Abstract
Maternal obesity accentuates offspring obesity in dams bred to develop diet-induced obesity (DIO) on a 31% fat, high-sucrose, high-energy (HE) diet but has no effect on offspring of diet-resistant (DR) dams. Also, only DIO dams become obese when they and DR dams are fed HE diet throughout gestation and lactation. We assessed glucose and oleic acid (OA) sensitivity of dissociated ventromedial hypothalamic nucleus (VMN) neurons from 3- to 4-wk old offspring of DIO and DR dams fed chow or HE diet using fura-2 calcium imaging to monitor intracellular calcium fluctuations as an index of neuronal activity. Offspring of DIO dams fed chow had ∼2-fold more glucose-inhibited (GI) neurons than did DR offspring. This difference was eliminated in offspring of DIO dams fed HE diet. At 2.5 mM glucose, offspring of chow-fed DIO dams had more GI neurons that were either excited or inhibited by OA than did DR offspring. Maternal HE diet intake generally increased the percentage of neurons that were excited and decreased the percentage that were inhibited by OA in both DIO and DR offspring. However, this effect was more pronounced in DIO offspring. These data, as well as concentration-dependent differences in OA sensitivity, suggest that genotype, maternal obesity, and dietary content can all affect the sensitivity of offspring VMN neurons to glucose and long-chain fatty acids. Such altered sensitivities may underlie the propensity of DIO offspring to become obese when fed high-fat, high-sucrose diets.
Keywords: long-chain fatty acids, glucosensing, development, metabolic sensing, epigenetic
the majority of neurons in the brain require glucose to fuel the metabolic demands imposed upon them by changes in their activity (19). However, in brain areas, such as the ventromedial hypothalamic nucleus (VMN), populations of specialized glucosensing neurons also utilize glucose as a signaling molecule to alter their activity in response to changes in ambient glucose concentrations. Glucose-excited (GE) neurons increase and glucose-inhibited (GI) neurons decrease their activity as glucose levels rise (2, 11, 16, 18, 33, 42). Some of these same glucosensing neurons also respond to long-chain fatty acids such as oleic acid (OA), as signaling molecules in a glucose-dependent fashion (19). Such “metabolic sensing” neurons enable the brain to regulate glucose and energy homeostasis in the body because of their ability to sense and respond to phasic changes in peripheral metabolic substrates (2, 16, 18, 32, 34, 35, 39, 41, 46).
These regulatory processes are also affected by long-term changes in metabolic state in a manner that is often dependent upon the genotype of the individual. For example, the offspring of dams selectively bred to develop diet-induced obesity (DIO) when fed a 31% fat, high-energy (HE) diet develop increased obesity as adults when their dams are fed HE diet through gestation and lactation (3, 4, 9, 22, 36). We showed that such interactions between genotype and maternal environment directly alter the sensitivity of VMN metabolic sensing neurons to regulatory hormones such as leptin (14). These findings suggested that similar manipulations of the perinatal environment might also have a long-term effect on the responsiveness of VMN metabolic sensing neurons to the signaling properties of glucose and fatty acids.
For this reason, we used DIO and diet-resistant (DR) dams fed low-fat chow or HE diet throughout gestation and lactation to assess the interactions of maternal genotype and diet on the responses of dissociated VMN neurons in their 3- to 4-wk-old offspring to glucose and OA. We used fura-2 calcium imaging to monitor changes in intracellular calcium ([Ca2+]i) as an index of neuronal activity in response to glucose and OA (16, 18).
MATERIALS AND METHODS
Dams and breeding.
All experiments were reviewed and approved by the Animal Care and Use Committee of the East Orange Department of Veterans Affairs Medical Center and were in compliance with the guidelines of the American Physiological Society (1). All breeding pairs were derived from our in-house colonies of rats bred selectively for their propensity to develop DIO or DR (23). All rats were housed at 23–24°C on 12:12-h light-dark cycle (lights off at 1800) with food and water available ad libitum. Dams were housed in cages in which the food was placed in bins in the wire cage tops to limit access of food to the 2–3 wk old pups.
One month prior to breeding, DIO and DR dams were divided into 4 groups: 1) DR Chow (n = 7) were fed Purina rat chow ad libitum which contains 3.30 kcal/g with 23.4% as protein, 4.5% as fat, and 72.1% as carbohydrate (27). 2) DR HE (n = 5) were fed a high-energy (HE) diet (no. C11024F; Research Diets, New Brunswick, NJ), which contains 4.47 kcal/g with 21% of the metabolizable energy as protein, 31% as fat, and 48% as carbohydrate, 50% of which is sucrose (27). 3) DIO Chow (n = 7) were fed Purina rat chow; and 4) DIO HE (n = 5) were fed HE diet. After 1 mo on their respective diets, DR HE and DIO HE dams underwent tail bleeding for plasma glucose, insulin, and leptin levels. Because we have previously shown that DIO rats do not develop obesity until the caloric density and fat content of their diet are increased, we took blood samples only from dams fed HE diet (22). All dams were then mated with males of the same genotype and were kept on their respective diets throughout gestation and weaning. A second blood sample was drawn on the 2nd wk of gestation for all dams on the HE diet. At birth, all litters were adjusted to 10 pups (5 males: 5 females). The final blood drawing was carried out in DR and DIO HE dams at 2 wk into the lactation period. The morphometric data from the dams used here (as provided in Table 1) were previously reported (14). The data for the pups used in the current studies were litter mates of those in that prior paper and have not been previously reported.
