Abstract
Anticancer agents, such as cisplatin, stimulate nausea, vomiting, and behaviors indicative of malaise. Rats and mice do not possess a vomiting response, and, therefore, in these species, the ingestion of kaolin clay (a pica response) has been used as an index of malaise. In the rat, cisplatin-induced kaolin intake is inhibited by antiemetic treatments. In addition, cisplatin activates vagal afferent fibers in the gut, and kaolin intake induced by cisplatin is largely dependent on an intact vagus. Nevertheless, little is known about the brain pathways controlling pica. We investigated the role of the lateral parabrachial nucleus (lPBN), a major visceral afferent link between the hindbrain and forebrain, in cisplatin-induced c-Fos expression and pica. Injection of cisplatin (6 mg/kg ip) produced c-Fos expression in the ventrolateral (external) lPBN, a region receiving viscerosensory input. In rats with bilateral ibotenic acid lPBN lesions, cisplatin treatment substantially increased kaolin intake compared with controls (∼30 g vs. ∼5 g, respectively, over 24 h). Food intake was reduced by cisplatin treatment and by apomorphine, an emetic agent that acts centrally. Unlike cisplatin, however, apomorphine stimulated kaolin intake to a similar degree in both the lesioned and control rats, suggesting that lPBN damage neither produces nonspecific effects nor enhances malaise in general. These data suggest that lPBN-lesioned animals not only demonstrate pica after cisplatin treatment, but, in fact, show an exaggerated response that is greatly in excess of any treatment known to produce kaolin intake in rats.
Keywords: emesis, nausea, pica, vagus, anorexia
cancer chemotherapy agents, such as cisplatin, stimulate nausea, vomiting, anorexia, and other behaviors indicative of malaise (for a review, see Refs. 2, 21, 27, 51). Several species, including dogs, cats, ferrets, pigs, and shrews, have been used to study emesis after treatment with chemotherapy agents (16, 18, 31, 33, 58). In contrast, laboratory rats and mice appear to lack a vomiting response (see Ref. 2 for review). For these species, kaolin intake has been used as a proxy for emesis (60, 64). A wide variety of stimuli, including cisplatin, induce kaolin consumption in rats (36, 53, 59, 60, 65). Like emesis, geophagia appears to be a defensive response. Clay may serve to bind or dilute a toxin in the gastrointestinal (GI) tract, thus reducing its adverse effects (45, 46). After injection with cisplatin, rats consuming kaolin show less body weight loss and a smaller reduction in food intake than rats without kaolin access (12). The consumption of clay by humans is also recognized as a potential detoxification strategy (48).
Despite the use of kaolin intake as an index of malaise in rats, very little is known about the neural systems that generate this behavior. Similar to the action of cisplatin on emesis (1, 7, 50), cisplatin-induced kaolin consumption in the rat is largely dependent on an intact subdiaphragmatic vagus (10) and is inhibited by common antiemetic drugs, such as 5-HT3 and NK1 receptor antagonists, as well as by corticosteroids (29, 49, 60). Cisplatin also activates GI vagal afferent fibers in the rat and ferret, a response that is blocked by antagonism of 5-HT3 receptors (15, 24). Furthermore, cisplatin stimulates c-Fos expression in brain areas receiving vagal afferent input—the nucleus of the solitary tract (NTS) and area postrema (AP)—and the extended amygdala of the rat (22, 23) and musk shrew (11), suggesting that a distributed neural system is involved in the behavioral effects of this drug.
Here, we investigated the role of the lateral parabrachial nucleus (lPBN) in cisplatin-induced kaolin consumption because this pontine area serves as an important connection between hindbrain and forebrain components of the gut-brain axis. The PBN receives projections from the NTS and AP and has outputs to the extended amygdala, including the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST) (25, 28, 39, 41, 62). In earlier studies, we reported brain c-Fos expression after cisplatin treatment, but we did not analyze the pons and midbrain (22, 23). Therefore, in the current report, we conducted an experiment to assess c-Fos expression in the lPBN after cisplatin injection. We also made bilateral ibotenic acid lesions that included the lPBN areas in which c-Fos was concentrated after cisplatin and compared the behavioral responses of lesioned and control animals after identical drug treatment. We assessed the impact of lPBN lesions on cisplatin-induced kaolin intake, anorexia, water consumption, and body weight. We also compared the behavior of lPBN-lesioned animals with controls after apomorphine injections because this emetic drug and cisplatin apparently stimulate kaolin intake by different routes, blood-born and vagal afferent activation, respectively, as suggested by our previous work (10). On the basis of the position of the lPBN to connect hindbrain and forebrain viscerosensory pathways, we hypothesized that bilateral lesion of the lPBN would reduce both cisplatin- and apomorphine-induced kaolin intake.
MATERIALS AND METHODS
Subjects
Forty-five male Sprague Dawley rats (Charles River; Kingston, NY) were housed individually in mesh-floored stainless-steel hanging cages (25 × 18 × 19 cm) and maintained in a temperature-controlled vivarium (∼23°C), with a 12:12-h light-dark cycle (lights on at 0600). All rats had ad libitum access to water. Animals also had free access to standard rat chow during testing in experiments 1 and 2 (LabDiet 5001, PMI Nutrition) and prior to and several days after surgery in experiment 2 (Rodent Diet-W 8604; Harlan Teklad, Madison, WI). The protocol was approved by the Institutional Animal Care and Use Committees at the Monell Chemical Senses Center and The Pennsylvania State University College of Medicine.
