Abstract
To investigate the role of brain angiotensin II (ANG II) in the pathogenesis of injury following ischemic stroke, mice overexpressing renin and angiotensinogen (R+A+) and their wild-type control animals (R−A−) were used for experimental ischemia studies. Focal brain ischemia was induced by middle cerebral artery occlusion (MCAO). The severity of ischemic injury was determined by measuring neurological deficits and histological damage at 24 and 48 h after MCAO, respectively. To exclude the influence of blood pressure and local collateral blood flow, brain slices were used for oxygen and glucose deprivation (OGD) studies. The severity of OGD-induced damage was determined by measuring indicators of tissue swelling and cell death, the intensity of the intrinsic optical signal (IOS), and the number of propidium iodide (PI) staining cells, respectively. Results showed 1) R+A+ mice showed higher neurological deficit score (3.8 ± 0.5 and 2.5 ± 0.3 for R+A+ and R−A−, respectively, P < 0.01) and larger infarct volume (22.2 ± 1.6% and 14.1 ± 1.2% for R+A+ and R−A−, respectively, P < 0.01); 2) The R+A+ brain slices showed more severe tissue swelling and cell death in the cortex (IOS: 140 ± 6% and 114 ± 10%; PI: 139 ± 20 cells/field and 39 ± 9 cells/field for R+A+ and R−A−, respectively, P < 0.01); 3) treatment with losartan (20 μmol/l) abolished OGD-induced exaggeration of cell injury seen in R+A+ mice. The data indicate that activation of ANG II/AT1 signaling is harmful to brain exposed to ischemia.
Keywords: AT1 receptor, mouse, losartan, middle cerebral artery
ischemic stroke, the third leading cause of death in the United States, is a severe complication of hypertension and arteriosclerosis. There are limited avenues for prevention and treatment of ischemic stroke. Accumulating evidence suggests that the angiotensin II (ANG II)/AT1 receptor pathway participates in the pathophysiology of ischemic stroke (8, 32). Blockade of the ANG II/AT1 pathway has been shown to have beneficial effect on cerebral ischemia in animal studies (11, 30) and clinical trials (10, 23). However, the mechanisms of ANG II/AT1 signaling in conjunction with stroke are still elusive.
The protective effect of blocking ANG II/AT1 receptor pathway on ischemic stroke has been related to blood pressure control and improvement of cerebral blood supply. However, the benefits can go beyond those (10, 38). The ANG II/AT1 pathway exists in neural/glial cells as well as cerebrovascular cells. AT1 receptors located in the cerebral vasculature participate in the regulation of the cerebrovascular circulation (31) and platelet-leukocyte-endothelium interactions (19). The ANG II/AT1 receptors located in the neural/glial cells participate in ischemic damage by inducing inflammation and oxidative stress (16, 33, 40).
Genetically modified mice models offer a useful tool for studying the role and mechanism of the ANG II/AT1 receptor pathway on ischemic stroke. Experimental stroke studies in ANG/AT1a receptor subtype deficient (AT1a−/−) mice and angiotensinogen knockout (AGN−/−) mice demonstrated that a lower level of ANG II or the lack of AT1 receptors was associated with less ischemic injury (28, 38). The smaller size of ischemic injury in AGN−/− mice was associated with better collateral blood supply. A smaller lesion area and better cerebral blood flow (CBF) were found in AT1a−/− mice after cerebral ischemia. In the most recent report, Inaba et al. (18) showed that reduction of CBF in the penumbra region contributes, at least in part, to exaggerated ischemic damage in human renin and angiotensinogen double transgenic (R+A+) mice. However, other potential mechanisms independent of pressure and CBF were not explored in that study. Interestingly, in cultured embryonic neurons of AT1−/− mice, in vitro oxygen and glucose deprivation (OGD), which offers a useful tool to exclude the influence of blood pressure and CBF on ischemic stroke, showed less neural injury when assessed by measuring the release of lactate dehydrogenase (38). This finding suggests that the ANG II/AT1 pathway located in the neuron is involved in ischemic damage.
