Abstract
Chronic heart failure (HF) is characterized by increased sympathetic drive. Enhanced angiotensin II (ANG II) activity may contribute to the increased sympathoexcitation under HF condition. The present study examined sympathoexcitation by 1) the effects of ANG II in the paraventricular nucleus (PVN) on renal sympathetic nerve activity (RSNA), and 2) the altered ANG II type 1 (AT1) receptor expression during HF. Left coronary artery ligation was used to induce HF. In the anesthetized Sprague-Dawley rats, microinjection of ANG II (0.05–1 nmol) into the PVN increased RSNA, mean arterial pressure (MAP), and heart rate (HR) in both sham-operated and HF rats. The responses of RSNA and HR were significantly enhanced in rats with HF compared with sham rats (RSNA: 64 ± 8% vs. 33 ± 4%, P < 0.05). Microinjection of AT1 receptor antagonist losartan into the PVN produced a decrease of RSNA, MAP, and HR in both sham and HF rats. The RSNA and HR responses to losartan in HF rats were significantly greater (RSNA: −25 ± 4% vs. −13 ± 1%, P < 0.05). Using RT-PCR and Western blot analysis, we found that there were significant increases in the AT1 receptor mRNA (Δ186 ± 39%) and protein levels (Δ88 ± 20%) in the PVN of rats with HF (P < 0.05). The immunofluorescence of AT1 receptors was significantly higher in the PVN of rats with HF. These data support the conclusion that an increased angiotensinergic activity on sympathetic regulation, due to the upregulation of ANG II AT1 receptors within the PVN, may contribute to the elevated sympathoexcitation that is observed during HF.
Keywords: angiotensin, sympathetic activity, blood pressure, AT1 receptor
it has been well established that increased sympathetic nerve activity is a characteristic feature of chronic heart failure (HF) (37, 41). This elevated sympathetic activity induces an increase in peripheral resistance that increases cardiac afterload and preload. Furthermore, continued elevation of cardiac sympathetic activation results in high levels of norepinephrine, which contributes to myocardial toxicity and apoptosis (49), resulting in a decrease in myocardial contractility. Some studies have shown that various cardiovascular reflex functions are impaired in HF (15, 23). Using an animal model of HF produced by coronary artery ligation, studies from this laboratory and work by others have revealed that renal sympathetic nerve activity (RSNA) is elevated in rats with HF (7, 43). However, peripheral blockade has been shown only partially reducing the effects of sympathoexcitation under HF condition (38). An increasing number of studies have suggested that altered central mechanism(s) may be responsible for the elevated neurohumoral drive in HF (6, 7, 42, 58).
The paraventricular nucleus (PVN) of the hypothalamus is an important central site for the integration of sympathetic nerve activity (53). Morphologic and electrophysiological studies have shown that the PVN is reciprocally connected to other areas of the central nervous system, such as the nucleus tractus solitarii and the rostral ventrolateral medulla (RVLM) in the brain stem. These brain sites are involved in cardiovascular regulation, which makes them the areas of interest for this study (13, 27, 51). Using retrograde tracing techniques, various studies have shown that the PVN is a major source of forebrain input to the sympathetic nervous system (50, 51). Stimulation of PVN has been shown to elicit increased discharge of several sympathetic nerves, including renal (18) and adrenal (20). Specifically, studies showed that the PVN plays an essential role in the mediation of RSNA under resting and reflex conditions (14). Our previous studies have shown increased neuronal activity, as measured by hexokinase activity, in the PVN during HF (44), which points to the involvement of the PVN in the altered sympathetic activity in HF. However, the specific mechanisms within the PVN that are involved in regulating sympathetic nervous outflow are still unknown.
In the PVN, a number of inputs that use different neurotransmitters converge to influence its neural activity (53). Angiotensin II (ANG II) has been found to act as a neurotransmitter in the central nervous system and is involved in the regulation of sympathetic activity to the cardiovascular system (22). In autonomic areas of the brain, such as PVN, RVLM, and nucleus tractus solitarii, ANG II has been shown as a contributor to sympathoexcitation and sympathoexcitatory reflexes, such as the cardiac sympathetic afferent reflex, baroreflex, and arterial chemoreflex in HF (1, 10, 54). Components of the angiotensin system including angiotensinogen, angiotensin-converting enzyme, and ANG II type 1 (AT1) receptors have been found to exist in the PVN (24, 46). Electrophysiological studies have demonstrated that ANG II influences neurons in the PVN (3, 32) and is also involved in cardiovascular reflexes (59), which suggests that ANG II within the PVN plays a role in regulating sympathetic nerve activity and cardiovascular function. Our recent study (30) has shown that injection of ANG II into the PVN significantly increased RSNA. AT1 receptors in the PVN have also been found to be involved in central mechanisms regulating cardiovascular function during hypertension and chronic HF (26, 58). Therefore, we hypothesize that altered ANG II activity and ANG II receptor expression in the PVN is involved in sympathetic dysfunction in HF. To test this hypothesis, we examined whether 1) the endogenous ANG II is partially responsible for the elevated RSNA in rats with HF, and 2) AT1 receptor mRNA and protein levels are increased in the PVN of rats with HF.
METHODS
Animals.
This study was approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee, and conformed to the guidelines for the care and use of laboratory animals of the National Institutes of Health and the American Physiological Society. Male Sprague-Dawley rats, weighing 200–220 g, were obtained from SASCO Breeding Laboratories (Omaha, NE) and were assigned to two groups (sham-operated and HF group).
