Abstract
The relationship between maternal plasma volume (PV) expansion and fetal growth is well established, but the underlying mechanisms remain unclear. Here, we examined the influence of maternal body weight and fetoplacental mass on gestational PV increment in the rat. Because IGF-I and IGF-II have growth-promoting and vasoactive properties, their relationship to PV expansion and fetoplacental growth was also studied. In normal rats, the gradual expansion of PV (+35% at day 22, i.e., term) was accompanied by a rise in circulating IGF-II (+45%) and a considerable drop in IGF-I (−73%). Increased maternal body weight induced by an obesogenic diet did not influence PV and circulating IGFs compared with rats on the standard diet. Combining the results from both diets, circulating IGF-II was the principal correlate of PV. A second experiment examined the effect of fetoplacental mass reduction by surgically removing half of the gestational sacs at day 16. This procedure reduced maternal PV and circulating IGF-II at term by 14% and 20%, respectively. We then investigated the effect of a constant infusion of IGF-II (1 mg·kg−1·day−1) from day 16, which raised circulating IGF-II by 38% and found increased PV (+19%) and a larger placental trophospongial area (+29%) at term. Our results indicate that the placenta, the primary source of IGF-II synthesis in pregnancy, drives PV expansion, and that IGF-II is among the regulatory factors of the gestational PV increment. Further studies should clarify whether IGF-II directly affects vascular function and/or indirectly promotes the secretion of placenta-derived vasoactive substances.
Keywords: insulin-like growth factors, placenta, plasma volume
fetal growth is the result of a complex interaction between the fetus (genetic constitution), the mother (nutrient and oxygen provision, uteroplacental perfusion), and the placenta (morphology, transplacental transport) (32). Evidence shows that placental morphology and transport can adapt to environmental conditions through endocrine pathways in which glucocorticoids and IGF play an important role (17). Both IGF-I and IGF-II regulate intrauterine growth, with deletion of the fetal Igf1 or Igf2 gene expression in mice resulting in a 40% reduction in birth weight (16). Placental growth is primarily regulated by IGF-II: deletion of the placental and/or the fetal Igf2 gene in mice leads to 30–40% smaller placentas, whereas fetal Igf1 knockout has no effect on placental growth (12, 17). In addition, IGF-II infusion during middle to late gestation in guinea pigs increases fetal weight at term through placental morphologic changes and improves transplacental nutrient transport (33).
The maternal concentrations of IGF-I and IGF-II are increased in human pregnancy (39, 40). In rodents, the rise in maternal IGF-II during gestation is even more prominent (26), reflecting the abundant secretion of this peptide by the placenta (21). These changes in IGF concentrations could affect maternal physiology through their angiogenetic (8) or vasomotor (7, 27, 36) properties, thereby establishing an additional regulatory pathway for fetal growth.
Maternal plasma volume (PV) expands by 40% in normal human pregnancy (10). The ensuing increase in cardiac output is required to sustain the high uteroplacental perfusion demand of the fetus. The interaction between PV expansion and fetal growth is shown by the following observations: PV at 30 wk gestational age (GA), and the gestational PV increment is correlated with birth weight (19); both PV expansion and birth weight are higher in second than in first pregnancies (6); PV is subnormal from the second trimester onward in pregnancies complicated by fetal growth restriction (30); PV is increased in overweight women (4) whose babies have a higher birth weight (18). Of note, PV expansion occurs gradually and continues up to 37 wk GA (30), mirroring the fetoplacental growth pattern (3).
In the current study, we explored how the maternal hemodynamic adaptation to pregnancy relates to maternal circulating IGFs, maternal body weight, and fetoplacental growth in rats. The first experiment examined the effect of weight gain provoked by an obesogenic diet on maternal hemodynamic variables and circulating IGFs, and fetoplacental growth. Although the experiment failed to show an effect of maternal weight gain on maternal hemodynamics, the placenta and circulating IGF-II were identified as correlates of PV expansion. The second experiment examined the surgical removal of gestational sacs (fetoplacental mass reduction) with PV and circulating IGFs as primary outcome variables. Because both PV and IGF-II were reduced through this intervention, suggesting a potential causative role for IGF-II, the third experiment examined the effect of a constant infusion of IGF-II on PV. This experiment confirmed that IGF-II infusion in rat dams increased PV at term.
MATERIALS AND METHODS
Animal Experiments
All protocols were approved by the Ethics Committee for Animal Experimentation of the Faculty of Medicine of the KU Leuven, Leuven, Belgium. Female Sprague-Dawley rats were purchased from Janvier Breeding (Le Genest Saint-Isle, France) and housed in a temperature- and humidity-controlled environment with a standard light-dark cycle. All animals had free access to tap water and a standard laboratory chow (Ssniff, Soest, Germany). The animals were allowed to acclimatize for 1 wk before the start of the experiments, and all experiments were done between 7:00 and 11:00 AM to minimize influence of diurnal patterns.
Experiment 1: effect of excess body weight on gestational PV expansion and IGF concentrations through pregnancy.