Experiment 1: Cisplatin-Induced c-Fos Expression
Thirteen rats were used for testing c-Fos expression after injection of saline (0.15 M NaCl; n = 7) or cisplatin (6 mg/kg; n = 6). The animals received an intraperitoneal injection of 2 ml/kg body wt. To adapt the animals to handling and injection, rats received a mock injection for the 2 days prior to testing, in which a syringe needle was inserted but no fluid was injected. On the test day, the animals were injected with saline or cisplatin between 0900 and 1145. Food cups were removed 2 h prior to euthanization to eliminate the possibility of increased brain c-Fos expression by eating behaviors. At the time of euthanization, rats weighed 441 ± 13 g (means ± SE) in the saline group and 445 ± 11 g in the cisplatin group.
Perfusion and fixation.
Twenty-four hours after treatment with saline or cisplatin, the rats were deeply anesthetized with sodium pentobarbital (50 mg ip, total per animal). The thoracic cavity was opened and, to assure a thorough fixation of the brain, rats were given 0.3 ml of heparin (1000 IU/ml) intracardially. This was followed by transcardial perfusion (∼20 to 50 ml/min) with 300 ml of 0.2 M PBS (pH 7.4) and then 500 ml of 2% acrolein-4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The animals were then perfused with an additional 150 ml of PBS to remove excess fixative. The brains were removed and placed in 10% sucrose/PBS followed by 20% and 30% sucrose/PBS, each for 24 h. After cryoprotection in sucrose, the brains were blocked, frozen on dry ice, and cut coronally in three series at 50 μm using a freezing microtome. After perfusion, stomachs were also removed and weighed because reduced gastric emptying is an indicator of the efficacy of the cisplatin treatment (29).
c-Fos immunohistochemistry.
Immunohistochemistry was conducted as previously described (38). Briefly, after rinses in 0.1 M PBS, the sections were treated with 0.5% NaBH4 (Sigma-Aldrich, St. Louis, MO) in 0.1 M PBS (pH 7.3–7.4), rinsed in PBS several times, then put in 5% goat serum (catalog no. G-6767; Sigma Chemical) in 0.4% Triton X-100/PBS for 1 h. They were then incubated for 24 h at room temperature in primary polyclonal IgG rabbit c-Fos antibody (1:3,000; catalog no. SC-52, Lot#D1508 Santa Cruz Biotechnology, Santa Cruz, CA) along with goat serum [5.0 μl/ml] in 0.4% Triton X-100/PBS. After further rinses with PBS, the sections were incubated for 2 h in secondary biotinylated goat anti-rabbit IgG and 0.4% Triton X-100/PBS (1:500; catalog no. 62-6140; Zymed Laboratories, San Francisco, CA), and mixed with goat serum [10.0 μl/ml]. After three more PBS rinses, the sections were exposed for 1 h to conjugated avidin-biotin complex [3.0 μl/ml each of solutions “A” and “B” from a Vectastain Elite kit (#PK-6100; Vector Laboratories, Burlingame, CA)] in 0.4% Triton X-100/PBS. Following three more PBS rinses, a visible immunoreactivity product was produced after 5 min of incubation in a 0.175 M sodium acetate (CH3COONa) solution containing 3,3-diaminobenzidine tetrahydrochloride [DAB; 0.35 mg/ml], nickel ammonium sulfate [NiSO4(NH4)2 6H2O, 0.01g/ml] and 30% H2O2 [0.3 μl/ml] (Sigma-Aldrich). The tissue then went through final PBS rinses and was mounted onto slides. The c-Fos immunohistochemical reaction product appears black in the nuclei of neurons.
Fos distribution and quantification.
The sections were examined with a light microscope (Nikon Optiphot, Tokyo, Japan), equipped with a digital video camera (Diagnostic Instruments, Sterling Heights, MI). With the objective focused on the upper surface of the section, specific regions were captured with video-imaging software (Spot for Windows, version 4.0.4) and subsequently analyzed with Optimas (Bioscan, Edmonds, WA). c-Fos cell staining was counted in the NTS and subareas of the PBN. The NTS was used as a positive control since we know that cisplatin consistently induces c-Fos expression in this area (22–23). Within these areas, the c-Fos immunoreactivity profiles were counted by Optimas based on a grayscale reference. Whenever possible, the same grayscale threshold was maintained for all areas within each brain. In any event, counting was not automatic. The program outlines each counted area, and this permitted the investigator to inspect it both in the digitized image and through the microscope. This reduces counting artifacts and undercounting-labeled nuclei that are stacked on one another. Most areas required counting over multiple sections, but the same number of sections was counted for each area in all brains. The results are presented as the average number of labeled cells per area. See Mungarndee et al. for further details (38).
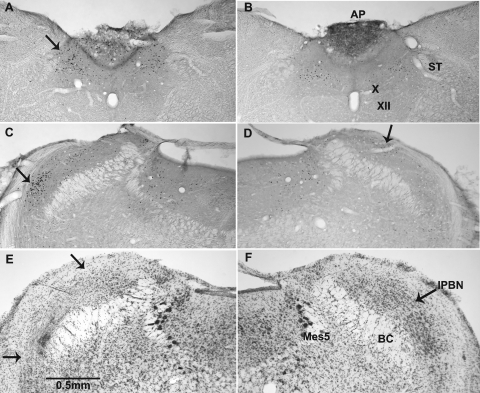
On the basis of visual inspection, three areas were identified for counting c-Fos expression, one in the caudal nucleus of the solitary tract (NTS; Fig. 1 A, arrow) and two in the lPBN (Fig. 1, C and D, arrow). In both the NTS and lPBN, the area in which c-Fos profiles were counted was determined by a computer-generated circle. The size of circle was held constant for each comparable section across all the experimental and control brains. The placement and size of the circle varied slightly from one level to the next because the relative size and position of the NTS and lPBN changes in the anterior-posterior plane. Relative to the clusters of c-Fos-positive neurons, the size of the circles was made large enough that slight differences in their placement did not materially affect the counts.
Fig. 1.
