Abstract
The mechanisms by which aging progressively depletes testosterone (Te) availability in the male are unknown. Accordingly, the objective was to estimate brain gonadotropin-releasing hormone (GnRH) outflow (release and action), which cannot be observed directly, on the basis of downstream effects on pituitary luteinizing hormone (LH) secretion. LH, in turn, feeds forward on (stimulates) gonadal Te secretion, which then feeds back on (inhibits) GnRH-driven LH secretion. LH and Te concentrations were measured repetitively (every 10 min) over 18 h during graded pharmacological blockade of endogenous GnRH outflow in 24 healthy 20- to 72-yr-old men. Data were analyzed using a new age-dependent regression model of GnRH-LH-Te interactions to estimate pulsatile LH secretion and elimination, GnRH outflow, LH feedforward, and Te feedback. By incorporating regression on age within the dose-response model, we show that aging erodes all three primary forward and reverse pathways linking the brain, pituitary gland, and testes. Aging is associated with concomitant deficits in GnRH → LH feedforward, LH → Te feedforward, and Te → GnRH/LH feedback. The analytical formalism should be generalizable to other ensemble regulatory systems, such as those that control growth, reproduction, stress adaptations, and glucose metabolism.
Keywords: gonadotropin, men, human, testosterone, testis, model
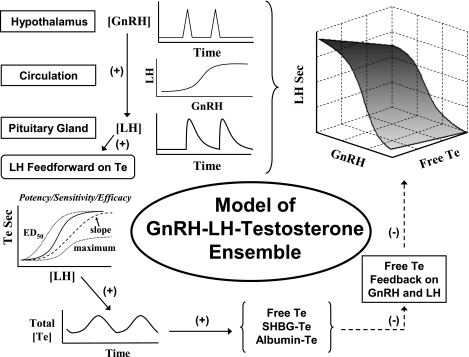
aging, undernutrition, protracted illness, and environmental toxins reduce production of major anabolic hormones such as testosterone (Te) in men and animals (3, 7, 22). Chronically diminished androgen availability, in turn, predicts increased all-cause mortality, cardiovascular risk, visceral adiposity, long bone fractures, muscle wasting, cognitive impairment, and sexual dysfunction (20, 35). Nonetheless, dissection of the root cause(s) of Te deprivation has been thwarted by the strongly interlinked nature of brain-pituitary-gonadal regulation (27). In principle, such dynamics are controlled via repeated incremental adjustments in feedforward (stimulation) by hypothalamic gonadotropin-releasing hormone (GnRH) and pituitary luteinizing hormone (LH) and feedback (inhibition or repression) by gonadal Te (11, 13, 27) (Fig. 1). In particular, neuronal bursts of GnRH evoke pulses of LH from pituitary gonadotropes (30). LH pulses stimulate time-delayed Te secretion by Leydig cells, as verified by direct spermatic vein sampling in humans (11). Te undergoes diffusion, advection, elimination, and binding to two plasma proteins, sex hormone-binding globulin (SHBG) and albumin (11, 17). Te feeds back to inhibit GnRH outflow (secretion and action) (28, 31).
Fig. 1.
Model of ensemble connectivity among hypothalamic gonadotropin-releasing hormone (GnRH), pituitary luteinizing hormone (LH), and gonadal testosterone (Te) signals mediated via nonlinear interface functions. Te feedback is imposed on virtual GnRH outflow, defined as combined secretion (amount) and action (efficacy) of GnRH. SHBG, sex hormone-binding globulin. Te Sec, Te secretion.
Quantification of ensemble GnRH-LH-Te dynamics remains difficult for several reasons. First, there are strong nonlinear dependencies among 1) hypothalamic GnRH → LH feedforward, 2) LH → Te feedforward, and 3) Te → GnRH/LH feedback. Second, primary pathophysiology putatively induces secondary adaptations at other sites, thereby obscuring the nature of the original defect (11, 13, 17, 27, 30). Third, hypothalamically secreted GnRH cannot be measured accurately in the general circulation. Only LH and Te concentrations can be observed directly. Hence, a strategy is needed to reconstruct GnRH's stimulation of LH secretion and Te's inhibition of GnRH secretion and action. The present analyses introduce a new age-dependent regression structure embedded within the dynamic model of conjoint GnRH-LH-Te regulation.
METHODS
We employ a dual strategem: 1) formulation of a mathematical model of the entire system (observed and unobserved signals), which incorporates age as a dependent variable, and 2) experimentation, in which graded amounts of a selective GnRH receptor antagonist are used to inhibit endogenous GnRH action competitively.
Clinical experiments and overview of modeling.
Twenty-four healthy adults, ages 20–72 yr, provided voluntary written informed consent to participate in this study, which was approved by the Mayo Clinic Institutional Review Board. Ten young and 8 older subjects were evaluated earlier using a nonpopulational model (14), and 23 subjects were assessed using an approximate-entropy construct (19). Subjects were healthy community-dwelling men within 20% of ideal body weight with unremarkable medical inventories and physical examinations. Each subject underwent four separate randomly ordered, overnight sampling studies ≥10 days apart. Blood was withdrawn from a forearm intravenous catheter every 10 min for 18 h beginning at 1800, and LH and Te concentrations were assayed. At 2 h after the start of sampling, ganirelix acetate (GRX) was injected subcutaneously at 0 (saline), 0.1, 0.3, or 1.0 mg/m2. GRX is a potent, selective long-acting competitive antagonist of the GnRH receptor (2). GRX, SHBG, and albumin concentrations were measured in a 2-h serum pool (16–18 h). Serum concentrations of LH, Te, GRX, SHBG, and albumin were determined as described previously (23, 29). Assay sensitivities were 0.010 IU/l for LH (First International Reference Preparation) and 8 ng/dl for total Te. No samples were undetectable. The general clinical paradigm of graded GnRH receptor antagonism was presented earlier and used for different purposes in a subset of the larger cohort studied here (14, 19, 21). One study involved in vivo validation (14), another showed model free estimation of the orderliness of LH secretion (19), and yet another assessed LH-Te feedforward via a linear estimation method (21).
