Abstract
Chronic restriction of a basic biological need induces adaptations to help meet requisites for survival. The adaptations to chronic restriction of sleep are unknown. A single episode of 10 days of partial sleep loss in rats previously was shown to be tolerated and to result in increased food intake and loss of body weight as principal signs. The purpose of the present experiment was to investigate the extent to which adaptation to chronic sleep restriction would ameliorate short-term effects and result in a changed internal phenotype. Rats were studied during 10 wk of multiple periods of restricted and unrestricted sleep to allow adaptive changes to develop. Control rats received the same ambulatory requirements only consolidated into periods that lessened interruptions of their sleep. The results indicate a latent period of relatively stable food and water intake without weight gain, followed by a dynamic phase marked by enormous increases in food and water intake and progressive loss of body weight, without malabsorption of calories. Severe consequences ensued, marked especially by changes to the connective tissues, and became fatal for two individuals. The most striking changes to internal organs in sleep-restricted rats included lengthening of the small intestine, decreased size of adipocytes, and increased incidence of multilocular adipocytes. Major organs accounted for an increased proportion of total body mass. These changes to internal tissues appear adaptive in response to high energy production, decomposition of lipids, and increased need to absorb nutrients, but ultimately insufficient to compensate for inadequate sleep.
Keywords: sleep rebound, adaptation, metabolism, adiposity, connective tissues, visceral organs
chronic deficiencies in basic biological requirements produce disease. This basic principle has been well established for nutrition, hydration, and oxygen. It also is believed to be true for sleep. However, the specific physiological properties and outcomes of chronic sleep deficiency are not well elucidated. Most studies on the effects of inadequate sleep have employed previously well-rested, young, normal human and animal subjects under conditions of acute and short-term sleep loss. As may be expected, early physical signs of deprivation seem nonspecific, while subject complaints and poor cognitive performance are pronounced (e.g., reviewed in Refs. 7, 38, 48, and 52). Recently, findings in humans undergoing sleep restriction prolonged for six nights suggest sleep loss produces risk factors for obesity, diabetes, and hypertension (45, 71), but questions arise about the extent to which outcomes can be extrapolated to conditions of chronic deficiency and actually become realized. Epidemiological studies show strong linkages between sleep loss and comorbid conditions and mortality (56, 73, 80; reviewed in Refs. 1 and 44) but do not establish causal relationships between regulatory processes and outcomes. If the requirement for sleep behaves like other basic biological requirements, then its chronic restriction should produce adaptive responses that would be believed to possess survival benefits but which also may produce detrimental components and medical implications.
The purpose of the present investigation in rats was to induce repeated restriction of sleep amount and processes over a long span of time to allow physiological adaptations to take place and thereby permit study. Rodents are champion sleepers, and the physiological correlates of sleep stages and wakefulness are comparable between rodents and humans (reviewed in Ref. 83). Already well established is the fact that acutely sustained total sleep deprivation in laboratory rats results in a progressive negative energy balance (2, 20, 28), suppression of major anabolic hormones (21, 24), and immune-related abnormalities (18, 19, 26, 27) that turn lethal after an average of 16 to 21 days (20, 53, 55, 62). However, rats that obtain nearly half-normal sleep amounts during the same time period do not develop severe pathology and do not die (20, 53, 55, 62). They typically do, however, show abnormalities in metabolic, hormonal, and immune-related parameters that are less severe than those observed under total sleep deprivation conditions (2, 18, 20, 21, 24, 28). Whether these changes are clinically important and whether an individual can adapt to sub-par sleep amounts are issues that bear on the relevance of sleep to physical health and its role in development and recuperation from disease.
The experimental paradigm in the present experiments was composed of long-term repetition of reduced and disrupted sleep followed by opportunities for unrestricted sleep. Each sleep-restriction period lasted 10 days, which is known to be tolerated in rats under acute sleep-loss conditions (2, 18, 20, 21, 24, 28, 53, 55). Each intervening period of sleep ad libitum lasted 2 days, which previously has been shown to normalize energy expenditure and antioxidant parameters in totally sleep-deprived rats (22, 23). The 10-day sleep-restriction and 2-day sleep-recuperation periods were repeated consecutively six times during 10 wk, which is a duration of 72 days determined a priori. This duration is 10 to 13% of the expected lifespan of the subjects (34a). The ambulatory requirements imposed on the sleep-restricted rats to produce arousals were also imposed on a group of ambulation controls, consolidated into sessions to provide greater opportunities for uninterrupted sleep. Because colony rodent diets are optimized for good health, the experimental rats in this study were provided with an atherogenic diet containing 12% fat (normal is 5%) and 0.26% sodium cholate to help increase the likelihood that subclinical changes might be identified if clinical changes proved elusive. The present results show that sleep restriction produced marked metabolic dysfunction after an initial period of relative clinical quiescence marked only by thirst during the recovery periods. The metabolic dysfunction that evolved was manifested by progressive hyperphagia, polydipsea, and body weight loss. The trajectory of food intake appeared only momentarily arrested by sleep recovery. Two subjects died during the final sleep-restriction cycle despite early tolerance and apparent adaptive increases in food and water consumption. Abnormalities in white adipose tissue and pathology of the skin developed in association with the metabolic abnormalities in all sleep-restricted subjects. These findings demonstrate both that adaptations occur in response to inadequate sleep and that chronically insufficient sleep produces detrimental effects to which some individuals are profoundly susceptible.
METHODS
Animals and Environmental Conditions
All procedures were carried out in accordance with protocols for animal care and use approved by IACUCs at The Medical College of Wisconsin and the Zablocki Veterans Administration Medical Center. Subjects were 20 male Sprague-Dawley rats obtained from Harlan Laboratories (Madison, WI) that weighed 452 (32 SD) g and were 28 (1 SD) wk old at the time of study. Environmental conditions included constant light to diminish the amplitude of the circadian rhythm, thereby decreasing the effects of sleep deprivation on both the amplitude and phase of the circadian rhythm (50, 78), which otherwise would be unequal among groups. Thermostatic control of heat lamps maintained temperatures in the apparatuses at 26.9 (1.1 SD)°C to offset evaporative cooling in the apparatuses and to maintain the thermoneutral zone for rats (72). Rats were fed ad libitum a purified (modified AIN-76A) diet (Zeigler Brothers, Garners, PA). The diet was composed of 20% protein, 45% carbohydrate, 13% fat, 10% fiber, 3.5% minerals, and 1% vitamins by weight, with 0.26% sodium cholate added to increase atherogenic properties. The caloric density was the same as normal laboratory chow at 3.7 kcal/g. Food pellets were stacked vertically in tube feeders designed to minimize waste with an opening to expose one pellet at a time for biting and a catch receptacle for crumbs.
Rats were surgically implanted with macro electrodes for recording electroencephalographic and electromyographic activity to distinguish wakefulness and individual sleep stages. Anesthesia and analgesia were induced by ketamine·HCl (100 mg/kg ip), xylazine·HCl (11 mg/kg im), and atropine sulfate (0.05 mg/kg im) after brief inhalant anesthesia with isofluorane. Supplementary doses of ketamine·HCl (21 mg/kg ip) were administered as needed to maintain the surgical plane of anesthesia. Electrodes were placed to obtain signals corresponding to cortical electroencephalogram, cortical theta (important in distinguishing paradoxical sleep; aka, rapid eye movement or REM sleep), and temporalis muscle electromyogram (EMG) activity, as previously described (3). The electrode implants were insulated and bound to the skull with anchors and dental acrylics. The head plug assembly on each rat was fastened to a long recording cable and counterbalanced boom assembly that permitted freedom of movement vertically and horizontally within large enclosures (described below). During surgery recovery, rats were housed on solid floors within the enclosures. At least 7 days were allotted for recovery from surgery and acclimation to the apparatuses.
