Abstract
Renin release from the juxtaglomerular (JG) cell is stimulated by the second messenger cAMP and inhibited by calcium. We previously showed JG cells contain a calcium sensing receptor (CaSR), which, when stimulated, decreases cAMP formation and inhibits renin release. We hypothesize CaSR activation decreases cAMP and renin release, in part, by stimulating a calcium calmodulin-activated phosphodiesterase 1 (PDE1). We incubated our primary culture of JG cells with two selective PDE1 inhibitors [8-methoxymethil-IBMX (8-MM-IBMX; 20 μM) and vinpocetine (40 μM)] and the calmodulin inhibitor W-7 (10 μM) and measured cAMP and renin release. Stimulation of the JG cell CaSR with the calcimimetic cinacalcet (1 μM) resulted in decreased cAMP from a basal of 1.13 ± 0.14 to 0.69 ± 0.08 pM/mg protein (P < 0.001) and in renin release from 0.89 ± 0.16 to 0.38 ± 0.08 μg ANG I/ml·h−1·mg protein−1 (P < 0.001). However, the addition of 8-MM-IBMX with cinacalcet returned both cAMP (1.10 ± 0.19 pM/mg protein) and renin (0.57 ± 0.16 μg ANG I/ml·h−1·mg protein−1) to basal levels. Similar results were obtained with vinpocetine, and also with W-7. Combining 8-MM-IBMX and W-7 had no additive effect. To determine which PDE1 isoform is involved, we performed Western blot analysis for PDE1A, B, and C. Only Western blot analysis for PDE1C showed a characteristic band apparent at 80 kDa. Immunofluorescence showed cytoplasmic distribution of PDE1C and renin in the JG cells. In conclusion, PDE1C is expressed in isolated JG cells, and contributes to calcium's inhibitory modulation of renin release from JG cells.
Keywords: angiotensin, calmodulin, phosphodiesterase, cAMP, adenylyl cyclase
renin is the rate-limiting enzymatic step in the production of angiotensin, a hormone that integrates cardiovascular and renal function in the control of blood pressure as well as salt and volume homeostasis (33). Renin is produced by, stored in, and released from juxtaglomerular (JG) cells, which are derived from renin progenitor cells (42) and are located in the afferent arteriole near the hilus of the glomerulus (2, 43, 35). Two main intracellular second messenger systems are known to regulate renin synthesis and secretion: the cyclic nucleotide, cyclic adenosine monophosphate (cAMP) (8, 41), and intracellular calcium (Ca) (21).
Renin secretion by the JG cells demonstrates a paradoxical inverse relationship with intracellular calcium concentration, such that millimolar changes in extracellular Ca or micromolar elevations in intracellular calcium retard renin release (1, 9, 14, 20, 22). The primary second messenger involved in the regulation of renin release is cAMP (8), and the concentration of cAMP in cells is determined by a balance between the rate of cAMP generation by adenylyl cyclases and cAMP hydrolysis by phosphodiesterases (PDE) (7, 16). We (30, 31), and also Grünberger et al. (18), have reported JG cells express calcium-inhibitable adenylyl cyclase (27). Furthermore, we have identified the JG-specific isoform-regulating renin release to be adenylyl cyclase type V (30). Thus, increases in the JG cell intracellular Ca retard the synthesis of cAMP, and therefore inhibit the release of renin from the JG cells (30, 31).
While adenylyl cyclase synthesizes cAMP, PDEs are enzymes that regulate the levels of cAMP by controlling their degradation (12). The nonselective PDE inhibitor IBMX increases renin release in isolated JG cells (18, 23, 31), suggesting PDE degradation of cAMP is an important regulating factor.
There are 11 PDE families differing in their structure, kinetics, localization, cellular expression, and inhibitor sensitivities (5). Inhibition of PDE3 (38), PDE4 (15), and PDE5 (39) increase renin release, implying participation of these isoforms in the regulation of cAMP levels, and therefore renin secretion from JG cells. PDE3 and PDE4 have been reported to be expressed in JG cells (15). However, none of these studies address how PDEs interact with Ca as a negative modulating signal.
The Ca-calmodulin-dependent PDE1 family comprises three isoforms: PDE1A, PDE1B, and PDE1C (3). PDE1 is regulated by Ca and is tightly associated with calmodulin, such that a rise in intracellular Ca rapidly reduces cAMP concentration (16) by increasing PDE1 activity. The PDE1A and PDE1B isoforms have a high affinity for cGMP, while PDE1C has an equally high affinity for both cAMP and cGMP (17). Furthermore, PDE1 and Ca-inhibitable adenylyl cyclases are usually found colocalized in the same tissues (17). Thus, PDE1C could be a perfect candidate, working in concert with adenylyl cyclase V, for calcium-calmodulin regulation of cAMP in the JG cell. Previously, we have shown (29) that calcium-sensing receptors (CaSR) are expressed in JG cells. Furthermore, these CaSR sense changes in extracellular Ca and initiate intracellular calcium-mediated changes in cAMP formation and renin release from JG cells (29). Thus, we hypothesized that the activation of the JG cell CaSR decreases cAMP and subsequently renin release, in part, by stimulating a Ca-calmodulin-activated PDE1C.