Modeling consisted of the following sequential strategy: 1) estimate multiple sets of potential LH pulse times by a validated diffusion-based incremental-smoothing methodology (10, 12), 2) estimate the biexponential kinetics of LH elimination and the LH secretion function simultaneously by a conditional maximum-likelihood (pulse number-penalized) methodology using each 18-h LH concentration-time series (5, 13), 3) estimate the free Te secretion rate via a logistic LH concentration → Te secretion feedforward model (17), and 4) use the estimated LH secretion rates (from step 2) and the free Te concentration (from step 3) to reconstruct the virtual GnRH signal and GnRH → LH dose-response function, which could have produced the estimated LH secretion rates (5, 14). The new feature is the inclusion within the overall model of age-dependent regression of each key regulatory parameter of interest. The mathematical concepts are summarized in the appendix.
RESULTS
On the control day, concentrations of total Te (408 ± 61 ng/dl, n = 24 subjects) and LH (4.1 ± 0.38 IU/l) were age invariant. Linear extrapolation to the age extrema of 20 and 80 yr yielded respective concentrations of SHBG of 19 and 42 nmol/l, bioavailable Te of 287 and 206 ng/dl, and free Te of 11.2 and 6.3 ng/dl and fraction of Te bound to SHBG of 35% and 56% (P ≤ 0.05 for each slope measure). By analysis of variance, GRX dose (P < 0.001), but not age (P = 0.44), determined LH concentrations (P < 0.001).
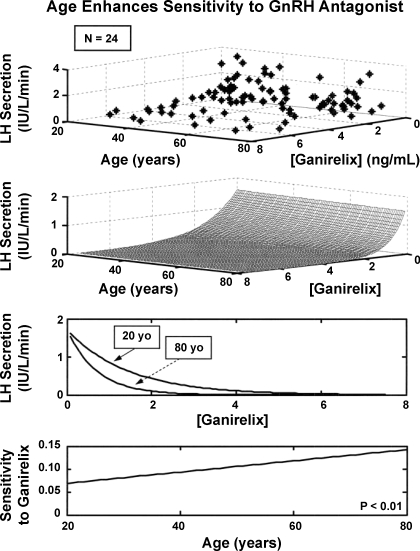
Figure 2 depicts the impact of age (independent variable) on the inhibitory relationship between measured GRX concentrations (independent variable) and calculated pulsatile LH secretion rates (dependent variable). The three-dimensional contour was determined by combined analyses of 10,368 individual-sample LH measurements and 96 individual-sample GRX measurements in the group of 24 men. Inhibitory sensitivity to GRX increased linearly and doubled over the regression-projected age span of 20–80 yr (P < 0.01). Inhibitory potency (1 half-maximally inhibitory GRX concentration) did not differ over the same age extrema (1.1 and 0.85 ng/ml, respectively).
Fig. 2.
Observed distribution (top) and analytical reconstruction (middle) of the tripartite relationship among calculated pulsatile LH secretion rates, serum ganirelix (GRX) concentrations, and age in 24 healthy men. A 3-parameter exponential model was used to represent the GRX-LH inhibitory interface. Estimated inhibitory functions projected to ages 20 and 80 yr and age-dependent linear increase in inhibitory sensitivity (bottom).
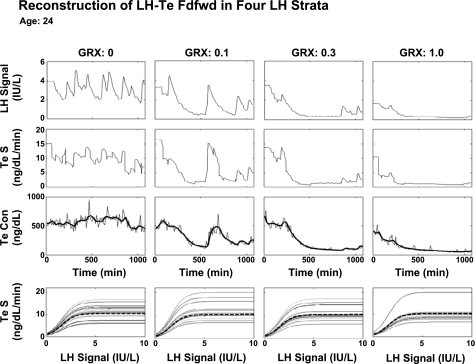
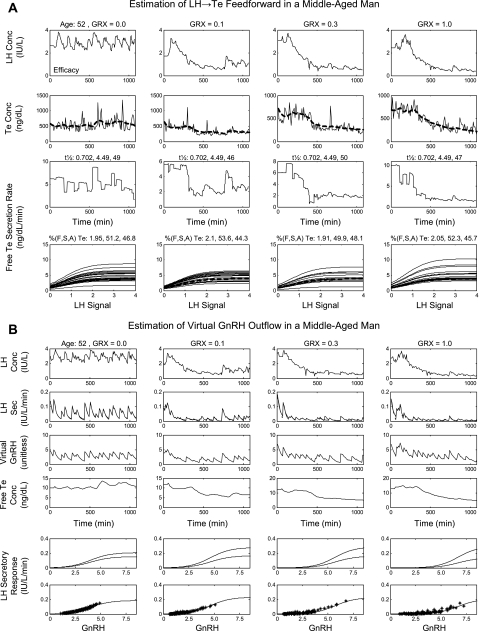
Figure 3 illustrates 18-h LH and Te concentration and secretion time series in one 24-yr-old subject. The GnRH receptor antagonist reduced pulsatile LH concentrations, calculated Te secretion rates, and measured total Te concentrations. Analytically estimated multiple LH → Te feedforward dose-response functions at any given GRX dose illustrate inferred pulse-by-pulse variability in LH efficacy. The latter, as quantified by the coefficient of variation, decreased linearly from 40% to 22% between projected (model-extrapolated) ages of 20 and 80 yr (P < 0.01).
Fig. 3.
Time profiles of predicted LH concentrations (LH signal), calculated Te secretion (Te S) rates (2nd row), measured (continuous lines) and estimated (interrupted curves) Te concentrations (Te Con), and analytically reconstructed LH-Te feedforward functions (Te S, bottom row). Columns (left to right) present data obtained after injection of GRX (0, 0.1, 0.3, and 1.0 mg/m2) at 120 min (x-axis) in 1 healthy 24-yr-old man. Multiple dose-response curves at any GRX concentration (bottom) reflect pulse-by-pulse random effects on efficacy of LH pulses observed at each GRX dose. Dashed lines, mode of each family of LH → Te dose-response functions estimated at each GRX dose.