The experimental apparatus was designed and refined by Bergmann, Rechtschaffen and colleagues (3, 62). Under baseline conditions, each of two rats is housed on half of a large, round platform (45 cm diameter) divided into two sides by Plexiglas within a large open-air enclosure. Beneath and extending outward from the platform on each side is a shallow pan of water (2–3 cm deep) that serves as a deterrent to leaving the platform. Each rat can move freely and perform normal behaviors of eating, grooming, and exploring and also can lie fully on the platform to sleep. Each rat also is free to step down into, sit in, and walk around in the pans; however, they almost always remain on the platform after initial exploration. During a 7-day baseline period the housing platform is rotated once per hour (3.3 rpm) for 6 s to clear it of debris and to let the rats become accustomed to the movement of the platform on which they stand. The usual response to each 6-s rotation is to walk against the rotation to remain comfortably at the widest part of the platform. The rats can also ride for a few seconds, but then are required to move away from the sides as the platform rotates beneath the dividing wall. Contact with the water most often occurs when a rat places forepaws in the water to support its torso while keeping hind paws on the disk. There are no specific prohibitions of the rats sitting in the pan water to sleep, but, historically, this rarely has been encountered.
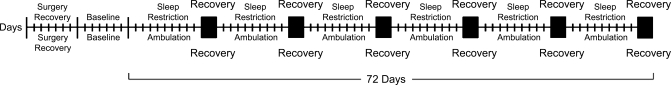
During the experimental phase, each pair of rats housed in an apparatus served as either two sleep-restricted or two ambulation-control rats. To produce chronic sleep restriction, the disk was rotated for 6 s according to a pattern of disk rotations that had reliably produced reduced and highly fragmented sleep under acute conditions (23, 26). In these prior studies, the housing platform had been rotated for 6 s each time sleep onset was detected in a rat to be totally deprived of sleep, which reduced and fragmented the sleep of a paired rat housed on the same platform. The reduced and fragmented sleep of the latter paired rat was replicated by means of electronic capture of the disk rotation patterns of each of several prior experiments to create a master disk rotation program. In this way, the disk rotation patterns replicated the acute conditions and could be repeated to create chronic conditions. During each 10-day sleep-restriction period, the disk rotations each lasted 6 s and comprised 26% of time. The minimum interval between rotations was 1 to 5 s, and the maximum interval occasionally exceeded 10 min. The modal interval between rotations was 5 to 10 s, and the average duration was 20 s. Each 10-day sleep-restriction period was followed by 2 days during which sleep was nearly ad libitum, and the platform was rotated for 6 s once per hour, as during baseline conditions. The sleep restriction-sleep recovery cycle was repeated six times during 72 days, determined a priori and illustrated by the schematic in Fig. 1.
Fig. 1.
Schematic representation of the experimental periods. After 7 days of recovery from surgery and 7 days of baseline monitoring, rats were either restricted of sleep or served as ambulation controls. Each tick mark represents 1 day. During 10-day sleep-restriction periods, subject sleep was fragmented and reduced in amount. During 10-day ambulation periods, subjects were administered an ambulatory requirement equivalent in total time to that of the sleep-restricted group but consolidated to increase undisturbed sleep opportunities. Each 10-day period of sleep restriction or ambulation was followed by 2 days of recovery in each group (■), during which sleep was permitted near ad libitum.
Procedure for Producing Ambulation Controls
An ambulation-control group consisted of pairs of rats in identical apparatuses and treated in an identical manner to those of the sleep-restricted group, but the disk rotations totaling 26% of time were consolidated to increase opportunities for uninterrupted sleep. The schedule of disk rotations was composed of 90-min cycles of 150 s of continuous disk rotation, followed by 30 s during which the disk was stationary. After each 90-min ambulation cycle, the disk then was stationary for 198 min. This 288-min schedule was repeated five times per day. Every 10-day period of ambulatory requirements was followed by 2 days during which sleep was permitted nearly ad libitum, except for hourly 6-s rotations of the platform, identical to the schedule of sleep restriction. Ambulation-control rats also were studied over a 10-wk period.
Assessment of Sleep Stages and Wakefulness
Electroencephalographic signals were recorded to validate the amount of sleep obtained in sleep-restricted rats compared with ambulation-control rats, and to investigate changes to sleep stages during sleep-recovery periods. Cortical EEG, cortical theta, and EMG signals (collectively the EEG) of a subset of rats from each group were recorded either continuously or on a rotating basis of 48 h to enable periodic recordings of the electroencephalophic signals of each subject with shared instrumentation. Signals were filtered and displayed online as analog signals (Grass Telefactor, Gamma PSG Software, West Warwick, RI) and as digital measurements of the signal amplitudes. Digital recordings were rectified, and the scaled sums in 30-s epochs were stored for analysis. Wakefulness and sleep stages were scored by means of a computer-assisted scoring routine developed and validated by Bergmann et al. (4) and subsequently refined by him. Scoring of each 30-s epoch was based on whether a sleep stage or wakefulness occupied most of the epoch. That is, an epoch could be scored as sleep even if it contained arousals. Scores were compared with analog recordings for verification. Data were excluded during cycle 6, by which time deterioration of the electrodes, especially the EMGs, precluded valid measurements in several subjects. For sleep-restricted rats, the data for six rats that had the best recordings throughout were scored. For ambulation-control rats, the data for a cohort of six rats that had the best recordings per cycle were scored, because high-quality recordings throughout were not necessarily attained for each subject. Two 24-h recordings were analyzed per subject for baseline and each 10-day sleep-restriction or ambulation cycle. One or both 24-h periods of each recovery cycle were captured for each subject analyzed for sleep during that cycle, and these data represent the recovery period aside from whether the first, second, or both days were recorded. A technical error resulted in delivery of an ambulation requirement of 33%, rather than 26%, of total time to three of six rats during cycle 2, and these data were included in the scoring for that cycle. Data were first averaged within subjects for baseline and each phase of each cycle, and then averaged across subjects within a group.
Tissues Harvested
After the planned duration of study, each rat was gently and deeply anesthetized by isoflurane inhalation, followed by ketamine·HCl (50 mg/kg ip), xylazine·HCl (8 mg/kg im), and atropine sulfate (0.03 mg/kg im). Supplemental doses of ketamine·HCl were administered, if needed, to obtain deep anesthesia. Rats were exsanguinated by cardiac puncture. Most major tissues were dissected, parceled, and preserved for current and potential studies in up to three ways including fast frozen in liquid nitrogen, frozen embedded, or formalin fixed. Sites for adipocyte collection included the omentum, perirenal, retroperitoneal, and epididymal depots, and surrounds of the mesenteric and popliteal lymph nodes. Adipose tissue was fixed in 4% paraformaldehyde. The small intestine was dissected at the pylorus and at the junction with the cecum, and washed three times through with saline to remove contents. External membranes among sections of the intestine were cut to lay the intestine straight for measurement with a ruler.
Adipocyte Morphometrics
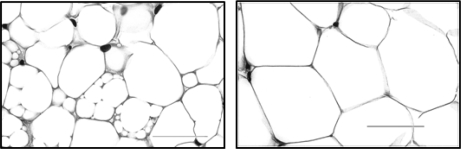
Fixed adipose tissue was blocked in paraffin, sectioned in 4-μm thickness, and stained with hematoxylin and eosin. Slides were coded to keep the examiner blind to the experimental conditions of each subject during morphometric analysis by brightfield microscopy (Olympus BX51 microscope and DP71 camera, ImagePro Plus image analysis software; Media Cybernetics, Bethesda, MD). Unilocular adipocytes were measured and counted at ×400 magnification in a representative region approximating 0.5 mm2 and composed of an average of 275 unilocular adipocytes (range: 64 to 635 adipocytes). The frequencies of the different sizes of unilocular adipocytes were tallied in increments of 200 μm2. Multilocular regions were defined as clusters of notably smaller yet relatively uniformly-sized lipid droplets encased within a single membrane, as shown in Fig. 2, compared with unilocular adipocytes. The incidence of multilocular cells was calculated as the proportion of microscopy fields per rat that contained multilocular cells. The area occupied by multilocular cells was calculated as a proportion of the total area measured per field. Two specimens (one perirenal and one popliteal lymph node site) in sleep-restricted rats were excluded from analysis due to insufficiency of the specimen for reliable measurements.
