Abstract
Leptin is thought to reduce food intake, in part, by increasing sensitivity to satiation signals, including CCK. Leptin action in both forebrain and hindbrain reduces food intake, and forebrain leptin action augments both the anorexic and neuronal activation responses to CCK. Here, we asked whether leptin signaling in hindbrain also enhances these responses to CCK. We found that food intake was strongly inhibited at 30 min after a combination of 4th-intracerebroventricular (4th-icv) leptin injection and intraperitoneal CCK administration, whereas neither hormone affected intake during this period when given alone. Leptin injections targeted directly at the dorsal vagal complex (DVC) similarly enhanced the anorexic response to intraperitoneal CCK. Intra-DVC leptin injection also robustly increased the number of neurons positive for phospho-STAT3 staining in the area surrounding the site of injection, confirming local leptin receptor activation. Conversely, the anorexic response to 4th-icv leptin was completely blocked by IP devazepide, a CCKA-R antagonist, suggesting that hindbrain leptin reduces intake via a mechanism requiring endogenous CCK signaling. We then asked whether hindbrain leptin treatment enhances the dorsomedial hindbrain, hypothalamus, or amygdala c-Fos responses to IP CCK. We found that, in contrast to the effects of forebrain leptin administration, 4th-icv leptin injection had no effect on CCK-induced c-Fos in any structures examined. We conclude that leptin signaling in either forebrain or hindbrain areas can enhance the response to satiation signals and that multiple distinct neural circuits likely contribute to this interaction.
Keywords: nucleus of the solitary tract, satiety signals, adiposity signals, food intake
leptin, a circulating hormone secreted by adipose tissue in proportion to fat mass, is a key humoral signal involved in the control of food intake and body weight (19). Leptin signaling in the brain reduces food intake primarily by reducing the size of meals (5, 11, 16), suggesting an augmentation of mechanisms that promote satiation. This idea is supported by several studies that have demonstrated an interaction between leptin and the gut peptide CCK (14), a prototypical satiation hormone that is secreted in response to the presence of nutrients in the gastrointestinal tract. In rats and mice, central or peripheral leptin pretreatment enhances CCK-induced anorexia (2, 4). Conversely, the reduction of plasma leptin induced by fasting causes a decrease in rats' responsiveness to CCK (12). Furthermore, the feeding response to CCK is blunted in rodents lacking functional leptin receptors, such as obese fak/fak Koletsky rats, and can be rescued by central leptin receptor gene therapy directed to the hypothalamic arcuate nucleus (ARC) of these animals (16).
Little is known about whether leptin-responsive brain regions beyond the ARC can also augment the response to CCK. Several studies have involved systemic administration of leptin, which may act at any number of central nervous system (CNS) sites and/or on peripheral vagal afferent fibers (2, 18). Others have injected leptin into the 3rd cerebral ventricle (3rd-icv), in the vicinity of the hypothalamus (4), but the caudal flow of cerebrospinal fluid dictates that such treatment delivers leptin to the midbrain and hindbrain, as well. Although leptin detection within the ARC can enhance CCK-induced anorexia (16), the hypothalamus is clearly not the only CNS site with the ability to transduce input from leptin into a decrease in food intake. Neurons in several feeding-relevant caudal brain stem nuclei, including the nucleus of the solitary tract (NTS, an area thought to be involved in satiety), express the long (signaling) form of the leptin receptor (7, 13). Peripheral injection of leptin induces phosphorylation of STAT3 (pSTAT3, a marker of leptin receptor activation) in NTS cells (9, 10), as well as in hypothalamic populations, adding strength to the suggestion that leptin acts directly on hindbrain sites. Furthermore, leptin injected into the dorsal vagal complex (DVC, which contains the NTS, area postrema, and dorsal motor nucleus of the vagus nerve) reduces food intake and body weight at doses that are subthreshold for such effects following ventricular administration (7).
These considerations support the hypothesis that neurons involved in the control of food intake are widely distributed throughout the CNS and interact with one another in an extensive and redundant manner (6). To test this hypothesis, we first asked whether injection of leptin into the 4th cerebral ventricle (4th-icv), which targets the caudal brain stem more selectively than lateral- or 3rd-icv treatment, enhances peripheral CCK-induced anorexia. Second, we asked whether injection of leptin directly into the DVC similarly augments the response to CCK and whether this effect is associated with activation of local leptin receptors, as judged by the induction of pSTAT3. Next, we investigated whether anorexia induced by hindbrain leptin signaling is dependent on endogenously released CCK, by coadministering leptin (4th-icv) and the CCK-1 receptor antagonist devazepide (DVZ, given intraperitoneally). In an effort to identify the neuronal substrates underlying this interaction, we also evaluated the profile of neuronal activation (as measured by c-Fos-like immunoreactivity) in response to combined administration of hindbrain leptin plus peripheral CCK compared with either treatment alone. Our findings indicate that while hindbrain leptin receptor activation enhances CCK-induced satiety, the neural circuitry underlying this effect is distinct from that engaged by forebrain leptin receptor activation.