HF was produced by left coronary artery ligation, as previously described (57). The degree of left ventricular dysfunction and HF was determined by using both hemodynamic and anatomic criteria. Left ventricular end-diastolic pressure (LVEDP) was measured by using a Mikro-Tip catheter (Millar Instruments, Houston, TX) at the time of the terminal experiment. To measure infarct size, the heart was dissected and the atria and right ventricle were removed. A digital image of the left ventricle was captured using a digital camera (Kodak, Rochester, NY). The percentage of infarct area to total left ventricle area was quantified using SigmaScan Pro (Aspire Software International, Ashburn, VA). Rats with both LVEDP > 15 mmHg and infarct size > 30% of total left ventricular wall were considered to be in HF.
General surgery for hemodynamic and RSNA measurement and microinjection.
On the day of the experiment (6–8 weeks after cardiac surgery), the rat was anesthetized with urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip) and instrumented for recording arterial pressure (AP) and heart rate (HR) as previously described (57). The rat was then placed in a stereotaxic apparatus, and a cannula connected to a microsyringe (0.5 μl) was introduced into the PVN (1.5 mm posterior, 0.4 mm lateral to the bregma, and 7.8 mm ventral to the dura).
The left kidney was exposed through a left retroperitoneal flank incision, and a branch of the renal nerve was isolated from the adipose and connective tissues. The distal end of the nerve was ligated, and the nerve was placed on a bipolar platinum electrode. The nerve-electrode junction was insulated electrically from the surrounding tissues with gel (Wacker, St. Louis, MO). The electrical signal from the electrode was linked via a high-impedance probe (H1P5) to a Grass P511 band-pass amplifier (gain, 10,000) with high- and low-frequency cutoffs of 1,000 Hz and 100 Hz. The output from the Grass amplifier was directed to a Grass integrator, which rectifies the signal and integrates the raw nerve discharge. The output of the Grass integrator was displayed as an integrated voltage that is proportional to the renal nerve discharge. The average rectified signal [resistor-capacitor circuit filtered with a time constant of 0.5 s] was then recorded and stored for later analysis in a computer-based data acquisition system (Mac-Lab). Efferent RSNA at the beginning of the experiment was defined as basal nerve discharge. The RSNA recorded at the end of the experiment (after the rat was injected with hexamethonium, 30 mg/kg iv) was defined as background noise. The value of RSNA was calculated by subtracting the background noise from the actual recorded value, and changes found in integration of the nerve discharge during the experiment were expressed as a percentage from basal value. Responses of mean AP (MAP) and HR were expressed as the difference between the basal value and the value after each dose of a drug.
Experimental protocols.
In six sham-operated rats and six HF rats, three doses of ANG II (0.05, 0.5, and 1 nmol in 100 nl) in a random order were injected into the PVN at intervals of 30 min. The responses in RSNA, AP, and HR over 30 min were recorded after each dose of ANG II. The vehicle control was performed using artificial cerebrospinal fluid (100 nl, composition in mM: 132 NaCl, 3.0 KCl, 0.65 MgCl2, 1.5 CaCl2, 24.6 NaHCO3, and 3.3 glucose, pH 7.4) microinjected into the PVN. To substantiate the concept that any responses of RSNA, AP, and HR to ANG II were not a peripheral action, intravenous injection of 0.5 nmol (in 100 nl) of ANG II were examined (sham, n = 5; HF, n = 5).
In five sham-operated rats and five HF rats, the AT1 receptor antagonist losartan was injected (50 nmol in 100 nl) into the PVN. The responses in RSNA, AP, and HR over the 30 min were recorded after each dose administration. Intravenous injection of 50 nmol (in 100 nl) of losartan were examined (sham, n = 5; HF, n = 5).
Brain histology.
For all of the microinjection placements in the PVN, at the end of the experiments, monastral blue dye (50 nl) was injected into the brain for histological verification of the location. The brains were removed, frozen, sectioned, and processed for histology as described previously (57). The location of the center of dye spot was transferred to a histological map based on the rat atlas (45).
Micropunch of the PVN.
In the other group of sham-operated and HF rats, after the rat was euthanized by pentobarbital (65 mg/kg ip), the brain was removed and frozen at −80°C. Six consecutive 100-μm thick coronal sections were cut with a cryostat (−18°C). The PVN and the supraoptic nucleus (SON) were punched bilaterally with a blunt needle (ID: 0.5 mm) according to the method of Palkovits and Brownstein (39). The punched tissue was put in 0.5 ml of TRI Reagent (Molecular Research Center) or protein extraction buffer (1% SDS, 0.1% Triton, 10% PMSF, 1 mM EDTA, 10 mM Tris). The total RNA and protein in the homogenate were extracted, respectively.
Semiquantitative RT-PCR for the measurement of AT1 receptor mRNA.
