Abstract
Glucagon-like peptide-2 (GLP-2) is a nutrient-regulated intestinotrophic hormone derived from proglucagon in the distal intestine. Enteral nutrients (EN) potentiate the action of GLP-2 to reverse parenteral nutrition (PN)-induced mucosal hypoplasia. The objective was to determine what enteral protein component, casein, soy, or whey protein, potentiates the intestinal growth response to GLP-2 in rats with PN-induced mucosal hypoplasia. Rats received PN and continuous intravenous infusion of GLP-2 (100 μg/kg/day) for 7 days. Six EN groups received PN+GLP-2 for days 1–3 and partial PN+GLP-2 plus EN for days 4–7. EN was provided by ad libitum intake of a semielemental liquid diet with different protein sources: casein, hydrolyzed soy, whey protein concentrate (WPC), and hydrolyzed WPC+casein. Controls received PN+GLP-2 alone. EN induced significantly greater jejunal sucrase activity and gain of body weight, and improved feed efficiency compared with PN+GLP-2 alone. EN induced greater ileal proglucagon expression, increased plasma concentration of bioactive GLP-2 by 35%, and reduced plasma dipeptidyl peptidase IV (DPP-IV) activity compared with PN+GLP-2 alone, P < 0.05. However, only whey protein, and not casein or soy, potentiated the ability of GLP-2 to reverse PN-induced mucosal hypoplasia and further increase ileal villus height, crypt depth, and mucosa cellularity compared with PN+GLP-2 alone, P < 0.05. The ability of whey protein to induce greater mucosal surface area was associated with decreased DPP-IV activity in ileum and colon compared with casein, soy, or PN+GLP-2 alone, P < 0.05. In conclusion, whey protein potentiates the action of GLP-2 to reverse PN-induced mucosal hypoplasia in association with decreased intestinal DPP-IV activity.
Keywords: mucosal growth, proglucagon, dipeptidyl peptidase-IV, whey protein concentrate
glucagon-like peptide-2 (glp-2) is a 33-amino acid intestinotrophic hormone derived from tissue specific post-translational processing of proglucagon in the endocrine L cells of the ileum and colon (12). GLP-2 is secreted following nutrient ingestion and is rapidly inactivated by dipeptidyl peptidase IV (DPP-IV) with a biological half-life of ∼7 min in humans (8). GLP-2 is considered a key mediator of intestinal adaptive growth through stimulation of epithelial cell proliferation and inhibition of apoptosis, leading to an enhanced absorptive surface area (1, 5, 10, 19). Moreover, both exogenous GLP-2 and the degradation-resistant analog of GLP-2, teduglutide, successfully stimulated intestinal adaptation in humans with short bowel syndrome in clinical trials (13, 17). Current evidence suggests that GLP-2 action requires an indirect signal, perhaps functioning through a paracrine mechanism involving insulin-like growth factor-I or epidermal growth factor to stimulate intestinal growth, because neither crypt epithelial cells nor enterocytes express the GLP-2 receptor (8).
The primary stimulus for GLP-2 secretion is the presence of luminal nutrients or enteral nutrition (EN) (2, 33). Our previous research has shown that a small amount of EN synergistically increases the intestinotrophic action of a low-dose of GLP-2 in parenterally fed rats in two separate models (23, 24). These models include parenteral nutrition (PN)-induced mucosal atrophy and a model of human short bowel syndrome that requires PN for survival. This finding that a small amount of EN potentiates GLP-2 action to stimulate mucosal growth during PN is consistent with the view that GLP-2 acts through downstream mediators as EN stimulate intestinal growth through multiple indirect signals. Moreover, this finding has clinical relevance because patients with short bowel syndrome and other intestinal diseases that require PN usually tolerate and are encouraged to consume at least partial EN to maintain mucosal integrity and prevent immune dysfunction due to exclusive PN (18, 20, 24). Thus, it is important to know what types of nutrients are most effective in potentiating GLP-2 action to optimize the EN formulation.
The enteral formula used in our previous studies (23, 24) was a liquid, partially hydrolyzed, semielemental, low-residue medical food designed for humans with impaired gastrointestinal function. The ability of this semielemental liquid diet to potentiate GLP-2 action appears to be unique because studies in our laboratory comparing this formulation with the AIN-93G diet (30) showed that the AIN-93G diet did not induce synergistic intestinal growth with GLP-2 administration, as did the semielemental liquid diet. This formula, which has a unique protein composition, but more typical fat and carbohydrate components, was previously shown to protect the intestine from radiation injury in human subjects and dogs (25). Moreover, evidence suggests that whey protein, but not fat, provided as oleic acid, increases secretion of GLP-1, a product of the proglucagon gene secreted in parallel with GLP-2, and inhibits DPP-IV activity in the proximal small intestine (15). These studies suggest that the protein component of this semielemental liquid diet interacted with GLP-2.