Fig. 2.
Representative histological images of multilocular and unilocular adipocytes. Left: A section of adipose tissue from the perirenal depot in a chronically sleep-restricted rat illustrative of heterogeneity of the morphology and prevalence of multilocular adipocytes in some regions. Multilocular regions are clusters of notably small yet relatively uniformly sized lipid droplets encased within a single membrane. Right: section of adipose tissue from the perirenal depot of an ambulation-control rat showing a unilocular phenotype with a comparatively homogeneous cell size. Stained with hematoxylin and eosin. Bar = 50 μm.
Post Hoc Assessment of Waste Products
The rate of food removed from feeders by sleep-restricted rats was so great as to raise concerns that an increased proportion of food considered as intake was instead unmetabolized as feeder waste or in fecal matter. To address this issue, waste was collected from age-matched rats treated in an identical manner through cycle 6. Forty-eight-hour accumulations of waste products were collected midway through each of the fifth and sixth cycles, corresponding to the time of the highest average food consumption in the present results, provided below. The water and waste products were separated first by course straining to collect feces and then through filter paper to collect food particles. An aliquot of feces and all of the filter papers containing food slurry were dried in a food dryer, and the dry weights were obtained. Bomb calorimetry of feeder and fecal waste products was performed by a commercial lab (Covance, Madison, WI).
Data Analyses
A mixed-effects change point model was used to analyze each parameter of food and water intake, and changes in body weight to account for such factors as alternating cycles of treatment and recovery and repeated measurements in the same subjects and to allow separation of common trends and animal-specific variability. The individual raw data on food and water intake first were expressed relative to the individual baseline means and were log transformed. This provided symmetric distribution of values around the no-change value. Then, three time-trend structures were considered: linear, one change point, and two change points. Selection was guided by the Bayesian Information Criterion, which is a statistical criterion for model selection in time series and linear regression that balances model complexity and goodness of fit. The final selections were two change-point models for the slopes in water and food intake, and a one-change-point model for the slope in body weight. Confidence intervals of the model parameters were set at 95%. Formulas and details for these mixed-effects models may be found in the appendix. The average proportion of time spent in total sleep, paradoxical sleep, and non-REM (NREM) sleep were analyzed separately, also by mixed-effect models. Taken into account were multiple measurements in each animal even when not all animals were measured during every cycle, as well as random animal effects. The model was fitted for each outcome with group-specific effects for treatment (i.e., sleep restriction or ambulation) and for sleep-recovery periods, as well as linear trends in time.
Data collected at discrete time points, such as the weight of an organ, energy in waste products, or average size of adipocytes were compared by means of Student's t-tests. The experiment-wise error rate was set at P < 0.05 for all planned and post hoc comparisons. Measurements of adipose tissue were compared by means of a one- tailed t-test, based on the hypothesis that adipose tissue in sleep-restricted rats would show more characteristics of degeneration than would that of ambulation controls. Values are means (SD) for individual values composing a group. Values are means ± SE, if data were first averaged within subjects and then averaged for the group (e.g., adipocyte size).
RESULTS
Mortality
Despite tolerance to sleep loss under acute conditions, the physiological effects of chronically inadequate sleep mounted and eventuated in the death of two subjects during cycle 6 on days 7 and 9. On the backdrop of changes to appearance and metabolism described below, two sleep-restricted rats separately passed into a severe state marked by a distinctly feeble appearance, stiff movements, and difficulty negotiating rotations of the platform, whereupon the rats were removed from the experimental conditions. Just 48 h beforehand, basal food intake for each animal was 235% and 124%, and respective water intakes were 249% and 364%. Twenty-four hours beforehand, the food intake values were 48% and 52% of baseline, and respective water intakes were 115% and 146% of baseline, testifying to late appetite and agility, as well as to the rapidity of demise. After removal of the experimental conditions, EEG-defined sleep was not observed in either rat, and death ensued within 6 h. These two animals were not studied postmortem.
Sleep Amounts
Sleep in sleep-restricted rats was characterized by fragmented and reduced sleep during sleep-restriction phases and by a rebound in paradoxical sleep (i.e., REM sleep) and near-baseline NREM sleep amounts during the sleep recovery phases, as shown in Table 1. The baseline percentages of wakefulness were lower than those previously observed under similar experimental conditions (19, 25, 28), suggesting a dietary-induced effect. Sleep amounts under chronic conditions remained within a range comparable to that of acute sleep restriction (19, 25, 28). Most sleep opportunities in sleep-restricted rats were < 1 min long. Besides heavy fragmentation of sleep, the amount of NREM sleep assessed by 30-s epochs in sleep-restricted rats was significantly reduced from baseline. The average amount of disrupted paradoxical sleep was stable across time at 2.4% to 3.4% of total time in sleep-restricted rats, reduced by 4.5 percentage points from the average baseline amount. The experimental routine for ambulation-control rats allowed opportunities for undisturbed, consolidated sleep lasting 3.3 h, five times per day. Although more paradoxical sleep occurred during recovery days than on ambulation days, paradoxical sleep amounts were not significantly different from the baseline value for ambulation controls, as shown in Table 1. On average, NREM sleep during the recovery periods in ambulation-control rats was similar to the baseline levels; however, there was an increasing trend of 1.8 percentage points per cycle (P = 0.003), and by recovery cycle 5 the NREM sleep amount occupied 61.6% of time.
Table 1.