Total RNA extracted from the punched tissue was subjected to reverse transcription for 40 min at 37°C in the presence of 400 units of Moloney murine leukemia virus reverse transcriptase (USB). Each 1 μl of complementary DNA was used as a template for PCR amplification. The following primers were used: for AT1 receptors (GenBank accession no.: NM 030985) the sense primer was 5′-AGAGGATTCGTGGCTTGAG-3′, the antisense primer was 5′-AGGGATCATGACAAATATG-3′; for β-actin (GenBank accession no.: NM 031144) the sense primer 5′-CACGGCATTGTAACCAACTG-3′, the antisense primer was 5′-TCTCAGCTGTGGTGGTGAAG-3′. A 25-μl PCR reaction mixture contained 0.7 μM forward and reverse primers and 1 unit of Taq polymerase (Invitrogen). The amplification was performed with 30 thermal cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were then fractionated in a 1% agarose gel and visualized by ethidium bromide staining and UV transillumination. The visualized DNA bands were captured by a digital camera. Density analysis of DNA bands was performed with Kodak 1D image analysis software. Data were expressed as the ratio of AT1 receptors and β-actin densities.
Western blot analysis.
The protein extraction from homogenates mentioned above was used for Western blot analysis. The protein concentration was measured using a protein assay kit (Pierce). Samples were adjusted to the same concentration of protein and treated at 95°C for 5 min before loading on the 7.5% SDS-PAGE gel (5 μg protein/30 μl per well) to be subjected to electrophoresis at 40 mA/each gel for 60 min. The fractionized proteins on the gel were then electrophoretically transferred onto a PVDF membrane (Millipore) at 300 mA for 90 min. The membrane was probed with primary antibody [rabbit polyclonal AT1 receptor and ANG II type 2 (AT2) receptor antibody, 1:1,000; rabbit polyclonal β-tubulin antibody, 1:2,000; all from Santa Cruz Biotechnology, Santa Cruz, CA] and secondary antibody (peroxidase-conjugated goat anti-rabbit IgG, 1:5,000; Pierce). AT1 receptor primary antibody is rabbit polyclonal affinity purified antibody raised against a peptide mapping within an NH2-terminal extracellular domain of AT1 of human origin. The signals were visualized using an enhanced chemiluminescence substrate (Pierce) and detected by a digital image system (UVP). AT1 receptor and AT2 antibody protein level were normalized using β-tubulin. As a test for the specificity and sensitivity of the AT1 receptor antibodies we used two different antibodies from two different companies, the two different antibodies were targeted at different ends of the protein, to show that they result in similar bands, the antibody from Santa Cruz Biotechnology for protein detection, and the antibody from Abcam for protein detection and immunostaining. These two antibodies target different ends of AT1 receptor protein. Santa Cruz Biotechnology AT1 receptor primary antibody is raised against a peptide mapping within an NH2-terminal extracellular domain of AT1 of human origin, and Abcam AT1 receptor primary antibody is corresponding to COOH-terminal amino acids of human AT1 receptor.
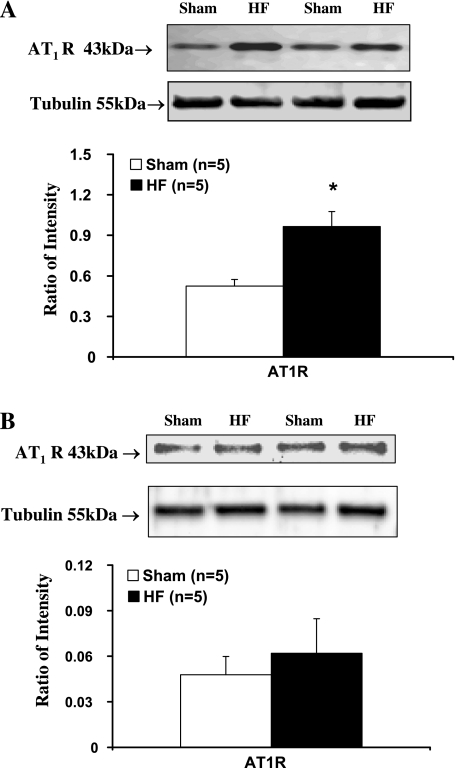
We also performed Western blot analysis on different tissues including brain, kidney, heart, and muscle, and found varying amounts of the same molecular band in various tissues. We examined specific areas in the brain suspected of having varying amounts of AT1 receptors, and the results showed that the level of AT1 receptor protein varies from tissue to tissue (incidentally we saw a 10-fold difference in protein levels in the SON compared with PVN) and close to undetectable levels in the optic tract tissue (negative control).
Immunofluorescent staining.
The rats were anesthetized with pentobarbital (65 mg/kg) and were perfused transcardially with 150 ml of heparinized saline followed by 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. The brain was removed and postfixed at 4°C for 4 h in 4% paraformaldehyde solution and then placed in 20% sucrose. The brain was blocked in the coronal plane, and sections 30 μm in thickness were cut with a cryostat. The sections were incubated with 10% normal donkey serum in PBS for 1 h at room temperature and were then incubated with primary antibody against AT1 receptor (mouse monoclonal antibody, 1:200, Abcam) overnight at 4°C. This antibody recognizes isoforms AT1A and AT1B of the AT1 receptor in rat. After being washed with PBS, the sections were incubated with Cy3-conjugated donkey anti-mouse secondary antibody (1:400, Jackson ImmunoResearch) for 2 h at room temperature. The nuclei were stained by Hoechst 33258 (Molecular Probes). After being washed with PBS and dried, the sections were coverslipped with fluoromounting-G (Southern Biotech). Distribution of AT1 receptor immunofluorescence within the PVN and SON was viewed using an Olympus fluorescence microscope equipped with a digital camera (Qimaging). Openlab software 4.0.3. (Improvision) was used to identify the total intensity of positive staining with Cy3. Three alternate sections (1.80 ± 0.1 mm posterior to bregma) representing the PVN and the SON were analyzed in this way, and then the mean data was calculated.