Table 1.
Body weights, plasma glucose, leptin, and insulin levels of DR and DIO dams fed HE diet during gestation and lactation
| DR HE | DIO HE | |
|---|---|---|
| Initial body weight, g | 243±7.00 | 315±7.05* |
| One month of HE diet | ||
| Body weight, g | 261±10.0 | 385±9.45* |
| Body weight gain, g | 15.0±3.60 | 69.7±4.06* |
| Glucose, mg/dl | 124±3.70 | 132±5.49 |
| Leptin, ng/ml | 4.38±1.40 | 9.90±1.88* |
| Insulin, ng/ml | 0.91±0.18 | 3.26±0.22* |
| Second week of gestation | ||
| Body weight, g | 307±11.0 | 433±15.9* |
| Body weight gain, g | 45.0±5.40 | 48.8±4.95 |
| Glucose, mg/dl | 121±8.50 | 133±4.82 |
| Leptin, ng/ml | 5.00±1.02 | 9.22±0.86* |
| Insulin, ng/ml | 1.79±0.45 | 5.39±0.17* |
| Second week post gestation | ||
| Body weight, g | 293±5.90 | 350±8.03* |
| Body weight gain, g | −20.0±5.80 | −58.0±2.00* |
| Glucose, mg/dl | 125±6.30 | 135±9.21 |
| Leptin, ng/ml | 1.28±0.23 | 2.88±0.59* |
| Insulin, ng/ml | 0.43±0.08 | 0.51±0.07 |
Values are presented as means ± SE. Diet-resistant dams (DR) and diet-induced obesity (DIO) dams were fed high-energy (HE) diet (n = 5/group) for 1 mo before breeding and continued on HE diet during gestation and lactation. Body weight gain represents the changes in body weight during 1 mo on HE diet, the first 2 wk of gestation and from gestation to second week of lactation, respectively.
P < 0.05 when data from DIO dams were compared to that of DR dams (data are from Ref. 14).
Pups and diet manipulations.
At 3 wk of age, all male pups were weaned to the diet groups of their respective dams. After 2–3 days on their respective diets, 1 male pup/dam was used to assess effects of increasing concentrations of OA on dissociated VMN neurons. Pups might also have accessed food crumbs dropped by their dams prior to weaning so, although we refer to the effects of “maternal diet” below, it is likely that pups did have some intake of the same diets of their dams for a week or more prior to being studied.
Measurement of glucose- and OA-induced changes in [Ca2+]i concentrations in dissociated VMN neurons.
The VMN was punched out of slices made through the ventrobasal hypothalamus, and single VMN neurons were dissociated by papain digestion and trituration, as previously described (16, 18, 29). Evaluation of glucose- and OA-induced alterations in [Ca2+]i oscillations in individual VMN neurons derived from bilateral VMN punches was assessed using fura-2 acetoxy-methyl ester (Molecular Probes, Eugene, OR), as previously described (18, 19). Neurons were classified as GE, GI, and nonglucosensing using previously established criteria for glucose-induced changes in [Ca2+]i area under the curve (AUC).
Criteria for quantitating changes in [Ca2+]i fluctuations in response to OA.