Digital photomicrographs of 50-μm coronal sections through the caudal medulla (A and B) and dorsal pons (C–F). A–D: photomicrographs from brains processed for immunohistochemical identification of c-Fos protein in experiment 1. E and F: photomicrographs from brains stained with cresyl violet in experiment 2. A and C: photomicrographs from rats treated with cisplatin. B and D: photomicrographs from those treated with saline. Arrows in A, C, and D indicate the nucleus of the solitary tract (NTS), the ventrolateral, or external, lateral parabrachial nucleus (lPBNv), and the lateral, dorsal parabrachial nucleus (lPBNd), respectively. Panel E depicts the extent of the ibotenic acid lesion in the lPBN (between the arrows). F: section from a control rat at the same rostrocaudal level of the PBN. The value of the line in E is approximate because the immunohistochemical processing shrinks sections 15–20% more than cresyl violet. AP, area postrema; BC, brachium conjunctivum; Mes5, mesencephalic trigeminal nucleus; ST, solitary tract; X, dorsal motor nucleus of the vagus; XII, hypoglossal nucleus.
Experiment 2: Cisplatin-Induced Pica in Lateral PBN-Lesioned Rats
Excitotoxic brain lesions.
Before surgery, animals assigned to the groups weighed 329.6 ± 14.7 g (means ± SE), unoperated controls; 324.5 ± 15.3 g, operated controls; and 320.8 ± 27.1 g, lPBN lesioned animals. The lesions were produced following a standard protocol and, thus, are abbreviated here (for details see Ref. 17). After being anesthetized with pentobarbital sodium (Nembutal, 50 mg/kg ip), the rat was mounted in a stereotaxic instrument, and the cerebellum was exposed via bilateral burr holes in the interparietal bone. Gustatory responses were identified in the pontine parabrachial nuclei (PBN) using glass insulated tungsten electrodes (∼1.0 MΩ), conventional amplification, and 0.1 M NaCl as a sapid stimulus. Once taste responses were identified bilaterally, the recording electrode was replaced with a 1.0-μl microsyringe (Hamilton) with a sturdy glass micropipette glued to the end. This assemblage was filled with ibotenic acid (IBO, 20 μg/μl in PBS, pH 7.4) and repositioned 0.5 mm anterior, lateral, and deep to the taste coordinates to approximate the center of the lateral PBN. With the pipette in place, 160–170 nl of IBO was infused over 10 min. The pipette was then left in place for an additional 10 min. The entire procedure was then repeated on the contralateral side. Bilateral lPBN lesions (lPBNx) were completed in 14 rats. Two of these died before the experiment was completed, so the experimental group had an n of 12. In addition, there were two control groups. The surgical controls (n = 10) were treated identically to the lPBNx group except that the recording electrode penetrated only into the cerebellum and no pipette (or injection) was used. The remaining 10 rats were naïve controls. After surgery, the rats had a minimum of 1 wk to recover before transport from Hershey to Philadelphia, PA. Once there, an additional recovery week was given before testing began.
Behavioral testing.
At the beginning of behavioral testing, the animals weighed 491.2 ± 10.1 g (means ± SE), unoperated controls; 494.8 ± 15.7 g, operated controls; and 491.3 ± 11.9 g, lPBN lesioned. Rats were given access to kaolin pellets (∼50 g), placed in a food hopper hung from the back of the animal cage. Standard powdered rat chow (∼100 g) was available in an open-topped glass jar attached to the front panel of the animal cage; water was provided ad libitum. Body weight, water bottles, food cups, and kaolin hoppers, as well as spillage from the latter two, were weighed daily at ∼0900. Chow and kaolin spillage was easily separated because the former is brown, the latter white. After a week of adaptation to these circumstances, all animals received an injection of apomorphine (10 mg·kg−1·ml−1 ip). Four days later, they received an injection of saline (2 ml/kg ip). After three more days, they received an injection of cisplatin (6 mg·kg−1·2 ml−1). The timing and order of treatments was based on our previous studies showing no effects of the control injections on kaolin intake, no lasting effect of apomorphine treatment, and a significant long-term effect of cisplatin injection on food intake and body weight (10, 12). Three days after the cisplatin injection, the animals were anesthetized (pentobarbital sodium, 50 mg ip, total per animal) and then euthanized by exsanguination and perfused using the method described for experiment 1.
Histology.
As in experiment 1, the brains were blocked, frozen, and cut at 50 μm in 3 coronal series. Different series of sections were stained for NeuN, a neuron-specific protein (38), and with cresyl violet. The NeuN series was of poor quality, low contrast, and variable staining, and thus were of limited use in determining the extent of the lesions. The adequacy of the lesions was judged from the cresyl violet series by comparing the areas without neurons with comparable areas in the brains of the PBS-injected controls. The PBN was defined from neuroanatomical criteria (39, 42, 52).
Statistical analysis.
c-Fos cell counts, body weight, food, water, and kaolin intake data are expressed as means ± SE. c-Fos cell counts were compared using independent sample Student's t-tests. For behavioral measures and body weight data, three-way (2 × 3 × 4) ANOVA were conducted using treatment (saline or drug), surgical condition (unoperated control, operated control, or lPBN lesioned), and time (4 days; 1 day prior and 3 days after injection) as factors. Body weight measures were transformed to percent baseline body weight (baseline = body weight at 2 days prior to an injection). Planned comparisons were performed using the Benjamini-Hochberg procedure to control Type I error rate (6). Differences were considered statistically significant if P < 0.05 for ANOVA or P < 0.025 to 0.001 for Benjamini-Hochberg tests. Statistical analyses were computed with Statistica (ver. 8.0, StatSoft, Tulsa, OK) and Excel (Microsoft).
RESULTS
Experiment 1: Cisplatin-Induced c-Fos Expression
c-Fos expression.