Statistical analysis.
Data are presented as means ± SE. Differences between groups were determined by a two-way ANOVA followed by the Newman-Keuls test for post hoc analysis of significance (StatView II; Berkeley, CA). P < 0.05 was considered statistically significant.
RESULTS
Tables 1 and 2 summarize the salient morphological and hemodynamic characteristics of sham-operated and HF rats utilized in the present study. For all of the experiments, any rats subjected to coronary artery ligation that displayed myocardial infarcts in < 30% of the left ventricular wall, were excluded from the study (7 out of 36 rats with coronary artery ligation surgery). Accordingly, the infarction area in the chronic HF group was ∼43% of the left ventricle. Conversely, sham rats had no observable damage to the myocardium. Heart weight was significantly greater in HF rats than in sham rats (P < 0.05), suggesting compensatory hypertrophy of noninfarct regions of the myocardium. LVEDP was significantly elevated in HF rats compared with sham rats. Rats with LVEDP > 15 mmHg were chosen for the HF group, while sham rats did not exhibit an increased LVEDP.
Table 1.
Baseline values of morphology and hemodynamics in rats with heart failure and sham-operated rats in the microinjection experiments
| Sham | HF | |
|---|---|---|
| Body weight, g | 365±;17 | 360±;20 |
| Heart weight, mg | 1138±;64 | 1521±;88* |
| Heart weight/body weight, mg/g | 3.1±;0.3 | 4.2±;0.4* |
| Infarct size, %LV | 0 | 44.5±;5.5* |
| LVEDP, mmHg | 2±;2 | 21±;4* |
| Basal mean arterial pressure, mmHg | 91±;9 | 87±;8 |
| Basal heart rate, beats/min | 334±;31 | 368±;45 |
| Basal integrated RSNA μVolt/s | 44.2±;8.8 | 51.3±;11.2 |
Values are means ±; SE; n = 11 rats/group. LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; RSNA, renal sympathetic nerve activity.
P < 0.05 vs. sham rats.
Table 2.
Characteristics of sham and heart failure rats in the molecular biological experiments
| Sham | HF | |
|---|---|---|
| Body weight, g | 378±;26 | 393±;24 |
| Heart weight, mg | 1159±;78 | 1614±;87* |
| Heart weight/body weight, mg/g | 3.1±;0.4 | 4.1±;0.3* |
| Infarct size, %LV | 0 | 40.2±;6.8* |
| LVEDP, mmHg | 3±;1 | 26±;7* |
Values are presented as mean ±; SE; n = 8 rats/group.
P < 0.05 vs. sham rats.
The dynamic characteristics of basal MAP, HR, and RSNA are also presented in Table 1. Although the level of raw RSNA in rats with HF tends to be higher than sham-operated rats, it did not reach statistical significance. Similarly, there were no statistically significant differences in basal MAP or HR between the sham and HF groups.
Figure 1 illustrates the brain histological data. Among the 22 injection sites that were in the PVN, 12 injection sites belonged to microinjection of ANG II, and 10 injection sites belonged to microinjection of losartan. Six out of 28 injections missed PVN, four injections belonged to microinjection of ANG II, and two injections belonged to microinjection of losartan.
Fig. 1.
A–C: schematic representations of serial sections from the rostral (−1.4) to the caudal (−2.1) extent of the region of the paraventricular nucleus (PVN). The distance (in mm) posterior to bregma is shown for each section. Each black circle is the site of termination of an injection considered to be within the PVN region in the ANG II injection experiments; each “+” symbol represents that in losartan injection experiments. D: histological photo showing the injection site (arrow) in the PVN of one rat. AH, anterior hypothalamic nucleus; f, fornix; 3V, third ventricle; OX, optic tract; SO, supraoptic nucleus.
Effects of microinjection of ANG II and losartan into the PVN on RSNA, MAP, and HR in HF and sham rats.
An example of the responses in RSNA, MAP, and HR to the administration of ANG II (1 nmol) into the PVN in a sham-operated rat and a rat with HF is illustrated in Fig. 2A. Microinjections of 0.05, 0.5, and 1 nmol of ANG II elicited increases in RSNA, MAP, and HR, reaching 33 ±; 4%, 7 ±; 2 mmHg and 18 ±; 6 beats/min, respectively, at the highest dose in sham-operated rats. The RSNA and HR responses were significantly elevated in the HF rats compared with the sham-operated rats, reaching 64 ±; 8% and 37 ±; 8 beats/min, respectively, at the highest dose (P < 0.05) (Fig. 2B).
Fig. 2.
A: segments of original recordings from an individual sham-operated rat and a rat with heart failure (HF), demonstrating the representative response to HR, mean arterial pressure (MAP), integrated renal sympathetic nerve activity (int. RSNA) and RSNA to the microinjections (arrows) of ANG II into the PVN. B: mean data of changes in HR, MAP, and RSNA after microinjections of ANG II and artificial cerebrospinal fluid (aCSF) into the PVN and intravenous injections in sham and HF rats. *P < 0.05 vs. group of sham.
The vehicle control, 100 nl of artificial cerebral spinal fluid microinjected into the PVN, had no effects on RSNA, MAP, and HR both in HF or sham rats (Fig. 2B). Intravenous injection of 0.5 nmol of ANG II had also no effect on these parameters (Fig. 2B). Similarly, microinjection of ANG II outside the PVN did not change RSNA, MAP, or HR (data is not shown).
