Abstract
The present studies evaluated intrarenal hemodynamics, pressure natriuresis, and arterial blood pressure in rats following recovery from renal ischemia-reperfusion (I/R) injury. Acute I/R injury, induced by 40 min of bilateral renal arterial occlusion, resulted in an increase in plasma creatinine that resolved within a week. Following 5 wk of recovery on a 0.4% NaCl diet, the pressure-natriuresis response was assessed in anesthetized rats in which the kidney was denervated and extrarenal hormones were administered intravenously. Increasing renal perfusion pressure (RPP) from 107 to 141 mmHg resulted in a fourfold increase in urine flow and sodium excretion in sham control rats. In comparison, pressure diuresis and natriuresis were significantly attenuated in post-I/R rats. In sham rats, glomerular filtration rate (GFR) averaged 1.6 ± 0.2 ml·min−1·g kidney weight−1 and renal blood flow (RBF) averaged 7.8 ± 0.7 ml·min−1·g kidney weight−1 at RPP of 129 mmHg. Renal cortical blood flow, measured by laser-Doppler flowmetry, was well autoregulated whereas medullary blood flow and renal interstitial hydrostatic pressure increased directly with elevated RPP in sham rats. In contrast, GFR and RBF were significantly reduced whereas medullary perfusion and interstitial pressure demonstrated an attenuated response to RPP in post-I/R rats. Further experiments demonstrated that conscious I/R rats develop hypertension when sodium intake is increased. The present data indicate that the pressure-natriuretic-diuretic response in I/R rats is blunted because of a decrease in GFR and RBF and the depressed pressure-dependent increase in medullary blood flow and interstitial pressure.
Keywords: kidney, renal blood flow, renal function
ischemic injury to the kidney is one of the leading causes of acute kidney injury (AKI) and is associated with a rapid decline in glomerular filtration rate (GFR) (14). Recovery from AKI occurs by restoration of GFR and tubular repair. The injury and recovery process of AKI has been studied extensively in several animal models. In the ischemia-reperfusion (I/R) model of AKI, damage to the proximal tubule can be severe. Repair of the proximal tubule is characterized by remodeling of the basement membrane, cellular proliferation, hypertrophy, and differentiation of new functional proximal tubule cells (14, 24). Despite the restoration of tubular morphology and the normalization of serum creatinine in the weeks following I/R injury, functional and structural recovery of the kidney is not complete. Evidence indicates that postischemic animals have a deficit in renal concentrating ability (2, 3), and renal microvessels are lost and do not regenerate in the postischemic kidney (3, 6). Postischemic kidneys therefore have sustained hypoxia and are predisposed to chronic kidney disease (CKD).
Recent experiments from our laboratory have demonstrated that postischemic rats have a normal level of arterial pressure, normal plasma creatinine, and minimal renal histological changes when permitted to recover for 5 wk while fed 0.4% NaCl chow (20, 25). An increase of dietary salt to 4.0% NaCl in these animals, however, results in the development of sodium-sensitive hypertension that is accompanied by substantial kidney damage (20, 25). Moreover, the development of progressive renal dysfunction and/or hypertension has been reported following severe AKI in patients (1, 7).
The sodium sensitivity of blood pressure in I/R animals after 5 wk of recovery indicates that the post-I/R kidney has a deficit in sodium handling that is unmasked when the rats are challenged with increased sodium intake. Despite the apparent recovery from the original ischemic insult, these animals are not capable of handling an elevated sodium load, presumably because of an alteration in the pressure-natriuresis relationship. Impaired pressure natriuresis is a characteristic of several models of genetic and experimentally induced hypertension (13, 19, 21, 26, 27). Moreover, subtle changes in renal microcirculatory dynamics have been implicated as important mechanisms in the pressure-natriuresis response (18). Since the post-I/R kidney has a decrease in peritubular capillaries (3, 6), we speculate that the pressure-natriuresis relationship and the sensitivity of blood pressure to sodium intake is altered in these rats by changes in renal hemodynamics. In the present experiments we tested the hypothesis that the pressure-natriuretic relationship is attenuated because of alterations in renal cortical and/or medullary hemodynamics in rats that have recovered from I/R injury while fed a 0.4% NaCl diet. This alteration in pressure natriuresis is proposed to mediate the development of sodium-sensitive hypertension in rats that have recovered from acute renal I/R injury.