Percent total time of wakefulness and fragmented sleep amounts in sleep-restricted and ambulation-control rats during 72 days of repeated sleep restriction or ambulation-control conditions
| Wakefulness |
TL Sleep |
TL NREM Sleep |
PS |
|||||
|---|---|---|---|---|---|---|---|---|
| Sleep Restricted | Sleep Recovery | Sleep Restricted | Sleep Recovery | Sleep Restricted | Sleep Recovery | Sleep Restricted | Sleep Recovery | |
| Days | 10 | 2 | 10‡ | 2 | 10† | 2 | 10‡ | 2‡ |
| Cycle 1 | 56.2 (5.2) | 33.9 (4.2) | 43.8 (5.2) | 66.1 (4.2) | 40.4 (4.9) | 54.4 (3.9) | 3.4 (0.8) | 11.7 (2.9) |
| Cycle 2 | 56.5 (8.2) | 35.5 (3.6) | 43.5 (8.2) | 64.5 (3.6) | 40.1 (8.0) | 53.7 (2.9) | 3.4 (0.8) | 10.9 (1.3) |
| Cycle 3 | 58.9 (8.0) | 33.9 (7.6) | 41.1 (8.0) | 66.1 (7.6) | 37.8 (8.1) | 54.2 (5.8) | 3.3 (0.7) | 12.0 (3.0) |
| Cycle 4 | 61.8 (8.7) | 34.3 (5.0) | 38.2 (8.7) | 65.7 (5.0) | 35.5 (8.0) | 53.9 (6.8) | 2.7 (0.7) | 11.8 (4.2) |
| Cycle 5 | 63.7 (6.8) | 38.5 (9.6) | 36.4 (6.8) | 61.5 (9.6) | 33.9 (6.6) | 49.1 (9.3) | 2.4 (0.4) | 12.3 (2.3) |
| Baseline | 39.0 (6.3) | 61.0 (6.3)* | 53.7 (5.8)* | 7.3 (0.8)* | ||||
| Ambulation Control | Sleep Recovery | Ambulation Control | Sleep Recovery | Ambulation Control | Sleep Recovery | Ambulation Control | Sleep Recovery | |
|---|---|---|---|---|---|---|---|---|
| Days | 10 | 2 | 10 | 2 | 10 | 2 | 10 | 2 |
| Cycle 1 | 48.9 (4.2) | 39.4 (4.9) | 51.1 (4.2) | 60.6 (4.9) | 46.2 (4.5) | 54.4 (5.4) | 4.9 (1.4) | 6.3 (1.3) |
| Cycle 2 | 48.5 (4.4) | 35.6 (4.9) | 51.5 (4.4) | 64.4 (4.9) | 46.8 (4.2) | 57.9 (4.9) | 4.7 (1.1) | 6.5 (1.2) |
| Cycle 3 | 49.1 (4.4) | 35.0 (5.7) | 50.9 (4.4) | 65.0 (5.7) | 46.2 (4.7) | 58.6 (6.3) | 4.7 (0.7) | 6.4 (1.7) |
| Cycle 4 | 47.2 (4.5) | 34.3 (3.8) | 52.8 (4.5) | 65.7 (3.8) | 47.7 (4.6) | 58.7 (4.5) | 5.1 (0.5) | 7.0 (0.9) |
| Cycle 5 | 47.2 (3.7) | 31.8 (1.9) | 52.8 (3.7) | 68.2 (1.9) | 47.8 (3.2) | 61.6 (2.5) | 5.0 (0.9) | 6.6 (1.3) |
| Baseline | 39.4 (4.2) | 60.6 (4.2)* | 54.9 (4.9)* | 5.8 (1.4) | ||||
Values are daily means (SD) as %total time; n = 6/group. TL, total; NREM, nonrapid eye movement; PS, paradoxical sleep or REM sleep. Significant within-group differences of
P < 0.001 apply to comparisons between baseline and cycles 1–5, where cycles 1–5 of sleep restriction, ambulation control, or recovery are shaded. Significance of
P < 0.006 and
P < 0.001 are between-group differences during sleep restriction or ambulation-control treatments.
Food and Water Intake and Change in Body Weight
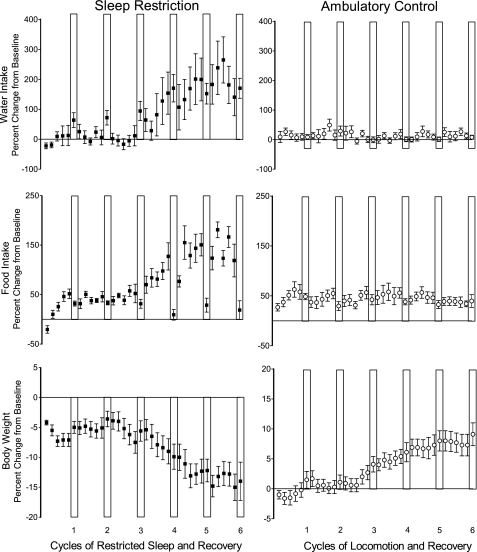
Food intake, water intake, and body weight losses were striking in rats chronically sleep deprived (shown in Fig. 3). Daily peak food consumption for individual sleep-restricted rats, calculated from sequential 48-h averages, ranged from 215% to 456% of individual amounts consumed during baseline. Peaks for two sleep-restricted rats of 259% and 386% of baseline were on the last sleep-restriction day of the sixth cycle, corresponding to notations that these two animals had a relatively robust appearance compared with others in the cohort. Peak 48-h food intake for each rat that died occurred during cycle 4, compared with cycle 5 or 6 for the survivors. The overall differences between sleep-restricted and ambulation-control rats in the slopes of food intake across time were dramatic. Food intake began to increase markedly during the third cycle of sleep restriction (detected change point 1 = cycle 3, day 5 ± 4 days) until during cycle 5 (detected change point 2 = cycle 5, day 2 ± 3.6 days), when food intake reached a plateau. The rise from change point 1 to change point 2, therefore, began after a long, 29-day quiescent period during which sleep was twice restricted and twice unrestricted. Between the two change points, the progression continued for 21 days before sustained high levels of food intake reached a plateau in association with advanced morbidity in some rats. Food intake increases during sleep restriction were halted by recovery sleep, discerned by a negative slope from the first to the second change point in sleep-restricted rats and levels that were not statistically significantly different from those of ambulation-control rats. The normalized food intake during the sleep recovery period did not reestablish food intake at a basal level. Instead, food intake was stepped up after each unrestricted sleep period after the second cycle, and a trajectory was resumed. Ambulation-control rats did not show a significant difference between ambulation periods and recovery periods; the data were without a significant slope.
Fig. 3.
The effect of chronically inadequate sleep on water and food intake and body weight. Percent change from baseline daily values for water intake (top), food intake (middle), and body weight (bottom), across 6 cycles of sleep restriction and sleep recovery in sleep-restricted rats (■) and across 6 cycles of ambulation requirements and sleep recovery in ambulation-control rats (○). Recovery periods are indicated by the white vertical bars. Values are means (SE). n = 10 per group.
Water intake showed a very similar pattern to food intake in sleep-restricted rats, but only on sleep-restriction days (see Fig. 3). Peak water consumption based on consecutive 48-h averages ranged from 223% to 814% of individual baseline amounts and occurred during cycles 5 and 6. Peaks for three rats occurred during the final 48-h recovery period of the study. Water intake began to increase markedly and progressively during cycle 3 of sleep restriction within 24 h of the detected change point for food intake (change point 1 on cycle 3, day 4 ± 2 days), even though these estimates were computed independently. Increases in water intake continued for the next 23 days until reaching a plateau (change point 2, cycle 5, day 4 ± 2 days, slope: P = 0.03), 2 days later than that for food intake. At the plateau, water intake was more than two times greater than the amount of water intake prior to the first days of cycle 3. This high level of consumption was maintained throughout the rest of the study. Cumulative food intake and cumulative water intake calculated over sleep-restriction days were highly correlated (r = 0.83). Water intake during recovery periods averaged 1.6-fold higher under sleep restriction than ambulation conditions (P < 0.005). Post hoc analysis of water intake in sleep-restricted rats during the initial two recovery periods, i.e., before the change in food intake was detected, indicated sleep-restricted rats drank more water then than during baseline (t9 = 3.58 or better; P < 0.006 or better). Ambulation-control conditions did not result in a change in water intake across time or between ambulation and recovery periods.
Body weight was stable in sleep-restricted rats until cycle 3, day 6 ( ± 0.8 days), after which the differences between groups became marked (P = 0.0002). This change point in body weight occurred 24 to 48 h after the change points for water and food intake. By cycle 6, day 8, the change in body weight from baseline values had decreased overall by 23% compared with the same duration of study for ambulation controls. In contrast, ambulation controls gained weight, averaging a 1.1-fold increase over the six cycles.
Caloric Value of Waste Products
Despite large group differences in the amount of food removed from feeders, the proportion of calories lost as feeder waste was not significantly different between sleep-restricted and ambulation-control rats. The proportion of calories in feeder waste averaged < 2% of food removed from the feeder for both sleep-restricted and ambulation-control rats during cycle 5, and also for ambulation-control rats during cycle 6. Feeder waste averaged < 8% during cycle 6 in sleep-restricted rats, based on the following individual values: 2%, 1%, 29%, 11%, 2%, 9%, and 2%. The outlying feeder waste values during cycle 6 were signs of advanced morbidity, in that some of the food removed from the feeder was neglected and was swept away during rotations of the platform. In association with increased food consumption, sleep-restricted rats produced more grams of fecal waste [t13 = 3.17, P < 0.007] than did ambulation controls. However, the calories per gram of fecal waste were nearly identical between sleep-restricted and ambulation-control groups, and between cycle 5 and cycle 6 [kcal/g feces (SD): sleep-restricted = 4.2 ± 0.1 and 4.4 ± 0.1; ambulation-control = 4.3 ± 0.1 and 4.3 ± 0.1, respectively]. These data indicate that food intake values reflect metabolized food, rather than waste or malabsorption.