An example of the responses in RSNA, MAP, and HR to administration of losartan (50 nmol) into the PVN in a sham-operated rat and a rat with HF is illustrated in Fig. 3A. Microinjection of losartan into the PVN decreased RSNA, MAP, and HR in both sham and HF rats. The responses of RSNA and HR to losartan in HF rats were significantly greater than the responses of sham rats (RSNA: −25 ±; 4% vs. −13 ±; 1%; HR: −21 ±; 3 beats/min vs. −10 ±; 1 beats/min, P < 0.05). Fig. 3B shows the mean data of RSNA, MAP, and HR responses to losartan microinjection in the PVN. Intravenous injection of 50 nmol of losartan also had no effect on these parameters (Fig. 3B). Microinjection of losartan outside the PVN did not change RSNA, MAP, or HR (data is not shown).
Fig. 3.
A: segments of original recordings from an individual sham-operated rat and a rat with HF, demonstrating the representative response to HR, MAP, int. RSNA, and RSNA to the microinjections of losartan into the PVN. B: mean data of changes in HR, MAP, and RSNA after microinjections of losartan into the PVN and venous in sham and HF rats. *P < 0.05 vs. group of sham.
Measurements of AT1 receptor expression in the PVN.
Results of RT-PCR experiments indicated that AT1 receptor mRNA expression in the punched PVN tissues from the HF rats was significantly increased compared with sham rats (P < 0.05) (Fig. 4). Western blot analysis showed that AT1 receptor protein levels were also significantly higher in HF rats compared with sham rats, which was consistent with the previous results. (P < 0.05) (Fig. 5A).
Fig. 4.
A: gene expression (mRNA) of ANG II type 1 (AT1) receptors in the PVN and supraoptic nucleus (SON) tissues measured. Top: example of visualized bands of AT1 receptors and β-actin of sham and HF rats. Bottom: mean data of band densities of AT1 receptors normalized by β-actin in sham and HF. *P < 0.05 vs. sham.
Fig. 5.
Protein level of AT1 receptors in the PVN (A) and SON (B) tissues. Top: example of visualized bands of AT1 receptors and β-tubulin. Bottom: mean data of band densities of AT1 receptors normalized by β-tubulin in sham and HF. *P < 0.05 vs. sham group.
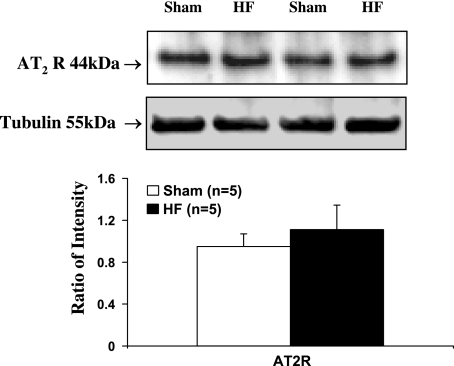
In contrast to the PVN, however, PCR and Western blot analysis results did not show significant changes in AT1 receptor mRNA (Fig. 4) and protein level (Fig. 5B) in the SON, another important hypothalamic area in control of cardiovascular and body fluid homeostasis (52), in HF rats compared with sham rats. This suggests that the change in AT1 receptors within the PVN in HF is region specific. Moreover, Western blot analysis results did not show a significant change in AT2 receptor protein level within the PVN in HF rats compared with sham rats (Fig. 6). The AT1 receptor/AT2 receptor ratio within the PVN is significantly higher in HF rats compared with the sham rats (0.87 ±; 0.18 vs. 0.55 ±; 0.09, P < 0.05).
Fig. 6.
Protein level of AT2 receptors in the PVN tissues. Top: example of visualized bands of AT2 receptors and β-tubulin. Bottom: mean data of band densities of AT2 receptors normalized by β-tubulin in sham and HF.
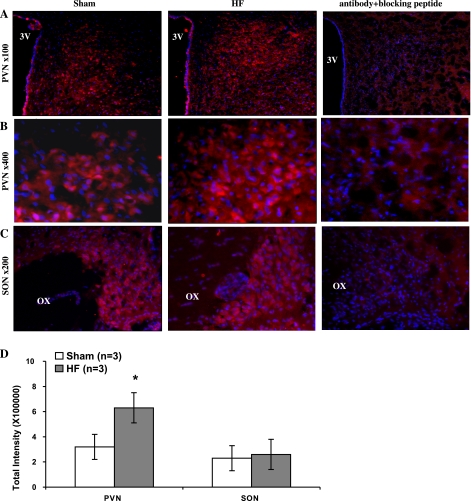
As an in situ confirmation of the alteration in AT1 receptors within the PVN, the immunofluorescence for AT1 receptors was significantly increased in the PVN from rats with HF compared with sham rats (Fig. 7, A and B). The immunofluorescence for AT1 receptors within the SON (Fig. 7C) was not altered from rats with HF compared with sham rats. Fig. 7D shows the quantification data of immunofluorescence signal in the PVN and the SON.
Fig. 7.
Immunofluorescent photomicrographs from the sections of the PVN (A and B) and SON (C) region stained for AT1 receptors with/without AT1 receptors blocking peptide. The intensity of AT1 receptor staining (red) is increased within the PVN in HF rats compared with sham rats. Blue spot shows the nucleus stained by Hoechst 33258. D: quantification data of immunofluorescence signal in the PVN and the SON. 3V, third ventricle; OX, optic tract. *P < 0.05 vs. sham group.