METHODS
Subjects
Naïve male Wistar rats were obtained from Harlan Laboratories (Indianapolis, IN). All subjects were individually housed in Plexiglas cages in a temperature-controlled room under a 12:12-h light-dark cycle. Water and standard rat chow (PMI Nutrition International, Gray Summit, MO) were available ad libitum, except where otherwise noted. All subjects were handled daily and habituated to intraperitoneal injection of 1 ml saline and measurement of food intake throughout the dark phase on at least three occasions before the experiments began. Body weight and food intake (FI) were measured daily. Study procedures were approved by the Animal Care Committee at the University of Washington and conformed to standards described in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Drugs
CCK octapeptide (Bachem, Torrance, CA) was dissolved in 0.1% BSA in sterile 0.9% saline. Recombinant rat leptin (National Hormone & Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. Parlow) was dissolved in sterile 0.01 M NaHCO3. DVZ (Tocris Bioscience, Ellisville, MO) was initially dissolved at high concentration in 100% DMSO (Sigma-Aldrich, St. Louis, MO) but was diluted for injections to 80% sterile normal saline, 5% Tween 80 (Sigma-Aldrich, St. Louis, MO), and 5% DMSO.
Surgery
Each rat received a guide cannula (Plastics One, 26-G, Roanoke, VA), implanted 2.0 mm above either the 4th ventricle or left NTS under 2 to 4% isoflurane in 1 liter oxygen/minute inhaled continuously during surgery. Stereotaxic coordinates for 4th-icv cannula placement were on the midline, 2.5 mm anterior to the occipital suture, and 5.2 mm ventral to the skull surface. The coordinates for cannulating the NTS were 0.7 mm lateral to midline, on the occipital suture, and 6.3 mm ventral to the skull surface, targeting the medial NTS at the level of the area postrema (AP). Because of the close proximity of the NTS, AP, and dorsal motor nucleus of the vagus nerve, any solution injected through this DVC cannula may be expected to diffuse to all three of the DVC nuclei. The cannula was cemented to three jeweler's screws attached to the skull, and closed with an obturator. Buprenorphine hydrochloride (0.3 mg/kg im) (Rickett Colman Pharmaceuticals, Richmond, VA) was administered at completion of surgery, and the rats recovered for at least 5 days while daily food intake and body weight were recorded. Cannula placement was verified before the start of the experiment through the measurement of a sympathetically mediated increase in plasma glucose 60 min after injection of 5-thio-d-glucose at a dose of either 210 μg into the 4th ventricle or 50 μg into the NTS (20, 21).
Study Protocols
Effect of 4th-icv leptin pretreatment on CCK-induced anorexia.
Rats were assigned to 1 of 2 weight-matched groups (n = 6–7/group, mean body weight = 435 g), receiving either 4th-icv vehicle (2 μl of 0.01M NaHCO3) or leptin (5 μg/2 μl) injections. On experimental days, all rats were weighed, and food was removed 4 h before the onset of the dark phase. At 1 h before dark onset, rats received 4th-icv injections of vehicle or leptin, delivered via 33-gauge injectors (Plastics One, Roanoke, VA) extending 2 mm beyond the end of the implanted cannulas. Immediately before dark cycle onset, rats were injected with saline vehicle or a dose of CCK (0.5 μg/rat ip, 1 ml volume) that is subthreshold for feeding effects when given alone. After the IP injections, preweighed food was returned to all subjects. FI was measured 0.5, 1, 4, and 24 h later. After 3 days for recovery from treatments, the same protocol was followed again with the IP injection conditions reversed among subjects, for a mixed between/within-subjects design.
Effect of DVC leptin pretreatment on CCK-induced anorexia.
This experiment used a within-subjects design, in which each rat (n = 7) received each of four conditions in counterbalanced order separated by at least 3 days: DVC vehicle/intraperitoneal vehicle; DVC leptin/intraperitoneal vehicle; DVC vehicle/intraperitoneal CCK; DVC leptin/intraperitoneal CCK. On experimental days, the protocol was nearly identical to that described for the 4th-icv experiment. DVC injection of either vehicle (0.5 μl) or leptin (0.25 μg/0.5 μl) was administered over 5 min at a rate of 0.1 μl/min via motorized syringe pump (KD Scientific, Holliston, MA), and took place 1 h before dark onset, and IP injections of vehicle or CCK (0.3 μg/rat, 1 ml) were administered immediately before the dark cycle. Food intake was then measured 30 min, 4 h, and 24 h later, as well as body weight at 24 h. We chose the dose of 0.25 μg leptin based on previously published 4th-icv and DVC dose-response functions (7). We lowered the CCK from 0.5 μg to 0.3 μg ip with the intention that it be below threshold for a feeding response when delivered alone. Cannula placement was histologically verified at the end of the experiment (see below), and only rats with correct placements were included for analyses.
Effect of intra-DVC leptin injection on hindbrain pSTAT3.
At the end of the above DVC leptin/CCK experiment, rats were killed and brains were examined to histologically verify cannula placement. Half of the rats received an intra-DVC injection of 0.5 μl vehicle, and the remaining 3 received 0.25 μg leptin. At 10 min postinjection, rats were deeply anesthetized (180 mg/kg ketamine and 30 mg/kg ip xylazine) and then transcardially perfused with PBS followed by 4% paraformaldehyde (PFA). Brains were removed and placed in 70% EtOH for 1 wk before being embedded in paraffin. Coronal microtome sections (8 μm) through the NTS were slide-mounted and stored at room temperature.
pSTAT3 immunohistochemical staining.
