Abstract
The aims of this study were to determine optimal pacing parameters of electrical stimulation on different gut segments and to investigate effects and possible mechanisms of gastrointestinal electrical stimulation on gut slow waves. Twelve female hound-mix dogs were used in this study. A total of six pairs of electrodes were implanted on the stomach, duodenum, and ascending colon. Bilateral truncal vagotomy was performed in six of the dogs. One experiment was designed to study the effects of the pacing frequency on the entrainment of gut slow waves. Another experiment was designed to study the modulatory effects of the vagal and sympathetic pathways on gastrointestinal pacing. The frequency of slow waves was 4.88 ± 0.23 cpm (range, 4–6 cpm) in the stomach and 19.68 ± 0.31 cpm (range, 18–22 cpm) in the duodenum. There were no consistent or dominant frequencies of the slow waves in the colon. The optimal parameters to entrain slow waves were: frequency of 1.1 intrinsic frequency (IF; 10% higher than IF) and pulse width of 150–450 ms (mean, 320.0 ± 85.4 ms) for the stomach, and 1.1 IF and 10–20 ms for the small intestine. Electrical stimulation was not able to alter colon slow waves. The maximum entrainable frequency was 1.27 IF in the stomach and 1.21 IF in the duodenum. Gastrointestinal pacing was not blocked by vagotomy nor the application of an α- or β-adrenergic receptor antagonist; whereas the induction of gastric dysrhythmia with electrical stimulation was completely blocked by the application of the α- or β-adrenergic receptor antagonist. Gastrointestinal pacing is achievable in the stomach and small intestine but not the colon, and the maximal entrainable frequency of the gastric and small intestinal slow waves is about 20% higher than the IF. The entrainment of slow waves with gastrointestinal pacing is not modulated by the vagal or sympathetic pathways, suggesting a purely peripheral or muscle effect.
Keywords: gastrointestinal motility, slow wave dysrhythmia, sympathetic modulation, vagal nerve modulation
along the gut are rhythmic myoelectrical activities called slow waves (8). The function of slow waves is to determine the frequency and propagation of contractions of the gut. Uncoupled or dysrhythmic gastrointestinal slow waves lead to a lack of coordinated contractions or peristalsis, and are associated with various gastrointestinal motility disorders (2). Slow wave activity in the gut arises from the defined pacemaker area in each region. In the stomach these pacemakers function as a unit, and the pacemaker cells along the greater curvature of the corpus usually provide the dominant frequency because these cells pace at the most rapid frequency (11, 24). The small intestine has intrinsic pacemaker activity from the duodenum to the terminal ileum, and there is also a proximal-to-distal pacemaker frequency gradient in this organ (7). However, there is no consensus regarding slow waves in the colon, such as origin, frequency, and propagation (3, 9, 25, 16). Recently, it has been proposed that gastrointestinal slow waves are generated by interstitial cells of Cajal (10, 18).
Gastrointestinal electrical stimulation or pacing has been advocated as a possible treatment for gastrointestinal motor disorder (11, 33). A number of previous studies reported that the gastric and small bowel electrical stimulation or pacing could not only entrain (control) normal intrinsic slow waves but also suppress ectopic pacemakers and restore normal slow waves (16). Generally, long pulses are needed for electrical stimulation to pace or entrain slow waves, which is important when electrical stimulation is applied in patients with gastroparesis (14, 16). While a number of studies have reported the entrainment of slow waves with gastrointestinal electrical stimulation (11), there is a lack of systematic study investigating the optimal parameters of electrical stimulation of the entire gut and possible modulatory effects of the vagal and sympathetic pathways.
Recently, electrical stimulation has also been proposed for treating obesity (4, 5). It is hypothesized that gastric electrical stimulation at a frequency higher than the normal slow waves may induce gastric dysrhythmia, delay gastric emptying, and thereby reduce food intake (33, 34). However, there is a lack of systematic studies investigating the induction of slow wave dysrhythmias along the gut with electrical stimulation at a frequency higher than the normal slow waves.
While electrical stimulation has been proposed for treating motility disorders as well as obesity, little information is available on the optimization of stimulation parameters. Due to the invasive nature of electrical stimulation, systematic and comprehensive studies are not realistic in humans, and therefore a canine study is needed.