In this study, we examined the effect of the ANG II/AT1 pathway on ischemic stroke using the R+A+ mouse model. First, we determined the severity of focal ischemic damage induced by middle carotid artery occlusion (MCAO). Next, we examined the possible role of the ANG II/AT1 pathway on ischemic injury by conducting OGD studies using brain slices to exclude the effects of hemodynamic factors on tissue swelling and cell death induced by ischemia.
METHODS
Experimental animals.
R+A+ and R−A− mice were produced using colonies maintained in our laboratory (founders were from the production colony by Dr. Curt D. Sigmund at the University of Iowa) and genotyped as previously described (17). Male R+A+ mice and R−A− control mice aged 16 to 20 wk were used for all experiments in this study. Mice were fed standard chow and water ad libitum. All experimental procedures were approved by the Wright State University Laboratory Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health.
CBF measurement.
Regional CBF was measured during the MCAO surgery as described previously (6, 14). Briefly, an incision was made in the scalp to expose the skull. Relative CBF was determined using a laptop computer-controlled laser Doppler flowmeter (model PF2B; Perimed, Sweden) with a fiberoptic probe (tip diameter, 0.5 mm) fixed on the skull of the MCAO side (2 mm posterior, 6 mm lateral to bregma). This site reflects the vascular territory supplied by distal segments of the middle cerebral artery.
Middle cerebral artery occlusion for inducing focal ischemic stroke.
MCAO surgery was performed as previously described (4, 9). Briefly, mice were anesthetized by using a ketamine/xylazine mixture (100:8 mg/kg), and the animal's body temperature was maintained using a water-jacketed heating pad. The left common carotid artery was exposed and ligated distal to the bifurcation. The left external carotid artery was ligated and cut for exposure of the left internal carotid artery. A suture was placed under the carotid internal artery and lightly lifted to prevent blood backflow from the head. Then, a small incision was made on the common carotid artery between the ligation and carotid bifurcation. A 7-0 nylon monofilament suture with a rounded head coated with poly-l-lysine was inserted through the small incision and advanced into the internal carotid artery until resistance was detected (about 10 mm distal to the bifurcation). A rapid fall of > 70% in regional CBF was used for verification of the success of MCAO. The suture was left in place with a ligation for permanent MCAO.
Evaluation of neurological deficits.
The evaluation of neurological deficits was carried out 24 h after MCAO according to a five-point scale (39): 0, normal motor function; 1, flexion of contralateral torso and forelimb upon lifting the whole animal by the tail; 2, circling to the contralateral side but normal posture at rest; 3, leaning to the contralateral side at rest; 4, no spontaneous motor activity.
Blood pressure and heart rate.
A radiotelemetry system (model TA11PA-C20; Data Science International) was used for recording arterial pressure in conscious mice as we described previously (7). The telemetric probe was implanted during MCAO surgery in the ipsilateral carotid artery. Arterial pressure was recorded continuously for 24 h starting 24 h after MCAO.
Quantification of infarct volume.
The extent of ischemic infarction was evaluated 48 h after MCAO by staining with 2% 2,3,5-triphenyltetrazolium chloride (Sigma) as reported previously (3, 27). Coronal brain sections (1 mm) were cut on a mouse brain matrix. Images of all stained slices were captured using a flatbed scanner, and the border between the infarct (unstained) and noninfarct (stained) tissue was outlined and calculated with Image J software (National Institutes of Health). The area of infarction was measured in each section by subtracting the area of the nonlesioned ipsilateral hemisphere from the total area of the contralateral hemisphere. The volume of infarction was calculated by summing the product of the area of infarct [mm2] × thickness [1 mm] over all sections.
Plasma and brain tissue ANG II levels.