We selected NTS sections for staining by identifying the injection sites. Slides were dewaxed in two incubations with xylene of 5 min each, then hydrated with a series of two 3-min incubations in 100% EtOH and 1 min in 95% EtOH, followed by a rinse with dH2O. Our technique for pSTAT3 staining is adapted from previously published methods (10). We first used an antigen-retrieval technique, where slides were incubated in sodium citrate buffer (Vector Laboratories, Burlingame, CA) in a 100°C water-bath for 40 min, then allowed to cool at room temperature for 20 min. After rinsing with 10 mM PBS and blocking with 10% normal donkey serum in PBS for 1 h at room temperature, slides were incubated with the primary antibody, rabbit anti-pSTAT3 (Sigma), at 1:200 in 0.1% BSA in PBS for 48 h at 4°C. Sections were then rinsed and incubated for 2 h at room temperature with donkey anti-rabbit Biotin SP (Jackson Immunoresearch, West Grove, PA) diluted at 1:200 in PBS. After rinsing with PBS, streptavidin-Cy3 (Jackson Immunoresearch) at a dilution of 1:200 in PBS was applied for 30 min at room temperature. Control sections incubated only with normal serum did not show staining. The slides were examined using a 10× objective on a Nikon Eclipse E600 fluorescence microscope, and digital RGB images were acquired with a Diagnostic Images SPOT RT Color camera and SPOT software. pSTAT3 staining was not quantified.
Effect of DVZ on 4th-icv leptin-induced anorexia.
This experiment used a within-subjects design, where each rat (n = 5) received each of 4 conditions in counterbalanced order separated by at least 3 days: 4th-icv vehicle/intraperitoneal vehicle (5% DMSO, 5% Tween 80, 80% normal saline); 4th-icv vehicle/intra-DVZ (0.5 mg/kg); 4th-icv leptin (5 μg/2 μl)/intraperitoneal vehicle; 4th-icv leptin/intra-DVZ. We chose the 0.5 mg/kg ip dose of DVZ based on a pilot study, in which we found that this dose had little or no effect on food intake when given alone (data not shown). The protocol for experimental days was similar to those described above, with the difference being that here, intraperitoneal injections of vehicle or DVZ were administered immediately after the 4th-icv injection of vehicle or leptin, ∼60 min before the onset of the dark cycle. Food was returned to the rats within 5 min before dark cycle onset, and intake was measured 1, 4, and 24 h later, along with body weight at 24 h.
Effect of 4th-icv leptin on CCK-induced c-FLI in the brain.
Cannulas targeting the 4th-ventricle were implanted and placement was verified as described above. Rats were then divided into four weight-matched groups: vehicle/vehicle, leptin/vehicle, vehicle/CCK, and leptin/CCK. On the experiment day, food was removed 4 h before dark phase onset. 4th-icv injections of either leptin (5 μg/2 μl) or vehicle were administered 1 h before the dark cycle, and rats were intraperitoneally injected with CCK (0.5 μg/rat) or vehicle immediately before dark onset. Ninety minutes after the intraperitoneal injection, rats were deeply anesthetized (180 mg/kg ketamine and 30 mg/kg xylazine ip) and then transcardially perfused with PBS followed by 4% PFA. The brains were removed and placed in 25% sucrose/PBS overnight, then frozen in isopentane at −37°C. Coronal cryostat sections (14 μm) through the NTS, PVN, and CeA were slide-mounted and stored at −80°C (n = 6–7/group for NTS; n = 4/group for forebrain regions).
Immunohistochemical staining.
The technique that we employed for c-Fos staining of anatomically matched sections through the NTS at the level of the AP and rostral to the AP, PVN, and CeA has been published previously (3). Our primary antibody was rabbit polyclonal anti-c-Fos (Calbiochem, San Diego, CA) diluted 1:5,000 in 0.1% BSA in 10 mM PBS, and the secondary antibody, donkey anti-rabbit IgG-Cy-3 (Jackson Immunoresearch) was diluted 1:200 in 0.1% BSA in 10 mM PBS. Control sections incubated with normal serum did not show staining.
One-half of the hindbrain sections was double-labeled for both c-Fos and dopamine beta-hydroxylase (DbH), a marker for catecholamine neurons, using a technique that has been previously published (25). The primary and secondary antibodies for c-Fos were the same as above, and we used a mouse monoclonal anti-DbH primary antibody (Millipore, Billerica, MA) at 1:10,000. The secondary antibody was goat anti-mouse Alexa 488 (Invitrogen, Carlsbad, CA).
Quantitative analysis of immunostaining.
Slides were examined using a 10× objective on a Nikon Eclipse E600 fluorescence microscope, and digital RGB images were acquired with a Diagnostic Images SPOT RT Color camera and SPOT software. National Institutes of Health Image software was used to count cells positive for c-Fos-like immunoreactivity (c-FLI). Labeling in the NTS was counted bilaterally on 4–6 sections per rat at two different anatomical levels: one rostral to the AP and one at the mid-AP level. Labeling in PVN and CeA was counted on 3 or 4 sections per rat. For each brain nucleus and anatomical level, mean values were determined for each subject. On double-labeled sections through NTS, DbH-positive cell bodies and cells positive for both DbH and c-FLI were counted unilaterally by eye, and mean values were taken for each subject.