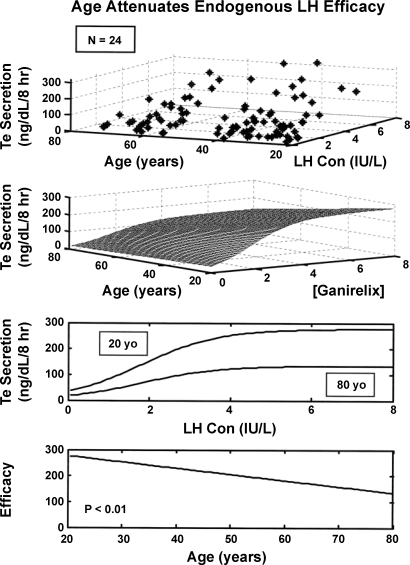
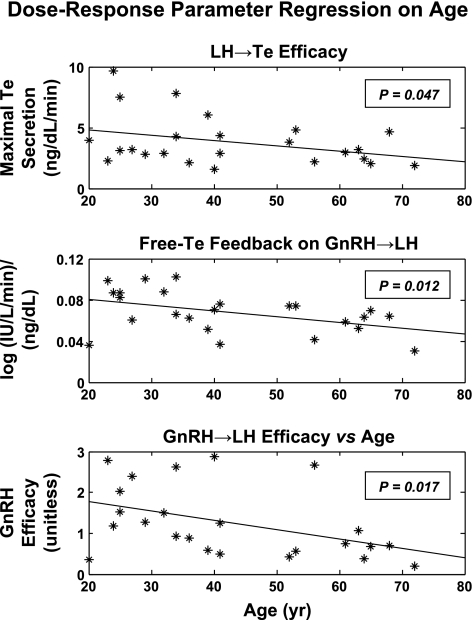
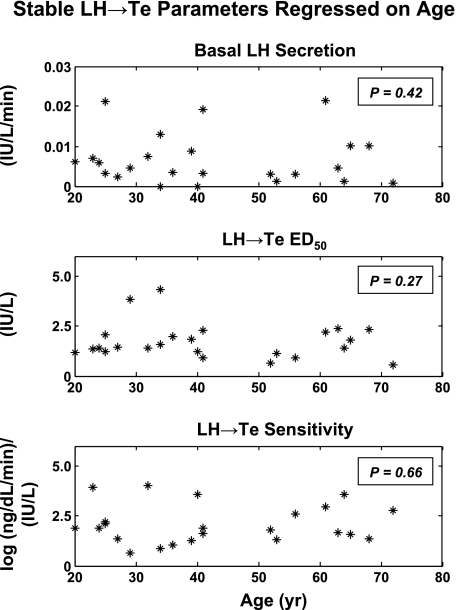
Four-parameter logistic regression analysis of LH → Te feedforward disclosed that age attenuates LH efficacy (P = 0.047; Table 1, Fig. 4). Over the model-extrapolated age range of 20–80 yr, LH efficacy declined by 49% (from 268 to 136 ng/dl per 8-h pulsatile Te secretion). Estimated testis sensitivity and LH EC50 (half-maximally effective LH concentration) did not differ significantly with age.
Table 1.
Summary statistics for age effects
| Dependent Variable | Slope | SE of Slope | t Value | df | One-Sided P Value* |
|---|---|---|---|---|---|
| LH → Te feedforward | |||||
| LH ED50, IU/l | −0.0071 | 0.0120 | −0.62 | 22 | 0.270 |
| LH efficacy, ng·dl−1·min−1 | −0.0440 | 0.025 | −1.75 | 22 | 0.047 |
| Testis sensitivity, slope units | 0.0055 | 0.0130 | 0.43 | 22 | 0.66 |
| Basal Te secretion, ng·dl−1·min−1 | 0.0024 | 0.0020 | 1.44 | 22 | 0.92 |
| GnRH and Te → LH | |||||
| Basal LH secretion, IU·l−1·min−1 | −0.0001 | 0.0000 | −0.20 | 22 | 0.42 |
| GnRH efficacy, IU·l−1·min−1 | −0.0227 | 0.010 | −2.26 | 22 | 0.017 |
| Te feedback on GnRH/LH, slope units | −0.0006 | 0.0002 | −2.41 | 22 | 0.012 |
Data are based on regression of the indicated dependent variable on age (n = 24 subjects). LH, luteinizing hormone; df, degrees of freedom; Te, testosterone; GnRH, gonadotropin-releasing hormone.
Against null hypothesis that age does not decrease the indicated dose-response parameter.
Fig. 4.
Observed distribution (top) and analytical 3-parameter logistic relationship (middle) among pulsatile Te secretion, LH concentrations, and age in 24 men. Age significantly reduced estimated LH efficacy (asymptotically maximal Te secretion rate; bottom).