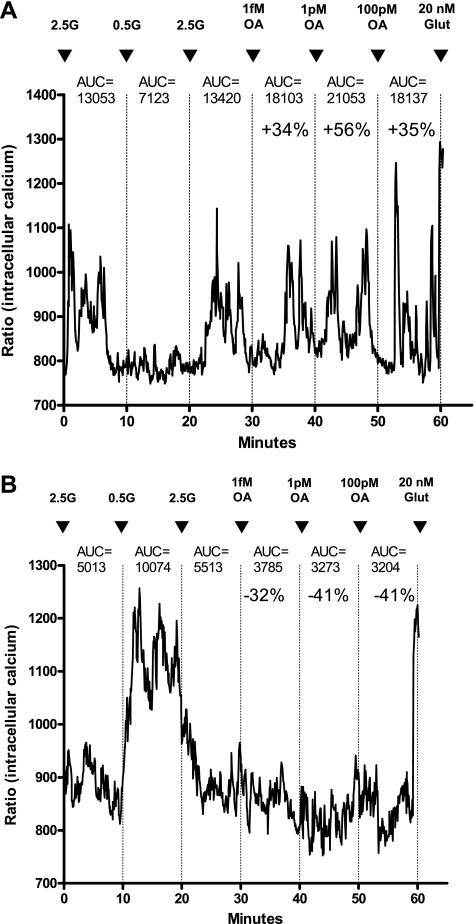
All experiments began with neurons held at 2.5 mmol/l glucose unless otherwise specified, as this concentration is equivalent to brain levels in the postprandial state (40). Changes in [Ca2+]i fluctuations in response to glucose and OA were assessed over 10-min periods after the addition of each substance. Significant changes in [Ca2+]i fluctuations were determined by first calculating the integrated AUC for every 10-min period using Origin 7.0 software (OriginLab, Northampton, MA). As previously described (19), the neurons were then classified as OA-excited (OAE) or OA-inhibited (OAI) when [Ca2+]i oscillations increased or decreased by ≥30% relative to the previous condition (Fig. 1). Other neurons demonstrated a biphasic response to increasing concentrations of OA such that the AUC for [Ca2+]i oscillations increased or decreased by >30% during exposure to low concentrations and reversed that pattern on exposure to higher concentrations. Neurons that did not meet these criteria were classified as OA nonresponsive. Concentration-dependent responses were determined by first characterizing the neurons as either GE or GI by their responses to changes in glucose from 2.5 to 0.5 to 2.5 mM glucose (18), and then neurons were next exposed to sequential addition of increasing doses of OA from 0.1 fM to 500 nM for DIO and DR rats on chow diet and 1 fM, 1 pM, and 100 pM of OA for DIO and DR on HE diet. All neurons were incubated with 20 nM glutamate terminally to ensure viability (19).
Fig. 1.
Representative changes in [Ca2+]i oscillations following exposure to incremental concentrations of oleic acid (OA) in freshly dissociated ventromedial hypothalamic nucleus (VMN) neurons from 3- to 4-wk-old male rats. The neurons were first characterized by their glucosensing category in response to altering glucose concentrations from 2.5 (2.5G) to 0.5 (0.5G) to 2.5 mmol/l (2.5G) followed by two or more concentrations of OA. Neurons were tested terminally with 20 nmol/l glutamate (Glut) to ascertain viability. A: Glucose-excited/OA excited neuron showing progressively increasing [Ca2+]i oscillations at 1 fM and 1 pM with a decrement at 100 pM OA; B: glucose-inhibited/OA-inhibited neuron showing decreasing [Ca2+]i oscillations at 1 fM to 1 pM with a plateau at 100 pM OA. Areas under the curve (AUC) for [Ca2+]i oscillation changes relative to the previous 10 min AUC values in response to changes in conditions are given over each 10-min segment of tracing.
Plasma glucose, leptin, and insulin assays.
Plasma from tail blood samples from DR and DIO dams fed chow or HE diet during gestation and lactation were assayed for glucose by automated glucose oxidase method (Beckman Coulter, Fullerton, CA). Insulin and leptin levels were analyzed by RIA (Linco, St. Charles, MO) using antibodies to authenticate rat insulin and leptin, respectively.
Statistical analysis.
Comparisons of body weights, plasma hormones, and glucose levels for the dams were carried out by two-way ANOVA (genotype, diet). Glucosensing properties of OA-responsive neurons were compared using two-way ANOVA. In offspring, the percentage of OAE and OAI neurons and the percent changes in AUC (an index of OA sensitivity) were compared by two-way ANOVA (genotype, maternal diet). When significant differences were found by two-way ANOVA, further comparisons were made by one-way ANOVA with post hoc Bonferroni corrections (P < 0.05). All data are expressed as means ± SE. (GraphPad Prism, La Jolla, CA).
RESULTS
Dam body weight gain and plasma hormone levels.
These data for dams fed an HE diet during gestation and lactation (Table 1) were previously reported (14). In this study, we did not assess the body weights or other morphometric parameters from DR and DIO dams fed chow since we have made such comparisons in previous studies. Those studies showed that DR and DIO chow-fed dams gain comparable amounts of body weight and have comparable levels of leptin, insulin, and carcass adiposity during gestation and lactation (13, 26). On the other hand, in this study, after 1 mo on the HE diet, DIO dams gained 367% more weight, had 126% higher plasma leptin and 258% higher insulin levels than did DR dams. At 2 wk of gestation, DIO dams gained comparable amounts of weight, had 84% higher plasma leptin and 201% higher insulin levels than did DR dams. Two weeks after delivery, DIO dams lost 190% more body weight than did DR dams. However, DIO dams remained significantly heavier and had 125% higher plasma leptin, but not insulin levels than did DR rats. Glucose levels did not differ significantly between the two groups during gestation or lactation (Table 1). Finally, we did not assess the effects of genotype × maternal diet on offspring body weights here. However, we previously showed that, although DIO offspring are heavier than DR offspring, these factors have no effects on offspring birth weight (13) or carcass adiposity at weaning (26, 37).