Statistical analysis.
Statistical comparisons between and within groups were made by two-way ANOVA. Post hoc and planned comparisons were made with Tukey's honestly significant difference tests. P values lower than 0.05 were considered to be statistically significant.
RESULTS
Effect of 4th-icv Leptin Pretreatment on CCK-Induced Anorexia
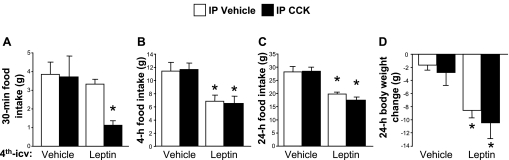
Hindbrain leptin pretreatment strongly enhanced the effect of CCK to reduce food intake. The combination of 4th-icv leptin and intraperitoneal CCK treatments significantly suppressed food intake relative to the vehicle/vehicle condition 30 min after dark onset (P < 0.05), whereas neither 4th-icv leptin plus vehicle nor 4th-icv vehicle plus CCK had any effect on intake at this time point (see Fig. 1A). There were significant main effects of both leptin [F (1, 11) = 4.93, P < 0.05] and CCK [F (1, 11) = 9.52, P < 0.05], as well as a significant interaction [F (1, 11) = 7.47, P < 0.05]. The results at 1 h post-dark onset were similar (data not shown). At 4 h into the dark phase, 4th-icv leptin markedly reduced intake relative to 4th-icv vehicle regardless of subsequent IP treatment [main effect F (1, 11) = 15.95, P < 0.01], while intraperitoneal CCK had no effect on its own, and the combination of leptin plus CCK did not reduce intake further than leptin alone (see Fig. 1B). Similarly, food intake at the 24-h time-point was significantly suppressed by 4th-icv leptin [F = (1, 11) = 48.59, P < 0.001], with no additional effect of CCK (see Fig. 1C). Consistent with these results, significant body weight loss over the course of 24 h was observed in rats that received leptin injections [F (1, 11) = 18.38, P < 0.01], and there was no interaction with CCK (see Fig. 1D).
Fig. 1.
Rats were pretreated with either vehicle or leptin (5 μg) administered 4th-icv and received IP injections of either vehicle or CCK (0.5 μg) 60 min later, immediately before the onset of the dark cycle. Food intake was measured at 30 min (A), 4 h (B), and 24 h (C) after intraperitoneal injections. Body weight change (D) over the 24 h following treatment was also measured. Data are expressed as means ± SE. A: *P < 0.05 vs. all other conditions. B–D: *P < 0.05 vs. vehicle/vehicle and vehicle/CCK conditions.
Effect of DVC Leptin Pretreatment on CCK-Induced Anorexia
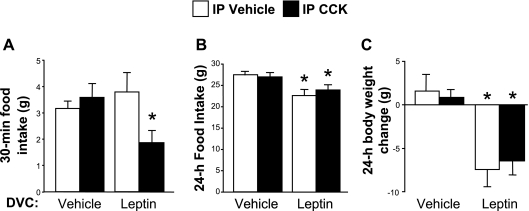
We observed a significant interaction between DVC leptin and IP CCK at 30 min after dark cycle onset [F (1, 5) = 12.12, P < 0.05]. DVC leptin followed by IP CCK significantly suppressed 30-min food intake (P < 0.05) by about 50%, whereas intake after DVC leptin/intraperitoneal vehicle and DVC vehicle/intraperitoneal CCK did not differ from that observed after DVC vehicle/intraperitoneal vehicle treatment (see Fig. 2A). There were no significant main effects or interaction for food intake at 4 h, but closer examination revealed a trend toward intake suppression with DVC leptin. Comparing only the DVC vehicle/intraperitoneal vehicle with DVC leptin/intraperitoneal vehicle conditions, there was significant anorexia after leptin treatment (mean intakes 9.08 ± 0.72 g vs. 7.6 ± 0.5 g, P < 0.05), but because of greater variability across the other conditions, we saw no significant anorexia with DVC leptin/intraperitoneal CCK treatment at the 4-h time point. At 24 h, DVC leptin significantly suppressed food intake both when delivered as a pretreatment to intraperitoneal vehicle and when delivered as a pretreatment to CCK [main effect of leptin F (1, 5) = 18.77, P < 0.01] (see Fig. 2B). As expected, intraperitoneal CCK had no effect on its own at 24 h, and there was no interaction between leptin and CCK at this time-point. Significant overnight body weight loss resulted from DVC leptin injections [main effect of leptin F (1, 5) = 18.17, P < 0.01], but there was no interaction with CCK (see Fig. 2C).
Fig. 2.
Rats were pretreated with either vehicle or leptin (0.25 μg) administered to the dorsal vagal complex (DVC) and then received intraperitoneal injections of either vehicle or CCK (0.3 μg) 60 min later, immediately before the onset of the dark cycle. Food intake is shown for 30 min (A) and 24 h (B) after the intraperitoneal injections. Body weight change (C) over the 24 h following treatment was also measured. Data are expressed as means ± SE. A: *P < 0.05 vs. all other conditions. B and C: *P < 0.05 vs. vehicle/vehicle and vehicle/CCK conditions.