METHODS
Experiments were performed on male Sprague-Dawley rats obtained from Harlan Sprague Dawley (Madison, WI). Animals were fed standard laboratory rat chow (AIN76A, Dyets, Bethlehem, PA) containing 0.4% NaCl and tap water ad libitum. The 0.4% NaCl diet was chosen on the basis of previous observations demonstrating that rats recover from I/R injury and remain normotensive when fed this diet (20, 25). All protocols were approved by the Institutional Animal Care and Use Committees at the Medical College of Wisconsin and Indiana University.
AKI induction.
To induce acute kidney injury, rats were anesthetized with ketamine (100 mg/kg ip) and pentobarbital sodium (25 mg/kg ip) and placed on a heated surgical table, and a midline abdominal incision was made. The blood supply to the kidneys was interrupted for 40 min by applying microvascular clamps on the renal pedicles of both kidneys. The clamps were then released, and the kidneys were reperfused. Control rats were subjected to sham surgery in which the kidneys were exposed but not touched.
Acute evaluation of renal function.
After 35 days of recovery from I/R injury, animals were anesthetized with ketamine HCl (13 mg/kg im), acepromazine maleate (0.25 mg/kg im), and Inactin (100 mg/kg ip). Rats were placed on a heated surgical board to maintain body temperature at 37°C. The trachea was cannulated to facilitate respiration, and catheters were placed in the carotid and femoral artery to measure renal perfusion pressure (RPP). The femoral vein was cannulated for intravenous infusions. A midline abdominal incision was made, and a flow probe was placed around the renal artery for measurement of renal blood flow (RBF) via an ultrasonic Doppler flowmeter (model T206, Transonic Systems, Ithaca, NY). Adjustable clamps were placed around the aorta above and below the renal arteries, and a loose ligature was placed around the celiac and mesenteric arteries so RPP could be adjusted during the experiment. The left ureter was cannulated for urine collection in some rats. In other animals, the left kidney was placed in a holder and optical fibers for laser Doppler flowmetry were implanted to a depth of ∼4.0 mm beneath the surface for measurements in the renal medulla and ∼0.5 mm for measurements in the renal cortex as previously described (16). Finally, in an additional study, renal interstitial hydrostatic pressure measurements were made by using a catheter modified with a polyethylene matrix tip and implanted into the renal cortex as described (11).
Animals were volume expanded with an intravenous infusion of 2% bovine serum albumin in 0.9% NaCl at a rate of 2 ml·h−1·100 g body wt−1. The volume status of the anesthetized rats provides urine flow and sodium excretion rates in the range of those observed in rats fed a 4.0% NaCl diet. Neural influences on the kidney were eliminated by renal denervation; the kidney was denervated by stripping the renal artery of all visible nerves and coating the artery with 5% phenol in ethanol. Circulating levels of extrarenal sodium and water-retaining hormones were set at maximal physiological levels by intravenous infusion of aldosterone (6.67 ng·min−1·100 g body wt−1, Sigma Chemical, St. Louis, MO), vasopressin (16.67 pg·min−1·100 g body wt−1, Sigma Chemical), norepinephrine (33.3 ng·min−1·100 g body wt−1, Sigma Chemical), and corticosterone (3.33 μg·min−1·100 g body wt−1, Sigma Chemical) as previously described (17, 22). Neural and hormonal influences on the kidney were fixed by denervation and hormone infusion since manipulation of RPP with aortic clamps (see below) may affect renal sympathetic activity and circulating levels of aldosterone and vasopressin (17, 22). [3H]inulin (PerkinElmer) was infused at a rate of 1 μCi·min−1·100 g body wt−1 for measurement of GFR.
Following the surgical preparation, the aortic clamp superior to the kidney was tightened to lower RPP to the lowest level (∼105–110 mmHg). After an equilibration period, two consecutive urine collection periods were made. RPP was then permitted to increase to ∼125–130 mmHg by releasing the aortic clamp. Renal excretory parameters were assessed while RPP was maintained at this level following an equilibration period in two consecutive urine collection periods. Finally, the superior mesenteric and celiac arteries were occluded and the aortic clamp below the kidney was tightened to increase RPP to the highest value. The renal excretory parameters were again assessed following an equilibration period at this elevated level of RPP. This method was also used to incrementally increase RPP from the lowest to the highest level in the laser-Doppler flowmetry experiments.