Effect of maternal genotype and diet on responses to glucose in VMN neurons.
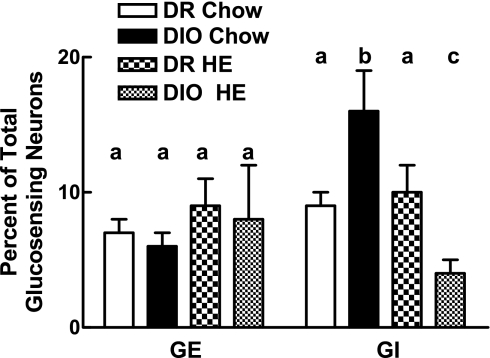
Overall, we assessed the physiological properties of 655 and 944 neurons from DIO and DR offspring of chow-fed (n = 7/genotype) dams and 361 and 324 neurons from DIO and DR offspring of dams fed HE diet (n = 5/genotype), respectively (Fig. 2). The major difference among the groups was that offspring of DIO chow-fed dams had almost 2-fold more GI neurons than did DR offspring (P < 0.05; Fig. 2). This difference was eliminated in offspring of HE diet-fed DIO dams where the percentage of GI neurons were lower than all other genotype and diet groups (P < 0.05; Fig. 2).
Fig. 2.
Dissociated VMN neurons from offspring of diet-induced obese (DIO) and diet-resistant (DR) dams fed chow or HE diet (n = 5–7 rats/group) through gestation and weaning were categorized as being glucose excited (GE) or glucose inhibited (GI) using calcium imaging. Total number of neurons analyzed for a given group is as follows: DR Chow = 944, DIO Chow = 655, DR HE = 324, DIO HE = 361. Data are expressed as means ± SE percentage of neurons in each group. For all of the data in the figure, bars with differing letters differ from each other by P = 0.05 or less by Bonferroni post hoc test after intergroup differences were found by two-way ANOVA.
Effect of maternal genotype and diet on responses of offspring VMN glucosensing neurons to OA.
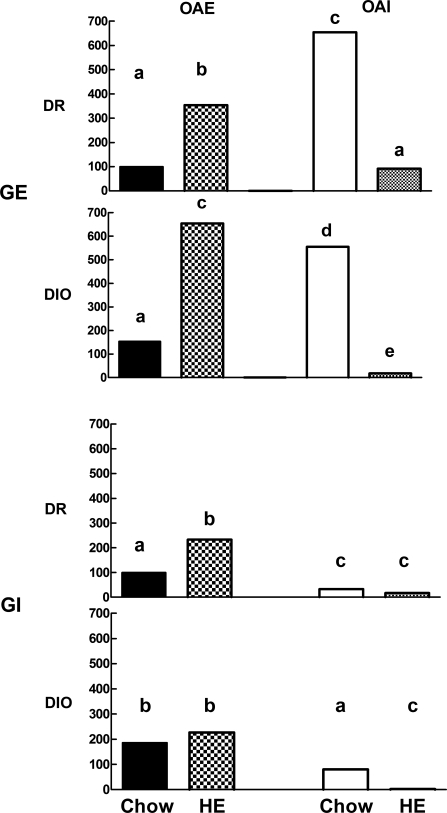
We previously showed that the responses to OA of VMN-glucosensing neurons were highly glucose dependent (19). Thus, we specifically assessed the effects of OA on VMN GE and GI neurons at 2.5 mM glucose and at OA concentrations from 0.1 fM to 500 nM (Figs. 1 and 3, Table 2). In DIO and DR offspring of chow-fed dams, OA excited 229% and 172% more GI than GE neurons, respectively (P < 0.05; Table 2). This preponderance of OA-induced excitation in GI neurons is likely due to the fact that GI neurons are mainly inhibited, whereas GE neurons are mostly activated at 2.5 mM glucose (11, 16, 18). Just as DIO offspring had more GI neurons, they also had 86% more GI neurons that were excited by OA than did DR offspring (P < 0.05; Table 2).
Fig. 3.
Dissociated VMN neurons from offspring of DIO and DR dams fed chow or HE diet through gestation and weaning (n = 5–7 rats/group) were categorized using calcium imaging as being GE or GI and then as being excited (OAE) or inhibited (OAI) by 15 mM oleic acid. This figure summarizes the data presented in Table 2, where each bar represents the mean percentage of GE or GI VMN neurons that were OAE or OAI in offspring of chow- and HE diet-fed DR vs. DIO dams. Bar heights are expressed as the mean percent of the DR chow-fed OAE neurons for all GE neurons (top two bar graphs) and all GI neurons (bottom two bar graphs). Bars for GE neurons from DIO and DR offspring with differing letters differ from each other by P = 0.05 or less. Similarly, bars for GI neurons from DIO and DR offspring with differing superscripts differ from each other by P = 0.05 or less.
Table 2.
