical concentrate (SPC) significantly reduced tumor MVD associated with reduced expression of bFGF in primary tumors. Values represent mean ± SEM. Asterisks indicate P at least <0.05 compared with the control.
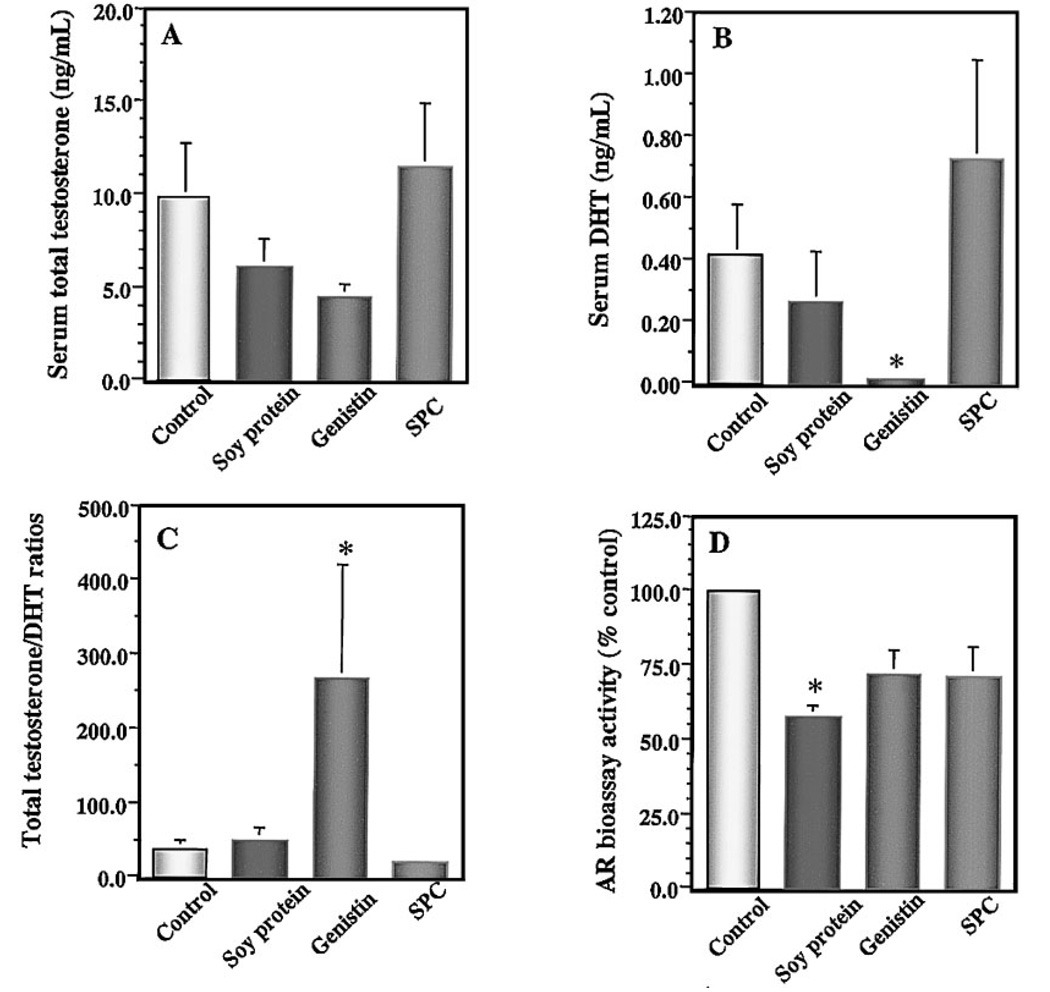
Effects of Dietary Soy Components on Serum Levels of Testosterone, DHT, Bioactive AR Ligand and the Expression of Tumor AR
A series of biomarkers were used to evaluate the effects of soy products on the modulation of serum sex hormone levels and serum AR bioactivity. Soy protein, genistin, and SPC did not significantly alter either the circulating levels of total testosterone (Fig. 4A) or tumor AR expression (data not shown). Interestingly, genistin treatment substantially reduced serum levels of DHT by 95% (P < 0.05; Fig. 4B), compared with the control group. Statistical analysis also indicated that serum levels of total testosterone and DHT in the genistin group were significantly lower than that in the SPC group (P < 0.05). Because SPC diet contained the same dietary level of genistein equivalents as that in the genistin diet, these results suggest that, although genistin is a potent soy component that down-regulates serum androgen levels, SPC may contain other soy phytochemical components that can counteract the androgen-lowering effect of genistin in vivo. Serum total testosterone to DHT ratio in genistin-treated mice was sevenfold higher than that in the control group (P < 0.05; Fig. 4C), suggesting that dietary genistin may also significantly inhibit conversion of testosterone to DHT in vivo.