Connective Tissue Pathology
Skin pathology.
Noteworthy changes in the appearance of sleep-restricted rats began during cycle 3. During cycles 3 to 6, the fur lost its normal sleekness and gained an oily cast along the sides, hips, and especially along the back, and generally lacked luster. During necropsy evaluation, the skin along the back and hips could be easily denuded by gently pulling on the fur. Skin dermatoses began on the hind paws typically as two small, raised, inflamed, and encrusted areas: one located on the main walking pad and the other situated further down the sole and laterally, beneath the calcaneus bone. By the end of cycle 6, these lesions typically had developed into brown plaques or ulcerations that had expanded (∼10 mm diameter), engulfed the interdigital pads on the palm and often an equally large region on the sole, and caused distortion of the normal structure of the foot. Lesions on the plantar surface of forepaws were less severe, but located similarly in two places: among the interdigital pads and just beyond the base of the palm near the wrist. Lesions on the apical pads of digits were uncommon. The tails typically had a few small papules scattered along mostly the dorsal and dorsolateral surfaces, considered mild compared with those observed on totally sleep-deprived rats (47). The paws and tails of ambulatory control animals appeared normal.
Biopsies of the skin of the main pad of the hind paw from each of two sleep-restricted and two ambulation-control rats that had been fixed in 10% neutral buffered formalin were sectioned in 4-μm thickness, stained with hematoxylin and eosin, and examined by a surgical pathologist (N. Markelova, Department of Pathology, The Medical College of Wisconsin). The morphology of the skin lesion from each sleep-restricted rat resembled decubitus ulceration, characterized by a central ulcer with necrotic material and underlying granulation tissue. The granulation tissue was composed of proliferative blood vessels lined by reactive endothelial cells, suggesting healing processes, as well as mixed acute and chronic inflammatory cell infiltrates. The acute inflammatory cells extended into the epithelial edges around the ulcer. The chronic inflammatory cell infiltrate extended into the collagenous stroma between the feeding thick-wall blood vessels and nervous bundles and to the skeletal muscles. Mild edema and occasional mast cells were present in the dermis. The epithelium near the ulcer showed regenerative hyperplasia and parakeratosis, while the uninvolved epithelium showed normal hyperkeratosis. No lipocytes were found in the subcutaneous tissue. The morphology of the skin of ambulation-control rats appeared normal.
Adipocyte morphology.
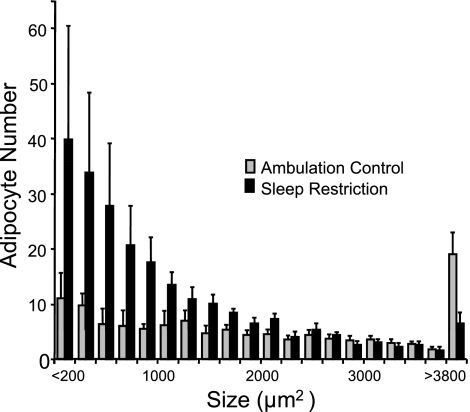
The average size of unilocular adipocytes was decreased significantly by 27% to 45% in five of six depots in sleep-restricted rats compared with ambulation controls. Especially large group differences in the size of unilocular adipocytes were found in the epididymal and omental sites (t16 = −4.37, P = 0.0002 and t16 = −3.83, P < 0.0007, respectively), followed by the retroperitoneal (t16 = −2.65, P < 0.009), popliteal (t15 = −2.82, P = 0.0006), and mesenteric (t16 = −2.10, P = 0.026) sites. Unilocular adipocytes in the perirenal site tended to be decreased by 28%. Changes to adipocyte size in the retroperitoneal depot are considered representative of the other adipose depots, as shown in Fig. 4, illustrating the preponderance of small adipocytes and the decrease in very large unilocular cells in the sleep-restricted group, compared with the ambulation-control group. Perirenal adipose tissue showed a high incidence of multilocular cells in sleep-restricted rats compared with ambulation-control rats (t15 = 3.53, P = 0.001). The omental and mesenteric adipose tissue also showed a higher incidence of multilocular cells in sleep-restricted compared with ambulation controls (t16 = 2.28, P < 0.02 and t16 = 2.00, P < 0.03). In microscopy fields where multilocular cells were found, they occupied from < 1% up to 95% of the field, typically in association with preponderance of small unilocular adipocytes.
Fig. 4.
Representative frequency distribution of adipocyte size in chronically sleep-restricted and ambulation-control rats. Bars represent the number of adipocytes standardized to 0.5 mm2 of retroperitoneal adipose tissue, tallied in size intervals of 200 μm2, from ambulation control (n = 10) and sleep-restricted (n = 8) rats. Values are means ± SE.
Visceral changes.
Despite a 17% lower absolute average body weight in sleep-restricted rats compared with that of ambulation-control rats, the average length of the small intestine was greater by 30% (t16 = 3.33, P = 0.004) and reflected by increased weight when measured. The absolute weight of hearts and lungs tended to be heavier in the sleep-restricted group compared with those of ambulation controls (Table 2), but group differences were not statistically significant. Spleens, livers, and kidneys did not differ in absolute weight between groups. Therefore, with the exception of the spleen, all of the major organs each accounted for a significantly greater proportion of body weight in sleep-restricted rats compared with ambulation-control rats (two-tailed t-tests, P < 0.02 or better). Besides greatly diminished adipose tissue depots, the normal fatty appearance of connective tissue membranes among the abdominal viscera was strikingly absent in sleep-restricted rats. For example, the mesenteric lymph nodes appeared packaged in nearly transparent membranes instead of sheathed in connective tissue that was creamy colored and fatty in appearance. Pallor of the liver was striking in both the sleep-restricted and the ambulatory-control groups. Therefore, sections of liver from two rats of each group were stained with Oil Red O for screening by brightfield microscopy, which revealed diet-induced fatty liver. Lipid droplets occupied 8% to 12% of the area surrounding the central veins and 20% to 21% of the portal areas, without apparent differences based on treatment group. The internal appearance of ambulatory control rats was considered healthy, except for fatty livers. The stomachs of sleep-restricted rats typically were packed with food. Visual inspection revealed thin walls in the fore stomach and no erosions in the corpus in sleep-restricted rats.
Table 2.
Weights of visceral organs and length of intestine in sleep-restricted and ambulation-control rats
| Experimental Group | No. Rats | Body Weight | Liver | Lung | Heart | Kidney | Spleen | Intestine, cm |
|---|---|---|---|---|---|---|---|---|
| Sleep restriction | 8 | 381±31† | 18.6±2.5 | 1.87±0.23 | 1.44±0.15 | 2.68±0.35 | 1.27±0.29 (n = 7) | 75.9±8.5* |
| Ambulation control | 10 | 459±24 | 20.0±3.0 | 1.66±0.21 (n = 8) | 1.33±0.10 | 2.68±0.18 | 1.34±0.29 | 58.1±13.0 |
Values are means ± SD in grams, unless marked.
P = 0.004,
P < 0.001 for group comparisons by two-tailed t-tests.