MATERIALS AND METHODS
Isolation of Mouse JG Cells
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and our protocol was approved by the Institutional Animal Care and Use Committee of the Henry Ford Health System. The JG cells were harvested from 8- to 10-wk-old C57/BL6 mice obtained from Jackson Laboratories (Bar Harbor, ME). JG cells were isolated using a technique derived from the method of della Bruna et al. (10) with numerous modifications previously described in detail (30, 31). To summarize, mouse renal cortex was dissected, minced, and digested with 0.25% trypsin (15,500 U/mg; Sigma-Aldrich, St. Louis, MO) and 0.1% collagenase (0.17 U/mg, type A; Roche Applied Science) at 37°C for 75 min. After enzymatic dissociation, the tissue was passed first through a 74-μ and then a 22-μ nylon mesh sieve. The retrieved cells were separated using 25 ml of a 35% isosmotic Percoll density gradient (Sigma-Aldrich) run with a volume-matched tube with marker beads. After 25 min of ultracentrifugation at 4°C and 17, 000 g with a 50.2-Ti rotor (Beckman Coulter), a layer rich in JG cells was obtained from a density layer of 1.07 g/ml. Percoll was washed from the cells, and the isolated JG cells were either used immediately or incubated in primary culture for 48 h at 70–80% confluence. The incubation medium was then replaced with serum-free medium to carry out the experimental protocols (as outlined below).
Inhibition of PDE Activity
After incubating isolated JG cells for 48 h in primary cultures, the culture medium was switched to serum-free reduced calcium medium (S-MEM + calcium added at 0.9 mM concentration) containing a nonselective PDE inhibitor, 100 μM IBMX (Sigma) (29–31). We used two different PDE1-selective inhibitors: either 20 μM of 8-methoxymethil-IBMX (8-MM-IBMX, Calbiochem) (3, 13, 36, 40) (n = 8), or 40 μM of vinpocetine (Biomol International) (3, 13, 34) (n = 8).
Since PDE1 is calmodulin-dependent, we used 10 μM of the calcium-calmodulin inhibitor W-7 (Sigma) (4, 32) (n = 8). We used both the normal 1.2 mM Ca in the media reflecting normal renal cortical interstitial free Ca concentration (25), as well as a reduced but still physiological Ca concentration of 0.9 mM as an established technique to amplify basal renin release (4) so as to exaggerate any inhibitory response to activation of the CaSR. In each case, compounds were tested using a primary culture of JG cells; either without or after the addition of 1 μM of the CaSR agonist cinacalcet (Sensipar; Amgen, Thousand Oaks, CA; n = 16) (28, 29). We ran additional experiments using combined W-7 and 8-MM-IBMX (n = 8). These inhibitors were dissolved in DMEM, and experiments were carried out without cinacalcet. After 2 h of incubation, medium was sampled to determine renin concentration, and cells were harvested for determination of intracellular cAMP content and protein concentration.
cAMP content.
After the incubation medium was removed for renin determination, JG cells were harvested by gently scraping the culture wells with 100 μl of PBS containing 1 mM IBMX plus 100 μl of 50% methanol. The cAMP content was determined from the harvested cells using an RIA kit (Biomedical Technology, Stoughton, MA). Values were corrected for JG cell total protein and expressed as pM/mg protein. The protein concentration in JG cell lysates was determined using the Coomassie plus Protein Assay Reagent kit (Pierce Biotechnology).
Renin release.
In each protocol, JG cells were incubated with serum-free medium for 2 h. The medium was then drawn off and centrifuged, and the supernatant recovered for assay of renin concentration (ANG I generation) using 6 h incubation with sheep angiotensinogen, and assayed using a Gamma Coat RIA kit (DiaSorin, Stillwater, MN) as previously described (30, 31). Values for renin concentration (μg ANG I generated·ml sample−1·h incubation−1) were corrected for JG cell total protein and are simplified hereafter to be expressed as μg ANG I/h·ml−1·mg protein−1.
PDE1 Expression in JG Cells
Western blot analysis for PDE1.
Freshly isolated JG cells were resuspended in lysis buffer [50 mM Tris, pH 6.8/5% (vol/vol) glycerol/2% SDS] containing a protease inhibitor cocktail and incubated for 10 min on ice, as previously described (30, 31). Then 2 μg of solubilized JG cell protein were heated to 95°C for 5 min, and the cell lysate was subjected to 7.5% PAGE under reducing conditions. Proteins were electrophoretically transferred to a PVDF membrane overnight at 4°C. The membranes were blocked for 1 h at room temperature and incubated with specific antibodies against PDE1A (Fabgennix International) (26), PDE1B (Novus Biologicals), or one of two antibodies against PDE1C (Fabgennix or Novus) (26), diluted 1:500 in 5% BSA for 2 h at room temperature. The presence of PDE1 was detected using an horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody at a dilution of 1:1,000. The bands were identified by chemiluminescence and exposed to X-ray film. As a positive control, 20 μl of mouse brain extract in SDS-PAGE (Santa Cruz Biotechnology) was used; all three PDE1 isoforms are known to be expressed in the brain (5). Alternately, homogenized mouse testis was used as a second positive control, as both PDE1A and PDE1C are highly expressed in mouse testis (44). Dissected mouse testis was homogenized with the lysis buffer referred to above, and processed as explained above. Then 20 μg were loaded into the 7.5% polyacrylamide gel.