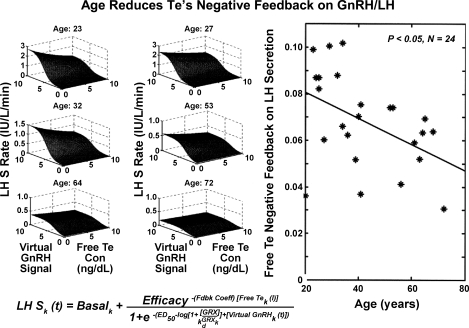
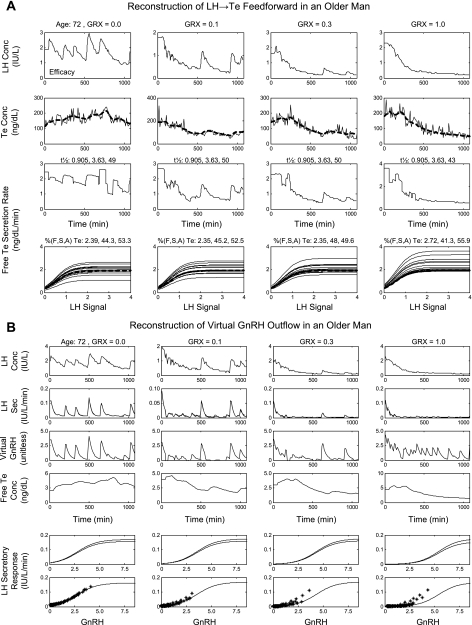
Figure 5 illustrates estimates of three-dimensional GnRH/LH/Te feedforward-feedback relationships in six subjects. Each surface plot represents model-predicted pulsatile LH secretion rates as a function of “reconstructed” virtual GnRH outflow (arbitrary units) and free Te concentrations (feedback signal). Age was a significant negative determinant of maximal virtual GnRH outflow (efficacy; P = 0.017; Table 1). Regression on age of all 24 individual exponential Te-feedback coefficients [(log IU·l−1·min−1)·(ng·dl−1)−1, defining the magnitude of free Te's inhibition of GnRH outflow], yielded a slope of −0.0006 ± 0.0002 (P = 0.012), predicting a 51% decrease across the model-extrapolated age extrema of 20 and 80 yr (Table 1). These outcomes indicate that age blunts maximal virtual GnRH drive and free Te's negative feedback on GnRH outflow-dependent LH secretion. Table 2 summarizes all parameters estimated for the entire cohort of 24 men, along with parameter SE.
Fig. 5.
Analytical estimation of GnRH's concentration-dependent feedforward on LH secretion (LH S) under feedback by free Te concentrations in 6 individual men (left). In the cohort of 24 men, age reduced free Te's feedback on GnRH efficacy (right). Virtual GnRH outflow (arbitrary units) is shown, defined by combined GnRH secretion and action.
Table 2.
Sample parameter estimates
| Parameter | Mean | SE |
|---|---|---|
| GnRH drive and free Te feedback on LH secretion | ||
| Basal LH secretion, IU·l−1·min−1 | 0.0671 | 0.0131 |
| η0, unitless | 0.0557 | 0.6429 |
| η1, unitless | 0.5331 | 0.1712 |
| GnRH potency, unitless | 1.6153 | 0.1119 |
| GnRH efficacy, IU·l−1·min−1 | 12.5336 | 1.7546 |
| Te feedback, exponential coefficient | 0.0680 | 0.0041 |
| β1, unitless | 11.6675 | 0.9604 |
| β2, unitless | 0.0066 | 0.0037 |
| β3, unitless | 0.2516 | 0.0112 |
| SD of residuals | 0.0608 | 0.0047 |
| SD of random effects | 15.6919 | 3.4938 |
| LH-driven Te secretion | ||
| t1/2, min | ||
| Fast free Te | 0.9224 | 0.0580 |
| Slow free Te | 4.6903 | 0.4363 |
| Total Te | 53.2500 | 3.0242 |
| Basal Te secretion, ng·dl−1·min−1 | 0.0330 | 0.0274 |
| LH potency, IU/l | 1.1314 | 0.1112 |
| Sensitivity, slope units | 2.0312 | 0.2013 |
| Efficacy, ng·dl−1·min−1 | 3.8440 | 0.4191 |
| SD of residuals | 1.6487 | 0.2767 |
| SD of random effects | 52.8094 | 5.7098 |
Potency = ln (−potency coefficient); t1/2, half-life. For sample (n = 24) and individual-parameter correlations, see supplemental information in the online version of this article.
Age affected placebo-associated, but not GRX-modified, GnRH pulse frequency, which was taken as the LH pulse frequency. For example, in relation to age, administration of GRX (1.0 mg/m2) increased GnRH/LH pulse number (per 24 h) to 29 ± 0.89 from the placebo value of 20.4 ± 1.4 in older men and to 28 ± 1.4 from the placebo value of 16.7 ± 0.67 in young men (P < 0.01 for age contrast after placebo, but P > 0.10 for age contrast after GRX). Age did not affect estimates of 1) the waveform mode of the virtual GnRH signal, 2) the rapid and slow half-lives of elimination of LH (means of 13 and 97 min, respectively), and 3) half-lives of free Te (0.86 and 4.9 min) or slow half-life of total Te (57 min) in this group of 24 men.
DISCUSSION
Healthy aging in male mammals, including humans, results in gradual Te depletion and impoverished anabolic drive to bone, muscle, brain, and sexual organs (1, 20). The fundamental basis of Te deprivation in this setting remains unknown. A major obstacle to establishing pathophysiology is that GnRH and free Te concentrations are not observed and can be estimated only via a mathematical model of the entire axis (5, 13, 14). Here, using data from all subjects considered together, we extend the ensemble construct to include regression of key dose-response parameters on age. This new analytical framework predicted that age significantly attenuates 1) virtual GnRH's outflow (secretion to and action on the pituitary gland), 2) endogenous LH's maximal stimulation of gonadal Te production (feedforward efficacy), and 3) Te's negative feedback on GnRH outflow-driven LH secretion. In contradistinction, age did not affect LH potency, testis sensitivity, basal LH secretion, the estimated waveform of virtual GnRH secretory bursts, Te's feedback on GnRH/LH pulse frequency, or the elimination kinetics of LH and Te. Accordingly, a population-based age-dependent regression model of interlinked control among GnRH, LH, and Te unveils multiple regulatory deficits in the aging male gonadal axis.
A salient inference was that virtual GnRH outflow (secretion and action) declines by ∼50% over the model-extrapolated age range of 20–80 yr. The decrease, estimated analytically, is consistent with the age-associated increase in LH's inhibitory susceptibility to graded incremental exposure to a specific competitive GnRH receptor antagonist (Fig. 2). The concept stated intuitively is that greater inhibition of LH pulse size by a fixed amount of a competitive GnRH antagonist denotes less endogenous GnRH drive, with the assumption that pituitary responses to GnRH are preserved with age, as strongly implied by existing data (2, 15, 29, 32, 36). The analytical outcome in the human also is consistent with evidence of altered GnRH synaptology (24), impaired release of GnRH from cultured hypothalamic fragments (8), preserved GnRH action in vitro and in vivo (9, 31), and decreased spontaneous and castration-induced pulsatile LH secretion (26) in the aged male rat. Proof of our inference would require direct measurement of GnRH secretion in (human) hypothalamopituitary portal blood.