At 8 wk of age, the animals were randomly assigned to 1) the control group fed the standard chow or 2) the experimental group that received ad libitum a highly palatable semisolid diet consisting of 33% ground chow, sucrose, and 33% unskimmed sweetened condensed milk which is presented in a plastic tray within the cage. We previously showed that this experimental diet boosts weight gain (5). From 14 wk of age onward, a cohort of animals was allowed to mate overnight just before estrus, as determined on a vaginal smear. Successful mating was confirmed by the presence of sperm cells on a smear and defined as day 1 of pregnancy; term is day 23. During pregnancy, the dams were kept on their respective diets. They were randomly assigned for hemodynamic measurements on either day 7, 14, or 22 of gestation (n = 8 at each time-point for each diet). Another cohort of virgin nonpregnant animals was used for comparison (n = 8 for each diet). Each of the eight subgroups of rats was weighed, and systolic blood pressure and heart rate were measured noninvasively in a heated chamber (32°C) using the tail-cuff method (IITC, Woodland Hills, CA). All animals were trained to undergo this procedure on 5 consecutive days before the actual measurement. The actual measurement consisted of four recordings, while the animal was visually relaxed and immobile, and an average value was calculated. After the blood pressure recordings, the dams were anesthetized with ketamine 50 mg/kg (Parke-Davis, Zaventem, Belgium) and xylazine 10 mg/kg (Bayer, Leverkusen, Germany), both intraperitoneally. Evans blue dye (0.4 ml of a 0.3 mg/ml solution; Sigma Aldrich, St. Louis, MO) was injected into the tail vein for PV determination. Syringes were weighed before and after injection to verify the injected volume (scale accuracy: 0.1 mg). After 10 min, a median laparotomy was performed, and blood was sampled from the descending aorta and collected into lithium-heparin tubes. For day 22 experiments, all fetuses and placentas were delivered, blotted dry, and weighed. Two placentas per animal (1 from the center of each horn) were fixed in a 4% paraformaldehyde solution for later histomorphometric examination. The animals were euthanized by cervical elongation while anesthetized.
Experiment 2: effect of fetoplacental mass reduction on maternal PV and IGFs.
Seventeen 14-wk-old rats were anesthetized on day 16 of pregnancy using the medication described for experiment 1. All animals underwent a median laparotomy, and the uterus was partially exteriorized. In 8 dams, we subsequently removed half of the gestational sacs, fetuses, and placentas from each uterine horn through 2 mm-long incisions on the antimesometrial side of the uterus. The other half of the gestational sacs were left untouched. The incisions were closed with nonresorbable purse string sutures. In the other nine dams, there were no further interventions beyond uterine manipulation (sham procedure). The duration of a typical procedure was 30 min for the fetoplacental mass reduction and 20 min for the sham procedure. All animals were allowed to continue their pregnancy undisturbed. On day 22, the animals were again anesthetized and PV determination, blood sampling, and fetoplacental prelevation were performed as described for experiment 1.
Experiment 3: effect of maternal IGF-II infusion on PV expansion.
Fourteen-week-old rats were anesthetized on day 16 of gestation and osmotic minipumps (Alzet 2001; Alzet, San Francisco, CA) were subcutaneously inserted in the interscapular region. The minipumps were loaded either with physiological saline solution (n = 9) or IGF-II (1 mg·kg−1·day−1 human recombinant protein in saline; n = 8; GroPep, Adelaide, Australia) and maintained a constant infusion rate of 0.9 μl/h for 7 days. IGF-II dosing was based on a previous experiment in pregnant guinea pigs (33). On day 22 of pregnancy, the dams were again anesthetized and PV determination, blood sampling, and fetoplacental prelevation were performed, as described for experiment 1.
Hematocrit, Plasma, and Blood Volume Measurements
Eighty-five microliters of blood was drawn into a heparin-coated capillary and centrifuged (Hawksley, Sussex, United Kingdom) for hematocrit determination. Lithium-heparin blood tubes were centrifugated at 1,200 g, plasma was aliquoted, 1 ml of which was immediately read at 620 and 740 nm on a Perkin Elmer Lambda 12 spectrophotometer (Waltham, MA) to determine the true Evans blue dye concentration; the measurements were done in triplicate and averaged. PV was calculated from this final concentration and the initially injected volume (14). Total blood volume was derived from PV and hematocrit, as previously described (14).
Hormone Radioimmunoassays
IGF-I was measured by radioimmunoassay, as described previously (38) after acid-ethanol extraction and the addition of excess IGF-II to separate IGF-I from the IGF-binding proteins. IGF-II was also measured by radioimmunoassay after extraction with formic acid-acetone, as described for human sera (38); a dilution curve confirmed adequate measurement of IGF-II in rat sera (data not shown).
Placental Histomorphometry
Placentas were embedded horizontally in paraffin; 3-μm-sections were obtained starting at the insertion of the umbilical cord, and hematoxylin-eosin stained. Two 3-μm-thick subsequent sections were assessed per placenta for each rat. The total placental cross-sectional area [and the area of the labyrinth and trophospongium (in mm2)] were measured at magnification ×2.5 using image analysis software (Axiovision version 4.5 and KS-400 version 3.0; Carl Zeiss, Oberkochen, Germany).