Effect of Intra-DVC Leptin Injection on Hindbrain pSTAT3
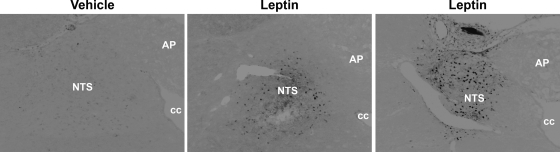
To confirm that our intra-DVC leptin administration protocol activates local leptin receptor signal transduction, we performed immunohistochemical staining to detect pSTAT3 in coronal sections of hindbrain collected 10 min after injection of leptin (0.25 μg) directly into this brain area. As expected, leptin-induced pSTAT3 induction was readily apparent in the NTS near the site of injection and was not observed in other hindbrain areas (Fig. 3). Vehicle-treated rats showed no pSTAT3 staining.
Fig. 3.
Representative images of hindbrain sections immunohistochemically stained for pSTAT3. Rats were injected into the DVC with either vehicle or leptin and euthanized 10 min later. Leptin injection produced a robust pSTAT3 response in the area surrounding the injection. NTS, nucleus of the solitary tract; AP, area postrema; cc, central canal. Images have been digitally converted from color to grayscale and inverted for better visual presentation. Black spots are pSTAT-positive nuclei.
Effect of DVZ on 4th-icv Leptin-Induced Anorexia
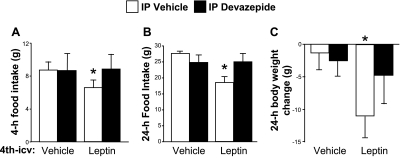
We observed no significant effects of leptin or DVZ at 1 h post-dark onset (data not shown). At 4 h, 4th-icv leptin reduced food intake significantly (P < 0.05) when given with intraperitoneal vehicle, and this effect of leptin was completely blocked by intra-DVZ (Fig. 4A). There was a near-significant interaction [F (1, 4) = 5.23, P = 0.08], and intake after 4th-icv leptin/intraperitoneal vehicle differed significantly from all other conditions (P < 0.05). Neither intra-DVZ condition differed from 4th-icv vehicle/intraperitoneal vehicle. The pattern of results at 24 h was similar, with a significant interaction detected between leptin and DVZ [F (1, 4) = 9.55, P < 0.05]. Here, 4th-icv leptin plus intraperitoneal vehicle reduced intake significantly (P < 0.05) compared with all other conditions, and intra-DVZ had no effect on intake regardless of the 4th-icv cotreatment (Fig. 4B). For body weight change, there was a main effect of leptin [F (1, 4) = 8.8, P < 0.05] but no significant interaction. However, planned comparisons revealed that 4th-icv leptin/intraperitoneal vehicle reduced body weight significantly (P < 0.05) compared with the 4th-icv vehicle/intraperitoneal vehicle and 4th-icv vehicle/intra-DVZ. Body weight change after 4th-icv leptin/intra-DVZ fell in between that seen after 4th-icv leptin/intraperitoneal vehicle and the two 4th-icv vehicle conditions, and 4th-icv leptin/intra-DVZ did not differ significantly from any of the other three conditions.
Fig. 4.
Rats were given 4th-icv vehicle or leptin (5 μg) injections followed by intraperitoneal vehicle or devazepide (DVZ; 0.5 mg/kg) 60 min prior to dark onset. Food was returned immediately before dark, and intake was measured 4 (A) and 24 h (B) later. Body weight change (C) over the 24 h following treatment was also measured. Data are expressed as means ± SE. A and B: *P < 0.05 vs. all other conditions. C: *P < 0.05 vs. vehicle/vehicle and vehicle/DVZ conditions.
Effect of 4th-icv Leptin on CCK-Induced c-FLI in the Brain
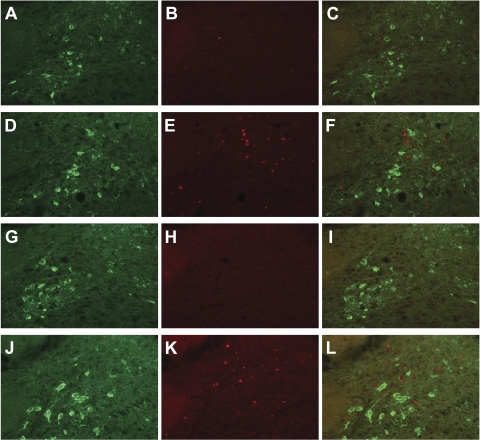
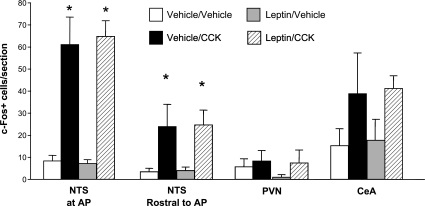
Surprisingly, although we observed the expected, robust response to CCK alone, we found no evidence of an interactive effect of 4th-icv leptin and CCK on brain c-FLI (see Fig. 6). Intraperitoneal CCK increased c-FLI in the NTS at both anatomical levels examined (mid-AP and rostral to AP) to the same extent regardless of 4th-icv pretreatment condition [main effect of CCK for mid-AP level: F (1, 20) = 71.02, P < 0.0001; main effect rostral to AP: F (1, 14) = 15.6, P < 0.01] (see Figs. 5 and 6). In the PVN, this dose of CCK was not sufficient to induce c-FLI, and 4th-icv leptin had no effect either on its own or in combination with CCK. We also observed a significant induction of c-FLI by CCK in the CeA [F (1, 12) = 5.7, P < 0.05] but again did not see an effect of leptin or an interaction between leptin and CCK. On some sections through PVN and CeA, the ARC was also present, but again, we saw no induction of c-Fos in this brain area in response to leptin, CCK, or the two hormones in combination (data not shown). In the subset of hindbrain sections that were double-labeled for DbH and c-Fos, we observed no significant colocalization of c-Fos with DbH-immunoreactivity at this low dose of CCK, nor was this lack of colocalization altered when rats were given 4th-icv leptin prior to CCK (mean number of DbH-positive cells/unilateral NTS section across all groups = 19.33 ± 1.3; mean number of double-labeled cells = 0.33 ± 0.2) (see Fig. 6). Thus, leptin does not act locally in the hindbrain to potentiate CCK-induced c-Fos expression in NTS catecholamine neurons, in contrast with the previously reported effect of 3rd-icv injection of leptin (25).