Immunolabeling of PDE isoforms in JG cells.
To confirm whether the PDE1A, PDE1B, and/or PDE1C isoforms are expressed in JG cells, we placed our primary cultures of JG cells on collagen IV-coated coverslips (Trevigen) for 48 h. The medium was then removed, and the cells fixed for 30 min with freshly prepared 4% paraformaldehyde diluted in PBS and then washed with Tris-buffered saline Tween (TBST) three times for 5 min each. The fixed cells were permeabilized with 0.1% Triton X-100 for 20 min, and then washed. Nonspecific binding was blocked with 5% BSA for 30 min. The cells were washed and then incubated for 2 h with the same antibodies detailed above against the PDE1A, PDE1B, or PDE1C isoforms diluted 1:200 in 5% BSA. Next, cells were washed, then incubated with a goat anti-rabbit antibody labeled with Alexa-Fluor 568 red-orange fluorescent dye (Invitrogen) diluted 1:100 in 5% BSA for 1 h. After incubation with the secondary antibody, cells were again washed, and the coverslips were mounted on slides with Fluoromount (Southern Biotech Associates). The preparations were examined by confocal microscopy (Visitech Confocal System) using excitation at 568 nm and emission measured at > 590 nm at magnification, ×100 using a 1,3 NA lens.
Coimmunolabeling of isoform PDE1C and renin.
To show PDE1C is present in JG cells, we performed immunofluorescence in JG cells grown on coverslips using the PDE1C antibody (Fabgennix International), combined with an antibody against renin (Innovative Research). The renin antibody was diluted 1:250 in 5% BSA, and fixed JG cells were incubated for 2 h and then incubated with a donkey anti-sheep secondary antibody labeled with Alexa-Fluor 488 fluorescent dye (Invitrogen) diluted 1:100 in 5% BSA for 1 h. Mounted samples were excited at 488 nm, and emission was measured at > 500 nm to obtain images of the renin antibody and at 568 nm, and emission was measured at > 590 nm for the PDE1C antibody.
In vivo immunohistochemistry for PDE1C.
Inactin-anesthetized Sprague-Dawley rats were instrumented to preserve the kidneys in situ by flushing the kidney with a retrograde perfusion of the aorta with 150 mM/l NaCl, followed by a 15-min perfusion with 4% paraformaldehyde in buffer containing 150 mM/l NaCl and 10 mM/l sodium phosphate (pH 7.4). Kidneys were stored in 4% formaldehyde until sectioning, at which point they were embedded in paraffin and cut into slices 5-μm thick. Longitudinal and transverse sections of the cortex were obtained from different kidneys. Paraffin-embedded slices were first deparaffinized with xylene and then hydrated gradually through 100% ethanol, then 95%, 70%, and finally distilled water. Each lasted for 5 min. Slides were incubated for 10 min with 0.01% Triton X-100 at 37°C, after permeabilization slides were incubated for 30 min with 3% BSA to block nonsepecific binding. Afterward, slides were incubated for 1 h at 37°C with a 1:40 dilution of an antibody against rabbit PDE1C protein (Fabgennix International) and then for 1 h at 37°C with a 1:100 dilution of secondary antibody (Alexa-Fluor 488 goat anti-rabbit IgG; Molecular Probes, Eugene, OR). Slides were then incubated for 1 h at 37°C with a 1:25 dilution of an antibody against sheep renin protein (Innovative Research) and then for 1 h at 37°C with a 1:100 dilution of secondary antibody (Alexa-Fluor 568 goat anti-sheep IgG). PDE1C fluorescence was detected with an inverted microscope (model IX81; Olympus America) with a digital camera (DP70) set at 488 nm excitation. The same settings were used to detect renin protein in the same cell, except that 568 nm excitation was used.
Statistical Analysis
The changes in both JG cell cAMP content and renin release were analyzed by paired t-tests, combining control and test data from the same individual preparation. Comparison between the treatments was evaluated by ANOVA with a Bonferroni post hoc test. We considered a P value < 0.05 to be significant. In the figures, for the sake of simplicity, all statistically significant changes are represented as P < 0.05, while the actual P values are presented in results.
RESULTS
PDE1 Inhibition
Incubation of JG cells in 0.9 mM Ca medium with the nonselective PDE inhibitor IBMX (Fig. 1) increased cAMP and renin release by 72% and 118%, respectively (both P < 0.001). In contrast, incubation with either of the PDE1 inhibitors 8-MM-IBMX or vinpocetine, had no effect on either JG cell cAMP content or renin release compared with untreated controls.