Assessment of sodium-sensitive hypertension in conscious rats.
A separate group of rats were prepared to evaluate the influence of a low- or high-sodium diet on arterial blood pressure and creatinine clearance. Following ∼28 days of recovery from I/R or sham surgery while fed 0.4% NaCl, the rats were anesthetized with ketamine HCl (50 mg/kg im) and acepromazine maleate (5 mg/kg im). Microrenathane catheters were implanted in the femoral artery and vein, tunneled subcutaneously, and exteriorized at the scapula in a stainless steel spring. Following recovery from anesthesia, all rats were placed in individual stainless steel cages that permit daily measurement of arterial blood pressure and overnight urine collection. The rats in this protocol were then fed 0.1% NaCl chow and were infused with isotonic NaCl (6 ml/day iv) to approximate the sodium intake achieved in rats fed 0.4% NaCl chow (9, 12).
After a week of recovery from catheter surgery while the rats were infused intravenously with isotonic saline at 6 ml/day, daily blood pressure measurements were obtained as we previously described (20, 25). An arterial plasma sample was obtained for measurement of plasma creatinine, and an overnight urine collection was obtained for quantification of urine creatinine and albumin. The intravenous infusion rate of isotonic NaCl was then increased to 60 ml/day to approximate the sodium intake of a 4.0% NaCl diet (9, 12), and blood pressure measurements were obtained after 5 days of infusion. A final arterial plasma sample and overnight urine sample were obtained for measurement of plasma and urine creatinine and urine albumin.
Analytical methods.
To assess recovery from I/R injury, plasma creatinine concentration was measured from tail blood samples 24 h, 7 days, and 28 days after injury. Creatinine concentration was determined by using standard assays (Sigma creatinine kit 555A). [3H]inulin concentration in urine and plasma was measured by liquid scintillation spectrophotometry (model 2450, Packard Instrument, Downers Grove, IL). Sodium concentrations in urine and plasma were measured with a flame photometer (model 943, Instrumentation Laboratory, Lexington, MA). Urine albumin was quantified with a fluorescent assay that utilized Albumin Blue 580 dye (Molecular Probes, Eugene, OR) and a fluorescent plate reader (FL600, Bio-Tek, Winooski, VT).
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed by an unpaired t-test or a one- or two-way analysis of variance with a Holm-Sidak post hoc test as appropriate. P < 0.05 was considered significant.
RESULTS
Acute I/R injury resulted in a transient decrease in renal function 24 h after surgery as indicated by a significant increase in plasma creatinine to 2.8 ± 0.4 mg/dl (n = 14) compared with 0.6 ± 0.1 mg/dl in sham control rats (n = 11). After 28 days of recovery, plasma creatinine in I/R rats (0.6 ± 0.1 mg/dl) returned toward control values and was not significantly different from that of sham controls (0.5 ± 0.1 mg/dl). Body weights were not different between the groups and averaged 392 ± 6 g in sham rats and 383 ± 13 g in post-I/R rats at the time of the acute study. The I/R rats experienced significant renal hypertrophy; left kidney weight averaged 1.84 ± 0.17 g in I/R rats compared with 1.37 ± 0.03 g in sham rats.
The acute pressure-natriuretic and diuretic relationship in sham and I/R recovery rats is illustrated in Fig. 1. In sham control rats (n = 8), increasing RPP from 107 ± 2 to 141 ± 5 mmHg resulted in a three- to fourfold increase in urine flow from 17.2 ± 6.8 to 70.5 ± 10.7 μl·min−1·g kidney wt−1 and sodium excretion from 3.0 ± 1.2 to 15.8 ± 2.2 μeq·min−1·g kidney weight−1. Both sodium excretion and urine flow rate significantly increased with each successive step in RPP. In contrast to the sham control group, the increase in urine flow and sodium excretion as RPP was elevated was attenuated in postischemic rats (n = 7). Significant differences in sodium and water excretion were noted between the sham and the I/R group at the greatest level of RPP, and the entire pressure-natriuretic relationship was significantly different between the groups as assessed by a two-way ANOVA.