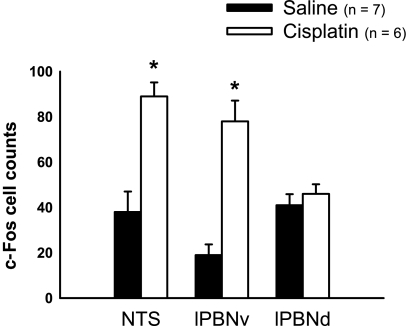
In the NTS, c-Fos expression was concentrated in areas adjacent to the AP (Fig. 1A). Within the NTS counting area, the rats injected with cisplatin showed significantly more c-Fos expression than saline-treated controls [t(11) = 4.6, P < 0.001; Fig. 2, NTS].
Fig. 2.
Average c-Fos-positive cell counts in the NTS, the lPBNv, and the lPBNd in rats injected with saline or cisplatin (6 mg/kg). The counts were made bilaterally, and the two sides were averaged. *P < 0.05, Student's t-test, saline vs. cisplatin. Values are expressed as means ± SE.
In the lPBN, two areas were counted, one ventrolateral (Fig. 1C, arrow), the other dorsal (Fig. 1D, arrow). In the ventrolateral area, cisplatin-treated rats displayed consistently strong c-Fos label compared with saline controls [t(11) = 6.0, P < 0.001; Fig. 2, lPBNv]. The more dorsal area in lPBN also had consistent, if less dense, c-Fos label, but it did not differ between treatment groups [t(11) = 0.80, P = 0.4; Fig. 2, lPBNd].
Stomach weights.
As an additional test for the potency of cisplatin, we also measured the weight of the excised stomach and its contents at the time of euthanization. Animals injected with cisplatin had significantly greater stomach weights than saline-treated rats [12.5 ± 1.9 vs. 8.0 ± 1.4 g; t(11) = 1.9, P < 0.05, one-tailed].
Experiment 2: Cisplatin- and Apomorphine-Induced Pica in lPBN Lesioned Animals
Lesion verification.
All 12 of the experimental animals had bilateral damage in the lPBN on both sides. Histology also was done on 6 of the 10 surgical controls, and none of them showed signs of neural damage in the PBN or elsewhere in the brain stem. The brain of one rat with lesions was poorly preserved. In this case, it was possible to determine that damage existed in the lateral PBN on both sides but not the extent of the damage. The behavioral data from this animal were consistent with that of the other rats with lesions, so they were included in the analysis. In these brains, the NeuN stain failed to work consistently, so the evaluation of the lesions relied primarily on the cresyl violet series (Fig. 1, E and F).
Eight of the twelve lesioned rats had sparing of the gustatory or medial PBN on at least one side, four unilaterally and four bilaterally. This sparing often included the most dorsomedial aspect of lPBN. This demonstrated that the damage to the medial PBN and the dorsomedial lPBN was not necessary to alter the effects of cisplatin. In all of the other brains, the lesions spread into the supratrigeminal area (between the PBN and the trigeminal motor nucleus), the Kolliker-Fuse nucleus (rostroventral to PBN), and in some cases the dorsal (intraoral) aspect of the principal trigeminal nucleus. Thus, it is difficult to rule out these structures as contributing to the effect. Nevertheless, one rat had lateral PBN damage confined to that subnucleus on one side (Fig. 1E). This animal consumed 24.4 g and 34.9 g of kaolin on the two test days: perfectly representative amounts for the PBNx group. Although only a single example, this militates against these adjacent structures as mediating the effect on pica. In fact, despite the damage in and near the central trigeminal apparatus, during the control period, the group with PBN lesions ate and drank amounts virtually identical to that of both control groups.
Cisplatin treatment.
There were significant three-way interaction effects of surgical condition with drug injection and test day on kaolin intake, food intake, water intake, and percent body weight [kaolin, F(6,87) = 12.4, P < 0.000001; food, F(6,87) = 3.9, P < 0.005; water, F(6,84) = 2.9, P < 0.05; body weight, F(6,87) = 12.3, P < 0.000001].
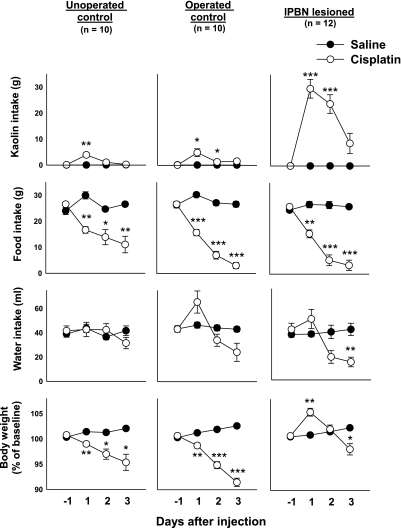
Figure 3 shows mean comparisons between saline and cisplatin at each time point. Compared with saline injections, cisplatin induced kaolin intake on days 1 and 2 in operated control and lPBN-lesioned rats and on day 1 in unoperated controls (Fig. 3; Benjamini-Hochberg tests). After cisplatin injection, food intake was significantly suppressed in all groups on days 1–3 (Fig. 3; Benjamini-Hochberg tests). On day 3, in lPBN-lesioned animals, cisplatin treatment reduced water intake (Fig. 3; Benjamini-Hochberg test). Percent baseline body weight was reduced on days 1–3 after cisplatin treatment in unoperated and operated control animals (Fig. 3; Benjamini-Hochberg tests). In comparison, lPBN-lesioned animals showed a significant increase in body weight from baseline on day 1 after cisplatin injection, but this declined by day 3 (Fig. 3; Benjamini-Hochberg tests).
Fig. 3.