Data Analysis
We used the JMP software version 7.0 (SAS Institute, Cary, NC) and Prism for windows version 5.0 (GraphPad Software, San Diego, CA). “Maternal body weight” was calculated as the total maternal weight subtracted by total blood volume in nonpregnant rats and by total weight of the litter (i.e., all fetuses and placentas) and total blood volume in pregnant rats. Normality of the data was assessed using the D'Agostino Omnibus test. All data followed a Gaussian distribution and are presented as means ± SE. Data obtained from two groups of rats were compared by two-sampled Student's t-test. Data obtained from more than two groups were compared by one- or two-way ANOVA with Bonferroni's post hoc test when P < 0.05. Correlations were assessed using Pearson's correlation coefficient, and multivariate regression analysis was done using the least squares method.
RESULTS
Experiment 1
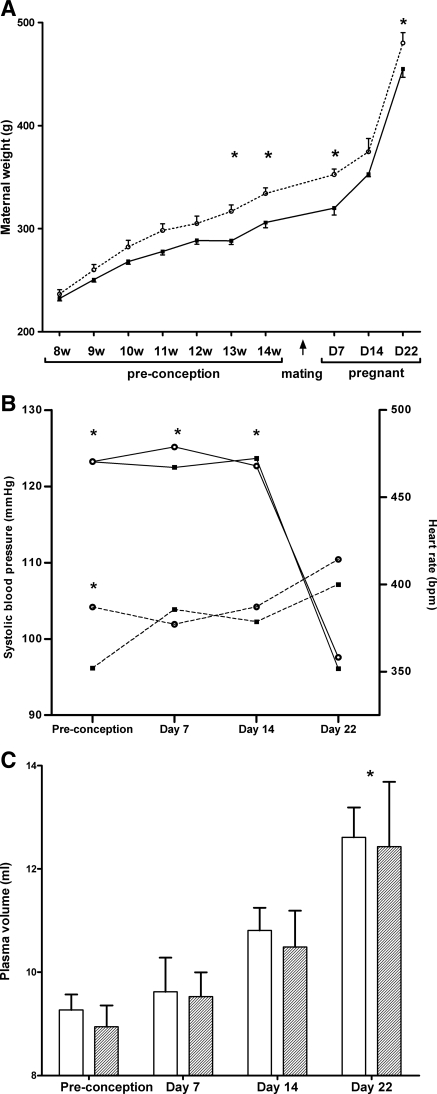
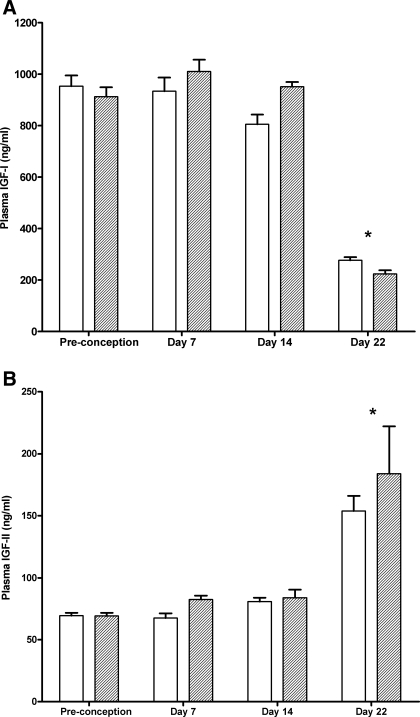
Pregestational maternal weight gain was stimulated by the experimental diet (Fig. 1A). At 14 wk (i.e., at preconception), the experimental rats weighed 9% more than did the controls (334 ± 16 g vs. 306 ± 14 g, respectively; P = 0.002); gestational weight gain, however, was comparable in both groups (p = 0.45). Term dams had increased heart rates and lower systolic blood pressure than nonpregnant or early-pregnant rats (Fig. 1B). PV increased gradually during gestation with maximum levels at term (35% higher than in nonpregnant rats; Fig. 1C). There were no differences between the two diet groups for any of the hemodynamic parameters. The changes in plasma concentrations of IGF-I and II during gestation are depicted in Fig. 2. IGF-I was strongly reduced (−73%) at day 22 of pregnancy, whereas IGF-II was increased (+45%) in late pregnancy. Again, no significant differences were observed between the two groups.
Fig. 1.
Maternal weight and hemodynamic parameters from dams in experiment 1. A: longitudinal weight data from dams on the control diet (circles) and dams on the experimental diet (squares) followed until term (n = 8 for each diet). Two-way ANOVA: influence of age and diet both P < 0.0001. No interaction. *P < 0.05 using Bonferroni post hoc test for comparison of experimental vs. control diet. B: longitudinal systolic blood pressure (full line) and heart rate (dotted line) data from dams on control diet (circles) and experimental diet (squares) followed until term (n = 8 for each diet). Two-way ANOVA: influence of age P < 0.0001. Diet nonsignificant. No interaction. *P < 0.05, using Bonferroni post hoc test, when compared with day 22. C: plasma volume in dams on the control diet (empty bars) and on the experimental diet (hatched bars). n = 8 for each diet at each time point. Two-way ANOVA: influence of age P < 0.0001. Diet nonsignificant. No interaction. *P < 0.05 using Bonferroni post hoc test when compared with preconception.