Fig. 6.
Representative images of sections through NTS that were double-labeled for DbH and c-Fos. Each image shows the A2 region of the left NTS. A–C: images from a vehicle/vehicle-treated rat. D–F: images from a vehicle/CCK-treated rat. G–I: images from a leptin/vehicle-treated rat. J–L: images from a leptin/CCK-treated rat. A, D, G, J: images show DbH-like immunoreactivity, which appears green and cytoplasmic. B, E, H, K: images show c-Fos-like immunoreactivity, which is red and nuclear. C, F, I, L: images merged to identify colocalization.
Fig. 5.
Mean ± SE number of c-FLI-positive nuclei counted bilaterally per section through NTS at the level of the AP, NTS rostral to AP, PVN, and CeA, after rats were treated with 4th-icv vehicle or leptin (5 μg) and IP vehicle or CCK (0.5 μg). *P < 0.05 vs. vehicle/vehicle and leptin/vehicle conditions.
DISCUSSION
Our results strongly support the hypothesis that hindbrain leptin receptor activation augments the anorexic response to systemically administered CCK. We have shown that following administration of leptin into either the 4th ventricle or directly into the DVC at doses that have no independent effect on short-term feeding, the anorexic response to intraperitoneal CCK is strongly enhanced. We also observed that intra-DVC leptin induced pSTAT3 in the area immediately surrounding the injection site, a clear demonstration that NTS leptin receptors were activated by the treatment. In many of these respects, our results parallel those observed following the combination of peripheral CCK and forebrain leptin administration. Specifically, 3rd-icv leptin enhances the feeding-suppressive effect of CCK (4), while inducing pSTAT3 expression in hypothalamic regions (15). Moreover, restoration of leptin receptors to the hypothalamic ARC of Koletsky (fak/fak) rats that otherwise lack these receptors is sufficient to reduce meal size and increase the satiation-inducing actions of CCK (16). These observations support the idea that leptin's ability to potentiate the response to meal-related signals involves actions in multiple forebrain and hindbrain areas.
The present data also support the hypothesis that the anorexic effect of hindbrain leptin receptor activation is dependent on input from endogenously released CCK. We showed that 4th-icv leptin-induced anorexia was blocked by an intraperitoneal dose of DVZ that had no effect on intake when delivered alone. Thus, the interaction between hindbrain leptin and CCK is not merely a pharmacologic effect observed only with acute CCK administration, but rather reflects the normal physiology of food intake control. Although previous studies have shown an effect of intra-DVZ to blunt anorexia induced by IP leptin treatment (2), which could potentially involve leptin receptors in any area of the brain or periphery [perhaps including those on the afferent vagus (18), which responds directly to CCK], we specifically targeted the hindbrain via the 4th-ventricle. To our knowledge, this is the first demonstration of an interaction between endogenously released CCK and leptin receptor activation in any specific CNS location, and it strengthens the physiological importance of the interaction between CNS leptin receptor signaling and the response to meal-related signals such as CCK.
In previous studies, the ability of 3rd-icv or peripherally administered leptin to enhance the anorexic effects of IP CCK was correlated with a synergistic effect of the two treatments on c-Fos expression in several brain areas, including the NTS and PVN (4, 23). Furthermore, in genetically obese fak/fak rats, ARC-specific leptin receptor gene therapy restored both the behavioral and dorsal hindbrain c-Fos response to CCK (16). On the basis of the hypothesis that leptin-sensitive hindbrain neurons might enhance the anorexic response to CCK by increasing CCK-induced neuronal activation, we expected to see an interactive effect of 4th-icv leptin and CCK on c-Fos expression in the brain. However, we found no effect of 4th-icv leptin on CCK-induced c-Fos expression in any of the hindbrain or forebrain nuclei examined, suggesting that the neurocircuitry that underlies leptin-CCK interactions depends on the brain area involved, even if the net effect on food intake appears similar. To investigate this hypothesis in greater detail, we performed a study in which hindbrain sections were double-labeled for DbH and c-Fos, based on our earlier report that forebrain leptin treatment increases the number of NTS catecholamine neurons that express c-Fos in response to CCK (25). Unlike our previous result, 4th icv leptin administration did not increase CCK-induced c-Fos in NTS catecholamine neurons. Our finding that hindbrain c-FLI faithfully reflects the interactive effects of leptin and CCK on feeding when leptin is administered into the forebrain, but not when infused in the proximity of the DVC, implies that this interaction can elicit a very similar behavioral response through distinct neural circuits involving multiple brain areas.