Fig 4.
Effects of soy components on serum levels of testosterone (A), dihydrotestosterone ; (DHT;B), testosterone to DHT ratio (C), and AR bioassay activity (D). Serum levels of total testosterone and DHT were measured by enzyme immunoassays. The AR bioassay activity was measured by yeast-based AR transactivation assay. Dietary supplement of genistin significantly reduced serum levels of DHT and increased total testosterone to DHT ratio, compared with the controls. Dietary soy protein significantly decreased bioactive androgen in vivo. Values represent mean ± SEM. Asterisks indicate P at least <0.05 compared with the control.
Bioassay levels of serum androgen were measured to determine the effect of soybean components on the modulation of AR ligands, by using a yeast-based AR transactivation assay. It should be noted that this method assesses the net bioactivity of all AR ligands available to the cell. Mice treated with soy protein had significantly reduced serum AR bioassay activity, by 42.1% (P < 0.05) compared with the control group (Fig. 4D). In addition, serum AR bioassay activity was positively correlated with tumor weight across all of the experimental groups (R2=0.147; P < 0.05).
Effects of Soy Components on Blood Isoflavone Levels
Serum levels of soy isoflavones and their metabolites and dietary soy isoflavones were measured by HPLC. As shown in Table I, mice in the genistin and SPC groups consumed similar amounts of genistein aglycone equivalents but significantly different amounts of the aglycone equivalents daidzein (P < 0.005) and glycitein (P < 0.005). Serum levels of genistein in mice treated with genistin and SPC diets were similar (1.572 and 1.839 µM, respectively) and were consistent with dietary intakes. Whereas serum levels of daidzein and glycitin and soy isoflavone metabolites (equol and DMA) in mice treated with SPC diet were 8- to 20-fold higher than that in mice treated with genistin diet. In particular, the serum level of equol in SPC group was fivefold higher than that of genistein or daidzein, the two abundant soy isoflavones in soy products.
TABLE I.
Dietary and Serum Levels of Soy Isoflavones and Metabolites
| Dietary intakea (mg/day/mouse) | Serum levelsb (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Genistein | Daidzein | Glycitein | Genistein | Daidzein | Glycitein | Equol | DMA |
| Control | 0.000 ± 0.000c | 0.000 ± 0.000c | 0.000 ± 0.000c | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 |
| Soy protein (20%) | 0.029 ± 0.000c | 0.014 ± 0.000c | 0.004 ± 0.000c | 0.063 | 0.017 | 0.004 | 0.0002 | 0.014 |
| Genistin (0.14%) | 1.741 ± 0.072d | 0.128 ± 0.005c | 0.006 ± 0.000c | 1.572 | 0.205 | 0.013 | 0.721 | 0.053 |
| SPC (0.5%) | 1.885 ± 0.201d | 1.502 ± 0.160d | 0.323 ± 0.034d | 1.839 | 1.641 | 0.261 | 9.361 | 0.554 |
Values are expressed as means ± standard errors. Within a column, the differences between values not having a common superscript letter (c,d) are statistically significant. See text for two-side P values for specific comparisons.
No standard deviations are provided for serum levels of isoflavones and metabolites because only a single sample was analyzed for each group.
DISCUSSION
The progression of prostate tumors from a localized disease occurs through a cascade of biological processes, including alterations in the proliferation, cell–cell adhesion, and invasive potential of the malignant cells. This study was designed to evaluate the effects of three well-characterized components of dietary soy, soy protein, SPC, and genistin, on the progression and metastasis of human prostate cancer. We used a xenograft mouse model, in which LNCaP was implanted orthotopically in an immune deficient (SCID) male host, because recent studies have shown that this model retains three important characteristics of the human disease: androgen sensitivity, PSA production, and frequent progression to metastasis [12]. In this study, the three soy dietary supplements were evaluated as potential interventions in adult animals that were raised on conventional diets. To the best of our knowledge, this is the first report in which this preclinical animal model has been used in the evaluation of the effect of nutritional manipulations on prostate cancer progression and metastasis.