Fig. 2.
Insulin-like growth factor (IGF) I (A) and II (B) during pregnancy in dams on the control diet (empty bars) and on the experimental diet (dashed bars). n = 8 for each diet at each time point. Two-way ANOVA: influence of age P < 0.0001. Diet nonsignificant. No interaction. *P < 0.05 using Bonferroni post hoc test when compared with preconception, day 7, and day 14.
The reproductive outcome of this cohort is displayed in Table 1. Litter size and fetal weight did not differ between both groups, but the mean placental weight was significantly lower in the overweight group. Placental histomorphometry showed a marked decrease in both labyrinth and trophospongium area in these dams compared with controls (Table 1), while the proportionality was preserved (standard diet: 76.8 ± 2.8% and 23.1 ± 2.8% of the placental area covered by labyrinth and trophospongium, respectively; experimental diet: 75.8 ± 3.7% and 23.9 ± 3.7%, respectively; P > 0.10).
Table 1.
Characteristics (day 22 of gestation) of rats on the standard and experimental diet
| Standard Diet (n = 8) | Experimental Diet (n = 8) | P Value | |
|---|---|---|---|
| Maternal hematocrit, % | 31.6±0.3 | 32.6±0.6 | 0.18 |
| Maternal systolic blood pressure, mmHg | 96.1±3.0 | 97.6±4.5 | 0.80 |
| Maternal heart rate, bpm | 400±8.1 | 414±8.1 | 0.23 |
| Maternal plasma volume/weight, ml/kg | 28.1±1.0 | 25.6±2.1 | 0.30 |
| Litter size, n | 14.4±0.5 | 15.0±1.2 | 0.64 |
| Mean fetal weight, mg | 5.38±0.1 | 5.27±0.15 | 0.61 |
| Total fetal weight/litter, mg | 77.5±4.0 | 78.0±4.8 | 0.94 |
| Mean placental weight, mg | 0.63±0.02 | 0.56±0.02 | 0.02 |
| Total placental weight/litter | 9.1±0.4 | 8.3±0.6 | 0.35 |
| Area total placenta, mm2 | 11.8±0.2 | 9.1±0.2 | <0.0001 |
| Area trophospongium, mm2 | 2.6±0.1 | 2.1±0.1 | <0.0001 |
| Area labyrinth, mm2 | 9.1±0.1 | 6.9±0.1 | <0.0001 |
Values are presented as means ± SE. Bolded P values indicate significance at P < 0.05.
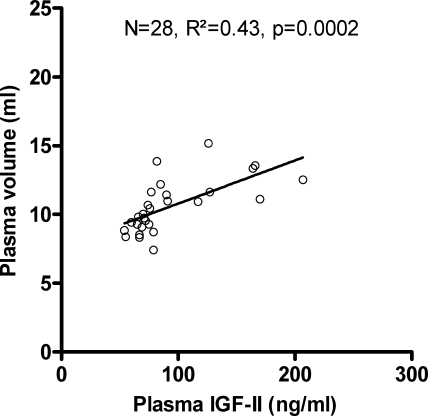
Because we found no differences in hemodynamic changes and IGF concentrations between the two diets, we performed regression analysis in the combined group. In nonpregnant rats (n = 16), no correlations were observed at the P ≤ 0.10 level between PV, maternal weight or maternal body weight, systolic blood pressure, heart rate, IGF-I, or IGF-II (data not shown). At term (n = 16), univariate analysis showed correlations between PV and IGF-II (R2 = 0.41; P = 0.01) and placental weight (R2 = 0.42, P = 0.01) but not between PV and maternal body weight, systolic blood pressure, heart rate, total fetal weight, litter size, or IGF-I (all P > 0.10).
Multivariate regression analysis in all animals of experiment 1 (n = 61) with PV as the dependent variable and maternal body weight, systolic blood pressure, heart rate, IGF-I, and IGF-II as the independent variables revealed that IGF-II was the only independent significant predictor of PV (P = 0.016, t ratio = 2.52, R2 of regression model = 0.44). All of the other variables had corrected P values > 0.10. Univariate regression analysis between PV and IGF-II in the entire cohort of experiment 1 is presented in Fig. 3.
Fig. 3.
Univariate regression analysis between plasma volume and plasma IGF II levels in the entire cohort of experiment 1.