DISCUSSION
In the present study, we observed that, in rats with HF, the responses of RSNA and HR to microinjection of ANG II into the PVN were augmented, accompanied by upregulated ANG II AT1 receptor message and protein in the PVN area. There is a significant difference in the response to AT1 receptor antagonist losartan in the PVN in HF, namely the fact that RSNA and HR responses to losartan in HF rats were significantly greater than the responses observed in sham rats. These results suggest that enhanced AT1 receptor-mediated angiotensin action in the PVN on sympathetic outflow may contribute to sympathetic dysfunction in HF. Although there was a tendency toward a change in responses of arterial pressure to ANG II, particularly at the high doses, they were not statistically significant. The changes in arterial pressure are dependent on a number of factors including RSNA-mediated renovasoconstriction. Activation of PVN produces a differential change in different sympathetic outflows (21) and that may account in part for the lack of changes in blood pressure responses in this study. To test whether RSNA burst frequency increased by increases in HR and reduced by falls in HR, we have conducted further experiments (data not shown) to prevent the change in HR and then monitored the changes in RSNA. After using β-receptor blocker metoprolol to prevent the changes of HR, we still saw the effects of ANG II in the PVN on the RSNA. Therefore, we think the effects of ANG II in the PVN are due to the direct effect on RSNA, not due to the effects from the changes in HR.
HF is characterized by elevated systemic sympathetic activity and salt and water retention (34). Previously, work from this laboratory has shown that inhibitory mechanisms of sympathetic regulation within the PVN via nitric oxide (NO) (56) and GABA (57) were reduced, while the excitatory mechanism regulated by N-methyl-d-aspartic type I receptor was enhanced (28) in HF rats. These alterations may induce an imbalance of the inhibitory and excitatory mechanisms in this area and influence sympathetic outflow. In the present study, we concentrated on the excitatory mechanism of renal sympathetic nerve regulation within the PVN. As a major excitatory neurotransmitter, ANG II has been found to regulate sympathetic nerve activity in several brain areas, including the hypothalamus. Using whole cell recording from hypothalamic slice preparations obtained from the rat, it was observed that ANG II induced depolarization of type II neurons in the PVN (pPVN) (3). AT1 receptor is the major ANG II receptor mediating the excitatory signal of ANG II in the PVN (25). We observed that stimulation of ANG II receptors induced a sympathetic excitation, further confirming that AT1 receptors in the PVN mediate sympathetic outflow. Dibona and Jones (6) also found in another brain area, that the angiotensin system influenced basal RSNA and arterial baroreflex regulation. Microinjection of AT1 receptor antagonists into the RVLM of anesthetized rats significantly improved the arterial baroreflex regulation of RSNA.
Although the angiotensin system does not appear to contribute significantly to the generation of resting tonic activity in neurons, it is shown to contribute significantly to the tonic activity of the neurons under certain conditions. These conditions include salt deprivation or spontaneous hypertension in which the endogenous levels of ANG II or AT1 receptors are upregulated (4). In the present study, after microinjection of AT1 receptor antagonist losartan into the PVN, the responses of RSNA and HR in HF rats were significantly changed compared with sham rats. This result supports the hypothesis that enhanced endogenous ANG II effects in the PVN contribute to the elevated sympathoexcitation in HF. The greater reduction of RSNA by losartan in the HF group suggests that there was increased angiotensinergic tone in the PVN stimulating RSNA. However, our basal measurements of RSNA did not show the significant increase in HF. It should be noted that comparison of basal RSNA levels between animals is wrought with a number of concerns, such as variability in number of fibers recorded from the nerve, the precision of nerve dissection from the surrounding tissue, the amount of fat surrounding the nerve, the proximity of the nerve segment to the electrode hooks, and nerve damage during handling and dissection (5). It is also possible that there are counterregulatory mechanisms including glutamatergic and GABA mechanisms that are offsetting the effect of the sensitized angiotensinergic mechanisms in the PVN.
In the present study, we did not measure the circulating ANG II levels. In the chronic HF state, plasma ANG II levels are found to be increased (33, 60). Although ANG II does not cross the blood-brain barrier, circulating levels of ANG II may effect the central nervous system including the PVN through some of the circumventricualr organs, such as the subfornical organ and the organum vasculosum of the lamina terminalis. The neurons in the circumventricualr organs have projections to the PVN. We think that through this pathway, the circulating ANG II could effect the local ANG II levels in the central nervous system (36). Many studies also show AT1 receptor upregulation under the condition of a higher level of circulating ANG II (10, 40, 55). Our results of AT1 receptor upregulation are consistent with these previous observations in the PVN. However, one observation indicated that the AT1 receptor protein level, not mRNA, increased in the PVN in rats with HF (17). Our data shows both AT1 receptor mRNA and protein are upregulated in the PVN, consistent with the observation that losartan can block the enhanced ANG II activity through the effects on the AT1 receptors.
As an in situ confirmation of the alteration in AT1 receptors within the PVN, the results of immunofluorescence for AT1 receptors showed a significant increase in the PVN from rats with HF compared with sham rats. According to the study of Li et al. (25), the immunoreactivity of AT1 receptors was colocalized with a presynaptic marker, synaptophysin, in the PVN. However, according to a study by Oldfield et al. (35), AT1 receptor immunostaining appears to occur in hypophysiotrophic neurons in the PVN that subserve anterior pituitary function but not in preautonomic cells. Further confirmation is needed regarding the immunoreactivity of AT1 receptors in the PVN.