Cisplatin-induced kaolin intake and alterations in food intake, water intake, and body weight in lPBN-lesioned and control animals (unoperated and operated). Rats were injected (ip) with saline or cisplatin (6 mg/kg) after 24 h of baseline measurements (day −1). *P < 0.01, **P < 0.0005, ***P < 0.000001, Benjamini-Hochberg-corrected test, saline vs. cisplatin at each time point. Note that the y-axis for kaolin intake has been expanded to include the lPBN lesion group data. However, the unoperated controls had an increase from 0.1 ± 0.0 to 3.9 ± 0.9 g, and the operated controls had an increase from 0.1 ± 0.0 to 4.9 ± 1.5 g from day −1 to day 1 after cisplatin injection. Values are expressed as means ± SE.
Mean comparisons between only cisplatin-treated lPBN-lesioned and control animals were also conducted. After cisplatin treatment, rats with lPBN lesions ingested significantly more kaolin than unoperated controls on days 1–3 and operated controls on days 1 and 2 (P ≤ 0.01, Benjamini-Hochberg tests). There were no significant differences between water and food intake measures across surgical conditions. Percent body weight also increased significantly from baseline in lPBN-lesioned rats compared with unoperated controls on days 1–3 and operated controls on days 1 and 2 (P ≤ 0.01, Benjamini-Hochberg tests).
Apomorphine treatment.
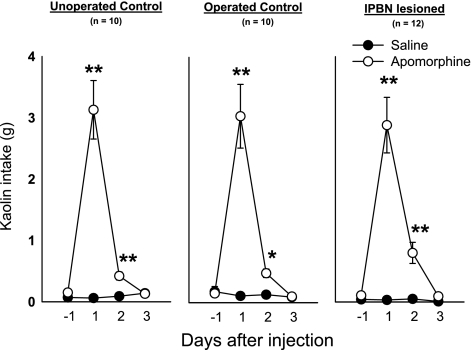
There were no significant effects of surgical condition on apomorphine-induced kaolin intake, food intake, water intake and body weight [all ANOVAs for interaction or main effects of surgical condition were nonsignificant]. For example, unoperated controls, operated controls, and lPBN-lesioned animals consumed 25.0 ± 3.1, 24.2 ± 3.0, and 23.1 ± 2.9 g of food, respectively, for the 24 h after injection. There were significant effects of apomorphine treatment on kaolin intake, food intake, and percent body weight over test days [kaolin, F(3,87) = 91.2, P < 0.000001; food, F(3,87) = 15.2, P < 0.000001; body weight, F(3,87) = 5.6, P < 0.005, injection by day interaction effect]. Apomorphine stimulated kaolin consumption on days 1 and 2 postinjection (Fig. 4; Benjamini-Hochberg tests) in all surgical conditions. The only significant effect of apomorphine on a mean comparison of food intake occurred in the operated control animals on day 1 postinjection (data not plotted; saline, 30.3 ± 0.9 vs. apomorphine, 24.2 ± 1.0, Benjamini-Hochberg test, P < 0.001). Although not statistically significant, there was also a trend in the other groups for apomorphine to reduce food intake on day 1 postinjection (unoperated control, saline, 30.0 ± 1.4 vs. apomorphine, 25.0 ± 1.0; lPBN-lesioned, saline, 26.8 ± 1.2 vs. apomorphine, 23.1 ± 1.1; nonsignificant Benjamini-Hochberg test). Although planned comparisons revealed no significant effects of apomorphine on body weight, there was a small tendency for apomorphine to reduce percent body weight on day 1 postinjection (unoperated control, saline, 101.4 ± 0.4 vs. apomorphine, 100.5 ± 0.4; operated control, saline, 101.2 ± 0.2 vs. apomorphine, 100.6 ± 0.7; PBN lesioned, saline, 100.9 ± 0.2 vs. apomorphine, 99.8 ± 0.5; nonsignificant Benjamini-Hochberg tests). There were no statistically significant effects of apomorphine on water intake using ANOVA or planned comparisons.
Fig. 4.
Apomorphine-induced kaolin intake in lPBN-lesioned and control animals (unoperated and operated). Rats were injected (ip) with saline or apomorphine (10 mg/kg) after 24 h of baseline measurement of kaolin intake (day −1). *P < 0.01, **P < 0.0005, Benjamini-Hochberg corrected test, saline vs. apomorphine at each time point. Values are expressed as means ± SE.
DISCUSSION
The current data reveal both behavioral and immunohistochemical evidence that the lPBN may function in the expression and modulation of chemotherapy-induced malaise. c-Fos expression results show that the lPBN (ventrolateral or external area) is activated by systemic injection of cisplatin. Furthermore, bilateral ibotenic acid lesions of the lPBN produced a dramatic increase in cisplatin-induced kaolin intake. Cisplatin treatment stimulated kaolin consumption in all groups, but lesioned animals showed a greater than 600% increase in kaolin intake by 24 h after injection compared with controls. Food intake was reduced to a similar degree by cisplatin treatment in lesioned and control animals, and all animals showed a similar level of kaolin intake after apomorphine, an emetic agent believed to act directly on the hindbrain (3, 19, 26).
After an injection of cisplatin, c-Fos expression in the NTS was present in areas that receive afferent input from the GI tract (Fig. 1A) (40). In turn, neurons from this region of the NTS project to the ventrolateral (external) lPBN (20), where c-Fos-positive cells were concentrated after cisplatin treatment in the current study. The lack of differential c-Fos label between the cisplatin and saline groups in the dorsal lPBN demonstrates that the effect of the chemotherapeutic treatment did not reflect generalized neural activation of the PBN. The parabrachial nuclei project monosynaptically to the limbic system, particularly to the hypothalamus, CeA and BNST (39), and these areas exhibit increases in c-Fos-positive neurons after intraperitoneal cisplatin injections (22, 23).