Of the three soy dietary supplements, SPC was the most effective in inhibiting prostate tumor weight and metastasis in the orthotopic SCID-LNCaP model. SPC is a rich source of soy isoflavones, such as genistein, daidzein, and glycitein, and other soy phytochemicals that are typically found in soy products or foods. Although genistein alone showed potent anti-prostate cancer activity in this model, that serum levels of genistein in mice treated with genistin and SPC diets were similar and were consistent with dietary intakes suggests that other soy phytochemical components are also important agents in inhibiting primary tumor growth. Moreover, SPC showed more potent anti-metastasis activity than genistin and soy protein. We do not know which component(s) of SPC are responsible for metastasis-suppression, but its identification is an important goal for future studies. It is interesting to find that dietary soy protein supplement also reduced tumor growth by 42%. Because the soy isoflavone level in soy protein diet is only 1/50 to 1/100 of that in genistin or SPC diet, the anti-growth effect of soy protein is not likely due to its isoflavone content but rather to soy protein or other unidentified soy components.
By using subcutaneous implantation of LNCaP cancer cells in SCID mice, we previously found that SPC and SPC plus soy protein significantly inhibited LNCaP tumor size by 30% (P < 0.05) and 40% (P < 0.005), respectively [11]. By using orthotopic LNCaP tumor model in this study, we found that SPC significantly inhibited tumor size by 70% (P < 0.005). These different responses are mainly due to use of different tumor models. These observations support that an orthotopic tumor model is more relevant than a subcutaneous tumor model for determining efficacy of treatment in vivo, because dietary treatment may alter organ-specific microenvironment that is critical for evaluation.
In this study, we applied automated immunohistochemistry and image analysis for the quantification of the expression of molecular markers in vivo. Several lines of evidence show that angiogenesis is essential for the growth of solid tumors and their metastases [19]. The angiogenic phenotype is the result of a net balance between these positive and negative regulators of neovascularization. Our results suggest that soy bioactive components inhibit the progression of LNCaP human prostate tumors in part by inhibiting the formation of new blood vessels. Soy protein, genistin, and SPC only moderately reduced tumor expression of the positive angiogenic factor bFGF, whereas VEGF expression was unchanged. This moderate reduction in tumor bFGF may contribute to the inhibitory effects of soy components on tumor angiogenesis. In addition, bioactive soy components may also inhibit angiogenesis by inducing the expression of angiogenesis inhibitors and/or by interfering with receptor signaling by means of bFGF or VEGF receptor tyrosine kinase activities. Genistein has been shown to be a protein tyrosine kinase inhibitor in vitro [20], and further analysis of the expression and/or phosphorylation of bFGF and VEGF receptors and on the expression of angiogenic inhibitors may provide important evidence of the molecular mechanisms by which bioactive soy components inhibit tumor angiogenesis in vivo.
In addition to anti-angiogenic activity, each of the soy dietary components inhibited the growth of human prostate tumors in vivo by means of induction of apoptosis. The underlying molecular mechanisms of apoptosis are complex, regulated by a network of inducers and repressors. p53 and p21/waf1 have been extensively studied as inducers of tumor cell apoptosis and bcl-2 as an important apoptosis repressor. It has been reported that genistein induces apoptosis of several types of tumor cells in vitro, including melanoma cells [21,22], breast cancer cells [23,24], and prostate cancer cells [25,26], by means of induction of p21/waf1 [21]. Genistein also induces apoptosis of human breast cancer cells in vitro by inactivating bcl-2 and inducing the expression of p53 [27]. However, the molecular mechanisms by which bioactive soy components inhibit the growth of human prostate tumors and induce apoptosis in vivo have not been described. We found that dietary SPC significantly increased the expression intensities of p53 in p53-positive cells in vivo, whereas dietary soy protein and genistin did not. LNCaP cells contain wild-type tumor suppressor p53; therefore, the increase of p53 expression intensity in SPC-treated LNCaP tumors suggests that soybean contains phytochemical components other than genistin that induce p53 expression in vivo. Our data suggest that the molecular mechanisms by which SPC induces apoptosis of LNCaP tumors in vivo differs from that of genistein and soy protein apoptosis induction. The detailed in vivo molecular mechanisms which underlie the observed induction of prostate tumor apoptosis by these three soy dietary supplements remain to be determined.
Androgens are a prerequisite for the development of both benign prostatic hyperplasia and prostate cancer. In the prostate, testosterone is rapidly and irreversibly converted to a more biologically active metabolite DHT by catalysis of 5α-reductase. The administration of 5α-reductase inhibitors inhibits rat prostate carcinogenesis [28], and men with prostatic cancer have an increased conversion rate of testosterone to DHT [29]. We observed that soy components inhibited LNCaP tumor growth without significant alterations of serum levels of testosterone. On the other hand, mice treated with genistin showed a significant decrease in serum DHT and an increase in the testosterone to DHT ratio, suggesting that genistin may inhibit conversion of testosterone to DHT, presumably by inhibiting 5α-reductase activity in vivo. The absence of such inhibition with SPC dietary supplements suggest that other soy phytochemicals in SPC can counteract genistin’s androgen-modulating effects.