This dissociation between the interactive effects of 4th-icv or DVC leptin and CCK at the level of food intake, but not c-Fos induction, also challenges the view that increased c-Fos expression in the NTS reliably reflects neuronal activation involved in the satiating response to meal-related stimuli. We recently reported a similar lack of leptin-induced enhancement of the c-Fos response to Exendin-4 (a glucagon-like peptide 1 receptor agonist), despite a strong interactive effect on feeding (24). These observations suggest that although elevated c-Fos expression in NTS is a reliable marker of both the central response to peripheral CCK and its enhancement by forebrain leptin action, this response does not capture other key pathways that also contribute to the behavioral effects.
Pertinent to this analysis are recent studies that address the mechanism by which hypothalamic leptin signaling may enhance hindbrain neuronal activation induced by peripheral CCK administration. CCK-induced c-Fos induction in the NTS is thought to involve the release of glutamate from vagal afferent terminals, which, in turn, depolarizes NTS cell bodies. Available data suggest that it is through augmentation of this effect that forebrain leptin action enhances the CCK-induced c-FLI. Several populations of leptin-responsive hypothalamic neurons project to the NTS, including POMC neurons in the ARC and oxytocin (OT) neurons in the PVN (3, 27). When leptin is administered either peripherally or 3rd-intracerebroventricularly, these cell types are activated and are thought to release neurotransmitters in the NTS. Recent studies from Wan et al. (22) suggest that the main effect of melanocortin 4 receptor (MC4R) activation in the NTS is to increase glutamate release by vagal afferent terminals. Similarly, OT acts presynaptically in the NTS to increase glutamate release from vagal afferent terminals and also increases neuronal excitability via a postsynaptic mechanism (17). Therefore, leptin's activation of the descending POMC and OT projections to the NTS would be expected to potentiate the release of glutamate by vagal afferents at NTS synapses induced by CCK stimulation. This effect could be construed as increasing the amount of viscerosensory stimulation to NTS neurons. If this model is correct, it is worth noting that forebrain leptin action does not necessarily increase NTS neuronal sensitivity to the incoming vagal signal evoked by CCK, but rather increases the intensity of that incoming vagal signal. Because increasing vagal stimulation is correlated with increased c-Fos expression (28), this model provides a viable explanation for the finding that forebrain leptin receptor activation enhances the c-Fos response to CCK.
Hindbrain leptin receptor activation appears to engage a set of neural pathways different from those recruited by hypothalamic leptin receptor stimulation. As evidenced by the lack of c-FLI in the ARC or PVN in response to 4th-icv leptin, hypothalamic POMC and OT projections to the NTS do not appear to be activated when leptin is administered exclusively to the hindbrain, unlike what is observed following 3rd-icv leptin injection (3, 15). Although hindbrain leptin injection clearly activates a subset of leptin-sensitive neurons in the NTS (as judged by pSTAT3 induction), the identity of those neurons and their relationship to cells that are activated by CCK remain uncertain. One possibility is that leptin acts not only on NTS neurons but also on vagal afferent terminals in that nucleus. Indeed, electrophysiological studies have established that leptin applied to hindbrain slices can affect glutamate transmission to NTS neurons. However, leptin in this system reduces glutamatergic transmission and also postsynaptically hyperpolarizes these cells (26), effects opposite to those seen following OT and MC4R receptor stimulation (17, 22). On the basis of this observation, leptin action exclusive to the hindbrain would not be expected to increase the CCK-induced vagal signal to NTS neurons. Similarly, administration of leptin by itself did not induce c-Fos in the dorsal medulla, despite the robust induction of pSTAT3 in NTS neurons when leptin was delivered to the DVC, and there was no further increase in CCK-induced c-Fos with 4th-icv leptin pretreatment. These findings suggest that leptin's activation of NTS neurons is not, in and of itself, sufficient to either induce local c-Fos expression or to enhance the NTS neuronal c-Fos response to CCK.
Although our data do not provide a clear candidate mechanism for how hindbrain leptin receptor activation enhances the anorexic response to CCK, one potential explanation is that in the NTS, leptin receptors are expressed on the same neurons that also express c-Fos in response to CCK, so there are no additional neurons recruited by the combination treatment. This possibility seems less likely in light of the recent report by Babic et al. (1) that CCK-induced activation of ERK signaling does not colocalize with either a substantial population of leptin receptor-bearing neurons or with leptin-induced pSTAT3 in the mouse, although it remains to be determined whether CCK-induced c-Fos and ERK activation occur in the same or distinct populations of NTS neurons. Alternative explanations include the possibilities that CCK engages leptin-responsive NTS neurons in a manner that is not detected as a rise in c-Fos or ERK activation, or that hindbrain leptin treatment and intraperitoneal CCK activate distinct neuronal populations within the NTS that then converge elsewhere in the CNS.