Thus, we hypothesized that the protein component of the semielemental liquid diet, possibly whey protein acting via modulation of DPP-IV activity, was responsible for potentiating the intestinotrophic action of GLP-2 to reverse PN-induced mucosal hypoplasia (23, 24). The objective of the current study was to determine what enteral protein component, casein, soy, or various forms of whey protein, potentiates the intestinal growth response to a low-dose of GLP-2 in parenterally fed rats.
MATERIALS AND METHODS
Animals and experimental design.
The animal facilities and protocols reported were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Male, Sprague-Dawley rats (Harlan, Madison, WI) initially weighing 175–200 g were housed in individual, stainless-steel cages with unlimited access to water in a room maintained at 22°C on a 12:12-h light-dark cycle. All rats were acclimated to the facility for 6 days while being fed a semipurified diet ad libitum (6).
Two experiments were conducted in rats receiving controlled PN in conjunction with partial EN as shown in Fig. 1. The EN formulation consisted of controlled modifications of a low-residue, semielemental liquid diet (Vital, donated by Ross Products Division, Abbott Laboratories, Columbus, OH). Experiment 1 tested the ability of a customized, rat version of the semielemental liquid diet (ABC) to show the same ability as the commercial human semielemental liquid diet (abc, Vital) to potentiate the intestinotrophic effects of GLP-2. The ABC and abc liquid diets were isocaloric and contained the same ingredients (Table 1) providing 16.7% energy from protein, 9.5% energy from fat, and 73.8% of energy from carbohydrate. Formulation of the liquid diet ABC was needed to design defined liquid diets that could be used to test the intestinotrophic effects of the specific protein components in experiment 2. In experiment 1, rats were randomly assigned to four treatment groups as follows: PN alone, PN+GLP-2, and two PN+ EN treatment groups: PN+abc+GLP-2 and PN+ABC+GLP-2. Rats in all groups were maintained with PN for 7 days. The three GLP-2 treatment groups received 100 μg GLP-2/kg body wt/day from days 1–7 coinfused continuously with PN solution. The final sample size for each group was 5 or 6. A nonsurgical group of rats fed a semipurified diet ad libitum was included for reference (Oral, n = 6).
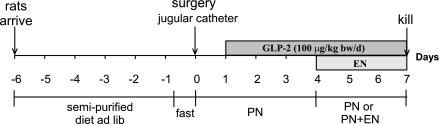
Fig. 1.
Timeline of the experimental protocol. Rats were maintained with parenteral nutrition (PN) for 7 days with continuous intravenous infusion of a low-dose of glucagon-like peptide-2 (GLP-2). Treatment groups were given partial enteral nutrients (EN) from a semielemental, low-residue liquid diet with different sources of protein for days 4–7 with a reduction in the volume of PN.
Table 1.
Experimental diets for the EN groups from experiment 2
| Ingredient | Casein | Aa | ABC | BC | B | C |
|---|---|---|---|---|---|---|
| Protein | ||||||
| Casein | 161.5 | 55.3 | 106.2 | 106.2 | 106.2 | |
| Hydrolyzed soy/collagenb | 106.2 | 106.2 | ||||
| Whey protein concentratec | 33.0 | 33.0 | 55.3 | |||
| Hydrolyzed WPC + caseind | 22.3 | 22.3 | 55.3 | |||
| L-amino acidse | 20.7 | 20.7 | 20.7 | 20.7 | 20.7 | 20.7 |
| Carbohydrate | ||||||
| Sucrose | 124.8 | 124.8 | 124.8 | 124.8 | 124.8 | 124.8 |
| Maltodextrinsf | 601.6 | 601.6 | 601.6 | 601.6 | 601.6 | 601.6 |
| Fat | ||||||
| High-oleic safflower oil | 21.15 | 21.15 | 21.15 | 21.15 | 21.15 | 21.15 |
| Median chain triglycerides | 17.44 | 17.44 | 17.44 | 17.44 | 17.44 | 17.44 |
| Monoglycerides + soy lecithin | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 | 1.62 |
| Vitamins and minerals | ||||||
| Mineral mix,g Ca-P Deficient | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Calcium phosphate, monobasic, monohydrate | 6.8 | 6.8 | 6.8 | 6.8 | 6.8 | 6.8 |
| Ingredient | ||||||
| Calcium carbonate | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 | 2.9 |
| Vitamin mixh | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Xanthan gum | 11.4 | 11.4 | 11.4 | 11.4 | 11.4 | 11.4 |
All values for the treatment groups are given in grams per kilogram.
Dietary treatment groups: A, hydrolyzed soy/collagen; B, whey protein concentrate; C, hydrolyzed whey protein concentrate + casein.
Significant hydrolysis, but not as much as the hydrolyzed whey protein concentrate + casein.
Primarily intact protein.
50/50 mixture of whey protein and casein that is heavily hydrolyzed by enzymatic digestion followed by filtration; contains ∼60% free amino acids, ∼33% <500D peptides, and no peptides >3 kDa.