Experiment 2
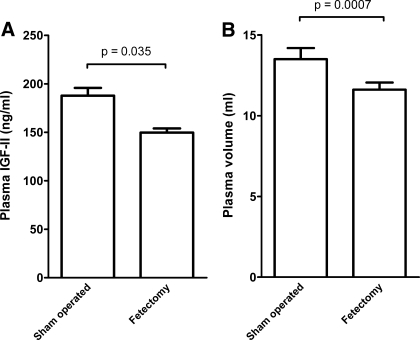
The surgical fetoplacental removal procedure reduced total fetal weight by 53% and total placental weight by 59% (Table 2). No additional fetal losses were observed following the intervention, and all dams carried to term. The mean weight of the surviving fetuses was unchanged compared with sham-operated animals, while the mean weight of the nonextracted placentas was decreased by 10%. Placental histomorphometry showed that trophospongium and labyrinth were proportionally reduced. Maternal PV and circulating IGF-II levels were diminished by 14% and 20%, respectively, compared with the sham-operated dams (Fig. 4). The decreased PV expansion in the fetectomy group was also reflected in a slightly higher hematocrit level. Maternal IGF-I levels were higher in dams that underwent fetoplacental mass reduction than in sham-operated controls (459.2 ± 69.4 ng/ml vs. 228.2 ± 35.7 ng/ml, respectively; P < 0.0001).
Table 2.
Characterististics of rats that underwent fetoplacental mass reduction vs. sham-operated rats (day 22 of gestation)
| Sham (n = 9) | Fetectomy (n = 8) | P Value | |
|---|---|---|---|
| Maternal weight, g | 463.5±6.9 | 377.8±10.6 | <0.0001 |
| Maternal body weight, g | 332.0±11.5 | 294.8±23.8 | 0.01 |
| Maternal hematocrit, % | 30.8±1.2 | 34.3±0.7 | 0.05 |
| Maternal plasma volume/body weight, ml/kg | 29.1±1.4 | 30.7±2.4 | 0.32 |
| Litter size, n | 13.2±0.8 | 6.0±0.7 | <0.0001 |
| Mean fetal weight, mg | 5.5±0.1 | 5.7±0.1 | 0.16 |
| Total fetal weight/litter, mg | 71.9±3.5 | 34.0±3.7 | <0.0001 |
| Mean placental weight, mg | 0.65±0.01 | 0.59±0.02 | 0.009 |
| Total placental weight/litter, mg | 8.6±0.5 | 3.5±0.4 | <0.0001 |
| Area total placenta, mm2 | 9.2±0.2 | 6.2±0.2 | <0.0001 |
| Area trophospongium, mm2 | 1.6±0.2 | 1.1±0.1 | <0.0001 |
| Area labyrinth, mm2 | 7.6±0.2 | 5.1±0.1 | <0.0001 |
Values are presented as means ± SE. Bolded P values indicate significance at P < 0.05.
Fig. 4.
Plasma IGF-II levels (A) and plasma volume (B) in dams that underwent partial fetoplacental reduction (n = 8) or a sham operation (n = 9).
Experiment 3
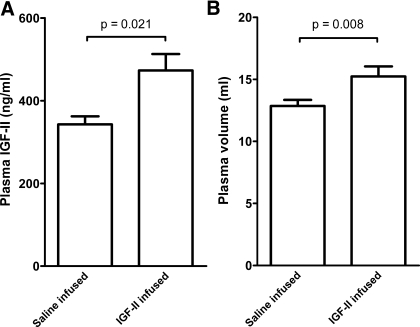
The constant IGF-II infusion raised circulating IGF-II levels by 38% (Fig. 5) but did not affect circulating IGF-I levels (336.9 ± 66.7 ng/ml in the saline group vs. 325.0 ± 43.3 ng/ml in the experimental group, P = 0.67). In IGF-II-infused dams, PV was increased by 19% (Fig. 5), while maternal weight and maternal body weight were unchanged (Table 3). While the mean placental weight was not different in saline- and IGF-II infused animals, histomorphometry showed that the total placental area and the area occupied by the trophospongium were increased by 6% and 29%, respectively, compared with saline. Fetal weight was unchanged, however.
Fig. 5.
Plasma IGF-II levels (A) and plasma volume (B) in dams that underwent IGF-II infusion (n = 8) or saline infusion (n = 9).
Table 3.
Characteristics of IGF-II-infused vs. saline-infused rats (day 22 of gestation)
| Saline (n = 9) | IGF-II (n = 8) | P Value | |
|---|---|---|---|
| Maternal weight, g | 467.6±12.7 | 467.6±15.7 | 1.00 |
| Maternal body weight, g | 331.2±8.7 | 321.7±9.3 | 0.59 |
| Maternal hematocrit, % | 31.3±0.7 | 31.8±0.8 | 0.70 |
| Maternal plasma volume/body weight, ml/kg | 27.9±1.0 | 31.5±0.8 | 0.01 |
| Litter size, n | 13.8±0.8 | 15.0±0.8 | 0.29 |
| Mean fetal weight, mg | 5.8±0.1 | 5.8±0.1 | 0.67 |
| Total fetal weight/litter, mg | 80.3±4.1 | 86.2±4.5 | 0.34 |
| Mean placental weight, mg | 0.62±0.01 | 0.59±0.02 | 0.14 |
| Total placental weight/litter, mg | 8.5±0.5 | 8.8±0.5 | 0.69 |
| Area total placenta, mm2 | 9.4±0.2 | 10.0±0.2 | 0.03 |
| Area trophospongium, mm2 | 1.7±0.1 | 2.2±0.1 | 0.0002 |
| Area labyrinth, mm2 | 7.6±0.2 | 7.8±0.2 | 0.48 |
Values are presented as means ± SE. Bolded P values indicate significance at P < 0.05.