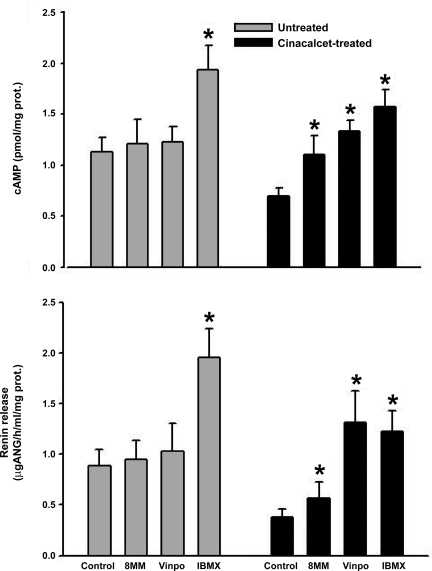
Fig. 1.
Juxtaglomerular (JG) cell cAMP content (top) and renin release (bottom) under basal conditions (grey bars) or after activation of the JG cell calcium sensing receptor (CaSR; black bars) with the calcimimetic cinacalcet incubated in 0.9 mM Ca medium. Nonselective inhibition of phosphodiesterase (PDE) with IBMX significantly increased both cAMP and renin under both basal conditions and after cinacalcet treatment. Cinacalcet reduced both cAMP and renin by half (P < 0.001). The selective PDE1 inhibitor 8-methoxymethil (8MM)-IBMX (8MM) had no effect under basal conditions, but significantly stimulated both cAMP and renin after activation of the CaSR. Another PDE1 inhibitor vinpocetine (Vinpo) had no effect under basal conditions, but also significantly stimulated both cAMP and renin after activation of the CaSR. prot., Protein. *P < 0.05 vs. respective control.
Similar to our previous report (29), activation of JG cell CaSR with cinacalcet lowered both JG cell cAMP content by 40% (P < 0.005) and renin release by 60% (P < 0.005). After activating CaSR with cinacalcet, nonselective PDE inhibition with IBMX increased both cAMP content and renin release (P < 0.005) to slightly above the initial baselines (without cinacalcet), but not back to the same magnitude seen with IBMX without activation of the CaSR (Fig. 1).
In JG cells incubated with cinacalcet, 8-MM-IBMX increased cAMP content 40% (P < 0.025) and renin release by 30% (P < 0.04). Similarly, vinpocetine increased JG cell cAMP by 60% (P < 0.005) and renin release by 80% (P < 0.006) (Fig. 1).
PDE1 and Ca-Calmodulin Inhibition
Figure 2 shows the renin responses to CaSR activation using JG cells incubated in either 0.9 mM Ca plus cinacalcet or in 1.2 mM Ca medium. To simplify data presentation, only renin as the end point is shown, although the responses of cAMP content paralleled those of renin. Similar to data in Fig. 1, the selective PDE1 inhibitor 8-MM-IBMX had no effect on renin from JG cells in 0.9 mM Ca media. Similarly, the calmodulin inhibitor W-7 (4, 32) had no effect (Fig. 2).
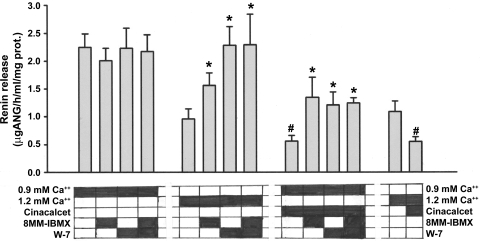
Fig. 2.
JG cell renin release after incubation with either 0.9 or 1.2 mM extracellular Ca under basal conditions or after activation of the JG cell CaSR with the calcimimetic cinacalcet. Incubation media contents are blocked out below each bar. The selective PDE1 inhibitor 8MM-IBMX, had no effect in 0.9 mM media, but significantly stimulated renin in 1.2 mM Ca or after activation of the CaSR. The calmodulin inhibitor W-7 had no effect under basal conditions in 0.9 mM Ca, but significantly stimulated renin in 1.2 mM Ca or after activation of the CaSR. A similar result occurred when 8MM and W-7 were combined, and this result was not additive. #P < 0.01 in either Ca media without cinacalcet. *P < 0.05 in response to activation of the CaSR with 1.2 mM Ca or with cinacalcet.
As before, addition of cinacalcet to 0.9 mM Ca media decreased JG cell renin release by 75% (P < 0.001). As shown in Fig. 2, after CaSR activation with cinacalcet, 8-MM-IBMX increased renin 141% (P < 0.02), W-7 increased renin release by 116% (P < 0.004), and the combination of both 8-MM-IBMX and W-7 increased renin similarly by 121% (P < 0.001). There were no differences in these three responses, and there was no additive effect with the combination of PDE1 and calmodulin inhibitors.
Increasing the incubation media Ca to 1.2 mM decreased basal renin by half (P < 0.001) compared with 0.9 mM Ca. In this medium, 8-MM-IBMX now increased renin release by 63% (P < 0.025). Also, W-7 increased renin release by 100% (P < 0.005), back to a similar level seen with the 0.9 mM Ca media. The combination of both 8-MM-IBMX and W-7 also increased renin release 100% (P < 0.025) but showed no additive effect by the two inhibitors (Fig. 2).
Finally, as shown in Fig. 2, adding cinacalcet to JG cells incubated in 1.2 mM Ca decreased renin release by 50% (P < 0.01).