Plasma and brain ANG II concentrations were measured using a commercial radioimmunoassay kit (ALPCO Diagnostics) as described previously (2, 7). Briefly, blood samples were obtained directly from the heart under anesthesia. Brain tissues were collected after saline perfusion following blood sampling to remove contaminating blood. Mouse plasma (100 μl) pretreated with EDTA and bestatin solution (ALPCO Diagnostics) was extracted through a phenysilylsilica column with methanol (ALPCO Diagnostics). Brain tissue was homogenized on ice in an acid ethanol (80% vol/vol, 0.1 N HCl) solution containing EDTA and bestatin solution. A sample of homogenate was taken to determine total protein content using the Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA). The homogenate was centrifuged at 30,000 g for 20 min, and the supernatant was added with 1% (vol/vol) heptafluorobutyric acid (Pierce, Rockford, IL). The solution was allowed to precipitate at 4°C overnight and was then centrifuged at 30,000 g for 20 min. The supernatant was extracted through phenysilylsilica column with methanol (ALPCO Diagnostics). After extraction, the sample was concentrated under a Savant vacuum centrifuge, reconstituted and incubated with ANG II antiserum and 125I-labeled ANG II at 4°C. After incubation, the antibody-bound fraction was precipitated using a secondary antibody. The samples were then centrifuged (1,000 g) for 5 min. Supernatants were discarded, and pellets were counted with a gamma counter. The assay was modified to use a smaller volume (20 μl) of antisera to increase the assay sensitivity.
OGD study in brain slices.
To exclude the possible influence of blood pressure and regional CBF, an in vitro OGD study was conducted on brain slices (1). Briefly, brains were obtained from R+A+ and R−A− mice by decapitation and placed in a chamber containing artificial cerebrospinal fluid (aCSF) bubbled with 95% O2-5% CO2. The aCSF contained (in mmol/l) 124 NaCl, 3.5 KCl, 2 CaCl2, 1 MgSO4, 1 NaH2PO4, 26 NaHCO3, and 10 glucose. Osmolarity was adjusted to 290 mOsm/l using small volumes of 3 mol/l NaCl. The brain was sectioned into 400 μm slices with a Vibratome Series 1,000 Sectioning System. The slices were placed onto an interface-type recording stage and superfused with aCSF equilibrated with 95% O2-5% CO2 for 60 min at 35°C. To induce OGD, slices were superfused with aCSF containing no glucose, and the bubbling gas was replaced with 95% N2-5% CO2 for 60 min. To determine the role of ANG II/AT1 receptor pathway on OGD-induced brain damage, losartan (20 μmol/l) was added in the aCSF at the time of OGD exposure to block AT1 receptors.
Evaluation of cellular swelling and cell death.
The magnitude of transmitted light through the slice [intrinsic optical signal (IOS)] was measured as an indirect measure of cellular swelling. Slices were transilluminated with white light from a stable incandescent light source via a randomized fiber optic bundle. Images of the slice were captured at 1-min intervals by using a fixed-gain black and white video camera and stored as 640 × 480 digital image files without compression. Regions of interest in cerebral cortex and hippocampal CA1 and CA3 regions were defined, and the average intensity in these regions was calculated for each image. Images also were processed to obtain images of the changes in light intensity. First, we averaged, pixel-by-pixel, five baseline images acquired immediately before the start of OGD exposure. Pixel intensities in all images before and after OGD exposure were then normalized to their respective pixel in the averaged baseline image. The results are expressed as the percent change from the average baseline image.
For analysis of cell death after OGD, brain slices were stained with propidium iodide (PI) and analyzed with confocal microscopy (35). Briefly, slices were washed with aCSF, incubated with PI (20 μg/ml) at 37°C for 15 min, fixed in 4% paraformaldehyde for another 15 min, and then mounted on glass slides. To avoid the influence of injuries caused by preparation of the brain slices, we only counted PI-positive nuclei between 80 and 90 μm of the slice surface. PI-positive cell nuclei were automatically counted using Image J software (National Institutes of Health).
Statistical analysis.
All data are represented as means ± SE. Changes in neurological scores, cell death, and effect of losartan treatment were analyzed using the nonparametric Mann-Whitney U-test. The parametric data of infarct volumes, physiological values, cellular swelling, and effect of losartan treatment were assessed using one- or two-way ANOVA, followed by Tukey's test. P < 0.05 was considered statistically significant.
RESULTS
Animal model.
As showed in Table 1, R+A+ mice had higher ANG II levels in plasma and brain, high blood pressure, and heart hypertrophy. These data confirmed the phenotype of the animals used for this study.
Table 1.