Regression Analyses in the Combined Experiments
For these analyses, we combined all day 22 pregnant dams evaluated in the study together (i.e., rats on the standard and experimental diet; sham-operated rats, and rats with surgical fetoplacental mass reduction; saline- and IGF-II infused rats; n = 50). Univariate analysis showed that PV correlated with maternal body weight (R2 = 0.09, P = 0.046), maternal plasma IGF-II (R2 = 0.17, p =0.003), total fetal weight (R2 = 0.19, P = 0.002), and total placental weight (R2 = 0.19, P = 0.002). Using PV as the dependent variable, and maternal body weight, total fetal weight, total placental weight, and maternal circulating IGF-I and IGF-II as the independent variables in a multivariate regression model, we found that only maternal circulating IGF-II predicted PV independently (P = 0.04, t ratio = 2.08, R2 regression model = 0.33).
DISCUSSION
The physiological pathways regulating gestational PV expansion are poorly understood. The traditional hypothesis is that vasodilatation, provoked by endocrine stimuli such as estrogens and/or the appearance of the uteroplacental vascular bed in early gestation, creates a state of relative vascular underfilling, which activates compensatory fluid retention mediated by the renin-angiotensin-aldosterone system (31). According to this scenario, PV expansion should mainly occur during the early stages of pregnancy (9). In most studies, however, PV expansion is a gradual phenomenon, continuing up to 37 wk GA (30) and mirroring fetoplacental growth (3), which suggests that other factors must also be at play. Animal and human data suggest that PV expansion is the result of an interaction between maternal and fetoplacental factors. Indeed, the close resemblance in hemodynamic adaptation between pregnant and pseudopregnant rats suggests a role for the maternal compartment, while the higher PV expansion in human twin pregnancies compared with singleton pregnancies supports a role for the fetoplacental unit (23).
In the current study, we examined the role of maternal body weight, the fetoplacental mass, and the IGF axis in PV expansion in the rat. We consider the rat to be an appropriate animal model because humans and rats share a hemochorial type of placentation (41). Also, PV expansion is initiated during the first third of pregnancy and continues until term in both species (2, 30). Finally, the IGF-II gene is abundantly expressed in the human and rodent placenta (21), both in the labyrinthine (the exchange area) and the trophospongial (also called junctional or basal zone) area of the rat placenta (41). This prominent expression causes a surge in circulating IGF-II in the last week of rat gestation (Fig. 2), which was also documented in human (40) and rabbit (26) pregnancy. Maternal IGF-I physiology however, is different in humans and rats. In rat dams, we confirmed previous data that IGF-I concentrations drop considerably in the last week of gestation, which has been attributed to both reduced hepatic synthesis and accelerated clearance (13). As expected, this decrease is less pronounced in rats with surgical removal of half of their gestational sacs from day 16. In humans, IGF-I concentrations may be somewhat reduced during early pregnancy, but there is a definite increase in the second half as a result of the exponential secretion of a placental variant of growth hormone (39).
Although the IGFs are small peptides (molecular weight: 7.5 kDa) that do not cross the placental barrier (13), the IGF production by the placental interface may affect both fetal and maternal physiology. On the fetal side, placental-specific deletion of the Igf2 gene in mice impairs placental growth, transplacental nutrient transport, and ultimately fetal growth (12). Regarding maternal physiology, we show for the first time a strong correlation between circulating IGF-II and PV during rat pregnancy (Fig. 3), which persists in multiple regression analyses. Surgical fetoplacental mass reduction predictably decreases maternal IGF-II concentrations, and also lowers PV at term (Fig. 4). We designed a further experiment in which exogenous IGF-II was infused from day 16 and documented that this constant infusion, which raises circulating IGF-II by 38%, increases PV at term, also when expressed per body weight (Fig. 5, Table 3). Taken together, the data indicate that the gestational IGF-II surge is involved in PV expansion.
Rosso and colleagues (28, 29) were the first to demonstrate a relationship between maternal PV and fetal growth in the rat. Food-restricted gravid rats showed decreased maternal PV and cardiac output together with smaller fetuses and placentas. The investigators suggested that fetoplacental growth restriction might be the result of impaired maternal hemodynamic adaptation. However, the current data would support another interpretation: any constraint on fetoplacental growth, imposed by an adverse maternal or placental environment, reduces placental IGF-II production and is accompanied by a blunted maternal PV expansion.
The IGFs, and IGF-I, in particular, are known to influence cardiovascular structure and function through activation of the type-1 IGF receptor. Liver-specific deletion of the Igf1 gene in mice results in an increased peripheral vascular resistance and blood pressure through increased endothelin-1 production (36). In rats, acute systemic administration of IGF-I produces a vasodilatory and blood pressure-lowering effect (7). IGF-I also increases blood flow in humans (27). The cardiovascular effects of IGF-II are not well documented to date, yet there is some evidence that IGF-II upregulates vascular endothelial growth factor expression and angiogenesis (8). Moreover, similar to IGF-I, IGF-II activates the type-1 IGF receptor in different tissues, including aortic smooth muscle cells (11). Hence, it is biologically plausible that IGF-II has cardiovascular effects comparable to those of IGF-I.