The following L-amino acids were added as grams per kilogram: tyrosine, 3.8; leucine, 3.4; valine, 3.3; isoleucine, 2.5; phenylalanine, 2.4; histidine (HCL) monohydrate, 2.0; methionine, 1.7; tryptophan, 0.62; and threonine, 0.97.
Maltodextrins from corn with a dextrose equivalent of 10–15 (150–451 g/kg).
AIN-76 Mineral mix, Harlan Teklad 79055, Madison, WI. Ammonium paramolybdate, Tetrahydrate was added at 0.7 g/kg diet.
Vitamin mix, Harlan Teklad 40060.
Experiment 2 determined which of the major protein components in the semielemetal, liquid diet—intact casein, hydrolyzed soy/collagen (A), whey protein concentrate (WPC) (B), or hydrolyzed WPC+casein (C)—potentiated intestinal growth in combination with a low dose of GLP-2, Table 1. Rats were randomly assigned to PN+GLP-2 and 6 PN+EN+GLP-2 treatment groups as follows: PN+casein diet+GLP-2 (Casein), PN+protein A diet+GLP-2 (A), PN+protein B diet+GLP-2 (B), PN+protein C diet+GLP-2 (C), PN+protein ABC diet+GLP-2 (ABC), and PN+protein BC diet+GLP-2 (BC). Rats in these seven groups were maintained with PN plus GLP-2 treatment for 7 days and received 100 μg GLP-2·kg body wt−1·day−1 from days 1 to 7 coinfused continuously with PN solution. The final sample size for each group was 6.
At each experiment, rats were fasted for 18 h before surgical placement of intravenous catheters. Rats were anesthetized by inhalation of isoflurane (IsoFlo; Abbot Laboratories, North Chicago, IL) via an anesthesia machine before surgery. Intravenous catheters were placed in the superior vena cava as previously reported (6, 22). Infusion of PN solution was initiated using a Harvard syringe pump (Harvard Apparatus, Holliston, MA) at 1.0 ml/h immediately following surgery (day 0), advanced to 1.67 ml/h on day 1, and maintained at full-strength infusion of 2.5 ml/h (60 ml/day) for groups not receiving EN (PN alone and PN+GLP-2) for days 2–6. Rats given PN alone and PN+GLP-2 on days 2–6 received the following daily nutrient intake: 64 kcal, 2.6 g protein (16.5% energy), 1.7 g fat (24% energy), and 11 g dextrose (59% energy). The composition and preparation of the nutritionally complete PN solution was previously reported (6). Treatment groups given EN received 2.5 ml/h PN solution for days 2–4, and then the infusion was gradually decreased to 1.67 ml/h on day 5 and 1.0 ml/h on day 6.
The semielemental liquid diets were offered as EN ad libitum in graduated feeding tubes on days 4–6. The liquid diets were designed to provide similar amounts of vitamins, minerals, energy, and macronutrients (Table 1). Two hundred-sixty-three grams of each diet were mixed with 737 g water to provided 1 kcal per ml with 16.7% energy from protein, 9.5% energy from fat, and 73.8% energy from carbohydrate. The amount of hydrolyzed soy/collagen in diet A is the same as in diet ABC. The same concentration of WPC or hydrolyzed WPC was tested in diets ABC, BC, B and C. Casein was used to offset the proteins that were eliminated in diets A, B, C, and BC compared with diet ABC to keep total protein content constant. The ABC diet provided a positive control and the casein diet a negative control. Approximately 43% of energy needs was provided by EN and 57% was provided by PN during the last 3 days of the study for PN+EN and PN+EN+GLP-2 groups.
Human GLP-2 (preproglucagon 126–158, California Peptide Research, Napa, CA) was diluted in PBS, pH = 7.4, 1 day before surgery and added to the PN solution daily. Vehicle was infused in rats not given GLP-2 (24). Body weights, PN solution infused, and the amount of EN consumed were recorded daily. After 7 days of PN or PN+EN, rats were anesthetized with isoflurane (IsoFlo, Abbott Laboratories, North Chicago, IL), and killed by exsanguinations within 10 min of stopping the continuous infusion feeding.
Intestinal composition, histology, and sucrase activity.
The entire small and large bowel and liver were removed for analysis. The bowel was sectioned into duodenum, defined as pylorus to ligament of Treitz; jejunum, defined as ligament of Treitz to ileum; ileum, defined as the final 25 cm of small bowel proximal to cecum; and colon. All sections of bowel were immediately flushed with ice-cold saline and put on a chilled glass plate to be sectioned. Three cm of proximal duodenum, jejunum, ileum, and colon were used for determining mucosal dry mass and concentrations of mucosal protein (bicinchoninic acid protein assay, Pierce Chemicals, Rockford, IL) and DNA (21). Three centimeters of proximal jejunum were used for determination of sucrase activity (4). One centimeter of jejunum and ileum was fixed in 10% buffered formalin for histology; fixed tissue was paraffin embedded, cut into 5-μm sections and stained with hematoxylin and eosin for histomorphology, as previously described (7).
Biochemical analyses.