Effect of genotype and maternal diet on VMN neuronal fatty acid sensing as a function of glucosensing neuron type
| OAE-C | OAE-HE | OAI-C | OAI-HE | |
|---|---|---|---|---|
| Glucose excited | ||||
| DR | 11±4a (13/83) | 39±10b (17/34) | 72±7a (55/83) | 10±8b (8/34) |
| DIO | 17±5a (9/52) | 71±1c (18/26) | 61±12a (28/52) | 2±2b (2/26) |
| Glucose inhibited | ||||
| DR | 30±3b (29/95) | 70±5c (24/31) | 10±5b (6/95) | 5±2b (4/31) |
| DIO | 56±12c (61/122) | 68±9c (21/30) | 24±5c (32/122) | 0b (0/30) |
Data are expressed as means ± SE percent of neurons in each category. The number of subtype neurons found is indicated in parenthesis as a function of the total number of neurons assessed in each group. DIO and DR dams were fed chow (C) or HE diet (HE) through gestation and lactation and dissociated ventromedial hypothalamic nucleus (VMN) neurons from their 3- to 4-wk-old offspring (5–7/group) were characterized as being glucose excited or glucose inhibited by their responses to altering glucose concentrations from 2.5 to 0.5 to 2.5 mM glucose. The percentage of those neurons from each group that were oleic acid excited (OAE) or oleic acid inhibited (OAI) by 15 nM OA was then determined. Intergroup differences were assessed by two-way ANOVA and then post hoc Bonferroni correction. For OAE neurons from each genotype, diet and glucosensing neuron type, data with differing letter superscripts differ from each other by P = 0.05 or less. The same holds true for OAI neurons, which were considered separately for purposes of statistical analysis.
As opposed to neurons excited by OA, offspring of chow-fed DIO and DR dams had 154% and 620% more GE than GI neurons that were inhibited by OA (P < 0.05; Fig. 3, Table 2). Again, this preponderance of OA inhibited GE neurons at 2.5 mM glucose is likely due to the fact that GE neurons are mostly activated, while GI neurons are predominantly inactivated at this glucose concentration. While DIO and DR offspring had comparable numbers of GE neurons inhibited by OA, DIO offspring had 140% more GI neurons inhibited by OA than did DR offspring (P < 0.05; Fig. 3, Table 2).
Maternal intake of HE diet markedly altered the responses of VMN neurons to OA in DIO and DR offspring.
Overall, it increased the percentage of neurons that were excited and decreased the percentage of neurons that were inhibited by OA (Fig. 3; Table 2). In their GE neurons, the effect was more marked in DIO than DR offspring (P = 0.05; Fig. 3, Table 2). Compared with comparable offspring of chow-fed dams, OA excited 317% more GE neurons in DIO and 254% more in DR offspring of dams fed an HE diet, respectively. For GI neurons, the effect of maternal HE diet intake was selective to DR offspring, where the percentage of OA-excited neurons was increased by 133%. In contrast, maternal intake of HE diet had the opposite effect on GE and GI neurons that were inhibited by OA where there were few detectable neurons of either type that were inhibited by OA in either DIO or DR offspring (Fig. 3, Table 2).
In addition to assessing the percentage of neurons that were excited or inhibited by OA, we also compared their concentration-dependent sensitivity to OA (Fig. 4, A and B). VMN GE neurons from offspring of chow-fed DIO dams were less sensitive to both the excitatory and inhibitory effects of OA than were those from DR offspring [F(2,26) = 5.209; P = 0.013]. Maternal intake of HE diet specifically increased the sensitivity of these same neurons in DIO offspring to OA [F(2,26) = 24.327; P < 0.0001; Fig. 4, A and B]. On the other hand, maternal intake of HE diet selectively decreased the sensitivity of GI neurons to the excitatory and inhibitory effects of OA selectively in DIO offspring [F(2,26) = 4.111; P = 0.037; Fig. 4, C and D].
Fig. 4.
Dissociated VMN neurons from offspring of DIO and DR dams fed chow or HE diet (n = 5–7 rats/group) through gestation and weaning were categorized using calcium imaging as being GE (A, B) or GI (C, D). They were then categorized as being excited (OAE) (A, C) or inhibited (OAI) (B, D) and their sensitivity to OA assessed by the percent change in [Ca2+]i oscillation AUC produced by successive exposure to 1 fM, 1 pM, and 100 pM OA. Data are expressed as means ± SE. Data points with differing letters differ from each other by P = 0.05 or less by Bonferroni post hoc test after intergroup differences were found by two-way ANOVA with repeated measures.