In the current study, we measured blood pressure noninvasively in awake, relaxed animals after multiple preliminary measurements. The procedure precluded blood pressure measurements in animals that were recovering from surgery (experiments 2 and 3). These animals were left undisturbed until term, and PV and circulating IGFs were considered to be the primary outcome variables.
Data from human volunteers show a correlation between body weight and PV, both in the nonpregnant (4) and pregnant state (19). Here, we explored the effect of maternal body weight in a dietary experiment of enhanced pregestational weight gain. Despite the increase in body weight provoked by the high-energy diet, there was no change in PV at any stage of pregnancy compared with animals on the standard diet (Fig. 1), confirming findings from a recent study in rats (1). In addition, maternal BW (corrected for fetoplacental mass and blood volume) was no correlate of PV in any of the regression analyses. A caveat is that our obesogenic diet increases fat mass but not lean mass (22), so that we cannot exclude that skeletal muscle mass is related to PV and the gestational PV expansion. Indeed, skeletal muscles are highly vascularized, and IGF-II plays a role in skeletal muscle regeneration (25).
In contrast to what is observed in obese women (20, 35), but in line with previous findings in murine models of obesity (1, 22, 34), fetal weight was not increased in overweight rats, and placental weight was even decreased. One explanation for this finding is that the increased transplacental transport documented in overfed rodents (24) may fulfill fetal requirements with smaller placental exchange areas.
The placenta is not only an important source of IGF-II but also a target tissue for IGF-II (15, 39). IGF-II infusion at midgestation in guinea pigs was found to increase the labyrinthine portion of the placenta (33). However, in the present study, IGF-II infusion during the last 5 days of pregnancy in rats mainly increased the trophospongial area. The reasons for this discrepancy need further study. Nonetheless, the data suggest that the effect of IGF-II on circulatory variables may be indirect, i.e., IGF-II may increase the availability of (yet to be characterized) vasoactive substances through enhanced growth of the trophospongium, which is the production site of many hormones and peptides.
While supraphysiological IGF-II concentrations did not improve fetal weight (Table 3), the effects could well be different in the setting of fetal growth restriction. Further research extending the current proof-of-concept study should examine the effect of IGF-II administration in animal models of fetal growth restriction. Indeed, preliminary experience in human pregnancies shows that circulatory expansion improves the hemodynamics of growth-restricted fetuses (37).
Perspectives and Significance
Clinicians are well aware of the relationship between the lack of maternal hemodilution during pregnancy, caused by subnormal PV expansion, and fetal growth restriction. Our data suggest that both maternal hemodynamics and fetal growth depend on the functional placental mass; in contrast, we have no evidence that maternal obesity influences PV expansion in rats. IGF-II, very likely of placental origin, emerges from our experiments as a regulator of the PV increment during pregnancy. Future studies should assess to what extent this is explained by a direct endocrine-type effect on vascular function, or an indirect stimulatory effect on placental growth and secretion of yet to be characterized vasoactive substances. Moreover, the effects of IGF-II administration should be assessed in pregnancies complicated by intrauterine growth restriction, in which PV expansion and placental growth are suboptimal.
GRANTS
This work was supported by grants G.0221.03 and G.0285.07 from the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (Belgium) and by a grant of the European Commission within its 6th Framework programme (EuroSTEC LSHC-CT-2006-037409). J. Deprest is the recipient of a “Fundamental Clinical Researcher” grant of the Fonds Wetenschappelijk Onderzoek-Vlaanderen (1.8.012.07.N.02).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank W. Coopmans and F. Vanderhoydonck for their assistance with the radio-immunoassays and L. Vercruysse for her help with the image analysis software.