PDE1 Expression in JG Cells
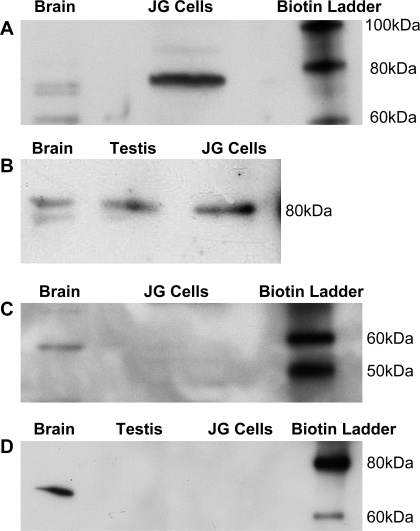
Western blot analysis with an antibody specific for the PDE1C isoform (Fabgennix) (26) identified a band at apparent 80 kDa as the expected size for mouse PDE1C (Fig. 3A). The antibody also identified PDE1C in the positive control of mouse brain (5). To confirm these results, we used a second antibody (Novus) with mouse testis and brain as positive controls (44). It also identified a band at 80 kDa (Fig. 3B), suggesting the PDE1C isoform is present in our preparation of JG cells.
Fig. 3.
Western blot analysis for PDE1C in freshly isolated JG cells. A: Western blot analysis for PDE1C using the Fabgennix antibody, which shows a positive band for PDE1C apparent at 80 kDa both in the positive control (brain) and also in our JG cells. B: Western blot analysis for PDE1C using the Novus antibody, which shows positive bands also apparent at 80 kDa in two positive controls (brain and testis), as well as in our JG cells. C: Western blot analysis for the PDE1A isoform was negative in JG cells, but shows a positive band at 64 kDa in the positive control (brain). D: Western blot analysis for the PDE1B isoform was also negative for JG cells, negative in the negative control of testis, but positive at 66 kDa for brain as a positive control.
Western blot analyses for the other two PDE1 isoforms were also run. An antibody selective for PDE1A (26) was negative for our JG cells but positive for the homogenized mouse brain control (5) at 64 kDa (Fig. 3C). An antibody selective for PDE1B (5, 44) was also negative for JG cells (Fig. 3D), positive for mouse brain controls at 66 kDa, and negative for the control of mouse testis (44). These results suggest that neither PDE1A nor -1B isoforms are detectable by Western blot analysis in JG cells.
Immunolabeling of PDEs in JG Cells
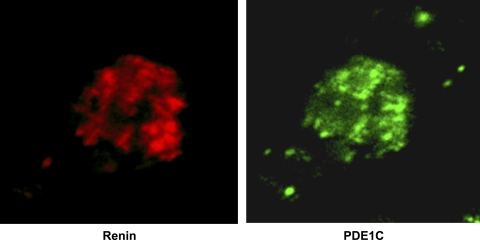
We used the Fabgennix PDE1C antibody to immunolabel and confocal microscopy to detect the PDE1C isoform in our primary culture of JG cells grown on coverslips. Figure 4 shows the PDE1C isoform localizing in a JG cell (shown in green). The same cell was positively labeled for renin (shown in red). To confirm these results, immunolabeling and confocal microscopy were repeated with a second, Novus PDE1C-selective antibody, with similar positive results (data not shown). However, immunolabeling with antibodies for either PDE1A or PDE1B failed to produce any detectable labeling in our primary cultures of JG cells (data not shown).
Fig. 4.
Immunoflourescence and confocal microscopy in a single JG cell using 2 antibodies; 1 specific for renin (red) to confirm that this is a JG cell, and another specific for the PDE1C (green) isoform. The JG cell is positive for both.
In Vivo Immunolabeling of PDE1C in JG Cells
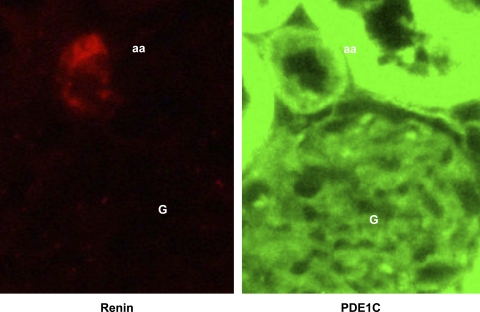
To show PDE1C in the JGA in vivo, renal cortical sections from live rat kidneys fixed in situ (Fig. 5) show immunolabeling with an antibody against the PDE1C (in green) in the afferent arteriole (aa) as well as in proximal tubules. The same sections labeled with an antibody against renin (in red) is positive only in the afferent arteriole. These results, in concert with our localization in primary cultures of mouse JG cells, the responses to activation of CaSR and pharmacologic inhibition of PDE1, and Western blot analysis, all support our hypothesis that the JG cells contain the Ca-activated PDE1C isoform.
Fig. 5.
Immunoflourescence of rat renal cortical sections fixed in situ showing (in red) renin in the afferent arteriole (aa) within the juxtaglomerular apparatus next to the glomerulus (G), as well as PDE1C localization in the JGA (green) using antibodies selective for each (see text).