This leads to the hypothesis that cisplatin treatment activates a pathway beginning at the level of GI vagal afferent fibers that extends through the NTS, PBN, and forebrain areas to produce c-Fos expression and perhaps pica. Our recent work demonstrated that ablation of the subdiaphragmatic vagus (SDX) blunted the effects of cisplatin treatment on c-Fos expression in the NTS and CeA (22), but the effects in the PBN were not assessed. Furthermore, we showed that cisplatin-induced pica is reduced by cutting the common hepatic branch (CHB) but not by section of the celiac, accessory celiac, or ventral gastric branches (10). These branches are sub-branches of the SDX, with the CHB innervating the duodenum and liver, the celiacs innervating the distal small intestine, and the ventral gastric innervating the stomach (44, 63).
These residual effects on c-Fos expression and pica after vagal section could be mediated via afferent neurons in the splanchnic nerves that synapse first in the spinal cord. At least some of the second-order neurons in this so-called sympathetic afferent system terminate in the most caudal aspect of the NTS (54), but cisplatin does not significantly increase c-Fos expression in the spinal cord (22). Another possible route of action could be via blood-born cisplatin, or another humoral factor released by cisplatin, acting directly in the hindbrain, perhaps in the AP. In this case, the subsequent rostral projections to the PBN, and other areas, would closely parallel or overlap those from the subjacent NTS. Indeed, cisplatin produces a strong c-Fos response in the AP (22, 23), and it is known that AP ablation can disrupt cisplatin-induced emesis in the cat (34); however, caution should be used in interpreting AP lesion experiments because the AP also receives vagal afferent input (40, 57).
If the presumed pathway through the PBN is important for the expression of cisplatin-induced pica, then lesion of this area should produce a reduction in this response. Sectioning only the CHB blunts the pica response (10) so, logically, if this afferent vagal activity is interrupted anywhere along its central course, the ingestion of clay should also be diminished. As is evident in experiment 2, this is far from the case.
In fact, lPBN lesions vastly increased cisplatin-induced pica. Indeed, regardless of the stimulus—a wide range of toxins and motion—the norm is kaolin consumption up to 6 g in 24 h (e.g., 36, 53, 59, 60, 65). We are not aware of any stimulus that produces the excessive kaolin intake reported here. lPBN-lesioned animals, when injected with 6 mg/kg cisplatin, ingested nearly 30 g of kaolin in 24 h. The body weight increase in lPBN-lesioned rats is likely the result of this excessive consumption of clay (Fig. 3). In lesioned animals, there was a close relationship between the amount of ingested clay, ∼30 g, on day 1 postinjection, and an increase in body weight, ∼5% (or ∼25 g), which suggests that much of the kaolin remained in the GI tract. Although kaolin intake is used as a metric for malaise, it appears unlikely that lPBN-lesioned animals experienced more malaise than control rats because they had similar levels of basal and reduced food intake after cisplatin treatment. Another possibility is that lPBN damage blocks the inhibition of intake normally signaled by the GI tract. Rats with large PBN lesions typically overconsume normally preferred fluids, such as sucrose, while exhibiting more or less normal rejection of aversive stimuli such as quinine (56). Also, in short-term tests, overconsumption is common after PBN lesions (see Fig. 4 in Ref. 37 for both mPBNx and lPBNx groups; CS and water intakes are almost double that of controls); the normal feeding behavior of the same rats does not vary significantly from controls. The lPBN contains neurons that respond to gastric stretch, and it is possible that destruction of these cells removed this inhibitory pathway (4, 5). Necropsies of lPBN-lesioned animals, at the end of day 3 postinjection, revealed that the stomach was exceptionally full with kaolin. Cisplatin also produces gastric stasis in normal control animals (e.g., see results and Ref. 29), which might enhance inhibitory feedback related to gastric fill. A lack of inhibition or gating might result from ablation to lateral parabrachial input to the ventrolateral medulla (52), which has an ascending projection to the CeA (43). Cisplatin treatment produces a robust increase in c-Fos expression in the CeA (11, 22, 23), but we have not examined the potential for facilitation of this response in lPBN-lesioned animals.
Cisplatin is well known for producing nephrotoxicity (66), and this can lead to mineral loss, such as excess secretion of sodium and magnesium (9, 30, 32). Reports indicate that lesion or antagonism of serotonin receptors in the lPBN produces enhanced drinking, including salt solution intake, in rats in response to hypovolemia or injection of angiotensin (13–14). Although kaolin is aluminum silicate and would likely not reduce mineral deficiencies produced by cisplatin treatment, it is possible that lPBN-lesioned animals overconsume kaolin because of an enhanced mineral appetite. Other measures of ingestion appear to be unaffected by combination of lPBN ablation and cisplatin injection. Water and food intakes after injection with cisplatin were nearly identical in both surgical groups, with less food intake and more disruption of normal water intake compared with unoperated control animals. There appears to be a small effect of the surgery on these measures (see Fig. 3). Body weight differences were difficult to evaluate because they were confounded by the amount of kaolin in the GI tract of lPBN-lesioned animals.
The present results show that an intact lPBN is not necessary for pica induced by apomorphine or cisplatin treatments. In species with a vomiting response, such as the ferret, apomorphine induces emesis by action on the hindbrain, possibly by targeting dopamine type 2 receptors, a standard emetic trigger (3, 19, 26). We have also reported that vagal lesions in the rat have no effect on apomorphine-induced kaolin intake (10), suggesting that apomorphine-induced pica is also the result of direct action of this drug on the hindbrain. Apomorphine treatment also induces c-Fos expression in the ventrolateral lPBN of the rat (8). Apomorphine produced the same level of kaolin intake in lPBN-lesioned and control animals, which indicates that the lPBN is not necessary for this response either. Although this is not compatible with the idea that lPBN-lesioned rats have a lack of inhibitory feedback related to gastric stretch, it is possible that the level of stimulation might be important, for example, lPBN-lesioned animals might ingest more kaolin relative to controls with a higher dose of apomorphine. This would provide further support for the hypothesis that lPBN lesions decrease the signals related to stretch of the GI tract. However, this could be difficult to test since apomorphine, a dopamine agonist, also produces a potent increase in locomotion (47), and it is possible that higher doses would produce additional movements that could interfere with ingestive behavior.