General characteristics of R+A+ mouse model
| Variants | R−A− | R+A+ |
|---|---|---|
| Body weight, g | 28±2 | 27±3 |
| Heart weight/body weight, mg/g | 3.7±0.3 | 5.9±0.3* |
| Mean arterial pressure, mmHg | 98±3 | 128±4* |
| Heart rate, beats/min | 565±25 | 581±32 |
| Plasma ANG II level, pg/ml | 78±34 | 227±53* |
| Brain ANG II level, fmol/mg | 36±12 | 114±25* |
Data are expressed as means ± SE; n = 5–6 mice/group. R+A+, mice overexpressing renin and angiotensinogen; R−A−, wild-type control animals.
P < 0.01, compared with R−A−.
Increased neurological deficits and stroke volume in R+A+ mice after MCAO.
MCAO surgery was guided by measuring CBF. An immediate fall (>70% of baseline) in CBF was considered to be a successful occlusion of the artery (Fig. 1A). After 24 h of ischemia, the neurological deficit score of R+A+ mice was much higher than that of R−A− mice (3.8 ± 0.5 and 2.5 ± 0.3 for R+A+ and R−A−, respectively, n = 6, P < 0.01; Fig. 1C). After 48 h of ischemia, the brain stroke volume was significantly greater in R+A+ mice (22.2 ± 1.6% and 14.1 ± 1.2% for R+A+ and R−A−, respectively, n = 6, P < 0.01; Fig. 1, B and C). Both neurological and histological data showed that MCAO-induced brain injury was more severe in R+A+ mice than in R−A− mice.
Fig. 1.
Enhanced neurological deficits and brain stroke volume in R+A+ mice after middle cerebral artery occlusion (MCAO). A: representative cerebral blood flow (CBF) trace of laser Doppler flowmetry shows an immediate fall (>70%) of CBF after MCAO, indicating success of the surgery. Arrow indicates MCAO was started after 1 min of baseline recording. B: representative brain 2,3,5-triphenyltetrazolium chloride (TTC) staining images in R+A+ and R−A− mice 48 h after MCAO. C: summarized neurological deficit scores and brain stroke volumes (%). R+A+, mice overexpressing renin and angiotensinogen; R−A−, wild-type control animals. Data are means ± SE. **P < 0.01, R+A+ vs. R−A−.
Increased tissue swelling in R+A+ mice after OGD.
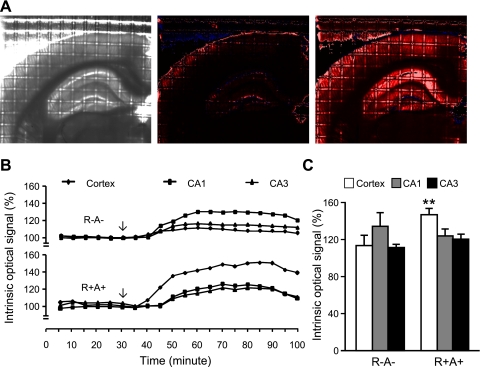
Tissue swelling was determined by measuring the IOS. Fig. 2, A and B show representative images and IOS before and during OGD. OGD for 30 min induced a higher percentage increase in IOS over the basal level in the cerebral cortex of R+A+ mice (140 ± 6% and 114 ± 10% for R+A+ and R−A− mice, respectively, n = 6, P < 0.01; Fig. 2C). However, IOS measurements were similar between the R+A+ and R−A− mice in the hippocampal CA1 region (125 ± 6% and 135 ± 14% for R+A+ and R−A− mice, respectively, n = 6, P > 0.05) and CA3 region (121 ± 4% and 112 ± 2% for R+A+ and R−A− mice, respectively, n = 6, P > 0.05).
Fig. 2.
Enhanced swelling of brain slices from R+A+ mice after oxygen and glucose deprivation (OGD). A: representative images of 1 brain slice captured using a fixed-gain black and white video camera at baseline prior to OGD exposure (left), and images of the same brain slice normalized pixel by pixel to baseline captured at 0 min (middle) and at 30 min (right) after the start of OGD. Increases in intrinsic optical signal (IOS) intensity are indicated by color from black to red and then white. Blue color indicates decreases in intensity. B: representative IOS traces at 5-min intervals (average of the 5 data points from 1-min interval recordings) in cerebral cortex, and hippocampal CA1 and CA3 regions. The arrow indicates that OGD was started after 30 min of baseline recordings. C: The summarized IOS data after 30 min of OGD. Data are expressed as means ± SE. **P < 0.01, R+A+ vs. R−A−.