Pulsatile LH secretion was modeled as the triple consequence of 1) competitive antagonism of GnRH action by a GnRH receptor inhibitor, 2) nonlinear GnRH-LH feedforward (stimulatory) dose-response properties, and 3) exponential noncompetitive feedback (inhibition) by free (protein-unbound) Te concentrations. The free Te concentration represents a plausible physiological signal, given its strong correlation with clinical features of anabolism and empirical evidence that free steroids can inhibit hypothalamopituitary drive (33). A key outcome of this analysis was that age significantly attenuates free Te's inhibition of GnRH outflow. In a subanalysis, age equally muted feedback by bioavailable and total Te concentrations (14). These outcomes were selective, because age did not significantly influence the feedback effect of Te concentrations on LH secretory-burst frequency. Although hypothalamopituitary androgen and estrogen receptor density may decline in aging animals (25), the biochemical basis of specific impairment of Te's negative feedback on GnRH outflow, but not GnRH/LH pulse frequency, is not known.
Trophic hormones such as LH direct target tissue responses via implicitly asymptotic dose-response functions. Analytical reconstruction of the nonlinear function transducing pulsatile LH's stimulation of Te secretion indicated that age diminishes (by 49%) LH efficacy (Fig. 4). Reduced testis responsivity to extrapolated maximal LH drive would be consistent with previous pharmacological data obtained using high doses of human chorionic gonadotropin (33). Taken together, noninvasive model-based dose-response estimation thus identifies impaired endogenous GnRH drive, decreased Te feedback on GnRH outflow (possibly compensatory to low GnRH output), and reduced LH efficacy in stimulating Te secretion.
Perspectives and Significance
A generalizable age-dependent regression model of the male gonadal axis provides an analytical formalism to quantify joint feedback and feedforward adaptations associated with aging in the human. Extensions of this basic construct to other interlinked physiological systems should have utility in probing age-dependent mechanisms of multipathway failure more generally. Moreover, proteomics research has unveiled a large repertoire of putative and confirmed physiological regulators. Given that direct measurements of multiple nonsystemic (locally active) mediators can be difficult in the intact animal and human, the concept of analytically estimating unobserved signals within a regulatory ensemble may have utility in investigating other networks. One approach to calibrating reconstructed virtual signals may be injection of a measurable homologous signal.
GRANTS
This work was supported in part by National Center for Research Resources Grant M01 RR-00585 to the Mayo Clinic, National Institutes of Health Grants RO1 AG-023133, R01 DK-060717, AG-031763, and K01 AG-019164, and National Science Foundation Award DMS-0107680.
Supplementary Material
ACKNOWLEDGMENTS
We thank Pamela Roebuck and Paul Takahashi for assistance with protocol implementation and Donna Scott and Ashley Bryant for manuscript and graphics support.
APPENDIX
Biomathematical Model of GnRH, LH, and Te Dynamics
The age-dependent regression model adds a coefficient for an age effect on any one physiological parameter in the model and then uses data from all subjects combined to solve the regression function, along with the physiological parameter set. The parameters of the overall model are summarized below.
Model of LH pulse times, secretion, and concentrations.
For each of the four GRX regimens (k = 0, 1, 2, 3), LH and Te concentrations were sampled every 10 min for 18 h, and GRX was administered after 120 min. GRX, SHBG (S), and albumin (A) concentrations were also measured in a 2-h serum pool (16–18 h). This protocol was described by Keenan et al. (14) in a subset of the larger group studied here.
One must first estimate the LH pulse times on the basis of observed LH concentration data. A recently published pulse detection method was employed (12). It utilizes a nonlinear diffusion equation, with a diffusion coefficient inversely related to the rate of increase, identifying putative pulse times as points of increase, which are not easily smoothed away (Fig. A1). The pulse times were denoted Tk,1, Tk,2,…, Tk,m, where the number of pulses (m) depends on k. LH secretion rates were assumed to be given by a model previously described (13)
| (A1) |
and accumulation of a given pulse mass (to be released) is assumed to be a weak linear function of the preceding IPI plus a random effect (A) that allows for biological variability in individual burst mass. An instantaneous normalized rate of secretion (ψL) is assumed to be a three-parameter generalized γ (probability density) function, with the concentrations given as
| (A2) |
One observes the following: , where ε is IID Gaussian. One obtains a Gaussian likelihood, in which there is an increasing number of random effects (m, a function of n). The asymptotic consistency and normality of the MLE was established previously (18). Then LH secretion rates were estimated as conditional expectations evaluated at the MLE: . Pulse number was used as a parameter penalty in calculating Akaike and Bayesian information criteria for objective model selection (5, 12), as illustrated in Fig. A2. A convolution of the secretion rates with the estimated biexponential kinetics produces “predicted” or fitted concentrations. The effects of unobserved GnRH and Te signals on LH pulse mass were implicitly captured by the η1,L(k) and the random effects AL, which could be more explicitly modeled as GnRH/Te → LH drive (4, 34).
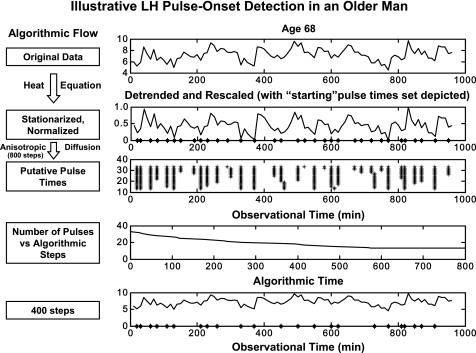
Fig. A1.