Blood was collected in chilled tubes containing a final concentration of 1 mg/ml EDTA, 0.1 mM Diprotin A (MP Biomedicals, Aurora, OH), and 0.01 mM aprotinin (Calbiochem, La Jolla, CA). Plasma was isolated by centrifugation at 1,800 g for 15 min at 4°C and was stored at −70°C until GLP-2 measurement. Plasma bioactive GLP-2 was measured by RIA using an antibody specific to the NH2 terminus of GLP-2 (16). DPP-IV activity was measured in plasma collected with 1 mg/ml EDTA and homogenates of ileal mucosa and intact colon using the discontinuous direct photometric method of Nagatsu et al. (26) as previous described (23). Proglucagon mRNA expression was measured in a two-step quantitative, real-time RT-PCR using SYBR Green detection method, as described previously (27). Sequences for forward and reverse primers (Integrated DNA Technologies, Coralville, IA) were reported (23). Data were analyzed using 7000 system software (Applied Biosystems, Foster City, CA).
Statistical analyses.
Treatment groups were analyzed using general linear models; differences among the treatment groups were assessed by one-way ANOVA followed by the protected least significant differences technique (SAS version 8.2; SAS Institute, Cary, NC). Changes in body weight were assessed by repeated-measures analysis. Statistics were performed on log-transformed data for results showing unequal variances among groups. All data are presented as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Experiment 1.
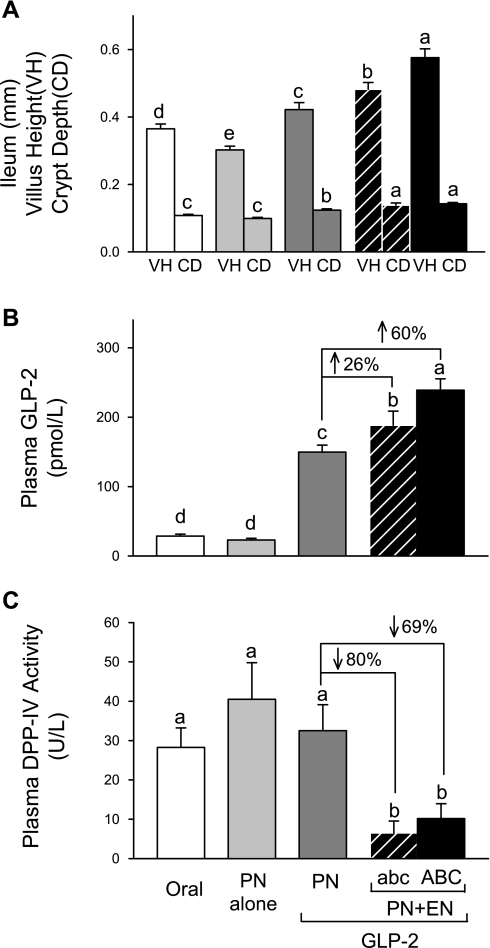
There were no significant differences in body weights among the four PN treatment groups on the day of surgery and during the period after surgery and before EN treatment (day 0 to day 4). GLP-2 in combination with EN (days 4–6) induced a significantly greater gain in body weight, as previously noted (24). Rats given PN alone exhibited significant mucosal atrophy throughout the small intestine that was reversed by GLP-2, as evidenced by histology and mucosal concentrations of protein and DNA (19). A synergistic effect of combination treatment with PN+abc+GLP-2 or PN+ABC+GLP-2 to further significantly increase the mucosa growth was noted in ileum for villus height and crypt depth (Fig. 2), sucrase activity, and mucosal concentrations of protein, and DNA (data not shown). In association with the greater mucosal growth, the plasma concentration of bioactive GLP-2 due to combination treatment with abc+GLP-2 or ABC+GLP-2 was significantly greater by 26% or 60%, respectively, compared with GLP-2 alone, Fig. 2. Moreover, combination treatment with abc+GLP-2 or ABC+GLP-2 significantly reduced plasma DPP-IV activity by 80% or 69%, respectively, compared with GLP-2 alone.
Fig. 2.
Data from experiment 1 comparing the human semielemental, low-residue liquid diet (abc) and a rat formulation of the diet (ABC). Results show that ABC has an even greater ability to potentiate ileal growth (A) and increase plasma GLP-2 concentration (B), compared with abc (C). Values are expressed as means ± SE; n = 6. Means with different letter superscripts are significantly different (P < 0.05).
In summary, data from experiment 1 establish that the ABC semielemental diet showed a similar, or in the case of ileum histology and plasma GLP-2, even greater ability compared with the commercial abc diet, to potentiate the intestinotrophic action GLP-2. Thus, the ABC semielemental liquid diet was used as a base in experiment 2 to manipulate the protein components.
Experiment 2: Body weight and food intake.