Fig. 1.
Relationship between renal perfusion pressure and urine flow rate (top) or sodium excretion rate (bottom) in anesthetized sham control or post-ischemia-reperfusion (I/R) rats with neural and hormonal influences on the kidney controlled. #P < 0.05 between groups, ‡P < 0.05 between groups, *P < 0.05 within a group compared with the lowest renal perfusion pressure (RPP), †P < 0.05 within a group compared with the middle RPP.
The effect of I/R injury on RBF and GFR is illustrated in Fig. 2. In sham-operated control rats, GFR remained constant over all levels of perfusion pressure and averaged 1.6 ± 0.2 ml·min−1·g kidney weight−1 at RPP of 129 mmHg. Compared with the sham control group, GFR was significantly reduced by ∼45–55% in postischemic rats at equivalent levels of perfusion pressure. RBF averaged 7.8 ± 0.7 ml·min−1·g kidney weight−1 at RPP of 129 mmHg in sham-operated controls and was not different at other levels of RPP. Compared with the sham group, RBF was significantly reduced in postischemic rats by ∼20–35% at the lower and midlevel RPP. Fractional sodium excretion, illustrated in Fig. 3, significantly increased from 1.1 ± 0.4% at the lower level of RPP to 6.3 ± 1.0% at the highest RPP in the sham control rats. There were no significant differences in this parameter between the sham and the I/R group.
Fig. 2.
Relationship between renal perfusion pressure and glomerular filtration rate (GFR; top) or renal blood flow (RBF; bottom) in anesthetized sham control or post-I/R rats with neural and hormonal influences on the kidney controlled. #P < 0.05 between groups; ‡P < 0.05 between groups; *P < 0.05 within a group compared with the lowest RPP.
Fig. 3.
Relationship between renal perfusion pressure and fractional sodium excretion in anesthetized sham control or post-I/R rats with neural and hormonal influences on the kidney controlled. *P < 0.05 within a group compared with the lowest RPP; †P < 0.05 within a group compared with the middle RPP.
The relationship between RPP and renal cortical and medullary blood flow was assessed in a separate group of sham and post-I/R rats. As illustrated in Fig. 4, both cortical and medullary blood flow were normalized to the value measured at ∼100 mmHg. The autoregulatory index (AI; the percent change in blood flow divided by the percent change in perfusion pressure) of cortical blood flow in the sham-operated control was 0.18 ± 0.20 from a RPP of 100–130 mmHg. In contrast, medullary blood flow in sham-operated control rats increased significantly as RPP was elevated (AI = 0.90 ± 0.24). Renal cortical blood flow was also well autoregulated over this range of pressures in postischemic rats with an AI of 0.19 ± 0.16. Interestingly, the ability of medullary blood flow of I/R rats to increase in response to RPP was significantly attenuated compared with the sham animals; the AI of renal medullary blood flow in the post-I/R rats averaged 0.12 ± 0.24.
Fig. 4.
Relationship between renal perfusion pressure and renal cortical blood flow (top) or renal medullary blood flow (bottom) measured by laser-Doppler flowmetry in anesthetized sham control or post-I/R rats with neural and hormonal influences on the kidney controlled. *P < 0.05 within a group from RPP of ∼100 mmHg; †P < 0.05 within a group from RPP of ∼110 mmHg.
The relationship between RPP and renal interstitial hydrostatic pressure (RIHP) was subsequently evaluated in a separate group of sham-operated and post-I/R rats (Fig. 5). At RPP values of ∼110 mmHg, RIHP values were similar in sham-operated and post-I/R groups. When RPP was elevated to ∼140 mmHg, RIHP values increased in sham-operated controls and this response was attenuated in post-I/R rats. The absolute values of RIHP were not different in post-I/R vs. sham-controls (P = 0.12). The average slope of RIHP-vs.-RPP relationship, however, was significantly reduced in post-I/R animals compared with sham-operated controls (Fig. 5, inset).
Fig. 5.
Relationship between renal perfusion pressure and renal interstitial hydrostatic pressure in anesthetized sham control (n = 14) or post-I/R rats (n = 11) with neural and hormonal influences on the kidney controlled. *P < 0.05 within a group from RPP of ∼110 mmHg in control group by Student's paired t-test. Inset indicates the evaluation of slopes derived from linear curve fitting for each animal between low- and high-pressure points. *P < 0.05 vs. sham-operated control.