Pica, emesis, and conditioned taste aversion (or avoidance) are responses that are often used to assess malaise in experimental animals (2). A common role for the PBN in these behaviors associated with malaise is not readily apparent. Although it is clear that the caudal hindbrain contains the motor circuitry sufficient for the vomiting response (35, 55), the role of the PBN in emesis has not been investigated. Furthermore, conditioned flavor aversion (or avoidance) learning is also a common method for determining the malaise produced by toxins. The PBN receives taste and visceral input required for this effect, and lesions of this area eliminate the acquisition of conditioned taste aversion (or avoidance) (e.g., 17).
Perspectives and Significance
The present work is the first to show the effects of an lPBN lesion on pica. A prior study demonstrated that ablation of more rostral sites, such as the amygdala or hippocampus, can reduce or enhance kaolin intake in rats exposed to motion (61). Although the prediction that lPBN lesion would block or decrease cisplatin- or apomorphine-induced kaolin intake was not supported by the current data, these results suggest that the lPBN might play an important role in modulating the amount of kaolin intake, at least when rats are injected with a highly toxic drug like cisplatin. The current data are important because they contribute to insight into the neural pathways that mediate pica, a common index of malaise. Unfortunately, animal models that are routinely used for biomedical research, such as laboratory strains of mice, rats, and rabbits, do not possess a vomiting response (2), which makes it difficult to determine the presence of visceral sickness. By investigating the role of the PBN and other brain regions in pica, we can more fully understand the neural pathways engaged in this behavioral test, particularly in research involving toxic drugs, such as cancer chemotherapy agents.
GRANTS
This work was supported by National Institutes of Health Grants DK065971, DC000240, and DC000014. Publication costs were funded by the University of Pittsburgh Cancer Institute, Biobehavioral Medicine in Oncology Program.
ACKNOWLEDGMENTS
We thank Nelli Horvath, Matthew Rosazza, and Liz Still for expert assistance. We also appreciate the helpful comments of the University of Pittsburgh Cancer Institute writing group and the reviewers on earlier versions of this manuscript.
REFERENCES
- 1.Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol 68: 325–345, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci 125: 100–115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews PL, Kovacs M, Watson JW. The anti-emetic action of the neurokinin(1) receptor antagonist CP-99,994 does not require the presence of the area postrema in the dog. Neurosci Lett 314: 102–104, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Baird JP, Travers JB, Travers SP. Parametric analysis of gastric distension responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1568–R1580, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125: 279–284, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bountra C, Bunce K, Dale T, Gardner C, Jordan C, Twissell D, Ward P. Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur J Pharmacol 249: R3–R4, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Chambers KC, Wang Y. Role of the lateral parabrachial nucleus in apomorphine-induced conditioned consumption reduction: cooling lesions and relationship of c-Fos-like immunoreactivity to strength of conditioning. Behav Neurosci 118: 199–213, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol 50: 147–158, 1993 [DOI] [PubMed] [Google Scholar]
- 10.De Jonghe BC, Horn CC. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol 294: R756–R765, 2008 [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48 h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 296: R902–R911, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jonghe BC, Lawler MP, Horn CC, Tordoff MG. Pica as an adaptive response: Kaolin consumption helps rats recover from chemotherapy-induced illness. Physiol Behav 97: 87–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca LA, Jr, Barbosa SP, Menani JV. Brain serotonin blockade and paradoxical salt intake in rats. Neuroscience 121: 1055–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Edwards GL, Johnson AK. Enhanced drinking after excitotoxic lesions of the parabrachial nucleus in the rat. Am J Physiol Regul Integr Comp Physiol 261: R1039–R1044, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Endo T, Nemoto M, Minami M, Yoshioka M, Saito H, Parvez SH. Changes in the afferent abdominal vagal nerve activity induced by cisplatin and copper sulfate in the ferret. Biogenic Amines 11: 399–407, 1995 [Google Scholar]
- 16.Florczyk AP, Schurig JE, Bradner WT. Cisplatin-induced emesis in the Ferret: a new animal model. Cancer Treat Rep 66: 187–189, 1982 [PubMed] [Google Scholar]
- 17.Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: III. The role of the nucleus of the solitary tract and the parabrachial nucleus in retention of a conditioned taste aversion in rats. Behav Neurosci 111: 180–187, 1997 [PubMed] [Google Scholar]
- 18.Gylys JA, Doran KM, Buyniski JP. Antagonism of cisplatin induced emesis in the dog. Res Commun Chem Pathol Pharmacol 23: 61–68, 1979 [PubMed] [Google Scholar]