There were no significant differences in body weights among oral, PN+GLP-2, and the 6 PN+EN+GLP-2 groups before surgery, and on the day of surgery, body weights ranged from 200 to 227 g. During the period after surgery and before EN treatment (day 0 to day 4), there was no difference in daily body weight among the groups given PN. In association with significantly greater energy intake on days 4–6, the 6 PN+EN+GLP-2 groups showed significantly greater gain of body weight after 7 days of PN compared with the PN+GLP-2 group, Table 2. Final body weights were not significantly different in the groups given partial EN compared with oral reference. Compared with PN+GLP-2, the PN+EN+GLP-2 groups showed 110–150% greater gain in body weight with only 14–28% greater total energy intake. Thus, feed efficiency was significantly improved when supplemental EN was provided compared with PN+GLP-2 alone. The groups fed WPC (ABC, BC, and B) consumed the most EN, and the group fed soy consumed the least amount of EN.
Table 2.
Body weight and energy intake in experiment 2
| Oral | PN+GLP-2 | PN+EN+GLP-2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Casein | A | ABC | BC | B | C | |||
| Body weight, g | ||||||||
| Initial | 216±4 | 217±2 | 214±3 | 213±3 | 214±1 | 218±1 | 217±2 | 218±1 |
| Day 4 | 246±3a | 228±2b | 224±2b | 222±2b | 223±2b | 228±3b | 229±2b | 227±1b |
| Final | 266±3a | 237±3b | 265±4a | 258±3a | 259±2a | 267±2a | 267±3a | 260±2a |
| Gain g/7 days | 51±3a | 20±2b | 51±2a | 45±4a | 45±2a | 50±1a | 50±2a | 42±2a |
| Enteral intake, ml | ||||||||
| Days 4–6 | 130±9bc | 116±11c | 150±6ab | 162±6a | 169±11a | 136±7bc | ||
| Total energy, kcal | ||||||||
| Days 4–6 | 192±6d | 263±9bc | 249±11c | 282±6ab | 295±6a | 302±11a | 269±7bc | |
| Days 0–6 | 389±5d | 459±9bc | 446±11c | 479±6ab | 492±6a | 498±11a | 466±7bc | |
Values are expressed as means ± SE; n = 5–6. Values in the same row with different superscripts are significantly different (P < 0.05). PN, parenteral nutrition; EN, enteral nutrition; GLP-2, glucagon-like peptide-2; A, hydrolyzed soy/collagen; B, whey protein concentrate; C, hydrolyzed whey protein concentrate + casein; ABC, rat version of human semielemental liquid diet.
Mucosal adaptive growth and sucrase activity.
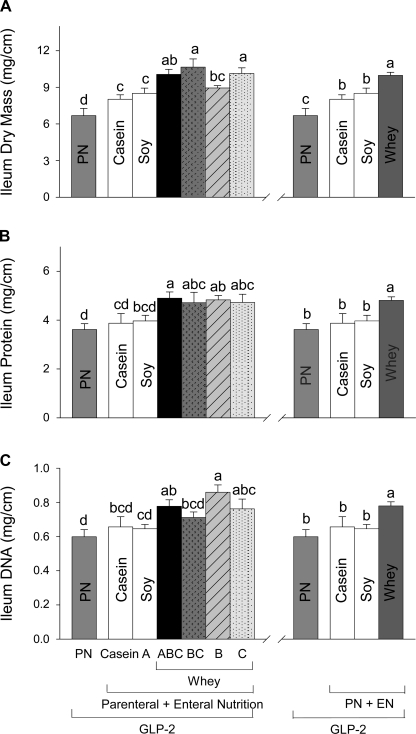
When EN included casein (PN+Casein+GLP-2) or soy (PN+A+GLP-2) compared with PN+GLP-2 alone, there were no significant differences in mucosal concentrations of protein or DNA in duodenum, jejunum, ileum, and colon, Fig. 3 (data for ileum are shown). In contrast, all of the 4 PN+EN groups that ingested WPC or hydrolyzed WPC+casein (ABC, BC, B, and C) showed greater mucosal dry mass and concentrations of protein and DNA in duodenum, jejunum, ileum, and colon compared with PN+GLP-2 alone (data for ileum are shown, Fig. 3). There were no significant differences in indices of mucosal cellularity among the four whey protein groups for all sections of intestine; thus, these groups were combined to examine the effects of whey protein within the EN treatments (Fig. 3). A significant effect of whey protein to further increase the mucosa growth beyond that induced by GLP-2 alone was noted in ileum for dry mass, protein, and DNA by 17–21%, 18–24% and 30–49% compared with casein, soy, or PN+GLP-2, respectively (Fig. 3). Consistent with increases in mucosal cellularity due to ingestion of whey protein, there was a significant effect of whey protein to increase villus height and crypt depth in ileum beyond that seen with GLP-2 alone compared with the soy and casein groups (data not shown).
Fig. 3.
Ileum mucosal dry mass (A), protein (B), and DNA (C) in rats maintained with PN+GLP-2 for 7 days as follows: PN alone, casein, A (hydrolyzed soy/collagen), B (whey protein concentrate, WPC), C (hydrolyzed WPC+casein), BC, and ABC. Values are expressed as means ± SE; n = 6. Means with different letter superscripts are significantly different (P < 0.05). The right panel shows the combined effects of the four whey diets compared with casein, soy, and PN+GLP-2 alone.