We used a yeast-based bioassay to determine the effects of soy dietary supplements on the net level of AR ligand in serum and found that the serum levels of bioactive AR ligand were positively correlated with tumor weight across all of the experimental groups (R2=0.147; P < 0.05). Of particular interest, soy protein- treated mice showed the most potent effect, with a reduction of AR-ligand bioactivity of 42% (P < 0.05). These results suggest that soy protein and/or some components associated with soy protein inhibit the growth of androgen-sensitive LNCaP tumors in part by competing with androgens in AR binding in vivo.
In summary, we have applied a clinically relevant orthotopic prostate tumor model to evaluate the effects of soy protein, genistin, and soy phytochemicals on tumor growth and metastasis of androgen-sensitive human prostate tumors and modulation of tumor biomarkers. We observed that the soy dietary supplement SPC significantly inhibited the orthotopic growth of LNCaP human prostate tumor in mice by 70%, and this tumor inhibition was associated with reduced tumor angiogenesis and enhanced tumor cell apoptosis. SPC also significantly inhibited metastases to lymph nodes and lungs. Supplementation of soy isoflavone genistin at the same dietary level as that in SPC-containing diet also significantly inhibited the tumor growth by 57%. Genistin tumor inhibition was associated with reduced tumor angiogenesis and enhanced tumor cell apoptosis, but without significant inhibition on tumor metastasis. Dietary soy protein also inhibited the tumor growth by 42%, again with a reduction of tumor angiogenesis and enhanced tumor apoptosis but without significant inhibition of lymph node or lung metastasis.
Further in vivo mechanistic assays indicated that these three soy components inhibited the tumor growth by means of multiple and interactive molecular mechanisms. Soy phytochemicals inhibited tumor growth in vivo associated with induction of wild-type p53 expression. In vivo genistein inhibition of tumor growth is associated with inhibition of bFGF and inhibition of DHT production. In vivo soy protein inhibition of prostate tumor growth is associated with inhibition of bFGF expression and inhibition of AR bioactivity. Our studies provide evidence that soy dietary supplements are effective agents for the prevention of prostate cancer growth and metastasis in an androgen sensitive, orthotopic animal model, and support the further investigation of similar dietary interventions in human prostate cancer patients.
ACKNOWLEDGMENTS
We thank Laurie Custer, Cancer Research Center of Hawaii, for her skillful performance of liquid chromatographic assays.
Grant sponsor: Hershey Prostate Cancer Research Program (Beth Israel Deaconess Medical Center); Grant sponsor: United States Public Health Service; Grant numbers: Grants F32 CA71161, RO1 CA 78521, R21 CA 086365; Grant sponsor: The Harvard Clinical Nutrition Research Center; Grant sponsor: NIH; Grant number: P30 DK40561.