To summarize, on the basis of the electrophysiological evidence described above (26), hindbrain leptin treatment is unlikely to enhance CCK-induced anorexia by increasing the intensity of the CCK-induced vagal input to NTS neurons. We favor the alternative hypothesis that the ability of hindbrain leptin action to enhance the anorexic response to CCK involves an integration that takes place at sites downstream, and studies are warranted to investigate this possibility. Such studies, however, should be informed by caveats about the use of c-Fos as a measure of neuronal activation. First, because activated neurons do not always express c-Fos (8), we may be missing an increase in neuronal activation in some or all of the examined nuclei because of the choice of c-Fos as our dependent variable. Second, there may be an important role for neuronal inhibition in the interaction between hindbrain leptin and CCK, which would not necessarily be detected as a change in c-Fos expression. Finally, the timing of injections and perfusions in this experiment may not have been optimal to observe an interactive effect of hindbrain leptin and CCK on c-FLI in feeding-relevant brain regions.
Perspectives and Significance
On the basis of our results, we conclude that hindbrain leptin receptor activation can enhance the anorexic response to CCK, and this may be one of the mechanisms by which endogenous leptin regulates food intake and body weight. Further research is required to identify the neural circuitry that mediates this interaction, but our data suggest that the relevant neural pathways differ from those recruited by forebrain leptin receptor activation. Endogenous leptin entering the brain from the periphery has access to leptin receptor populations throughout the brain, and increasing evidence suggests that the control of food intake and body weight involves nuclei distributed across all levels of the CNS (6). Therefore, a complete understanding of how leptin affects feeding requires additional studies that investigate the mechanisms of leptin action in both the forebrain and the hindbrain.
DISCLOSURES
M. W. Schwartz is a consultant for Merck and Pfizer.
GRANTS
These studies were supported by NIH grants K99DK078779 to DLW and DK52989, NS032273 and DK68384 to MWS. This material is also based upon work supported in part by the Office of Research and Development Medical Research Service, Department of Veterans Affairs. Dr. Baskin is Senior Research Career Scientist, Research and Development Service, Department of Veterans Affairs Puget Sound Health Care System, Seattle, WA. This research was also supported by the Clinical Nutrition Research Unit at the University of Washington, supported by NIH grant P30DK035816, and the facilities of the Cellular and Molecular Imaging Core of the University of Washington NIH Diabetes Endocrinology Research Center, supported by NIH grant P30DK17047.
ACKNOWLEDGMENTS
The authors would like to thank Iaela David, Nicole Lilly, Miles Matsen, Hong Nguyen, Loan Nguyen, and Eric Parise for their expert technical assistance.
REFERENCES
- 1.Babic T, Townsend RL, Patterson LM, Sutton GM, Zheng H, Berthoud HR. Phenotype of neurons in the nucleus of the solitary tract that express CCK-induced activation of the ERK signaling pathway. Am J Physiol Regul Integr Comp Physiol 296: R845–R854, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 94: 10455–10460, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287: R87–R96, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol Regul Integr Comp Physiol 276: R1545–R1549, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Flynn MC, Plata-Salaman CR. Leptin (OB protein) and meal size. Nutrition 15: 508–509, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity 14Suppl 5: 216S–221S, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14: 173–213, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology 143: 3498–3504, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes 55: 567–573, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol Regul Integr Comp Physiol 275: R180–R185, 1998 [DOI] [PubMed] [Google Scholar]
- 12.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141: 4442–4448, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Mercer JG, Moar KM, Findlay PA, Hoggard N, Adam CL. Association of leptin receptor (OB-Rb), NPY and GLP-1 gene expression in the ovine and murine brainstem. Regul Pept 75–76: 271–278, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Moran TH, Aja S, Ladenheim EE. Leptin modulation of peripheral controls of meal size. Physiol Behav 89: 511–516, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289: E1051–E1057, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 89: 477–485, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Porte D, Jr, Baskin DG, Schwartz MW. Leptin and insulin action in the central nervous system. Nutr Rev 60: S20–S9; discussion S68–S84, S85–S87, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213: 451–452, 1981 [DOI] [PubMed] [Google Scholar]
- 21.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 856: 37–47, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 28: 4957–4966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Martinez V, Barrachina MD, Tache Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res 791: 157–166, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55: 3387–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Williams DL, Schwartz MW, Baskin DG. Immunocytochemistry and laser capture microdissection for real-time quantitative PCR identify hindbrain neurons activated by interaction between leptin and cholecystokinin. J Histochem Cytochem 56: 285–293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams KW, Smith BN. Rapid inhibition of neural excitability in the nucleus tractus solitarii by leptin: implications for ingestive behaviour. J Physiol 573: 395–412, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289: R247–R258, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Zittel TT, Glatzle J, Kreis ME, Starlinger M, Eichner M, Raybould HE, Becker HD, Jehle EC. c-Fos protein expression in the nucleus of the solitary tract correlates with cholecystokinin dose injected and food intake in rats. Brain Res 846: 1–11, 1999 [DOI] [PubMed] [Google Scholar]