DISCUSSION
Adaptive responses to sleep restriction were dramatic and ultimately insufficient to maintain health and ensure survival. Despite the fact that sleep was only partially restricted during the sleep-restriction phases, the fragmentation and reduction in sleep eventually resulted in obvious pathology with a progressive course. This evidence indicates that deficits developed for which the allotments of recovery sleep were insufficient for recuperation. In truth, the eventual lethal nature of repeated sleep restriction was unexpected. The milder signs of acute sleep restriction were predicted to be ameliorated in large measure by adaptation so that it might appear as though the experimental conditions could be continued indefinitely. Signs began to become outwardly manifested during the third cycle of sleep restriction and sleep recovery, indicating that the first two cycles were not benign, but, rather, internal insufficiencies had accrued and reached some threshold. Ambulation-control rats remained healthy, with stable food and water intake and body weight and without signs of altered well-being.
The clinical course was marked by eventual dynamic increases in food and water intake and body weight loss. Calorimetry of waste products verified that nearly all food removed from feeders was metabolized. Lengthening of the intestine is consistent with increased surface area to absorb water and nutrients (6, 11, 57, 66, 77). Increases in multilocular adipocytes, known to be rich with mitrochondria (33, 35), further reflect high energy production. Pathology developed in the connective tissues, including atrophy of adipose tissue, loss of fattiness of membranes among abdominal organs, decubitous-like skin ulcers, and hair loss. In light of catabolism, these degenerative changes to connective tissues may be due to absorption of lipid content or other nutriment, which therefore is under study. Inanition is suspected, since chronic restrictions of other basic needs essential for survival result in the body's use of its own substrates to help meet the missing requirements (12, 29, 65).
Thirst during the initial recovery periods was the first and only significant sign of an imbalance during the first stages of chronic sleep restriction. Increased intake of water without an increase in food intake is thought to be due to cellular or extracellular hypovolic stimuli, which may include intake of water in anticipation of future needs (30). Considering an apparent absence of hemorrhage, the logical explanation for thirst in rats during a recuperation phase therefore is to rid the body of excess solutes that are produced by increased metabolism by yet unnamed sources. During phases of sleep restriction, the progressive nature of the increases in intake of water emerged along with those for food intake, indicating prandial drinking (30, 43), a regulatory response that maintains osmotic balance as food is absorbed (reviewed in Ref. 85).
The catabolic state implies that the internal work of maintaining physiological functions requires more energy when sleep is restricted than when sleep is replete. Energy output may be parceled into four components to include energy used for 1) carrying out essential metabolic functions of the body; 2) executing various physical activities; 3) digesting, absorbing, and processing food; and 4) maintenance of body temperature (reviewed in Ref. 34). In the case of muscle activity, the overall experimental requirements for locomotor behaviors imposed on ambulation-control rats closely resembled those imposed on sleep-restricted rats. While group differences most certainly existed in the muscle activity that goes into the field metabolic rate, such as muscle tone and the mechanics of food intake, these differences do not seem to constitute a degree of strenuousness that would result in substantial increases in metabolic rate due to external work. The maintenance of body temperature as a cause of catabolism has been investigated in acutely sustained total and paradoxical sleep deprivation in rats. Those results suggest a mild thermoregulatory shift early during sleep loss that does not appear to develop into a progressive inability to retain heat (61, 67, 82). Declines in body temperature late in the survival period of sleep-deprived rats are associated with bloodstream infection precipitating a moribund state (19) for which impaired vasoconstriction is a known consequence (15). Therefore, the changes in body composition and metabolic profile suggest more energy is required to sustain one or more functions at the tissue and cellular levels under conditions of chronically inadequate sleep.
At the tissue level, mass is related to its metabolism or functional workload (13, 42), contributing 55–80% to the basal metabolic rate (reviewed in Refs. 31, 46, 60, and 81). The weights of major organs were preserved in sleep-restricted rats and thereby accounted for an increased proportion of lean body mass. This is opposite to energy-restricted states produced by dietary limitations, wherein major organs, such as the liver and the intestine, almost always decrease in mass, sometimes before an appreciable decrease in adipose depots (32, 49; reviewed in Ref. 60). We and others have shown that brain metabolism is heterogeneously decreased during sleep loss in both laboratory rats (25) and humans (74, 75), making it an unlikely source of increased contribution to an energy deficiency under chronic conditions. The heterogeneous decreases in brain metabolism are indirectly associated with suppression of pituitary hormones, including growth hormone, prolactin, and thyroid-stimulating hormone, for which secretion depends on negative feedback control by the hypothalamus (21, 24). This apparent pan suppression of hormones appears to include the hypothalamic-pituitary-adrenal axis. Corticosterone has not been found increased in sleep-deprived animals studied by the Rechtschaffen-Bergmann method to levels associated with either distress or starvation (reviewed in Ref. 21), suggesting that metabolic outcomes in the present study are not likely due to excessive corticosterone. The remaining plausible explanation is the functional workload at the cellular level, presumably mitochondrial. About 20% of the expected energy utilization is attributed to the mitochondrial proton leak and ∼80% is coupled to ATP synthesis for several major functions, such as protein synthesis, Na+-K+-ATPase activity, Ca2+ ATPase activity, gluconeogenesis, ureagenesis, and substrate cycling, among others (reviewed in Ref. 63). In all likelihood then, failed consolidation of sleep processes renders one or more cellular functions as inefficient or ineffectual. Sleep is composed of a constellation of behavioral and physiological elements that may be expected to enable or facilitate such cellular processes. Elements include a decreased field metabolic rate, a decreased thermoregulatory set point, and frequent periods of muscle atonia and poikilothermy during which respiration and vascular blood flow are dynamic. The notion that sleep enables certain processes to occur is consistent with long-standing speculations that enforced rest serves an important function underlying sleep (69, 84). In the present study, these processes were repeatedly restricted and disrupted.
Much recent attention in the study of sleep has been focused on the central nervous system as the primary, if not sole, beneficiary of both sleep functions and consequences (36, 76). The present results of peripheral adaptations and pathological outcomes demonstrate that such conclusions are left wanting and appear to overlook the potent peripheral regulatory factors that provide feedback to the brain. A brain-centered approach runs the risk of fostering the notion that physical signs of sleep loss have their etiology in a dysfunctional brain. Contrarily, increased food and water consumption by sleep-restricted rats is a rational central nervous system-mediated response to a negative energy balance. These data demonstrate that the regulation of appetitive behaviors by the central nervous system remains intact during chronic sleep restriction, which also is the case for restriction of food or water. Abnormalities in neuroendocrine systems indeed point to failed negative feedback at the level of the brain (21, 24), but suppression of hormones may be considered adaptive, on balance, by decreasing mobilization of protein and limiting actions that may be lipolytic, anti-insulin, and inflammatory in the periphery.
Seemingly contradictory is the weight loss in sleep-restricted rats and reported weight gain in short-sleeping humans, based on recent reviews of clinical laboratory and epidemiological evidence (44, 54, 79). Linkage between short sleep and obesity in humans has been challenged, in part on the basis that shortened sleep is quite different than disturbed sleep, for which physical and behavioral problems are associated (37). It yet is unknown, too, for example, whether humans would lose weight under conditions of chronic sleep restriction, if they are not permitted to be sedentary or are permitted to drink only water rather than fluids containing calories. Where the scientific evidence is sufficient, normal humans and laboratory rats exhibit comparable signs of proinflammatory markers and hormonal changes. These comparisons include an early decline in circulating leptin, an adipokine that decreases under conditions of a negative energy balance before apparent changes in body mass (21, 70), and an increase in oxygen consumption during sleep itself in humans, if sleep has been fragmented (8). Weight loss and weight gain are two ends of a spectrum, and it should be borne in mind that dystrophies involve the similar, if not identical, intermediary factors in pathogenesis.