REFERENCES
- 1.Yatani R, Kusano I, Shiraishi T, Hayashi T, Stemmermann GN. Latent prostatic carcinoma: pathological and epidemiological aspects. Jpn J Clin Oncol. 1989;19:319–326. [PubMed] [Google Scholar]
- 2.Parkin DM, Muir CS. Cancer incidence in five continents. Comparability and quality of data. IARC Sci Publ. 1992;120:45–173. [PubMed] [Google Scholar]
- 3.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 4.Mandel JS, Schuman LM. Epidemiology of cancer of the prostate. Rev Cancer Epidemiol. 1980;1:1–65. [Google Scholar]
- 5.Boyle P, Kevi R, Lucchuni F, La Vecchia C. Trends in diet-related cancers in Japan: a conundrum? Lancet. 1993;342:752. doi: 10.1016/0140-6736(93)91748-b. [DOI] [PubMed] [Google Scholar]
- 6.Morton MS, Griffiths K, Blacklock N. The preventive role of diet in prostatic disease. Br J Urol. 1996;77:481–493. doi: 10.1046/j.1464-410x.1996.09361.x. [DOI] [PubMed] [Google Scholar]
- 7.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 8.Pollard M, Wolter W. Prevention of spontaneous prostate-related cancer in Lobund-Wistar rats by a soy protein isolate/isoflavone diet. Prostate. 2000;45:101–105. doi: 10.1002/1097-0045(20001001)45:2<101::aid-pros3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Aronson WJ, Tymchuk CN, Elashoff RM, McBride WH, McLean C, Wang H, Heber D. Decreased growth of human prostate LNCaP tumors in SCID mice fed a low-fat, soy protein diet with isoflavones. Nutr Cancer. 1999;35:130–136. doi: 10.1207/S15327914NC352_6. [DOI] [PubMed] [Google Scholar]
- 10.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–558S. doi: 10.1093/jn/132.3.552S. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J-R, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Gleave ME, Bruchovsky N, Rennie PS, Beraldi E, Sullivan LD. A metastatic and androgen-sensitive human prostate cancer model using intraprostatic inoculation of LNCaP cells in SCID mice. Cancer Res. 1997;57:1584–1589. [PubMed] [Google Scholar]
- 13.Purvis IJ, Chotai D, Dykes CW, Lubahn DB, French FS, Wilson EM, Hobden AN. An androgen-inducible expression system for Saccharomyces cerevisiae. Gene. 1991;106:35–42. doi: 10.1016/0378-1119(91)90563-q. [DOI] [PubMed] [Google Scholar]
- 14.Caplan AJ, Langley E, Wilson EM, Vidal J. Hormone-dependent transactivation by the human androgen receptor is regulated by a dnJ protein. J Biol Chem. 1995;270:5251–5257. doi: 10.1074/jbc.270.10.5251. [DOI] [PubMed] [Google Scholar]
- 15.Ham J, Thomson A, Needham M, Webb P, Parker M. Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumor virus. Nucleic Acids Res. 1998;16:5263–5276. doi: 10.1093/nar/16.12.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke AA, Custer LJ, Lynne R, Wilkens LR, Le Marchand L, Nomura AMY, Goodman MT, Kolonel LN. Liquid chromatographic analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B. 2002 doi: 10.1016/s1570-0232(02)00216-7. in press. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J-R, Mukherjee P, Gugger ET, Tanaka T, Blackburn GL, Clinton SK. The inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 1998;58:5231–5238. [PubMed] [Google Scholar]
- 18.Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2nd ed. New York: McGraw–Hill; 1980. [Google Scholar]
- 19.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1989;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 21.Casagrande F, Darbon JM. p21CIP1 is dispensable for the G2 arrest caused by genistein in human melanoma cells. Exp Cell Res. 2000;258:101–108. doi: 10.1006/excr.2000.4914. [DOI] [PubMed] [Google Scholar]
- 22.Kuzumaki T, Kobayashi T, Ishikawa K. Genistein induces p21(Cip1/WAF1) expression and blocks the G1 to S phase transition in mouse fibroblast and melanoma cells. Biochem Biophys Res Commun. 1998;251:291–295. doi: 10.1006/bbrc.1998.9462. [DOI] [PubMed] [Google Scholar]
- 23.Choi YH, Zhang L, Lee WH, Park KY. Genistein-induced G2/M arrest is associated with the inhibition of cyclin B1 and the induction of p21 in human breast carcinoma cells. Int J Oncol. 1998;13:391–396. doi: 10.3892/ijo.13.2.391. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 25.Choi YH, Lee WH, Park KY, Zhang L. p53-independent induction of p21 (WAF1/CIP1), reduction of cyclin B1 and G2/M arrest by the isoflavone genistein in human prostate carcinoma cells. Jpn J Cancer Res. 2000;91:164–173. doi: 10.1111/j.1349-7006.2000.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123–131. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- 27.Constantinou AI, Kamath N, Murley JS. Genistein inactivates bcl-2, delays the G2/M phase of the cell cycle, and induces apoptosis of human breast adenocarcinoma MCF-7 cells. Eur J Cancer. 1998;34:1927–1934. doi: 10.1016/s0959-8049(98)00198-1. [DOI] [PubMed] [Google Scholar]
- 28.Homma Y, Kaneko M, Kondo Y, Kawabe K, Kakizoe T. Inhibition of rat prostate carcinogenesis by a 5alpha-reductase inhibitor, FK143. J Natl Cancer Inst. 1997;89:803–817. doi: 10.1093/jnci/89.11.803. [DOI] [PubMed] [Google Scholar]
- 29.Meikle AW, Smith JA, Stringham JD. Production, clearance, and metabolism of testosterone in men with prostatic cancer. Prostate. 1987;10:25–31. doi: 10.1002/pros.2990100106. [DOI] [PubMed] [Google Scholar]