Changes in excitatory synaptic functions can occur by presynaptic mechanisms, such as altered neurotransmitter release and/or postsynaptic mechanisms. We observed that AT1 receptors were upregulated in the PVN in HF rats, which is associated with the enhanced ANG II receptor function. This may be one of the central mechanisms underlying the elevated sympathoexcitation in HF, as the upregulation of AT1 receptors has been observed in other studies in response to different challenges. For example, AT1 receptors have been shown to be involved in the integration of neuroendocrine function in the forebrain region. AT1 receptor antagonists inhibited osmotic-dependent vasopressin release from hypothalamic tissues. In the intact animal, intracerebroventricular application of losartan induced a significant reduction in drinking and plasma vasopressin level in dehydrated rats (1, 16). These observations suggest that alteration of the AT1 receptors in the hypothalamus may play a role in adaptation of the body to different homeostatic challenges. The significantly higher gene expression of AT1 receptors in the PVN also has been observed in some other disease states, such as spontaneous hypertension (47). The spontaneously hypertensive rat is found to have significantly higher numbers of AT1 receptor subtype. Central angiotensinergic pathways may use AT1 receptors and play a role in the function of sympathetic pathways maintaining arterial pressure.
The AT2 receptor is another important mediator for the action of ANG II. Both AT1 and AT2 receptor subtypes in the PVN are involved in ANG II-related sympathetic activity. AT1 receptors exert excitatory responses to administered ANG II into the PVN, while AT2 receptors exert inhibitory effects (2). The net effect of ANG II therefore may depend on the cellular AT1-to-AT2 receptor ratio (48). The functions and intracellular signaling pathways of the AT2 receptor in most peripheral tissues and organs are opposite to that of the AT1 receptor. For example, stimulating AT2 receptor induces vasodilation, stimulates NO production, and inhibits reactive oxygen species generation (19). Recently, Gao et al. (11) found that AT1 receptor protein expression in the RVLM of HF rats was upregulated, while AT2 receptor was significantly downregulated and therefore greatly increased the protein expression of AT1-to-AT2 receptor ratio in HF. These data led them to postulate that the balance between the AT1 receptor and AT2 receptor may be critical to maintain sympathetic tone in normal conditions, and that the imbalance of AT1 receptor and AT2 receptor may contribute to the sympathoexcitation in the HF state. In our study, we did not observe a significant change in the expression of AT2 receptors within the PVN in HF rats compared with sham rats. There was an increased AT1-to-AT2 receptor ratio in our study as well that could account for the enhanced excitatory effects of ANG II receptor in the HF state.
The cause(s) of upregulation of AT1 receptors within the PVN in HF remain to be examined. In the HF state, the cardiac sympathetic afferents and chemosensitive afferents are sensitized and result in an increased sympathetic drive (9, 31). The increased afferent input to the autonomic centers including the PVN may be part of the reason for the upregulation of AT1 receptors within the PVN in HF (54, 59). Also many central and peripheral humoral factors, such as aldosterone and TNF-α, are significantly altered in HF (8, 12). All of these alterations may induce the compensatory responses in the cardiovascular and autonomic centers. Further studies are also required to determine the cellular mechanisms of action of ANG II in the PVN and its interactions with other neurotransmitters in that region. For example, we have observed that ANG II reduces the level of neuronal NO synthase in cultured NG108 neuronal cells (29). This would indicate that the enhanced ANG II action, which produces excitatory effects can also exaggerate this effect by reducing the inhibitory effects of NO.
In conclusion, the results of the present study indicate that an altered ANG II system within the PVN due to the upregulation of the AT1 receptors in the PVN may contribute to the elevated sympathetic nerve activity in the HF state. Because sympathoexcitation contributes to the deterioration of the HF state, a comprehensive understanding of the sympathoexcitation will help to uncover new therapies for the sympathetic dysfunction in HF.
Perspective and Significance
In the PVN, there are multiple other excitatory neurotransmitters/substances that are involved in the sympathetic outflow and cardiovascular function, including ANG II, norepinephrine, serotonin, dopamine, cytokines, and reactive oxygen species (8, 12). Increased ANG II action in diseases, such as hypertension and HF, may contribute to increased sympathetic outflow. This study shows that altered ANG II system within the PVN due to the upregulation of the AT1 receptors may contribute to the elevated sympathetic nerve activity in the HF state. Similar alterations in the central ANG II system may contribute to sympathoexcitation in various other disease states, such as hypertension.
GRANT
This work was supported by National Heart Lung and Blood Institute Program Project Grant P01-HL-062222.
ACKNOWLEDGMENTS
The technical assistance of Xuefei Liu was greatly appreciated.