Losartan abolished the increased tissue swelling in R+A+ mice after OGD.
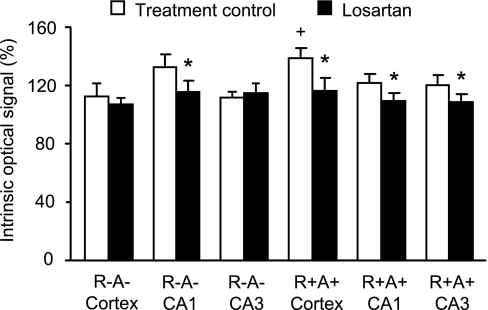
To study the possible role of the ANG II/AT1 receptor pathway on OGD hypersensitivity, the AT1 receptor blocker losartan (20 μM) was added to the perfusion solution during OGD. As shown in Fig. 3, losartan treatment reduced OGD-induced tissue swelling in the hippocampal CA1 region of R−A− mice (116 ± 5% and 132 ± 6% for losartan and treatment control group, respectively, n = 5, P < 0.05) and all regions (cortex, CA1 and CA3) of R+A+ mice. Losartan had no effect on OGD-induced tissue swelling in the cortex and hippocampal CA3 region of R−A− mice.
Fig. 3.
Effect of losartan on swelling in brain slices after OGD. Data are expressed as means ± SE. *P < 0.05, losartan vs. treatment control; +P < 0.01, R+A+ vs. R−A−.
OGD-induced cortex cell death increased in R+A+ mice and was abolished by losartan.
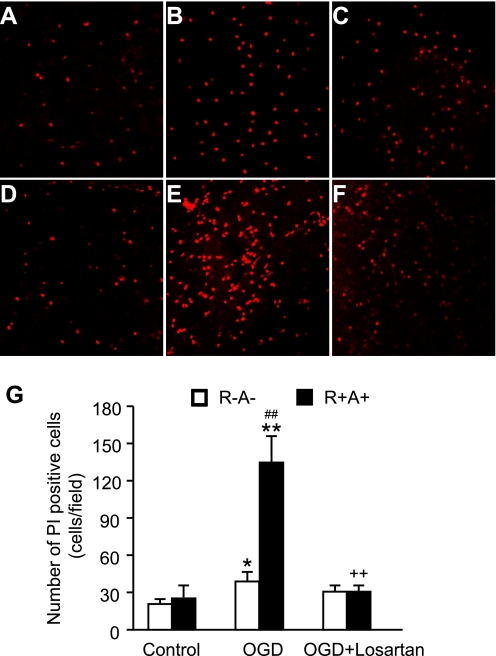
Cell death after OGD was determined by staining brain slices with PI and counting cells with confocal microscopy. As shown in Fig. 4, OGD resulted in cortex cell death in both R+A+ mice (25 ± 10 cells/field and 139 ± 20 cells/field for control and OGD, respectively, n = 5, P < 0.01) and R−A− mice (20 ± 3 cells/field and 39 ± 9 cells/field for control and OGD, respectively, n = 5, P < 0.05). There were significantly more PI-positive cells in the cerebral cortex of R+A+ mice than in R−A− mice after OGD (n = 5, P < 0.01). Losartan treatment abolished the higher PI staining of cells in the cerebral cortex of R+A+ mice (31 ± 4 cells/field, n = 5, P < 0.01) with no significant effect on PI- positive cell counts in R−A− mice (31 ± 5 cells/field, n = 5, P > 0.05).
Fig. 4.
Exaggerated cerebral cortex cell death in R+A+ mice after OGD and the effect of losartan. Top: representative laser confocal microscopic images of propidium iodide (PI)-labeled cells in R−A− mice (A: OGD control, B: OGD; C: OGD + losartan) and R+A+ mice (D: OGD control, E: OGD; F: OGD + losartan). G: summarized data in PI-labeled cells after OGD. Data are expressed as means ± SE. *P < 0.05, **P < 0.01, OGD vs. OGD control; ++P < 0.01, OGD + losartan vs. OGD; ##P < 0.01, R+A+ vs. R−A−.