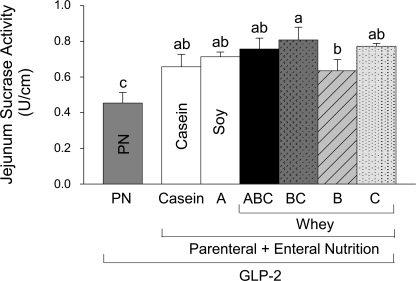
Mucosal sucrase activity reflects the digestive capacity of jejunum. All six PN+EN+GLP-2 groups showed significantly greater jejunal sucrase segmental activity (U/cm mucosa) compared with PN+GLP-2, Fig. 4. Ingestion of casein (PN+Casein+GLP-2) and WPC+casein (PN+B+GLP-2) showed the lowest sucrase segmental activity among the PN+EN+GLP-2 groups, and when sucrase activity was expressed as specific activity, these treatments did not induce significantly greater sucrase activity, compared with PN+GLP-2.
Fig. 4.
Jejunum mucosal sucrase segmental activity in rats maintained with PN + GLP-2 for 7 days as follows: PN alone, casein, A (hydrolyzed soy/collagen), B (whey protein concentrate, WPC), C (hydrolyzed WPC+casein), BC, and ABC. Values are expressed as means ± SE; n = 6. Means with different letter superscripts are significantly different (P < 0.05).
Proglucagon expression, plasma bioactive GLP-2, and DPP-IV activity.
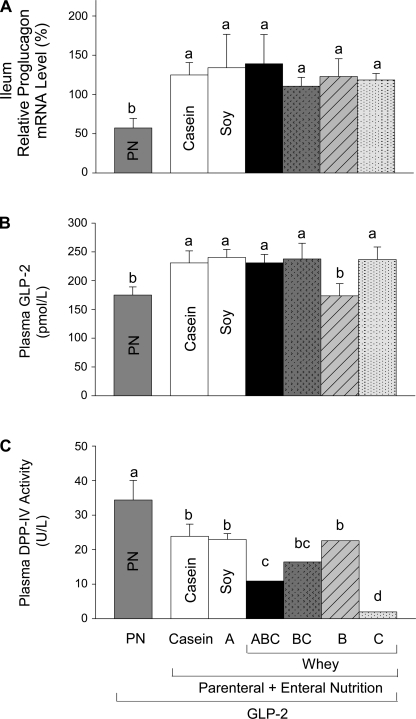
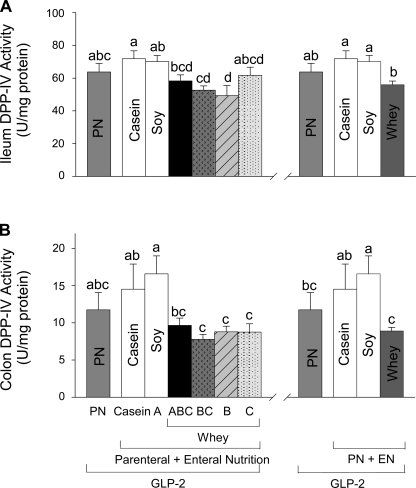
All groups given EN showed significantly increased proglucagon expression in ileum compared with PN+GLP-2 alone (Fig. 5). Five of the six groups given PN+EN+GLP-2 groups showed significantly, ∼35% greater, plasma bioactive GLP-2 compared with PN+GLP-2. Only the group given WPC+casein (PN+B+GLP-2) did not show an increase in plasma GLP-2. All 6 PN+EN+GLP-2 groups showed significantly lower DPP-IV activity in plasma compared with PN+GLP-2. The PN+EN+GLP-2 groups that ingested hydrolyzed WPC+casein (ABC, BC, and C) showed lower plasma DPP-IV activity compared with casein or soy, with the largest effect in the group fed the highest concentration of hydrolyzed WPC+casein (C). The four PN+GLP-2 groups given EN from whey protein showed significantly decreased DPP-IV specific activity in ileum and colon compared with casein or soy, Fig. 6.
Fig. 5.
Proglucagon expression in ileum (A), and plasma concentrations of bioactive GLP-2 (B), and DPP-IV activity (C) in rats maintained with parental nutrition (PN)+GLP-2 for 7 days as follows: PN alone, casein, A (hydrolyzed soy/collagen), B (whey protein concentrate, WPC), C (hydrolyzed WPC+casein), BC, and ABC. Values are expressed as means ± SE; n = 6. Means with different letter superscripts are significantly different (P < 0.05).
Fig. 6.
Mucosal DPP-IV specific activity in ileum (A) and colon (B) in rats maintained with parental nutrition (PN)+GLP-2 for 7 days as follows: PN alone, casein, A (hydrolyzed soy/collagen), B (whey protein concentrate, WPC), C (hydrolyzed WPC+casein), BC, and ABC.Values are expressed as means ± SE; n = 6. Means with different letter superscripts are significantly different (P < 0.05). The right panel shows the combined effects of the four whey diets compared with casein, soy, and PN+GLP-2 alone.