Perspectives and Significance
The outcomes of the present studies put emphasis on a vital role for sleep in physical health. The outcomes embody the same fundamental attributes as chronic restriction of other basic needs, which were debated and laid out a long time ago for food and water restriction (39, 51). In the present study, metabolic dysfunction was glaring among the outcomes and should be a starting point and cornerstone of study, as it is for nutritional studies. Not only does disease ensue when one basic need is severely restricted, but requirements of other basic needs are altered. The present outcomes of a catabolic state, malnutrition, and dynamic food and water consumption substantiate this point for sleep in concordance with evidence from acute, selective sleep-deprivation studies (61). Relationships between the amount of food consumed and sleep obtained repeatedly have been reported (e.g., 9, 14, 16, 17, 40, 41, 64, 68) but without coalescence or potential theoretical implications (10, 58). In the present study of chronic sleep restriction, the latency to produce unambiguous clinical manifestations was long, a phenomenon that could be construed as rendering the outcomes as somehow artificial and not germane to real life (38). Such a property of a long quiescent period occurs during food and water restriction, too. Nutritional stringency (“wear and tear”) does not become apparent for a long time, due to body reserves and to the fact that repair does not and cannot stop. Indeed, the latency to clinical effects during restriction of a basic need belies the importance of both meeting the need and the insidiousness of internal changes. And finally, the outcomes of the individual sleep-restricted rats demonstrated that there is an upper limit of chronic restriction for which death is inevitable and a lower limit wherein perfect recovery is theoretically possible, which conforms with the laws of nature for other basic needs (39, 51). Between these upper and lower limits is a variable degree of alteration in body phenotype for which only partial recovery may be possible. Insidious, yet potentially permanent, changes and susceptibilities may ensue. The exact manifestations would be expected to depend on individual vulnerabilities, the involvement of a secondary restriction or preexisting condition, and severity of the unmet need.
APPENDIX
Mixed Effects Change-Point Models
In the final two change-point models, the water intake (WI) was modeled as follows: log2[WIi(g)t] = treatmentg + αi + I[phase(t) = recovery]·(recoveryg + ρi) + timeg·t + I(g = SR) (b + βi) [min(c2−c1, t−c1)] + + εi(g)t, and food intake (FI) was modeled as follows: log2[FIi(g)t] = treatmentg + αi + I[phase(t) = recovery]·(recoveryg + ρi) + timeg·t + I(g = SR) {bTrt + I[phase(t) = recovery]bRec} [min(c2−c1, t−c1)] + + εi(g)t, where, i is the individual, g is the group, and t is time. I denotes the indicator function of an event, SR denotes the sleep-restriction group, b and bTrt measure the increase in sleep between c1 and c2 during the sleep-restriction/ambulation phase, while bRec is the additional increase in slope during the recovery phase.
In these formulas, treatmentg takes into account that one or both groups may have an offset from zero after the baseline period and prior to a meaningful change due to treatments, and that the offset could be different for the two groups. Recoveryg models the effect of the recovery period compared with the sleep-restricted/ambulation phase, and timeg is the slope of the overall trend before the first change point. α, ρ, and β are normally distributed random effects that consider individual differences in responses to treatment or recovery. For example, lower effects may be generally found for one particular rat, and also these lower effects may apply to the restriction phases but not the recovery phases of the cycle or vice versa. [x]+ = max (x,0) denotes the positive part of x. It is this change in slope from c1 to c2 that defines the change points of time-ordered data, and εi(g)t is the normally distributed error term with treatment group-specific variance.
Body weights (BW) in grams were expressed on a logarithmic scale and modeled by two time-trend structures. The final selection of a one change-point model was as follows: log2[BWi(g)t] = treatmentg + αi + (timeg + γi)·t + (bg + βi) (t−c1) + + εi(g)t.
Similar to the food and water intake data, αi, γi, and βi are independent, normally distributed random effects, and c1 is the change point. All models were fitted using the n/me package in R2.7.1. (59).
GRANTS
Research support was provided by the National Heart, Lung, and Blood Institute Grant HL-086447. Additional resources and facilities were provided by the Medical College of Wisconsin and the Department of Veterans Affairs.
ACKNOWLEDGMENTS
Appreciation is expressed to Nichole Nellessen, Dennis Baker, and Aletha Champine for technical assistance, Natalia Markelova for skin pathology reports, Bernard Bergmann for upgrades to instrumentation for collection and analysis of electroencephalographic signals for analysis of sleep stages and wakefulness, and Thomas Gardiner for programming a master disk rotation schedule to produce reduced and fragmented sleep.
A portion of this work was presented at a joint meeting of the American Academy of Sleep Medicine and the Sleep Research Society in 2008.
REFERENCES
- 1.Akerstedt T, Nilsson PM. Sleep as restitution: an introduction. J Intern Med 254: 6–12, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bergmann BM, Everson CA, Kushida CA, Fang VS, Leitch CA, Schoeller DA, Refetoff S, Rechtschaffen A. Sleep deprivation in the rat. V. Energy. Sleep 12: 31–41, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat. II. Methodology. Sleep 12: 5–12, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Bergmann BM, Winter JB, Rosenberg RS, Rechtschaffen A. NREM sleep with low-voltage EEG in the rat. Sleep 10: 1–11, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Boass A, Toverud SU. Enhanced nonsaturable calcium transport in the jejunum of rats during lactation, but not during pregnancy. J Bone Miner Res 12: 1577–1583, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH. Sleep deprivation. In: Principles and Practice of Sleep Medicine, edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA: Saunders, 1994, p. 50–67 [Google Scholar]
- 8.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol 71: 1112–1118, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Borbely AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res 124: 457–471, 1977 [DOI] [PubMed] [Google Scholar]
- 10.Bowersox SS, Baker TL, Dement WC. Rapid eye movement sleep and its relationship to feeding behavior in the adult cat. Physiol Behav 32: 907–909, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Boyne R, Fell BF, Robb I. The surface area of the intestinal mucosa in the lactating rat. J Physiol 183: 570–575, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breen HB, Espat NJ. The ubiquitin-proteasome proteolysis pathway: potential target for disease intervention. Jpen J Parenter Enteral Nutr 28: 272–277, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Burrin DG, Britton RA, Ferrell CL. Visceral organ size and hepatocyte metabolic activity in fed and fasted rats. J Nutr 118: 1547–1552, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Danguir J, Nicolaidis S. Dependence of sleep on nutrients' availability. Physiol Behav 22: 735–740, 1979 [DOI] [PubMed] [Google Scholar]
- 15.Delaney KA, Vassallo SU, Larkin GL, Goldfrank LR. Rewarming rates in urban patients with hypothermia: prediction of underlying infection. Acad Emerg Med 13: 913–921, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dewasmes G, Cohen-Adad F, Koubi H, Le Maho Y. Sleep changes in long-term fasting geese in relation to lipid and protein metabolism. Am J Physiol Regul Integr Comp Physiol 247: R663–R671, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Dewasmes G, Duchamp C, Minaire Y. Sleep changes in fasting rats. Physiol Behav 46: 179–184, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol 289: R1054–R1063, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol Regul Integr Comp Physiol 265: R1148–R1154, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat. III. Total sleep deprivation. Sleep 12: 13–21, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab 286: E1060–E1070, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: IX. Recovery. Sleep 12: 60–67, 1989 [PubMed] [Google Scholar]