REFERENCES
- 1.Barbella Y, Cierco M, Israel A. Effect of losartan, a nonpeptide angiotensin II receptor antagonist, on drinking behavior and renal actions of centrally administered renin. Proc Soc Exp Biol Med 202: 401–406, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Camargo LAD, Saad WA. Renal effects of angiotensin II receptor subtype 1 and 2-selective ligands injected into the paraventricular nucleus of conscious rats. Regul Pept 84: 91–96, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dampney RA, Fontes MA, Hirooka Y, Horiuchi J, Potts PD, Tagawa T. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 467–472, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Deka-Starosta A, Garty M, Zukowska-Grojec Z, Keiser HR, Kopin IJ, Goldstein DS. Renal sympathetic nerve activity and norepinephrine release in rats. Am J Physiol Regul Integr Comp Physiol 257: R229–R236, 1989 [DOI] [PubMed] [Google Scholar]
- 6.DiBona GF, Jones SY. Sodium intake influences hemodynamic and neural responses to angiotensin receptor blockade in rostral ventrolateral medulla. Hypertension 37: 1114–1123, 2001 [DOI] [PubMed] [Google Scholar]
- 7.DiBona GF, Sawin LL. Reflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 266: R27–R39, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Felder RB, Francis J, Weiss RM, Zhang ZH, Wei SG, Johnson AK. Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain. Ann NY Acad Sci 940: 444–453, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45: 1173–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Wang W, Li YF, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52: 708–714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-α blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol 293: H599–H609, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hardy SGP. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res 894: 233–240, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst 50: 1–11, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Hirsch AT, Dzau VJ, Creager MA. Baroreceptor function in congestive heart failure: effect on neurohumoral activation of vascular resistance. Circulation 75, Suppl IV: IV36–IV48, 1987 [PubMed] [Google Scholar]
- 16.Hogarty DC, Speakman EA, Puig V, Phillips MI. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res 586: 289–294, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovasc Res 79: 671–678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol 256: R1325–R1330, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12: 70–88, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Katafuchi TY, Oomura Y, Kurosawa M. Effects of chemical stimulation of paraventricular nucleus on adrenal and renal nerve activity in rats. Neurosci Lett 86: 195–200, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Kenney MJ, Weiss M, Mendes T, Wang Y, Fels RJ. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand 177: 7–15, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kumagai K, Reid IA. Angiotensin II exerts differential actions on renal nerve activity and heart rate. Hypertension 24: 451–456, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Lenkei Z, Corvol P, Llorens-Cortes C. The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Brain Res Mol Brain Res 30: 53–60, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Morris M, Diz DI, Ferrario CM, Ganten D, Callahan MF. Role of paraventricular angiotensin AT1 receptors in salt-sensitive hypertension in mRen-2 transgenic rats. Am J Physiol Regul Integr Comp Physiol 270: R1178–R1181, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Zhang W, Stern J. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience 118: 585–601, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Li YF, Vollmer A, Rebeller B, Patel KP. Inhibitory effect of NO on AT1 receptor expression in neuronal cells (Abstract). FASEB J 17: A1264, 2004 [Google Scholar]
- 30.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R1035–R1043, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD. Angiotensin II enhanced carotid body chemorefllex control of sympathetic outflow in chronic heart failure rabbit. Cardiovasc Res 71: 129–138, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Ferguson AV. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices: modulation of IA by angiotensin II. Neuroscience 71: 133–145, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure–A role for angiotensin II. Circulation 102: 1854–1862, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Marenzi G, Lauri G, Guazzi M, Assanelli E, Grazi M, Famoso G, Agostoni P. Cardiac and renal dysfunction in chronic heart failure: Relation to neurohumoral activation and prognosis. Am J Med Sci 321: 359–366, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13: 139–146, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721–730, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 334: 1349–1355, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Palkovits M, Brownstein M. Brain microdissection techniques. In: Brain Microdissection Techniques, edited by Cuello AE. Chichester, UK: Wiley, 1983 [Google Scholar]
- 40.Pan YX, Gao L, Wang WZ, Zheng H, Liu D, Patel KP, Zucker IH, Wang W. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertension 49: 519–527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel KP. Neural regulation in experimental heart failure. Baillieres Clin Neurol 6: 283–296, 1997 [PubMed] [Google Scholar]
- 42.Patel KP, Zhang K. Neurohumoral activation in heart failure: role of paraventricular nucleus. Clin Exp Pharmacol Physiol 23: 722–726, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Patel KP, Zhang PL, Carmines PK. Neural influences on renal responses to acute volume expansion in rats with heart failure. Am J Physiol Heart Circ Physiol 271: H1441–H1448, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol 265: R923–R928, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Orlando, FL: Academic, 1986 [Google Scholar]
- 46.Pfister J, Spengler C, Grouzmann E, Raizada MK, Felix D, Imboden H. Intracellular staining of angiotensin receptors in the PVN and SON of the rat. Brain Res 754: 307–310, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin Exp Pharmacol Physiol 33: 690–695, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Singh K, Communal C, Sawyer DB, Colucci WS. Adrenergic regulation of myocardial apoptosis. Cardiovasc Res 45: 713–719, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of the projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980 [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324, 1983 [DOI] [PubMed] [Google Scholar]
- 53.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410–417, 1980 [DOI] [PubMed] [Google Scholar]
- 54.Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W, Zhu GQ. AT1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Auton Neurosci 121: 56–63, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Yoshimura R, Sato T, Kawada T, Shishido T, Inagaki M, Miyano H, Nakahara T, Miyashita H, Takaki H, Tatewaki T, Yanagiya Y, Sugimachi M, Sunagawa K. Increased brain angiotensin receptor in rats with chronic high-output heart failure. J Card Fail 6: 66–72, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within the paraventricular nucleus of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995–H1004, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol 97: 1746–1754, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension 50: 904–910, 2007 [DOI] [PubMed] [Google Scholar]