REFERENCES
- 1.Akyol A, Langley-Evans SC, McMullen S. Obesity induced by cafeteria feeding and pregnancy outcome in the rat. Br J Nutr 22: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Barron WM. Volume homeostasis during pregnancy in the rat. Am J Kidney Dis 9: 296–302, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Bleker OP, Buimer M, van der Post JAM, van der Veen F. Ted (GJ) Kloosterman: on intrauterine growth. The significance of prenatal care studies on birth weight, placental weight, and placental index. Placenta 27: 1052–1054, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol Renal Fluid Electrolyte Physiol 247: F632–F636, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism 56: 1431–1438, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Campbell DM, MacGillivray I. Comparison of maternal response in first and second pregnancies in relation to baby weight. J Obstet Gynaecol Br Commonw 79: 684–693, 1972 [DOI] [PubMed] [Google Scholar]
- 7.Cao N, Lau S, Nguyen TT, White PJ. Characterization of the acute cardiovascular effects of intravenously administered insulin-like growth factor-I in conscious Sprague-Dawley rats. Clin Exp Pharmacol Physiol 33: 1190–1195, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chao D, D'Amore WA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Fact Rev 19: 111–120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54: 2056–2063, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol 112: 440–450, 1972 [DOI] [PubMed] [Google Scholar]
- 11.Chisalita S, Johansson G, Liefvendahl E, Bäck K, Arnqvist H. Human aortic smooth muscle cells are insulin resistant at the receptor level but sensitive to IGF-I and IGF-II. J Mol Endocrinol 2009[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417: 945–948, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Davenport ML, Clemmons DR, Miles MV, Camacho-Hubner C, D'Ercole AJ, Underwood LE. Regulation of serum insulin-like growth factor-I (IGF-I), and IGF binding proteins during rat pregnancy. Endocrinology 127: 1278–1286, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Foldager N, Blomqvist CG. Repeated plasma volume determination with the Evans Blue dye dilution technique: the method and a computer program. Comput Biol Med 21: 35–41, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Forbes K, Westwood M. The IGF axis and placental function: a mini review. Horm Res 69: 129–137, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta 24: 803–812, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. Placental efficiency and adaptation: endocrine regulation. J Physiol 57: 3459–3472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garn SM, Pesick SD. Relationship between various maternal body mass measures and size of the newborn. Am J Clin Nutr 36: 664–668, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Gibson HM. Plasma volume expansion and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. J Obstet Gynaecol Br Commonw 80: 1067–1074, 1973 [DOI] [PubMed] [Google Scholar]
- 20.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev 9: 140–50, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Han VK, Carter AM. Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta 21: 289–305, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Holemans K, Caluwaerts S, Poston L, Van Assche FA. Diet-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol 190: 858–865, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol 14: 601–612, 1985 [PubMed] [Google Scholar]
- 24.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23: 271–278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levinovitz A, Jennische E, Oldfors A, Edwall D, Norstedt G. Activation of insulin-like growth factor II expression during skeletal muscle regeneration in the rat: correlation with myotube formation. Mol Endocrinol 6: 1227–1234, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Nason KS, Binder ND, Labarta JI, Rosenfeld RG, Gargosky SE. IGF-II and IGF-binding proteins increase dramatically during rabbit pregnancy. J Endocrinol 148: 121–30, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Pendergrass M, Fazioni E, Collins D, DeFronzo RA. IGF-I increases forearm blood flow without increasing forearm glucose uptake. Am J Physiol Endocrinol Metab 275: E345–E350, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Rosso P, Kava R. Effects of food restriction on cardiac output and blood flow to the uterus and placenta in the pregnant rat. J Nutr 110: 2350–2354, 1980 [DOI] [PubMed] [Google Scholar]
- 29.Rosso P, Streeter MR. Effects of food or protein restriction on plasma volume expansion in pregnant rats. J Nutr 109: 1887–1892, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Salas SP, Marshall G, Gutiérrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 47: 203–208, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Schrier RW, Howard RL. A unifying hypothesis of sodium and water regulation in health and disease. Hypertension 18: 164–168, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Schröder HJ. Models of fetal growth restriction. Eur J Obstet Gynecol 110: S29–S39, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Sferruzzi-Perri AN, Owens JA, Pringle KG, Robinson JS, Roberts CT. Maternal insulin-like growth factors-I and -II act via different pathways to promote fetal growth. Endocrinology 147: 3344–3355, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294: R528–R538, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Swanson LD, Bewtra C. Increase in normal placental weights related to increase in maternal body mass index. J Matern Fetal Neonatal Med 21: 111–113, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Tivesten Å, Bollano E, Andersson I, Fitzgerald S, Caidahl K, Sjögren K, Skøtt O, Liu JL, Mobini R, Isaksson OG, Jansson JO, Ohlsson C, Bergström G, Isgaard J. Liver-derived insulin-like growth factor-I is involved in the regulation of blood pressure in mice. Endocrinology 143: 4235–4242, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Valensise H, Vasapollo B, Novelli GP, Giorgi G, Verallo P, Galante A, Arduini D. Maternal and fetal hemodynamic effects induced by nitric oxide donors and plasma volume expansion in pregnancies with gestational hypertension complicated by intrauterine growth restriction with absent end-diastolic flow in the umbilical artery. Ultrasound Obstet Gynecol 31: 55–64, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Verhaeghe J, Van Bree R, Van Herck E, Laureys J, Bouillon R, Van Assche FA. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in umbilical cord serum: correlations with birth weight. Am J Obstet Gynecol 169: 89–97, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Verhaeghe J. Does the physiological acromegaly of pregnancy benefit the fetus? Gynecol Obstet Invest 66: 217–226, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wilson DM, Bennett A, Adamson GD, Nagashima RJ, Liu F, DeNatale ML, Hintz RL, Rosenfeld RG. Somatomedins in pregnancy: a cross-sectional study of insulin-like growth factors I and II and somatomedin peptide content in normal human pregnancies. J Clin Endocrinol Metab 55: 858–861, 1982 [DOI] [PubMed] [Google Scholar]
- 41.Zhou J, Bondy C. Insulin-like growth factor-II and its binding proteins in placental development. Endocrinology 131: 1230–1240, 1992 [DOI] [PubMed] [Google Scholar]