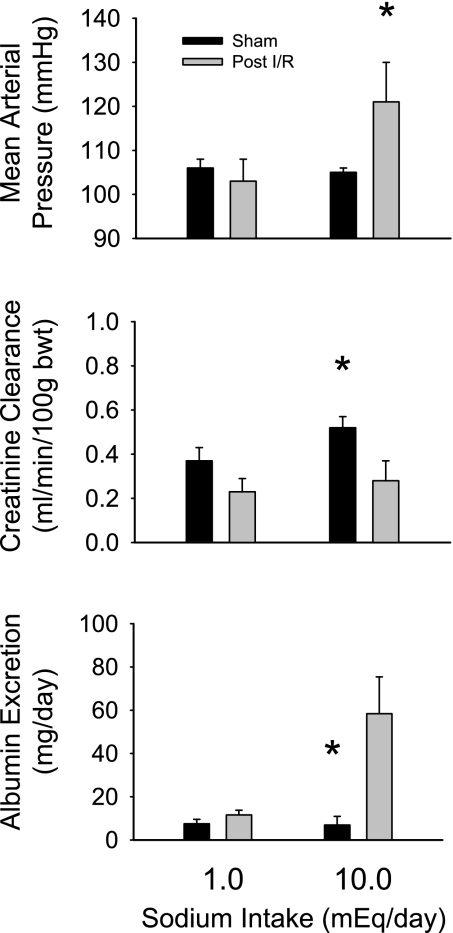
Further experiments were performed in conscious rats to determine the relationship between sodium intake and arterial blood pressure, creatinine clearance, and albumin excretion in rats that have recovered from the sham or I/R (n = 6/group) surgery while maintained on the 0.4% NaCl chow (Fig. 6). At the lower level of NaCl intake (∼1 mmol/day by iv saline infusion), mean arterial blood pressure was not different between the sham (106 ± 2 mmHg) and I/R rats (103 ± 5 mmHg). When NaCl intake was increased to ∼10 mmol/day, blood pressure was not altered in the sham rats but significantly increased by 18 mmHg in I/R rats after 6 days of infusion. Creatinine clearance in I/R rats tended to be lower than in sham control rats when the animals received 1 mmol NaCl per day. Following 5 days of 10 mmol/day NaCl intake, creatinine clearance was significantly different between the sham (0.52 ± 0.05 ml·min−1·100 g body wt−1) and I/R rats (0.28 ± 0.09 ml·min−1·100 g body wt−1). As an index of renal damage, albumin excretion was not different between I/R and sham rats at the lower level of sodium intake; when sodium intake was increased, however, albumin excretion rate significantly increased in I/R rats but was not changed in the sham group.
Fig. 6.
Relationship between sodium intake by intravenous infusion of isotonic saline and mean arterial blood pressure (top), creatinine clearance rate (middle), and albumin excretion rate (bottom) in sham or I/R rats 35 days after surgery. *P < 0.05 compared with I/R rats at the same sodium intake.
DISCUSSION
The present experiments demonstrate that rats recovered from acute renal I/R injury for 5 wk develop sodium-sensitive hypertension and have a blunted pressure-natriuretic diuretic relationship, a significant reduction in GFR and RBF, a blunted medullary blood flow response, and a blunted RIHP response to elevated RPP compared with sham-treated animals. Since post-I/R rats have normal blood pressure while fed 0.4% NaCl chow but develop hypertension when the salt content of the food is increased to 4.0% NaCl, the anesthetized studies were performed in a volume-expanded state to provide excretion rates similar to that of rats fed the 4.0% NaCl diet. To control for extrarenal factors that could alter function in the anesthetized animals, the kidneys were denervated, and the rats were administered high levels of several extrarenal hormones (aldosterone, vasopressin, norepinephrine, and corticosterone). The present experiments confirm our previous results demonstrating that post-I/R rats are normotensive when fed the 0.4% NaCl chow, that plasma creatinine is normal, and that there is minimal albumin or protein excretion (4, 6, 20, 25). The observed alterations in renal function in the post-I/R rats fed the 0.4% NaCl chow were therefore most likely mediated by changes in kidney function as a result of the I/R injury and not because of systemic hypertension or gross histological abnormalities.