- 19.Harding RK, Hugenholtz H, Kucharczyk J, Lemoine J. Central mechanisms for apomorphine-induced emesis in the dog. Eur J Pharmacol 144: 61–65, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 358: 2482–2494, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Horn CC. Brain Fos expression induced by the chemotherapy agent cisplatin in the rat is partially dependent on an intact abdominal vagus. Auton Neurosci 148: 76–82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci 132: 44–51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci 115: 74–81, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Jia HG, Rao ZR, Shi JW. An indirect projection from the nucleus of the solitary tract to the central nucleus of the amygdala via the parabrachial nucleus in the rat: a light and electron microscopic study. Brain Res 663: 181–190, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Knox AP, Strominger NL, Battles AH, Carpenter DO. Behavioral studies of emetic sensitivity in the ferret. Brain Res Bull 31: 477–484, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24: 2932–2947, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull 30: 163–172, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur J Pharmacol 555: 164–173, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Martinez F, Deray G, Dubois M, Beaufils H, Jacquiaud C, Bourbouze R, Benhmida M, Jaudon MC, Jacobs C. Comparative nephrotoxicity of carboplatin and cisplatin in euvolemic and dehydrated rats. Anticancer Drugs 4: 85–90, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Matsuki N, Ueno S, Kaji T, Ishihara A, Wang CH, Saito H. Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn J Pharmacol 48: 303–306, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Mavichak V, Wong NL, Quamme GA, Magil AB, Sutton RA, Dirks JH. Studies on the pathogenesis of cisplatin-induced hypomagnesemia in rats. Kidney Int 28: 914–921, 1985 [DOI] [PubMed] [Google Scholar]
- 33.McCarthy LE, Borison HL. Antiemetic activity of N-methyllevonantradol and nabilone in cisplatin-treated cats. J Clin Pharmacol 21: 30S–37S, 1981 [DOI] [PubMed] [Google Scholar]
- 34.McCarthy LE, Borison HL. Cisplatin-induced vomiting eliminated by ablation of the area postrema in cats. Cancer Treat Rep 68: 401–404, 1984 [PubMed] [Google Scholar]
- 35.Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res 647: 255–264, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell D, Laycock JD, Stephens WF. Motion sickness-induced pica in the rat. Am J Clin Nutr 30: 147–150, 1977 [DOI] [PubMed] [Google Scholar]
- 37.Mungarndee SS, Lundy RF, Jr, Norgren R. Central gustatory lesions and learned taste aversions: unconditioned stimuli. Physiol Behav 87: 542–551, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mungarndee SS, Lundy RF, Jr, Norgren R. Expression of Fos during sham sucrose intake in rats with central gustatory lesions. Am J Physiol Regul Integr Comp Physiol 295: R751–R763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976 [DOI] [PubMed] [Google Scholar]
- 40.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Papas S, Ferguson AV. Electrophysiological characterization of reciprocal connections between the parabrachial nucleus and the area postrema in the rat. Brain Res Bull 24: 577–582, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Boston, MA: Elsevier Academic, 2005 [Google Scholar]
- 43.Petrov T, Krukoff TL, Jhamandas JH. Branching projections of catecholaminergic brainstem neurons to the paraventricular hypothalamic nucleus and the central nucleus of the amygdala in the rat. Brain Res 609: 81–92, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. J Comp Neurol 384: 248–270, 1997 [PubMed] [Google Scholar]
- 45.Phillips TD. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol Sci 52: 118–126, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Phillips TD, Sarr AB, Grant PG. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Nat Toxins 3: 204–213; discussion 221, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Pucilowski O, Trzaskowska E, Kostowski W. Differential effects of chronic ethanol on apomorphine-induced locomotion, climbing and aggression in rats. Drug Alcohol Depend 20: 163–170, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Reid RM. Cultural and medical perspectives on geophagia. Med Anthropol 13: 337–351, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Saeki M, Sakai M, Saito R, Kubota H, Ariumi H, Takano Y, Yamatodani A, Kamiya H. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn J Pharmacol 86: 359–362, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Sam TS, Chan SW, Rudd JA, Yeung JH. Action of glucocorticoids to antagonise cisplatin-induced acute and delayed emesis in the ferret. Eur J Pharmacol 417: 231–237, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 129: 3–16, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317, 1980 [DOI] [PubMed] [Google Scholar]
- 53.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon OR, Schramm LP. The spinal course and medullary termination of myelinated renal afferents in the rat. Brain Res 290: 239–247, 1984 [DOI] [PubMed] [Google Scholar]
- 55.Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp Physiol 87: 563–574, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci 109: 939–954, 1995 [PubMed] [Google Scholar]
- 57.Strain SM, Gwyn DG, Rutherford JG, Losier BJ. Direct vagal input to neurons in the area postrema which project to the parabrachial nucleus: an electron microscopic-HRP study in the cat. Brain Res Bull 24: 457–463, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Szelenyi I, Herold H, Gothert M. Emesis induced in domestic pigs: a new experimental tool for detection of antiemetic drugs and for evaluation of emetogenic potential of new anticancer agents. J Pharmacol Toxicol Methods 32: 109–116, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Takeda N, Hasegawa S, Morita M, Horii A, Uno A, Yamatodani A, Matsunaga T. Neuropharmacological mechanisms of emesis. I. Effects of antiemetic drugs on motion- and apomorphine-induced pica in rats. Methods Find Exp Clin Pharmacol 17: 589–590, 1995 [PubMed] [Google Scholar]
- 60.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav 45: 817–821, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Uno A, Takeda N, Horii A, Sakata Y, Yamatodani A, Kubo T. Effects of amygdala or hippocampus lesion on hypergravity-induced motion sickness in rats. Acta Otolaryngol (Stockh) 120: 860–865, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Voshart K, van der Kooy D. The organization of the efferent projections of the parabrachial nucleus of the forebrain in the rat: a retrograde fluorescent double-labeling study. Brain Res 212: 271–286, 1981 [DOI] [PubMed] [Google Scholar]
- 63.Wang FB, Powley TL. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res 329: 221–230, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods Find Exp Clin Pharmacol 24: 135–138, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto K, Takeda N, Yamatodani A. Establishment of an animal model for radiation-induced vomiting in rats using pica. J Radiat Res (Tokyo) 43: 135–141, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci 334: 115–124, 2007 [DOI] [PubMed] [Google Scholar]