Candidate sets of LH pulse times (right) in a 68-yr-old man infused with saline. Top row: original 18-h LH concentration time series, during which data were obtained every 10 min. Second row: heat equation-detrended data rescaled to a uniform distribution on the interval [0,1]. * on x-axis denote all 32 initial local minima (potential pulse-onset times). Third row: remaining pulses after stepwise probabilistic dropout of the least contributory minima (*) across 800 iterations. Fourth row: algorithmic cycle-dependent (x-axis) estimates of LH pulse number (y-axis, per 24 h). Bottom 2 rows: locations of putative pulse onsets retained after 400 vs. 200 selective-smoothing operations (shown in relation to original LH series). Arrows identify the 4 pulse onsets removed between 200 and 400 iterations.
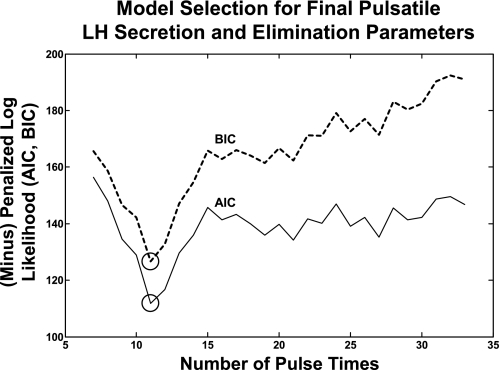
Fig. A2.
Maximum likelihood-based estimation of LH half-lives and LH secretory parameters, conditional on candidate LH pulse sets. Successively diminished sets of candidate pulse times are created, and each is analyzed separately. Estimates of LH dynamics are thus conditional on that particular pulse set. Penalized negative log-likelihood function is calculated for each pulse-set evaluation. Pulse number enters into the likelihood estimate via a parameter penalty for each pulse added. Optimal fitted pulse-time set is obtained using Akaike and Bayesian information criteria (AIC and BIC), as defined in Ref. 12 and verified statistically in Ref. 5.
Binding of Te to carrier proteins.
By the law of mass action
with association constants and (apparent) (11, 17). Also, takes into account SHBG primarily being realized as a dimer. The forward binding rates (κ∼1) were then calculated using subject- and strata (k)-specific SHBG and albumin concentrations and a literature-based estimate of free Te: 0.4 nM (28).
Model of (LH-driven) Te secretion and concentrations.
The LH feedforward signal, was taken to be a time-delayed time averaging of LH concentrations. The input signal feeds through a four-parameter logistic dose-response function to produce a time-delayed Te secretion rate, (11)
| (A3) |
Random effects (A) are allowed on the efficacy parameter to permit possible pulse-by-pulse variations in stimulus efficacy. The logistic function parameters do not depend on GRX stratum (k). Let denote the three primary Te compartments, with total Te concentration the sum (11)
| (A4) |
One can solve for (·) recursively with , and one can obtain total Te concentration as the sum. One observes the following: , where ε is IID Gaussian. One obtains a Gaussian likelihood, conditioned on the LH feedforward signals, and thereby MLEs.
The asymptotic consistency and normality of the MLEs were established previously (18). Te secretion rates were reconstructed as conditional expectations evaluated at the MLE: . A convolution of the secretion rates with the estimated biexponential kinetics produces “predicted” concentrations, and one can then obtain free Te concentrations: . These procedures in a young, a middle-aged, and an older man are illustrated in Figs. A3–A5.
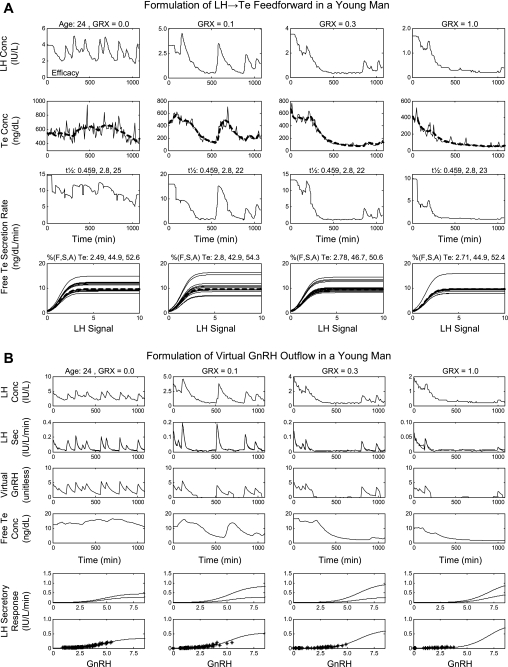
Fig. A3.
Reconstruction of LH → Te feedforward and estimation of GnRH → LH drive under Te repression using data from 0.0, 0.1, 0.3, and 1.0 mg/m2 GRX in a 24-yr-old man. A: reconstruction of LH → Te feedforward from the 4 GRX doses. Numerical half-life values given horizontally above each plot in the 3rd row are the fraction of total Te undergoing slow-phase elimination and the respective fast and slow half-lives (min). Numbers above each plot in the 4th row are percent free, SHBG-bound, and albumin-bound Te, respectively. B: estimation of GnRH → LH drive under Te repression using data from all 4 GRX doses. LH secretory response (bottom 2 rows) is the LH secretion rate driven by virtual GnRH outflow (unitless). *, LH secretion rates (y-axis). Bottom curve in each column is the mean GnRH → LH dose-response function, and the 2 curves in the penultimate row represent dose-response estimates at the lowest Te concentration (top curve) and the highest Te concentration (bottom curve) for that column and that GRX dose.
Fig. A4.
Reconstruction of LH → Te feedforward and estimation of GnRH → LH drive under Te repression using data from 0.0, 0.1, 0.3, and 1.0 mg/m2 GRX in a 52-yr-old man. See Fig. A3 legend for details.
Fig. A5.
Reconstruction of LH → Te feedforward and estimation of GnRH → LH drive under Te repression using data from 0.0, 0.1, 0.3, and 1.0 mg/m2 GRX in a 72-yr-old man. See Fig. A3 legend for details.
Estimation of gonadotrope sensitivity to GRX inhibition.
According to classical concepts of a competitive ligand-receptor interaction, the magnitude of an observed biological response is determined jointly by concentration(s) of the agonist and any competing antagonist and properties of the receptor-effector response pathway (15). These minimal assumptions are satisfied, because 1) GnRH is the exclusive or predominant physiological agonist of the cognate human pituitary receptor (Kd = 0.85 nM) and 2) GRX (Kd = 0.69 nM) acts strictly competitively in vitro and in vivo (2, 6, 29).