DISCUSSION
We hypothesized that activation of the JG cell CaSR decreases cAMP and therefore, renin release, in part by stimulating Ca-calmodulin-activated PDE1C. We present multiple lines of evidence for a novel presence of the Ca-dependent isoform PDE1C in JG cells, as well as its participation in regulating renin release by decreasing intracellular JG cell cAMP content. We found that inhibiting PDE1, after activation of the JG cell CaSR using the calcimimetic cinacalcet or by increasing the media Ca concentration, increased both cAMP content and increased renin release. We provide evidence by Western blot analysis using two different antibodies targeted to the PDE1C isoform, and confocal microscopy and immunolabeling of PDE1C in our primary culture of renin-containing isolated mouse JG cells. We also show PDE1C localized in the JGA of renal cortex fixed in situ. All together, these multiple lines of investigation all support our hypothesis that the calcium-sensitive isoform PDE1C is expressed in JG cells, and furthermore, that it mediates changes in cAMP levels and therefore in renin release.
We initially used the nonselective PDE inhibitor IBMX and observed in all of our protocols that JG cell cAMP content and renin release increased ∼50% and 100%, respectively, under basal condition. As has been reported previously (7, 15, 31, 39), these data support an important contribution of PDEs in controlling JG cell cAMP levels and renin release. We also used two different selective PDE1 inhibitors: 8-MM-IBMX and vinpocetine. However, under conditions without CaSR activation, neither inhibitor had any effect compared with untreated controls. It is possible that the elevation of endogenous cAMP formation, by reducing Ca, preempts the calcium-mediated role of PDE1 and that only when CaSR are activated (6, 28) can the calcium-mediated effect of PDE1 be disclosed.
Activation of the JG cell CaSR by either adding the calcimimetic cinacalcet or by increasing the extracellular Ca media concentration from 0.9 to 1.2 mM (or both) resulted in a significant inhibition of cAMP content and renin release from our primary culture of JG cells, consistent with our previous report (29) that the JG cells have CaSR that translate millimolar changes in extracellular Ca into Ca-mediated intracellular events. After this activation of the CaSR, both PDE1 inhibitors now resulted in significant reversal of the inhibition of cAMP and renin release. While the real selectivity of such drugs is always a concern, the fact that neither worked until CaSR were activated, while the nonspecific IBMX had a profound effect on both cAMP and renin, and that the effects of these, in general, were not as great as IBMX, suggest they are selective for Ca-stimulated conditions. Rich et al. (36) previously showed that concentrations of 8-MM-IBMX > 80 μM are needed to inhibit the other isoforms, such as PDE3 or PDE4. Also, it has been published that 8-MM-IBMX inhibits PDE5 (24), but furthermore, that this occurs at concentrations > 30 μM (26, 37). To increase the selectivity of the inhibitor, we used a concentration of only 20 μM of 8-MM-IBMX to reduce the influence on isoforms other than PDE1 (3, 24, 26, 37). Although vinpocetine may be a less selective inhibitor of PDE1 than 8-MM-IBMX, it was also only effective after CaSR activation, and its effect was only slightly greater than that of the highly selective 8-MM-IBMX. Combined, these results suggest CaSR activation leads to increased PDE1 degradation of cAMP and reduced release of renin.
Mupanomunda et al. (25) has shown that renal cortical interstitial calcium concentration may actually vary normally over surprisingly wide ranges, consistent with the concentrations we have used. Thus, our observations suggest that PDE1C may contribute to the overall balance of cAMP formation in the JG cell under normal physiologic circumstances and that the JG cell CaSR may serve as an important conduit pathway (29), linking changes in the extracellular calcium with a calcium-mediated balance of cAMP metabolism and synthesis within the cell. We have previously reported that cAMP synthesis and renin release are dependent upon the Ca-inhibitable isoform, adenylyl cyclase-V (30, 31). Ca-stimulated PDE1 and Ca inhibitable adenylyl cyclases are reported to be often found colocalized in the same tissues (17). While we have not yet tested the interaction of these two Ca-dependent pathways, our data suggests they may work symbiotically, such that as intracellular Ca increases, cAMP synthesis is diminished, while its degradation by PDE1C is enhanced. Conversely, as intracellular Ca is decreased, the activity of adenylyl cyclase V is enhanced, while the influence of PDE1C suppressed, exaggerating the effect of increased cAMP synthesis, and, consequently, renin release. Our data, as well as that previously presented (18, 29, 30, 31), are all consistent with such a relationship.