- 23.Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol 288: R274–R383, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Everson CA, Nowak TS., Jr Hypothalamic thyrotropin-releasing hormone mRNA responses to hypothyroxinemia induced by sleep deprivation. Am J Physiol Endocrinol Metab 283: E85–E93, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Everson CA, Smith CB, Sokoloff L. Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J Neurosci 14: 6769–6778, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol 295: R2067–R2074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol 278: R905–R916, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Everson CA, Wehr TA. Nutritional and metabolic adaptations to prolonged sleep deprivation in the rat. Am J Physiol Regul Integr Comp Physiol 264: R376–R387, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition 22: 830–844, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Fitzsimons TJ, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol 67: 273–283, 1969 [DOI] [PubMed] [Google Scholar]
- 31.Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, Elia M. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr 83: 1062–1067, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman MN, Ruderman NB. Starvation in the rat. I. Effect of age and obesity on organ weights, RNA, DNA, and protein. Am J Physiol Endocrinol Metab 239: E269–E276, 1980 [DOI] [PubMed] [Google Scholar]
- 33.Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab 285: E1230–E1236, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Guyton AC, Hall JE. Energentics and metabolic rate. In: Textbook of Medical Physiology Philadelphia, PA: Elsevier Saunders, 2006, p. 881–888 [Google Scholar]
- 34a.Harlan Laboratories HSD: Sprague Dawley SD Survival Curve Indianapolis, IN: Harlan Laboratories, 2009 [Google Scholar]
- 35.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 279: C670–C681, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature 437: 1254–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Horne J. Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol 77: 266–276, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Horne JA. Sleep function, with particular reference to sleep deprivation. Ann Clin Res 17: 199–208, 1985 [PubMed] [Google Scholar]
- 39.Jackson CM. The Effects of Inanition and Malnutrition Upon Growth and Structure Philadelphia, PA: Blakiston's, 1925, p. 616 [Google Scholar]
- 40.Jacobs BL, McGinty DJ. Effects of food deprivation on sleep and wakefulness in the rat. Exp Neurol 30: 212–222, 1971 [DOI] [PubMed] [Google Scholar]
- 41.Johansson GG, Elomaa E. Effects of partial food restriction on nocturnal meal size and feeding speed are counteracted by concurrent REM sleep deprivation in the rat. Behav Brain Res 20: 275–280, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Johnson DE, Johnson KA, Baldwin RL. Changes in liver and gastrointestinal tract energy demands in response to physiological workload in ruminants. J Nutr 120: 649–655, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Kissileff HR. Food-associated drinking in the rat. J Comp Physiol Psychol 67: 284–300, 1969 [DOI] [PubMed] [Google Scholar]
- 44.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev 11: 163–178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci 1129: 287–304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulstad R, Schoeller DA. The energetics of wasting diseases. Curr Opin Clin Nutr Metab Care 10: 488–493, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Kushida CA, Everson CA, Suthipinittharm P, Sloan J, Soltani K, Bartnicke B, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: VI. Skin Changes. Sleep 12: 42–46, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann NY Acad Sci 1129: 305–322, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Ma SW, Foster DO. Starvation-induced changes in metabolic rate, blood flow, and regional energy expenditure in rats. Can J Physiol Pharmacol 64: 1252–1258, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Mistlberger RE, Belcourt J, Antle MC. Circadian clock resetting by sleep deprivation without exercise in Syrian hamsters: dark pulses revisited. J Biol Rhythms 17: 227–237, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Morgulis S. Fasting and Undernutrition New York: Dutton, 1923, p. 407 [Google Scholar]
- 52.Naitoh P, Kelly TL, Englund C. Health effects of sleep deprivation. Occup Med 5: 209–237, 1990 [PubMed] [Google Scholar]
- 53.Obermeyer W, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: XIV. Comparison of waking hypothalamic and peritoneal temperatures. Sleep 14: 285–293, 1991 [DOI] [PubMed] [Google Scholar]
- 54.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity 16: 643–653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilcher JJ, Bergmann BM, Fang VS, Refetoff S, Rechtschaffen A. Sleep deprivation in the rat: XI. The effect of guanethidine-induced sympathetic blockade on the sleep deprivation syndrome. Sleep 13: 218–231, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health 15: 123–135, 1990 [DOI] [PubMed] [Google Scholar]
- 57.Popper DA, Shiau YF, Reed M. Role of small intestine in pathogenesis of hyperlipidemia in diabetic rats. Am J Physiol Gastrointest Liver Physiol 249: G161–G167, 1985 [DOI] [PubMed] [Google Scholar]
- 58.Porter JM, Horne JA. Bed-time food supplements and sleep: effects of different carbohydrate levels. Electroencephalogr Clin Neurophysiol 51: 426–433, 1981 [DOI] [PubMed] [Google Scholar]
- 59.R Development Core Team R: a language and environment for statistical computing [Online]. R Foundation for Statistical Computing. http://www.r-project.org [August 2009].
- 60.Ramsey JJ, Hagopian K. Energy expenditure and restriction of energy intake: could energy restriction alter energy expenditure in companion animals? J Nutr 136: 1958–1966, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep 25: 18–24, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science 221: 182–184, 1983 [DOI] [PubMed] [Google Scholar]
- 63.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758, 1997 [DOI] [PubMed] [Google Scholar]
- 64.Ruckebusch Y, Gaujoux M. Sleep-inducing effect of a high-protein diet in sheep. Physiol Behav 17: 9–12, 1976 [DOI] [PubMed] [Google Scholar]
- 65.Scarlatti F, Granata R, Meijer A, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ 16: 12–20, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Schedl HP, Wilson HD, Ramaswamy K, Lichtenberger L. Gastrin and growth of the alimentary tract in the streptozotocin-diabetic rat. Am J Physiol Gastrointest Liver Physiol 242: G460–G463, 1982 [DOI] [PubMed] [Google Scholar]
- 67.Shaw PJ, Bergmann BM, Rechtschaffen A. Effects of paradoxical sleep deprivation on thermoregulation in the rat. Sleep 21: 7–17, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Siegel JM. REM sleep predicts subsequent food intake. Physiol Behav 15: 399–403, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snyder F. Sleep of the body. Biol Psychiatry 1: 271–281, 1969 [PubMed] [Google Scholar]
- 70.Spiegel K, Leproult R, L'hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89: 5762–5771, 2004 [DOI] [PubMed] [Google Scholar]
- 71.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Szymusiak R, Satinoff E. Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav 26: 687–690, 1981 [DOI] [PubMed] [Google Scholar]
- 73.Tamakoshi A, Ohno Y. JACC Study Group. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep Med Rev 27: 51–54, 2004 [PubMed] [Google Scholar]
- 74.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9: 335–352, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Thomas ML, Russo MB. Neurocognitive monitors: toward the prevention of cognitive performance decrements and catastrophic failures in the operational environment. Aviat Space Environ Med 78: B144–B152, 2007 [PubMed] [Google Scholar]
- 76.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev 10: 49–62, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Townsend WF, Walter LG, Kinzie JL, Ammon HV. Effect of cholera toxin on ileal water and solute transport after resection of the proximal small intestine in the rat. Gut 22: 953–957, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsai LL, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: XVI. Effects in a light-dark cycle. Sleep 15: 537–544, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol 159: 59–66, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wallander MA, Johansson S, Ruigomez A, Garcia Rodrguez LA, Jones R. Morbidity associated with sleep disorders in primary care: a longitudinal cohort study. Prim Care Companion J Clin Psychiatry 9: 338–345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Webster AJ. The energetic efficiency of metabolism. Proc Nutr Soc 40: 121–128, 1981 [DOI] [PubMed] [Google Scholar]
- 82.Zenko CE, Bergmann BM, Rechtschaffen A. Vascular resistance in the rat during baseline, chronic total sleep deprivation, and recovery from total sleep deprivation. Sleep 23: 341–346, 2000 [PubMed] [Google Scholar]
- 83.Zepelin H. Phylogeny. In: Principles and Practice of Sleep Medicine, edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA: Saunders, 1994, p. 69–80 [Google Scholar]
- 84.Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. In: Brain, Behavior Evolution, edited by Riss W. Basel: Karger, 1974, p. 425–470 [DOI] [PubMed] [Google Scholar]
- 85.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol 288: R1450–R1467, 2005 [DOI] [PubMed] [Google Scholar]