Model of the GnRH feedforward signal on LH.
Because the elimination of GnRH occurs so rapidly, its concentration at the pituitary will be a “scaled” version of its secretion rate. Thus the following would approximate the unobserved GnRH signal
where ψG is a three-parameter generalized γ (normalized probability density) function and the amplitude is a linear function of the prior IPI plus a random effect . Given the estimated LH secretion rates and estimated free Te concentrations , the measured GRX concentrations, and the GnRH model G(k)(t) (see above), a plausible representation of free Te, acting as a noncompetitive inhibitor, is
| (A5) |
A Gaussian likelihood, analogous to that for Te and LH secretion (see above) is obtained, and the MLE was obtained. The GnRH signal is then estimated as (see Fig. 5)
Free Te negative feedback on the LH (and thereby GnRH) pulse timing.
A Weibull renewal process (16) was fitted from the optimal LH pulse-time sets, with frequency allowed to depend on subject, strata (k), and mean free Te: . The model also includes a regularity parameter (γ), which was allowed to be different for subject and strata (k).
Regression on age.
Parameter regressions on age are illustrated for the three significant regressions (all slopes negative) in Fig. A6 and for three nonsignificant regressions in Fig. A7 (see supplemental information in the online version of this article for individual parameter estimates and their correlations).
Fig. A6.
Significant parameter regressions on age.
Fig. A7.
Nonsignificant parameter regressions on age.
Standard errors of the model (n = 24 subjects) and of individual parameters in each subject.
The foregoing error terms can be estimated analytically as the inverse of the Fisher information matrix obtained via the likelihood method presented here. Table 2 gives SEs of model parameter estimates for the cohort (n = 24 men; see supplemental information for individual parameter SEs based on 432 observations per subject).
Footnotes
- aL and (1 − aL)
- Fast and slow fractions of biexponential LH elimination
- aTe and (1 − aTe)
- Fast and slow fractions of free Te elimination
- AGk,j
- Random-effect component of jth GnRH pulse mass for series k
- ALk,j
- Random-effect component of the jth LH pulse mass for series k
- ED50
- ED50 coefficient for LH secretion as a dose-response function of GnRH and free Te
- f1
- Coefficient for free Te inhibition of LH efficacy on GnRH
- FL(k)(t)
- LH feedforward signal (on Te secretion rate)
- G(k)(t)
- Unobserved GnRH signal, acting on the pituitary, at time t
- Ĝi(k)(i = 1,…, n)
- Reconstruction of unobserved GnRH signal, based on estimated LH secretion rate and estimated free Te concentrations
- [GRX(k)] and KdGRX
- GRX concentration (k = 0, 1, 2, 3) and GRX dissociation constant
- IPI
- Interpulse interval (min)
- k
- Index for k = 0, 1, 2, 3 (i.e., 4 levels of GRX for each subject)
- KaS
- Association constant for Te binding to SHBG (2 binding sites)
- MLE
- Maximum-likelihood expectation
- nAKaA
- (Apparent) association constant for Te binding to albumin (multiple binding sites)
- t
- Time (min)
- Tk,1, Tk,2,…, Tk,m
- LH pulse times (same as GnRH pulse times) for k = 0, 1, 2, 3
- [totalTe], [TeS], [TeA], and [FrTe]
- Concentrations at time t (left implicit) of total Te, Te bound to SHBG (S), Te bound to albumin (A), and free (Fr) Te
- XL(k)(t)
- LH concentration at time t for series k
- XTe(k)(t) = [XFrTe(k)(t), XTeS(k)(t), XTeA(k)(t)]
- 3 components of Te concentration for series k
- XTe(k)(t)
- True (i.e., without measurement error) Te concentration at time t for series k
- X̂FrTe,i(k)
- Estimated free Te concentration at time ti
- YTe,i(k)= XTe(k)(ti) + εTe,i(k)(1 = 1,…, n)
- Observed total Te concentrations
- ZL(k)(t)
- LH secretion rate (mass·unit volume−1·unit time−1)
- ZTe(k)(t)
- Te secretion rate (mass·unit volume−1·unit time−1)
- ẐL,i(k)(i = 1,…, n)
- Estimated LH secretion rate, reconstructed as the conditional expectation of the unobserved LH secretion rate (at the MLE), given the observed LH concentrations
- ẐTe,i(k)(i = 1,…, n)
- Estimated Te secretion rate, reconstructed as the conditional expectation of the unobserved Te secretion rate (at the MLE), given the observed Te concentrations
- αL1 and αL2
- Fast and slow LH elimination rates (min−1)
- αTe(1) and αTe(2)
- Fast and slow free Te elimination rates (min−1)
- βL(k)
- Basal LH secretion rate (mass·unit volume−1·unit time−1)
- βTe
- Basal Te secretion rate (mass·unit volume−1·unit time−1)
- εTe,i(k)(1 = 1,…, n)
- Measurement error at time ti for total Te concentration
- η0,L(k) and η1,L(k)
- Parameters for mean pulse mass, i.e., coefficients for a linear function of the preceding IPI [e.g., the jth IPI (Tk,j − Tk,j−1)]
- η0,Te, η1,Te, and η2,Te
- Potency, sensitivity, and efficacy parameters of the logistic dose-response function for Te secretion, driven by the LH feedforward signal
- η0,G and η1,G
- Parameters for mean GnRH pulse mass, i.e., coefficients for a linear function of the preceding IPI [e.g., the jth IPI (Tk,j − Tk,j−1)]
- κ1S, κ−1S, κ1A, and κ−1A
- On- and off-binding rate coefficients for free Te to SHBG (S) and albumin (A)
- κ˜1S and κ˜1A
- Forward binding rates for free Te to SHBG (S) and albumin (A)
- ψL(t − Tk,j)
- 3-Parameter γ (probability density) function representing normalized pulse mass secretion at time t for pulse beginning at time TLk,j
- ψG(t − Tk,j)
- 3-Parameter γ (probability density) function representing normalized GnRH pulse mass secretion at time t for pulse beginning at time Tk,j
- λ̂(k)
- Estimated frequency for IPI length under the Weibull model of IPI
REFERENCES
- 1.Allan CA, McLachlan RI. Age-related changes in testosterone and the role of replacement therapy in older men. Clin Endocrinol (Oxf) 60: 653–670, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Beckers T, Bernd M, Kutscher B, Kuhne R, Hoffmann S, Reissmann T. Structure-function studies of linear and cyclized peptide antagonists of the GnRH receptor. Biochem Biophys Res Commun 289: 653–663, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82: 407–413, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Blum JJ, Reed MC, Janovick JA, Conn PM. A mathematical model quantifying GnRH-induced LH secretion from gonadotropes. Am J Physiol Endocrinol Metab 278: E263–E272, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Chattopadhyay S, Veldhuis JD, Keenan DM. Probabilistic recovery of pulsatile, secretory and kinetic structure: an alternating discrete and continuous schema. Q Appl Math 66: 401–421, 2008 [Google Scholar]