Despite the general restoration of renal morphology following ischemic renal injury, the present data indicate that renal excretory function does not fully return to normal. Of interest, we previously reported a permanent loss of peritubular capillaries in the post-I/R kidney (3, 6). Under the conditions of the present experiment in animals following 5-wk recovery from renal I/R, we have shown that these rats have normal serum creatinine levels, generally restored tubular structure, and minimal renal fibrosis (4). Despite the apparent normality of structure, there is hypoxia associated with vascular rarefaction, renal hypertrophy associated with an increase in interstitial cells, and alterations in gene expression suggestive of a predisposition to CKD and hypertension (2, 5). The present experiments confirm our previous observations that rats exposed to increased sodium intake after 5 wk of recovery from I/R developed sodium-sensitive hypertension and exacerbated CKD (20, 25). The present study demonstrates that the pressure-natriuretic response is blunted in post-I/R rats, primarily because of alterations in renal hemodynamics.
The development of sodium-sensitive hypertension in the post-I/R rats suggests that the blunted pressure-natriuretic response may lead to an inability to excrete elevated sodium intake at a normal level of arterial blood pressure. The present data do not exclude, however, a role for other important factors (renal nerves, circulating hormones, etc.) that may be altered in I/R animals and participate in the development of sodium-sensitive hypertension. Since the pressure-natriuretic relationship was assessed in rats in which neural and hormonal influences were fixed whereas the conscious studies were performed in animals in which renal nerves and circulating hormones were not controlled, it is possible that other mechanisms mediate sodium-sensitive hypertension in I/R rats.
We propose that the alteration in sodium handling in rats apparently recovered from renal I/R injury is attributed to the loss of microvasculature in the kidney. The significant reduction in GFR and RBF and the altered response of the medullary circulation to increased RPP are consistent with the reduction in vascular density in the post-I/R kidney as the mediator of the impaired pressure-natriuretic relationship. The reduction in GFR likely reflects the dependence of GFR on RBF in conditions with a reduced blood flow (8). We wish to emphasize that RBF and GFR were impaired when normalized for kidney weight; this is likely due to the hypertrophy that occurs in I/R kidneys. Such differences are reduced, however, when normalized per body weight, providing a potential explanation for the recovery of serum creatinine values to normal or near-normal levels in postischemic animals. Nevertheless, the data underscore an important clinically relevant aspect of this model; the loss of peritubular capillaries is associated with moderate alterations in renal vascular function under unstressed conditions. When the post-I/R kidney is challenged with a stimulus requiring adept hemodynamic responses (i.e., increased NaCl intake), we hypothesize that homeostasis can only be achieved at an elevated level of arterial pressure.
We propose that the observed reduction in peritubular capillaries in post-I/R rats may by responsible for this impaired adaptive response post-I/R since the lack of medullary autoregulation, which is characterized by an increase in the number of perfused vasa recta capillaries in response to elevated RPP, represents an important component of the pressure-natriuresis response (23). Moreover, we recently demonstrated that VEGF-121 administration to postischemic rats preserved blood vessel density and attenuated the development of salt-sensitive secondary CKD (15). We suggest that the reduction in peritubular capillaries in response to I/R injury compromises the renal medullary hemodynamic responses associated with pressure natriuresis.
In addition to alterations in vascular structure, it is possible that the vascular and/or tubular injury may result in an imbalance of vasoactive factors. For example, there is a loss of normal endothelial nitric oxide synthase function up to 1 wk following ischemic injury in the I/R model (10), and I/R injury results in a reduction in kallikrein in the affected kidney (5). An alteration of vasoactive factors, along with the rarefaction of renal microvasculature, could potentially result in an increase in vascular resistance and mediate the decrease in filtered load of sodium and water and altered renal medullary blood flow observed in the post-I/R animals. A further potential explanation for the observed differences in GFR and RBF is a different sensitivity of the vasculature of the sham and I/R rats to the exogenously administered hormones or to volume expansion. Although these possibilities cannot be excluded, we previously reported that RBF normalized to kidney weight is reduced in anesthetized, euvolemic I/R rats in which the extraneous hormones were not infused (2). The exact mechanisms that lead to the alterations in renal hemodynamics remain to be elucidated.