We examined the correlations between DPP-IV activity and mucosa cellularity in ileum, the primary site where EN potentiated the intestinotrophic effects of GLP-2. Significant negative correlations were observed between ileal mucosa dry mass and DPP-IV specific activity (R2 = 0.24, P = 0.0007), ileal mucosa protein, and DPP-IV specific activity (R2 = 0.23, P = 0.0006), and ileal mucosa DNA and DPP-IV specific activity (R2 = 0.19, P = 0.003).
DISCUSSION
Luminal nutrients maintain intestinal cell turnover by acting directly to provide nutrients to the mucosa and triggering the release of hormones, such as GLP-2 to increase enteric blood flow. We have demonstrated that EN and GLP-2 have both unique and interrelated intestinotrophic actions, as reflected in the ability of EN to potentiate the intestinotrophic action of GLP-2 in parenterally fed rats with or without massive bowel resection (23, 24). This observation has clinical relevance to those who require PN but can often ingest only a small amount of food. Thus, we determined which of the enteral protein components in the semielemental liquid diet used in our studies—intact casein, hydrolyzed soy/collagen, WPC, and hydrolyzed WPC+casein—potentiates the ability of a low dose of GLP-2 to reverse PN-induced mucosal hypoplasia. Results indicate that ingestion of all forms of whey protein studied, but not intact casein or hydrolyzed soy/collagen, significantly potentiate mucosal growth in ileum based on histology and measures of mucosal cellularity, compared with treatment with PN+GLP-2 alone. The intestinotrophic effect of whey protein was most dramatic in ileum but present throughout the small intestine and colon.
The ability of whey protein to potentiate the intestinotrophic action of GLP-2 in parenterally fed rats was associated with a significant reduction in DPP-IV activity in ileum and colon compared with casein, soy, or PN+GLP-2 alone. GLP-2 is synthesized in ileum and colon and rapidly degraded by DPP-IV cleavage of the first two N-terminal amino acids (8) and removed from the circulation by the kidney (32). The half-life of GLP-2 in the circulation is ∼7 min, and reduced degradation of GLP-2 by DPP-IV, as occurs with the GLP-2 analog (Gly2)GLP-2, extends the half-life (8). We did not observe greater plasma concentration of bioactive GLP-2 in rats fed whey protein compared with casein or soy. However, the local concentration of bioactive GLP-2 in ileum may be higher with ingestion of whey due to reduced degradation by DPP-IV leading to greater paracrine stimulation of mucosal growth as was observed in ileum. Moreover, ileum DPP-IV-specific activity showed a significant negative correlation with indices of ileum mucosa growth. Consistent with this notion, mice fed whey protein show a 50% reduction in DPP-IV activity in the proximal small intestine in association with increased levels of the intact incretin hormones, GLP-1 and glucose-dependent insulinotropic polypeptide, as well as augmented insulin response and enhanced oral glucose tolerance (15). Humans fed a meal with whey protein compared with casein, cod, and wheat gluten also show a greater secretion of glucose-dependent insulinotropic polypeptide and insulin, but not GLP-1 (28, 29).
Rats fed the highest concentration of hydrolyzed WPC showed a dramatic decrease in plasma DPP-IV activity without an increase in plasma GLP-2 or greater intestinal growth compared with other groups fed whey protein. One explanation for this decrease in plasma DPP-IV activity given that rats were killed in a fed state is that peptide fragments from ingestion of hydrolyzed whey were absorbed (14) and acted as cosubstrates or competitive inhibitors for DPP-IV, which reduced activity overall. The hydrolyzed WPC used in this study contains ∼60% free amino acids, ∼33% <500D peptides, and no peptides >3 kDa. Chabance et al. (3) showed that several small peptides, as well as a 24 amino acid peptide, can cross the intestinal barrier and be detected in the plasma 20 min and 1 h after milk ingestion. Interestingly, rats fed soy protein as a hydrolysate of soy and collagen, which would also include peptide fragments, did not show enhanced ileal growth or reduced DPP-IV activity in plasma or in the intestine.
Consistent with the view that GLP-2 is regulated by luminal nutrients, all groups given partial EN that provided 43% of energy for days 4–7 showed significant induction of GLP-2 system responses, including significantly greater proglucagon expression in ileum, a 35% increase in plasma concentration of bioactive GLP-2, and reduced plasma DPP-IV activity compared with PN+GLP-2 alone. Moreover, all groups given EN showed improved digestive capacity as reflected in greater jejunal sucrase activity and gain in body weight, and improved feed efficiency. Ingestion of carbohydrate and fat, rather than protein, appears to be the major stimulus for secretion of GLP-2 from the intestinal endocrine L-cells in humans (9, 11, 33). However, peptones or protein hydrolysates stimulate GLP-1 secretion in isolated rat ileum (11) and the NCI-H716 human intestinal cell line (31). Our data confirm that five different forms of EN, containing a mixture of the same carbohydrate and fat, but different types of protein, showed a similar ability to increase the concentration of bioactive GLP-2 in plasma and stimulate enterocytes to express disaccharidase activity. However, only whey protein, and not casein and soy, induced greater mucosal cellularity and increased ileal villus height and crypt depth compared with PN+GLP-2 alone.