- 6.Cheng CK, Leung PC. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev 26: 283–306, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chubb CE, Desjardins C. Testicular function and neuroendocrine activity in senescent mice. Am J Physiol Endocrinol Metab 247: E410–E415, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Jarjour LT, Handelsman DJ, Swerdloff RS. Effects of aging on the in vitro release of gonadotropin-releasing hormone. Endocrinology 119: 1113–1117, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Kaler LW, Critchlow V. Anterior pituitary luteinizing hormone secretion during continuous perifusion in aging male rats. Mech Ageing Dev 25: 103–115, 1984 [DOI] [PubMed] [Google Scholar]
- 10.Keenan DM, Alexander S, Irvine CH, Veldhuis JD. Quantifying nonlinear interactions within the hypothalamo-pituitary-adrenal axis in the conscious horse. Endocrinology 150: 1941–1951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements. Proc Natl Acad Sci USA 101: 6740–6745, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan DM, Chattopadhyay S, Veldhuis JD. Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236: 242–255, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Keenan DM, Sun W, Veldhuis JD. A stochastic biomathematical model of the male reproductive hormone system. Soc Indust Appl Math J Appl Math 61: 934–965, 2000 [Google Scholar]
- 14.Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 147: 2817–2828, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Keenan DM, Veldhuis JD. Mathematical modeling of receptor-mediated interlinked systems. In: Encyclopedia of Hormones San Diego, CA: Academic, 2003, p. 286–294 [Google Scholar]
- 16.Keenan DM, Veldhuis JD. Disruption of the hypothalamic luteinizing-hormone pulsing mechanism in aging men. Am J Physiol Regul Integr Comp Physiol 281: R1917–R1924, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Keenan DM, Veldhuis JD. Divergent gonadotropin-gonadal dose-responsive coupling in healthy young and aging men. Am J Physiol Regul Integr Comp Physiol 286: R381–R389, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Keenan DM, Veldhuis JD, Yang R. Joint recovery of pulsatile and basal hormone secretion by stochastic nonlinear random-effects analysis. Am J Physiol Regul Integr Comp Physiol 275: R1939–R1949, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Liu PY, Pincus SM, Takahashi PY, Roebuck PD, Iranmanesh A, Keenan DM, Veldhuis JD. Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol Endocrinol Metab 290: E34–E41, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Liu PY, Swerdloff RS, Veldhuis JD. The rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab 89: 4789–4796, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Age-specific changes in the regulation of LH-dependent testosterone secretion: assessing responsiveness to varying endogenous gonadotropin output in normal men. Am J Physiol Regul Integr Comp Physiol 289: R721–R728, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Morley JE, Kaiser F, Raum WJ, Perry HM 3rd, Flood JF, Jensen J, Silver AJ, Roberts E. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor I to growth hormone. Proc Natl Acad Sci USA 94: 7537–7542, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 141: 257–266, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Romero MT, Silverman AJ, Wise PM, Witkin JW. Ultrastructural changes in gonadotropin-releasing hormone neurons as a function of age and ovariectomy in rats. J Neurosci 58: 217–225, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Roth GS. Hormone receptor changes during adulthood and senescence: significance for aging research. Fed Proc 38: 1910–1914, 1979 [PubMed] [Google Scholar]
- 26.Shaar CJ, Euker JS, Riegle GD, Meites J. Effects of castration and gonadal steroids on serum luteinizing hormone and prolactin in old and young rats. Fed Proc 66: 45–51, 1974 [DOI] [PubMed] [Google Scholar]
- 27.Smith WR. Qualitative mathematical models of endocrine systems. Am J Physiol Regul Integr Comp Physiol 245: R473–R477, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem Mol Biol 16: 801–810, 1982 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi PY, Liu PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Graded inhibition of pulsatile LH secretion by a selective GnRH-receptor antagonist in healthy men: evidence that age attenuates hypothalamic GnRH outflow. J Clin Endocrinol Metab 90: 2768–2774, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terasawa E. Control of luteinizing hormone-releasing hormone pulse generation in nonhuman primates. Cell Mol Neurobiol 15: 141–164, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev 9: 3–37, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Veldhuis JD, Iranmanesh A, Mulligan T. Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. J Clin Endocrinol Metab 90: 302–309, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Keenan DM, Iranmanesh A, Takahashi PY, Nehra AX. The ensemble male hypothalamo-pituitary-gonadal axis. In: Physiological Basis of Aging and Geriatrics, edited by Timiras PS. New York: Taylor & Francis, 2007, p. 185–203 [Google Scholar]
- 34.Yang R. Maximum Likelihood Estimation Asymptotics for Parameter-Dependent Mixed Effects Models with Applications to Hormone Data (Dissertation). Charlottesville, VA: University of Virginia, 1997 [Google Scholar]
- 35.Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol 146: 609–617, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and α-subunit release in young vs. older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol 135: 399–406, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.