DISCUSSION
We previously showed that feeding DIO dams an HE diet during gestation and lactation makes them obese and that their offspring becomes more obese as adults than do offspring of either chow-fed DIO dams or offspring of DR dams fed chow or an HE diet (22). On the other hand, these offspring of obese DIO dams do not weigh more than any of the other groups of offspring at weaning (13), the age at which VMN neurons were evaluated here for the interactions of genotype and maternal diet on the sensitivity to both glucose and OA. Overall, offspring of chow-fed DIO dams had almost two times more GI neurons and, when held at 2.5 mM glucose, more of their GI neurons were excited and inhibited by OA than were those of comparable DR offspring. On the other hand, while offspring of chow-fed DIO and DR dams had the same proportions of GE neurons that were excited or inhibited by OA, DIO GE neurons were less sensitive to the excitatory and inhibitory effects of increasing concentrations of OA than were those of DR offspring. Thus, in offspring of chow-fed dams, DIO offspring had a larger proportion of GI neurons and more of these GI neurons were responsive to OA, while their GE neurons were less sensitive to the effects of OA.
As expected (26), feeding DIO dams an HE diet during gestation and lactation made them obese and left DR dams relatively lean. A combination of HE diet intake by the dams, and by the pups themselves during the latter stages of lactation, markedly altered the responses of their VMN neurons to both glucose and OA. HE diet intake produced a selective, four-fold reduction in the proportion of detectable GI neurons in DIO offspring without affecting GE neurons in DIO or DR offspring. It is possible that no such effect was seen in DR offspring because of the small number of GI neurons present in their chow-fed offspring. HE diet intake also increased the overall percentage of both GE and GI neurons that were excited by OA and decreased the percentage that were inhibited by OA in DIO and DR offspring. This effect was more marked for GE than GI neurons, particularly for GE neurons in DIO offspring. Finally, HE diet intake increased the sensitivity of GE neurons to the excitatory and inhibitory effects of OA in DIO offspring but decreased the sensitivity to the excitatory effects of OA in their GI neurons.
These results have several potential implications. First, the VMN is a critical site for the regulation of both energy homeostasis (10) and the counterregulatory responses to hypoglycemia (5, 6, 45). We previously showed that DIO rats have abnormalities of both these regulatory processes (14, 24, 25, 44). Because the major differences in glucosensing neurons between DIO and DR rats here was in the proportion of GI neurons, this suggests that these specific neurons might be important contributors to these abnormalities in DIO rats. Second, the current results reinforce our previous demonstration of the critical interrelationship between glucose and fatty acid acting as signaling molecules in metabolic sensing neurons of the VMN (19). Finally, they also illustrate the marked effect that the interactions among genotype, diet, and obesity have on the way in which these critical regulatory neurons respond to both glucose and fatty acids.
Given the well-described effects of glucose on activating GE and inactivating GI neurons (2, 11, 16, 18, 33, 42), it was not surprising to find that OA inhibited more GE and excited more GI neurons when they were held at 2.5 mM glucose (16, 18). On the other hand, maternal HE diet intake had it greatest impact on the OA responses of GE neurons held at 2.5 mM glucose. This effect was especially marked in DIO offspring, which had greater percentages of GE neurons that were excited or inhibited by OA and increased sensitivity of these GE neurons to OA. Thus, while maternal intake of the moderately high-fat, high-sucrose HE diet affected fatty acid sensing in both DIO and DR offspring, the greatest impact was on DIO GE neurons. We previously showed that feeding DIO dams an HE diet makes them obese and selectively alters the fatty acid composition of their milk. Specifically, milk from obese DIO dams is extremely low in monounsaturated and polyunsaturated fatty acids, including OA (13). Their milk also has markedly elevated levels of insulin (13). Because pups receive most of their nutrients and much of their insulin from maternal milk during the first 1–2 wk of life (8, 38), the reduced oleic (and other) fatty acid milk content of their obese DIO dams may be an important cause of the relatively selective increases in the number and/or sensitivity of their VMN neurons to glucose and OA. These changes, in turn, might be important contributors to the increased obesity caused by fostering either DIO or DR offspring to obese DIO dams (28). Finally, because these abnormalities of maternal milk occur only in obese selectively bred DIO dams, the results here and in prior studies suggest an important genotype × diet interaction on neuronal metabolic sensing and energy homeostasis.
There are some caveats to our work. First, we used calcium imaging in dissociated neurons as a surrogate for the effects of glucose and OA on neuronal activity. Although there is not an absolute parallel between changes in glucose- and fatty acid-induced [Ca2+]i fluctuations and neuronal action potential frequency, we have shown that they do correlate well with changes in membrane potential in dissociated VMN neurons (16, 19) and with changes in action potential frequency using patch-clamp technique in hypothalamic slices (32, 42). Thus, because the use of calcium imaging in dissociated neurons provides a relatively high-throughput means of screening large numbers of neurons simultaneously, this method has proved to be very useful and is a reasonable surrogate for assessing glucose- and OA-induced changes in neuronal activity.
Second, we have previously demonstrated that the responsiveness to OA of VMN glucosensing neurons is dependent upon ambient glucose concentrations (19). However, here, we only assessed neurons here at 2.5 mM glucose. Thus, it is quite possible that additional differences between DIO and DR offspring and the effects of maternal diet would have been found if we had also assessed them at 0.5 mM glucose, the concentration of hypothalamic glucose present under fasting conditions (16, 18). Despite this omission, we believe that the current findings are sufficient to emphasize the importance of genotype and maternal diet in determining the ways in which VMN hypothalamic neurons respond to glucose and long-chain fatty acids as signaling molecules.