DISCUSSION
There are three major findings in the present study. First, brain injury is exaggerated in the R+A+ mice compared with their wild-type controls after MCAO, an in vivo focal cerebral ischemic model. This is evidenced from the higher neurological deficit score and larger stroke volume after MCAO in R+A+ mice. Second, the brain slices of R+A+ mice show exaggerated cellular damage to OGD, an in vitro model of ischemia. This is confirmed from the increased tissue swelling and cell death in the brain cortex after ischemia. Third, blockade of ANG II/AT1 receptor pathway abolishes the exaggerated cellular edema and cell death in the cortex of R+A+ mice. This study suggests that activation of brain ANG II/AT1 receptor pathway exaggerates cerebral ischemic injury. The in vitro data also suggest that the responsible mechanism might be, in part at least, independent of blood pressure and CBF.
In the in vivo ischemic stroke study, our finding of an increased cerebral ischemic injury in R+A+ mice is consistent with the data from other laboratories using the same or similar animal models (18, 38). Inaba et al. (18) showed that the reduction of CBF in the penumbra region and superoxide production after MCAO were exaggerated in R+A+ mice, and that the beneficial effect of AT1 receptor blocker on ischemic damage was at least partly dependent on a decrease in oxidative stress and an increase in CBF in the penumbra. Walther, et al. (38) found an enlarged infarct size in angiotensinogen transgenic (ANG +/+) mice and a smaller infarct size in AT1a−/− mice compared with their wild-type littermates after 24-h MCAO. However, Maeda et al. (28) found a smaller ischemic core and a larger penumbra at 1 h but not at 24 h after MCAO in AGN−/− mice.
There are several possible mechanisms for the effect of ANG II on ischemic stroke. First, ANG II causes cerebral vasoconstriction through its AT1 receptor pathway (34). Second, ANG II may regulate the microvascular density and collateralization in the cerebral vascular network. Maeda, et al. (28) showed that the collateral circulation was improved in ANG −/− mice. There are several reports that AT1 receptor blockade increases microvascular density and collateralization in the cerebral vascular network (13, 26, 29). Third, ANG II/AT2 pathway has been shown to protect brain from ischemic damage (20). Fourth, activation of ANG II/AT1 pathway in neuronal and glial cells may render them vulnerable to ischemia (38). We should mention here that the plasma and brain tissue ANG II levels in the R+A+ mice were about three times greater than those measured in R−A− mice. Thus, ANG II could activate both AT1 and AT2 receptor pathways. The data from others and from our study indicate that the protective mechanism of ANG II/AT2 does not overwhelm the detrimental mechanism of ANG II/AT1 in R+A+ mice. The beneficial effect of AT2 could be well displayed when AT1 receptors are blocked leading to enhanced interaction of ANG II with AT2 receptors.
Recent clinical evidence suggests that the beneficial effect of AT1 receptor blockade on ischemic stroke can be observed independent of the effects on blood pressure and flow (10). This conclusion is also supported by studies of cultured embryonic neurons which showed that cells from AT1−/− mice had less damage after OGD (38). However, direct evidence is lacking. To exclude the possible influence of blood pressure, CBF supply, vascular density, and collateral circulation, OGD in brain slices have been a useful in vitro model for ischemia. However, to our knowledge, this model has not been used to address the effect of the ANG II/AT1 pathway on ischemic damage. We designed experiments to evaluate the role of the ANG II/AT1 pathway in ischemic stroke on brain slices by measuring tissue swelling and cell death. In the present study, we found that brain slices of R+A+ mice had exaggerated swelling and cell death in the cortex after 30 min of OGD. Blockade of AT1 receptors with losartan abolished the enhanced damage seen in R+A+ mice. These data suggest that ANG II/AT1 activation contributes to cell injury and death independent of hemodynamic changes.