Enzymatic activity of the PDE1 family is calcium-calmodulin dependent (5), and its binding of the Ca-calmodulin complex stimulates cyclic nucleotide hydrolysis (16). We tested the involvement of calmodulin and found that the calmodulin inhibitor W-7, as well as the PDE1 inhibitor 8-MM-IBMX, both reversed the suppression of cAMP formation and renin release in response to CaSR activation. When we combined these two inhibitors, there was no additive effect. It has been shown repeatedly since 1983 that calmodulin is involved in some way with the Ca paradox and Ca-mediated inhibition of renin secretion (11, 19, 32). A variety of calmodulin inhibitors, including calmidazolium, W-7, W-13, and trifluoperazine, all similarly increased renin release (32) from dog and rabbit renal cortical slices. We previously showed in rat renal cortical slices that W-7 reversed the inhibition of renin induced by a high extracellular calcium medium (4). della Bruna et al. (11) found, using isolated mouse JG cells, calmodulin inhibition stimulates the exocytosis of renin. Calmodulin inhibition has also been found to stimulate renin secretion in isolated-perfused kidneys (19). We found that the inhibition of cAMP formation, and, consequently, renin release after stimulating the CaSR on JG cells was reversed by 8-MM-IBMX and by W-7 but showed no additive effect when combined, suggesting they work in concert under these conditions. W-7 had its effect when the CaSR was activated by cinacalcet or when we used 1.2 mM Ca media, but not with the 0.9 mM Ca media. As we and others have previously shown (11, 19, 32), W-7 increased basal renin release, as well as cAMP. Thus, our data supports the hypothesis that the influence of calmodulin on renin is coupled to PDE1C in the JG cells, as in other cell types (3, 5), and provides a novel explanation for the previously unexplained role of calmodulin in regulating renin.
Using Western blot analysis with two different PDE1C-specific antibodies, we confirmed the expression of PDE1C in freshly isolated JG cells. However, we did not find either the A or B isoforms in our preparation. Because PDE1A, PDE1B, and PDE1C are all expressed in brain and many peripheral neurons (5), we used homogenized brain tissue as our positive control for the Western blot analysis. To confirm this positive result for PDE1C, mouse testis was also used as positive control. PDE1A and PDE1C are highly expressed in testicular germ cells with different cellular patterns, while PDE1B showed a very low in situ hybridization signal in this tissue (44). Thus we also used mouse testis as a negative control in the Western blot analysis for PDE1B. It may be noted that in our positive controls, we see multiple bands. PDE1C has several splice variants (PDE1C1–PDE1C5) (44) which could account for these. Consistent with this observation, both suppliers of our antibodies (Novus, Fabgennix) suggested that in positive controls for PDE1C, several bands may appear.
Not much is known about PDE1 expression in the kidney. Dousa (12) reported that PDE1 was detected in a suspension of rat cortical tubules, in OK cells and the renal tubular cell line LLCPK, but not in rat glomeruli or in mesangial cells. Our studies with immunofluorescence confirmed expression of PDE1C isoform in JG cells, as well as the apparent absence of either the A or B isoforms. Additionally, we present data showing the same JG cell labeled with both an antibody against PDE1C and another specific antibody against renin. We also used in situ fixed cortical tissue to show the presence of PDE1C in the renin-positive JGA, as well as in the proximal tubules of live animals. Thus we provide multiple lines of evidence, in both freshly isolated JG cells and JG cells in primary culture, that PDE1C but neither the A nor B isoforms are expressed in these renin-containing cells.
In conclusion, we have found that PDE1 can be activated by a calcimimetic acting on the CaSR of the JG cell or by increasing the extracellular Ca concentration within a physiological range. We have found that different PDE1-selective inhibitors reverse the Ca-provoked inhibition of cAMP and of renin, and that this is mirrored by inhibition of calmodulin, suggesting that the PDE1C in the JG cell, as in other cell types, is Ca-calmodulin dependent. Furthermore, we report using multiple lines of investigation that the JG cell contains the Ca-activated isoform PDE1C, which degrades cAMP. Taken together, all of these results support our hypothesis; suggesting that Ca-activated PDE1C metabolism of cAMP, balanced with Ca-inhibitable adenylyl cyclase-V cAMP synthesis (30), is part of an intricate Ca-regulated interaction influencing the enzymatic synthesis and degradation of this second messenger and its modulation of renin secretion from the JG cell.
Perspectives and Significance
Our finding that CaSR-mediated activation of PDE1C in the JG cell and degradation of cAMP, in concert with our previous findings (31) that Ca inhibits JG cell adenylyl cyclase V synthesis of cAMP, define a novel integrated mechanism by which Ca can modulate renin release through it effects on the activities of the enzymes controlling the level of its second messenger cAMP. We also resolve a 25-yr-old question of how calmodulin fits in with the Ca-renin interaction. However, it is important to keep in mind that these changes in Ca don't themselves regulate renin secretion, but rather modify the activity of the enzymes that control the production and degradation of the second messenger cAMP. Thus, while stimulatory pathways such as the renal baroreceptor, renal nerves, or the macula densa still control the secretion of renin, the amplitude of those signals is modified by Ca's influence over the balance between the activity of adenylyl cyclase V and PDE1C.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grant RO1-HL-076469 (to W. H. Beierwaltes).
ACKNOWLEDGMENTS
This work was previously presented, in part, in abstract form at the 2008 American Heart Association Council of High Blood Pressure Research in Atlanta, GA (Hypertension 50: e75, 2008).