Finally, despite the major contribution of maternal milk to the metabolic milieu of the pups (8, 38), they also ingest some of the maternal diet themselves during the last week or so of the lactation period. Thus, the food pellets were placed on top of the cage lids where it was accessible to the pups only when they were able to climb up to the lids or eat crumbs left by their dams on the cage floor. However, it is possible that such limited exposure might have had effects independent of their exposure to maternal milk. HE diet exposure also affected VMN OA sensing in DR offspring and, because HE diet has little effect on maternal milk content or obesity in DR dams (13), it is quite possible that intake of HE diet by the DR pups themselves might have contributed to these diet-induced changes.
In conclusion, these studies confirm our previous findings (19) that VMN glucosensing neurons also utilize long-chain fatty acids such as OA as signaling molecules to alter neuronal activity in a glucose-dependent fashion. Importantly, we demonstrate for the first time that there are marked differences in the ways in which VMN neurons from DIO and DR pups respond to both glucose and OA. Furthermore, both genotype and maternal intake of a diet of moderate fat and high sucrose content interact to alter selectively this responsiveness in DIO more than in DR offspring. Given the role of both glucose and fatty acid sensing in the regulation of energy and glucose homeostasis (11, 16, 18, 20, 42, 43), these studies have major implications for our understanding of the ways in which heredity and environment interact to alter the development of the developing neonate.
Perspectives and Significance
Hypothalamic neurons respond to substrates, such as glucose and fatty acids, as signaling molecules by altering their activity (11, 15–19, 31). The mechanisms underlying the signaling effects of these substrates are largely separate from their utilization as primary substrates required to fuel the energetic needs of the neuron (30). All neurons use the high-affinity hexokinase I to initiate glycolysis and oxidation of glucose to produce ATP (30). However, a large proportion of hypothalamic glucosensing neurons also use the low-affinity enzyme glucokinase (GK) as a gatekeeper of glycolytic and oxidative flux to regulate neuronal activity (11, 16–18, 31). In GE neurons, increasing glucose levels act through GK to increase ATP/ADP levels, inactivates the ATP-sensitive K+ channel, and increases neuronal activity (30). In GI neurons, GK also acts as a critical regulator of substrate, nitric oxide, and reactive oxygen species production (7, 19), which alter neuronal activity. While glucose is the primary metabolic substrate for neurons (41), long-chain fatty acids are more important as a substrate for astrocyte metabolic activity (12). Similarly, while neuronal fatty acid sensing does require some intracellular metabolism, at least half of the signaling effects of fatty acids in VMN neurons is initiated by their binding to the fatty acid transporter-receptor, FAT/CD36 (15, 19).
Thus, differences in the function of these regulatory pathways between DIO and DR rats are likely to underlie the differences that we found here in their neuronal glucose and fatty acid sensing. For glucosensing neurons, although DIO rats have increased expression of GK in their arcuate nucleus, VMN GK levels do not differ between DIO and DR rats (11). Thus, it is more likely that some downstream portion of the regulatory pathway, such as AMP-activated protein kinase activity or nitric oxide or reactive oxygen species production might underlie the marked differences in the proportion of GI neurons between DIO and DR offspring that we found here. We know even less about the mechanisms of fatty acid sensing that might underlie the differences in the responses of VMN neurons demonstrated here between DIO and DR rats. Therefore, further exploration of the factors that affect these hypothalamic metabolic sensing neurons should provide important insights into the ways in which they affect the regulation of energy and glucose homeostasis and contribute to the growing epidemic of obesity in the world (21).
GRANTS
This work was supported by the Research Service of the Department of Veterans Affairs (to B. E. Levin and A. Dunn-Meynell), the National Institute of Diabetes, Digestive and Kidney Disorders (Grant DK-53181 to B. E. Levin), an award from the Fondation pour la Recherche Médicale, France (to C. Le Foll) and the American Heart Association (B. G. Irani).
Acknowledgments:
We thank Sunny Park for her expert technical assistance.