Perspectives and Significance
The presence of luminal nutrients regulates intestinal secretion of GLP-2. Our data show that ingestion of semielemental formulas with the same composition of carbohydrate and fat and different sources of protein show a similar ability to increase plasma concentration of bioactive GLP-2, digestive capacity, and weight gain. However, only whey protein, but not casein or soy, potentiates the ability of GLP-2 to reverse PN-induced mucosal hypoplasia leading to further increases in mucosal cellularity and absorptive surface area. Further studies are required to determine whether the ability of whey protein to promote adaptive intestinal growth is mediated by a reduction in intestinal DPP-IV activity that may improve the bioactivity of GLP-2 by prolonging its half-life. Given the trophic effects of supplemental oral feeding in many individuals with short bowel syndrome who require PN, formulas with whey protein should be encouraged when possible and especially when GLP-2 is administered.
GRANTS
This work was supported by National Institutes of Health grant R01-DK-42835 and by funds from the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service, project WISO 3433, University of Wisconsin-Madison.
ACKNOWLEDGMENTS
We thank Michael J. Grahn for his expert technical assistance. We acknowledge the generous contribution of Vital formula and the input of Dr. Jeff Baxter at Abbott Nutrition.
REFERENCES
- 1.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH, Jr, and Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr 71: 1603–1610, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Chabance B, Marteau P, Rambaud JC, Migliore-Samour D, Boynard M, Perrotin P, Guillet R, Jolles P, Fiat AM. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 80: 155–165, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem 7: 18–25, 1964 [DOI] [PubMed] [Google Scholar]
- 5.Dahly EM, Gillingham MB, Guo Z, Murali SG, Nelson DW, Holst JJ, Ney DM. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol 284: G670–G682, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr 132: 2010–2014, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol 285: R800–R808, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Dube PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab 293: E460–E465, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dube PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm Metab Res 36: 755–760, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology 131: 589–605, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139: 3780–3786, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Estall JL, Drucker DJ. Glucagon-like Peptide-2. Annu Rev Nutr 26: 391–411, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Ferrone M, Scolapio JS. Teduglutide for the treatment of short bowel syndrome. Ann Pharmacother 40: 1105–1109, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Gardner ML. Gastrointestinal absorption of intact proteins. Annu Rev Nutr 8: 329–350, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahren B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 147: 3173–3180, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides 21: 73–80, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen PB, Hartmann B, Thulesen J, Graff J, Lohmann J, Hansen BS, Tofteng F, Poulsen SS, Madsen JL, Holst JJ, Mortensen PB. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology 120: 806–815, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kang W, Gomez FE, Lan J, Sano Y, Ueno C, Kudsk KA. Parenteral nutrition impairs gut-associated lymphoid tissue and mucosal immunity by reducing lymphotoxin Beta receptor expression. Ann Surg 244: 392–399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmann MC, Nelson DW, Murali SG, Liu X, Brownfield MS, Holst JJ, Ney DM. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. JPEN J Parenter Enteral Nutr 32: 254–265, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg 223: 629–635; discussion 635–628, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102: 344–352, 1980 [DOI] [PubMed] [Google Scholar]
- 22.Lasekan JB, Rivera J, Hirvonen MD, Keesey RE, Ney DM. Energy expenditure in rats maintained with intravenous or intragastric infusion of total parenteral nutrition solutions containing medium- or long-chain triglyceride emulsions. J Nutr 122: 1483–1492, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Murali SG, Holst JJ, Ney DM. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am J Physiol Regul Integr Comp Physiol 295: R1794–R1802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr 84: 1142–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 25.McArdle AH, Wittnich C, Freeman CR, Duguid WP. Elemental diet as prophylaxis against radiation injury. Histological and ultrastructural studies. Arch Surg 120: 1026–1032, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Nagatsu T, Hino M, Fuyamada H, Hayakawa T, Sakakibara S. New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem 74: 466–476, 1976 [DOI] [PubMed] [Google Scholar]
- 27.Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol 294: R1175–R1184, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Nilsson M, Holst JJ, Bjorck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr 85: 996–1004, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 80: 1246–1253, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology 142: 4522–4528, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Grande C, Pintado J, Alarcon C, Castilla C, Valverde I, Lopez-Novoa JM. Renal catabolism of human glucagon-like peptides 1 and 2. Can J Physiol Pharmacol 68: 1568–1573, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Xiao Q, Boushey RP, Drucker DJ, Brubaker PL. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 117: 99–105, 1999 [DOI] [PubMed] [Google Scholar]