Perspectives and Significance
This study helps clarify the mechanisms by which incomplete recovery from renal ischemia-reperfusion results in a loss of normal homeostatic responses. The postischemic kidney manifests an impairment of the pressure natriuresis and diuresis relationship that likely leads to the sensitivity of blood pressure to elevated sodium intake in this model. The observed shift of the pressure-natriuresis relationship to higher pressures appears to be attributable to an alteration in renal vascular function as evidenced by a decrease in GFR and an inability to increase medullary blood flow and renal interstitial hydrostatic pressure as perfusion pressure is elevated. We hypothesize that the loss of vasculature observed in renal ischemia-reperfusion injury is the underlying cause of these functional effects.
GRANTS
This work was supported by National Institutes of Health Grants DK-63114 to D. P. Basile and HL-29587 and DK-62803 to D. L. Mattson.
REFERENCES
- 1.Askenazi D, Feig D, Graham N, Hui-Stickle S, Goldstein S. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Donohoe D, Roethe K, Mattson DL. Chronic renal hypoxia after acute ischemic injury: effect of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Donohoe D, Roethe K, Osborn J. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Basile DP, Donohoe DL, Phillips SA, Frisbee JC. Enhanced skeletal muscle arteriolar reactivity to Ang II following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 289: R1770–R1776, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, Greene AS, Cowley AW., Jr Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am J Physiol Renal Physiol 288: F953–F963, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bonomini V, Stefoni S, Vagelisat A. Long-term patient and renal prognosis in acute renal failure. Nephron 36: 169–172, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, Troy JL, Daugharty TM, Deen WM, Robertson CR. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol 223: 1184–1190, 1972 [DOI] [PubMed] [Google Scholar]
- 9.Cholewa BC, Meister CJ, Mattson DL. Importance of the renin-angiotensin system in the regulation of arterial blood pressure in conscious mice and rats. Acta Physiol Scand 183: 309–320, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Conger J, Robinette J, Schrier R. Smooth muscle calcium and endothelium derived relaxing factor in the abnormal vascular responses of acute renal failure. J Clin Invest 82: 525–537, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Estan J, Roman RJ. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am J Physiol Regul Integr Comp Physiol 256: R63–R70, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW., Jr Effects of daily sodium intake and angiotensin II upon cortical and medullary blood flow in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1317–R1323, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Gross V, Lippoldt A, Schneider W, Luft FC. Effect of captopril and angiotensin II receptor blockade on pressure natriuresis in transgenic TGR(mRen-2)27 rats. Hypertension 26: 471–479, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Humes HD, Liu S. Cellular and molecular basis of renal repair in acute renal failure. J Lab Clin Med 124: 749–754, 1994 [PubMed] [Google Scholar]
- 15.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson DL, Lu SH, Roman RJ, Cowley AW., Jr Relationship between renal perfusion pressure and blood flow in different regions of the kidney. Am J Physiol Regul Integr Comp Physiol 264: R578–R583, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Mattson DL, Raff H, Roman RJ. Influence of angiotensin II on pressure natriuresis and renal hemodynamics in volume-expanded rats. Am J Physiol Regul Integr Comp Physiol 260: R1200–R1209, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284: R13–R27, 2003 [DOI] [PubMed] [Google Scholar]
- 19.McLennan GP, Kline RL, Mercer PF. Effect of enalapril treatment on the pressure-natriuresis curve in spontaneously hypertensive rats. Hypertension 17: 54–62, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Roman RJ, Cowley AW., Jr Abnormal pressure-diuresis-natriuresis response in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 248: F199–F205, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Roman RJ, Cowley AW., Jr Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 248: F190–F198, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Roman RJ, Cowley AW, Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume-expanded rats. Cortical and medullary hemodynamics. Hypertension 12: 168–176, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol 4: 1387–1395, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Van der Mark J, Kline RL. Altered pressure natriuresis in chronic angiotensin II hypertension in rats. Am J Physiol Renal Fluid Electrolyte Physiol 266: F739–F748, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Wang CT, Chin SY, Navar G. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000 [DOI] [PubMed] [Google Scholar]