The ANG II/AT1 receptors are broadly distributed in various brain regions including cortex and hippocampus (24, 36) and in different cell types including neurons, glia, and cerebral endothelial cells (12, 22). AT1 receptors stimulate a cascade of signaling events that regulate a number of proinflammatory factors in neurons, glial, and endothelial cells (5, 25). AT1 receptor signaling also contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature (19). Hamai, et al. (16) found that AT1 receptor blockers against oxidative stress might contribute to their neuroprotective effect against cerebral ischemia. Blockade of the AT1 receptor even offers neural protection, including antiapoptosis and anti-inflammatory in the intracerebral hemorrhage rat model (21). Our data is supported by previous reports on the involvement of AT1 stimulation in apoptotic death of myocytes (15) and possible apoptotic pathways triggered by AT1 stimulation (37).
Interestingly, OGD-induced edema was similar in hippocampal CA1 and CA3 regions of R+A+ and R−A− mice. This regional effect could be related to the different pattern of receptor distribution or regulation in different brain areas. Losartan abolished the enhanced swelling and cell death in the cortex and eliminated the enhanced swelling of the hippocampal CA1 and CA3 regions of R+A+ mice. Losartan also decreased swelling in the CA1 region, but not in the CA3 region of R−A− mice during OGD. Again, these results highlight possible regional differences in the action of the angiotensin pathways.
Perspectives and Significance
The findings in this study suggest that overproduction of ANG II is harmful during ischemic stroke. The detrimental effect of ANG II appears to be mediated through its AT1 receptors. Increased sensitivity to ischemia-induced swelling and cell death could be one mechanism involved in the enlargement of ischemia-induced cerebral damage seen in R+A+ mice. However, the present in vitro model, while extremely useful, is not a physiologically relevant model. The effects described in our experiments may not faithfully explain the mechanisms effective in vivo. Observations with in vivo systemic or intracerebral administration of ANG II/AT1 receptor antagonists and inclusion of an animal group treated with a nonrenin angiotensin system interfering antihypertensive drug will be helpful in an in-depth study based on the current findings. Nevertheless, the major impact of this work is to demonstrate that the ANG II/AT1 receptor pathway can participate in ischemic injury independent of its effects on blood pressure and CBF. Overproduction of ANG II through activation of AT1 receptors in the brain renders brain cells sensitive to ischemia. The responsible downstream signaling mechanisms deserve further investigation. Finally, whether losartan may exert its effect through mechanisms not related to AT1 receptors also awaits a more complete analysis.
GRANTS
This work was supported by American Heart Association Grant SDG-0535201N (to Y. Chen), Wright State University research funds (to Y. Chen), and the National Heart, Lung, and Blood Institute Grants HL-48058 and HL-84207 (to C. D. Sigmund).
ACKNOWLEDGMENTS
The editor-in-chief of this journal, Curt D. Sigmund, is an author of this paper. For this reason, peer review of this paper was coordinated by Dr. Alberto Nasjletti, Editor-in-Chief of American Journal of Physiology-Heart and Circulatory Physiology.
REFERENCES
- 1.Aitken PG, Fayuk D, Somjen GG, Turner DA. Use of intrinsic optical signals to monitor physiological changes in brain tissue slices. Methods 18: 91–103, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 279: F636–F645, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17: 1304–1308, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-l-lysine: neurological and histological validation. Brain Res 833: 181–190, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Blume A, Herdegen T, Unger T. Angiotensin peptides and inducible transcription factors. J Mol Med 77: 339–357, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Borlongan CV, Lind JG, llon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res 1010: 108–116, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Chen H, Hoffmann A, Cool DR, Diz DI, Chappell MC, Chen A, Morris M. Adenovirus-mediated small-interference RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension 47: 230–237, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chrysant SG. Possible pathophysiologic mechanisms supporting the superior stroke protection of angiotensin receptor blockers compared to angiotensin-converting enzyme inhibitors: clinical and experimental evidence. J Hum Hypertens 19: 923–931, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res 19: 641–648, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359: 995–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Engelhorn T, Doerfler A, Heusch G, Schulz R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neurosci Lett 406: 92–96, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fogarty DJ, Matute C. Angiotensin receptor-like immunoreactivity in adult brain white matter astrocytes and oligodendrocytes. Glia 35: 131–146, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Forder JP, Munzenmaier DH, Greene AS. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am J Physiol Heart Circ Physiol 288: H1989–H1996, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol 294: H156–H163, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Shainberg A, Jacobson KA, Shneyvays V, Grossman E. Adenosine protects against angiotensin II-induced apoptosis in rat cardiocyte cultures. Mol Cell Biochem 252: 133–139, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamai M, Iwai M, Ide A, Tomochika H, Tomono Y, Mogi M, Horiuchi M. Comparison of inhibitory action of candesartan and enalapril on brain ischemia through inhibition of oxidative stress. Neuropharmacology 51: 822–828, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke 36: 1253–1258, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Inaba S, Iwai M, Tomono Y, Senba I, Furuno M, Kanno H, Okayama H, Mogi M, Higaki J, Horiuchi M. Exaggeration of focal cerebral ischemia in transgenic mice carrying human renin and human angiotensinogen genes. Stroke 40: 597–603, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol 292: H2306–H2315, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, Sakanaka M, Shiuchi T, Horiuchi M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 110: 843–848, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, Park DK, Sinn DI, Kim JM, Kim M, Roh JK. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther 322: 1051–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kjeldsen SE, Lyle PA, Kizer JR, Dahlof B, Devereux RB, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn SM, Harris KE, Wedel H. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J Clin Hypertens (Greenwich) 7: 152–158, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krizanova O, Kiss A, Zacikova L, Jezova D. Nitric oxide synthase mRNA levels correlate with gene expression of angiotensin II type-1 but not type-2 receptors, renin or angiotensin converting enzyme in selected brain areas. Physiol Res 50: 473–480, 2001 [PubMed] [Google Scholar]
- 25.Lebrun CJ, Blume A, Herdegen T, Möllenhoff E, Unger T. Complex activation of inducible transcription factors in the brain of normotensive and spontaneously hypertensive rats following central angiotensin II administration. Regul Pept 66: 19–23, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Li JM, Mogi M, Iwanami J, Min LJ, Tsukuda K, Sakata A, Fujita T, Iwai M, Horiuchi M. Temporary pretreatment with the angiotensin II type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke 39: 2029–2036, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24: 117–121, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, Hata R, Bader M, Walther T, Hossmann KA. Larger anastomoses in angiotensinogen-knockout mice attenuate early metabolic disturbances after middle cerebral artery occlusion. J Cereb Blood Flow Metab 19: 1092–1098, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Munzenmaier DH, Greene AS. Chronic angiotensin II AT1 receptor blockade increases cerebral cortical microvessel density. Am J Physiol Heart Circ Physiol 290: H512–H516, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 31: 2478–2486, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol 25: 485–512, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saavedra JM, Benicky J, Zhou J. Mechanisms of the anti-ischemic effect of angiotensin II AT1 receptor antagonists in the brain. Cell Mol Neurobiol 26: 1099–1111, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz R, Heusch G. Angiotensin II type 1 receptors in cerebral ischaemia-reperfusion: initiation of inflammation. J Hypertens Suppl 24: S123–S129, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Stenman E, Edvinsson L. Cerebral ischemia enhances vascular angiotensin AT1 receptor-mediated contraction in rats. Stroke 35: 970–974, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Fujikura K, Higashiyama T, Takata K. DNA staining for fluorescence and laser confocal microscopy. J Histochem Cytochem 45: 49–53, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Tonelli L, Johren O, Hoe KL, Hauser W, Saavedra JM. Gerbil angiotensin II AT1 receptors are highly expressed in the hippocampus and cerebral cortex during postnatal development. Neuroscience 95: 981–991, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Vivar R, Soto C, Copaja M, Mateluna F, Aranguiz P, Munoz JP, Chiong M, Garcia L, Letelier A, Thomas WG, Lavandero S, Díaz-Araya G. Phospholipase C/protein kinase C pathway mediates angiotensin II-dependent apoptosis in neonatal rat cardiac fibroblasts expressing AT1 receptor. J Cardiovasc Pharmacol 52: 184–190, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Walther T, Olah L, Harms C, Maul B, Bader M, Hortnagl H, Schultheiss HP, Mies G. Ischemic injury in experimental stroke depends on angiotensin II. FASEB J 16: 169–176, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 25: 165–170, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke 37: 1271–1276, 2006 [DOI] [PubMed] [Google Scholar]