REFERENCES
- 1.Antonipillai I, Horton R. Role of extra- and intracellular calcium and calmodulin in renin release from rat kidney. Endocrinology 117: 601–606, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol Renal Fluid Electrolyte Physiol 237: F333–F343, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Beierwaltes WH. Nitric oxide participates in calcium-mediated regulation of renin release. Hypertension 23: I40–I44, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Breitwieser GE, Gama L. Calcium-sensing receptor activation induces intracellular calcium oscillations. Am J Physiol Cell Physiol 280: C1412–C1421, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chiu YJ, Hu SH, Reid IA. Inhibition of phosphodiesterase III with milrinone increases renin secretion in human subjects. J Pharmacol Exp Ther 290: 16–19, 1999 [PubMed] [Google Scholar]
- 8.Churchill PC. Second messengers in renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 249: F175–F184, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Churchill PC, Churchill MC. Isoproterenol-stimulated renin secretion in the rat: second messenger roles of Ca and cyclic AMP. Life Sci 30: 1313–1319, 1982 [DOI] [PubMed] [Google Scholar]
- 10.della Bruna RD, Pinet F, Corvol P, Kurtz A. Regulation of renin secretion and renin synthesis by second messengers in isolated mouse juxtaglomerular cells. Cell Physiol Biochem 1: 98–110, 1991 [Google Scholar]
- 11.della Bruna RD, Pinet F, Corvol P, Kurtz A. Calmodulin antagonists stimulate renin secretion and inhibit renin synthesis in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 262: F397–F402, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Dousa TP. Cyclic-3',5'-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int 55: 29–62, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Dunkern TR, Hatzelmann A. Characterization of inhibitors of phosphodiesterase 1C on a human cellular system. FEBS J 274: 4812–4824, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fray JC, Park CS. Forskolin and calcium: interactions in the control of renin secretion and perfusate flow in the isolated rat kidney. J Physiol 375: 361–375, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skott O. Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res 90: 996–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Goraya TA, Cooper DM. Ca2+-calmodulin-dependent phosphodiesterase (PDE1): current perspectives. Cell Signal 17: 789–797, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Goraya TA, Masada N, Ciruela A, Willoughby D, Clynes MA, Cooper DM. Kinetic properties of Ca2+/calmodulin-dependent phosphodiesterase isoforms dictate intracellular cAMP dynamics in response to elevation of cytosolic Ca2+. Cell Signal 20: 359–374, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Grünberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradox on of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res 99: 1197–1206, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hackenthal E, Schwertschlag U, Taugner R. Cellular mechanisms of renin release. Clin Exp Hypertens A 5: 975–993, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Kurtz A, della Bruna RD, Pfeilschifter J, Bauer C. Role of cGMP as second messenger of adenosine in the inhibition of renin release. Kidney Int 33: 798–803, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Kurtz A, Penner R. Effects of angiotensin II on intracellular calcium and electrical function of mouse renal juxtaglomerular cells. Kidney Int Suppl 30: S51–S54, 1990 [PubMed] [Google Scholar]
- 23.Linseman DA, Lawson JA, Jones DA, Ludens JH. Glyburide attenuates calmodulin antagonist-stimulated renin release from isolated mouse juxtaglomerular cells. Am J Physiol Renal Fluid Electrolyte Physiol 269: F242–F247, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109: 366–398, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Mupanomunda MM, Tian B, Ishioka N, Bukoski RD. Renal interstitial Ca2+. Am J Physiol Renal Physiol 278: F644–F649, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 292: L294–L303, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Gαi3 and m3-mediated stimulation via Gβγq. J Biol Chem 272: 21317–21324, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC, Colloton M, Karbon W, Scherrer J, Shatzen E, Rishton G, Scully S, Qi M, Harris R, Lacey D, Martin D. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 308: 627–635, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension 50: 737–743, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform V mediates renin release from juxtaglomerular cells. Hypertension 49: 618–624, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension 49: 162–169, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Park CS, Honeyman TW, Chung ES, Lee JS, Sigmon DH, Fray JC. Involvement of calmodulin in mediating inhibitory action of intracellular Ca2+ on renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 251: F1055–F1062, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Persson PB. Renin: origin, secretion and synthesis. J Physiol 552: 667–671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips PG, Long L, Wilkins MR, Morrell NW. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 288: L103–L115, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Rasch R, Jensen BL, Nyengaard JR, Skott O. Quantitative changes in rat renin secretory granules after acute and chronic stimulation of the renin system. Cell Tissue Res 292: 563–571, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Rich TC, Tse TE, Rohan JG, Schaack J, Karpen JW. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J Gen Physiol 118: 63–78, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res 90: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Sandner P, Kornfeld M, Ruan X, Arendshorst WJ, Kurtz A. Nitric oxide/cAMP interactions in the control of rat renal vascular resistance. Circ Res 84: 186–192, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Sayago CM, Beierwaltes WH. Nitric oxide synthase and cGMP-mediated stimulation of renin secretion. Am J Physiol Regul Integr Comp Physiol 281: R1146–R1151, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation 115: 2331–2339, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand 181: 383–390, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wagner C, Hinder M, Kramer BK, Kurtz A. Role of renal nerves in the stimulation of the renin system by reduced renal arterial pressure. Hypertension 34: 1101–1105, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Yan C, Zhao AZ, Sonnenburg WK, Beavo JA. Stage and cell-specific expression of calmodulin-dependent phosphodiesterases in mouse testis. Biol Reprod 64: 1746–1754, 2001 [DOI] [PubMed] [Google Scholar]