REFERENCES
- 1.American Physiological Society Guiding principals for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B.Activity of single neurons in the hypothalamus feeding centers: effect of glucose. Am J Physiol 207: 1146–1154, 1964 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ.The fetal and infant origins of disease. Eur J Clin Invest 25: 457–463, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ.In utero programming of chronic disease. Clin Sci 95: 115–128, 1998 [PubMed] [Google Scholar]
- 5.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI.Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99: 361–365, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg WP, Tamborlane WV, Brines ML, Shulman GI, Sherwin RS.Chronic hypoglycemia and diabetes impair counterregulation induced by localized 2-deoxy-glucose perfusion of the ventromedial hypothalamus in rats. Diabetes 48: 584–587, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH.Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 292: R1418–R1428, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF.Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab 82: 4270–4273, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Morris MJ.Differential responses of orexigenic neuropeptides to fasting in offspring of obese mothers. Obesity 17: 1356–1362, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB.Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE.Glucokinase is the likely mediator of glucosensing in both glucose excited and glucose inhibited central neurons. Diabetes 51: 2056–2065, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Deglon N, Hantraye P, Pellerin L, Bonvento G.Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci 27: 7094–7104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE.Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol 291: R768–R778, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Irani BG, Le Foll C, Dunn-Meynell AA, Levin BE.Ventromedial nucleus neurons are less sensitive to leptin excitation in rats bred to develop diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 296: R521–R527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo YH, Su Y, Gutierrez-Juarez R, Chua S., JrOleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol 101: 2305–2316, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eikis J, Zhang BB, Levin BE.Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kang L, Dunn-Meynell AA, Routh VH, Liu X, Levin BE.Knockdown of GK mRNA with GK RNA interference (RNAi) blocks ventromedial hypothalamic (VMH) neuronal glucosensing (Abstract). Diabetes 53: A43, 2004 [Google Scholar]
- 18.Kang L, Routh VH, Kuzhikandathil EV, Gaspers L, Levin BE.Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE.Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol 297: R655–R664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin BE.Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 276: R382–R387, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Levin BE.Why some of us get fat and what we can do about it. J Physiol 583: 425–430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin BE, Dunn-Meynell AA.Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol 283: R1087–R1093, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE.Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Levin BE, Dunn-Meynell AA, Banks WA.Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling prior to obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE.Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab 285: E949–E957, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Levin BE, Govek E.Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol Regul Integr Comp Physiol 275: R1374–R1379, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Levin BE, Hogan S, Sullivan AC.Initiation and perpetuation of obesity and obesity resistance in rats. Am J Physiol Regul Integr Comp Physiol 256: R766–R771, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Keesey RE.Defense of differing body weight set-points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Levin BE, Magnan C, Migrenne S, Chua SC, Jr, Dunn-Meynell AA.The F-DIO obesity-prone rat is insulin resistant prior to obesity onset. Am J Physiol Regul Integr Comp Physiol 289: R704–R711, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA.Neuronal glucosensing: What do we know after 50 Years? Diabetes 53: 2521–2528, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE.Localization of glucokinase gene expression in the rat brain. Diabetes 49: 693–700, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Migrenne S, Cruciani-Guglielmacci C, Kang L, Wang R, Rouch C, Lefevre AL, Ktorza A, Routh VH, Levin BE, Magnan C.Fatty acid signaling in the hypothalamus and the neural control of insulin secretion. Diabetes 55: 139–144, 2006 [Google Scholar]
- 33.Oomura Y, Kimura K, Ooyama H, Maeo T, Iki M, Kuniyoshi N.Reciprocal activities of the ventromedial and lateral hypothalamic area of cats. Science 143: 484–485, 1964 [DOI] [PubMed] [Google Scholar]
- 34.Oomura Y, Nakamura T, Sugimori M, Yamada Y.Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav 14: 483–486, 1975 [DOI] [PubMed] [Google Scholar]
- 35.Oomura Y, Ono T, Ooyama H, Wayner MJ.Glucose and osmosensitive neurons of the rat hypothalamus. Nature 222: 282–284, 1969 [DOI] [PubMed] [Google Scholar]
- 36.Ounsted M, Sleigh G.The infant's self-regulation of food intake and weight gain. Difference in metabolic balance after growth constraint or acceleration in utero. Lancet 1: 1393–1397, 1975 [DOI] [PubMed] [Google Scholar]
- 37.Ricci MR, Levin BE.Ontogeny of diet-induced obesity in selectively-bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 285: R610–R618, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Sanchez J, Priego T, Palou M, Tobaruela A, Palou A, Pico C.Oral supplementation with physiological doses of leptin during lactation in rats improves insulin sensitivity and affects food preferences later in life. Endocrinology 149: 733–740, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG.Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Silver IA, Erecinska M.Extracellular glucose concentrations in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14: 5068–5076, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew O, Sakaruda O, Shinohara M.The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 23: 897–916, 1977 [DOI] [PubMed] [Google Scholar]
- 42.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH.Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus (VMN). Diabetes 50: 2673–2681, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Steffens AB, Scheurink AJW, Luiten PGM, Bohus B.Hypothalamic food intake regulating areas are involved in the homeostasis of blood glucose and plasma FFA levels. Physiol Behav 44: 581–589, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Tkacs NC, Levin BE.Obesity-prone rats have pre-existing defects in their counterregulatory response to insulin-induced hypoglycemia. Am J Physiol Regul Integr Comp Physiol 287: R1110–R1115, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB.Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5: 383–393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH.The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959–1965, 2004 [DOI] [PubMed] [Google Scholar]