The aims of this study were 1) to systematically investigate the effects of stimulation parameters on the entrainment of slow waves and the induction of slow wave dysrhythmias along the gut, and 2) to investigate possible modulatory effects of vagal and sympathetic pathways involved in gastrointestinal electrical stimulation.
MATERIALS AND METHODS
Animal Preparation
Twelve female hound-mix dogs (18–22 kg) were used in the study. After an overnight fast, each dog was operated on under anesthesia. Anesthesia was induced with intravenous Pentothal (sodium thiopental, 11 mg/kg; Abbott Laboratories, North Chicago, IL) and maintained on 2–4% IsoFlo (Abbott Laboratories) in oxygen (1 l/min) carrier gases delivered from a ventilator after endotracheal intubation. Midline laparotomy was performed. Two pairs of 28-gauge stainless steel cardiac pacing wires (A&E Medical, Farmingdale, NJ) were implanted on the serosal surface of the stomach along the greater curvature 2 cm and 14 cm above the pylorus. Another two pairs of electrodes were implanted on the serosal surface of the duodenum 5 cm and 10 cm below the pylorus, respectively. The last two pairs of electrodes were implanted on the serosal surface of the ascending colon 10 cm and 20 cm below the ileum-cecum junction, respectively. The proximal pair of electrodes in each organ was used for electrical stimulation, and the distal pair was for recording gastric slow waves.
In addition to implantation of electrodes, bilateral truncal vagotomy was performed at the level of the diaphragmatic hiatus in six of the 12 dogs (19). A segment (1 cm) of ventral and dorsal trunks of the vagus innervating the stomach was excised to prevent regeneration of these nerves. The branches to the stomach were also severed to ensure that all of these vagal nerves innervating the stomach were denervated.
Experiments were performed after the dogs were fully recovered from the surgical procedure (2 wk later). The experimental protocols were approved by the Institutional Animal Care and Use Committee at the Veterans Affairs Medical Center, Oklahoma City, OK and at the University of Texas Medical Branch, Galveston, TX.
Experimental Protocols
Two series of experiments were performed 1) to investigate the effects of electrical stimulation of the stomach, small intestine, and colon on slow waves; and 2) to investigate possible modulatory effects of the vagal and sympathetic nerves involved in the effects of electrical stimulation on slow waves.
Experiment 1: effects of electrical stimulation on slow waves.
The aims of this experiment were 1) to record and characterize slow waves in the stomach, small intestine, and colon; 2) to determine optimal stimulation parameters for gastric electrical stimulation (GES), duodenal electrical stimulation (DES), and colonic electrical stimulation (CES) to entrain the slow waves of the corresponding organ; 3) to determine the maximum driven frequency of the slow waves, defined as the maximum pacing frequency at which the intrinsic slow wave could be paced or entrained; 4) the effects of electrical stimulation at abnormally high frequencies on the impairment of normal slow waves or the induction of slow wave dysrhythmia.
The experiment was performed in three randomized sessions (GES, DES, and CES) with an interval of at least 3 days between two consecutive sessions. In each session, the proximal pair of the electrodes placed in the stomach (for GES), small intestine (for DES), or colon (for CES) was used for electrical stimulation, while the distal pairs of electrodes were used for recording slow waves. The recordings were made throughout the session. The protocol of each session included three periods: 1) a 20-min baseline recording without electrical stimulation; 2) a period of electrical stimulation of the stomach, small intestine, or colon with different parameters for the entrainment of slow waves; different values for two parameters (pulse width and frequency) were tested in this period, while the pulse amplitude was fixed; 3) a period of electrical stimulation at frequencies higher than the normal slow wave frequency for the impairment of normal slow waves. During this period, electrical stimulation was performed at various frequencies higher than the normal frequency of slow waves, while the pulse width and pulse amplitude were fixed. During periods 2 and 3, each set of GES, DES, or CES parameters was tested for 5–10 min, and sufficient time was given for the slow wave to recover before another set of parameters was tested.
Electrical stimulation was performed using an adjustable electrical stimulator (model A310; World Precision Instruments, Sarasota, FL). Repetitive long pulses were used for electrical stimulation with three parameters: pulse amplitude, pulse width, and pulse frequency. For each test, only one of the three parameters was altered. The pulse amplitude was fixed at 4 mA. The pulse widths tested were 100–500 ms for GES and 10–30 ms for DES. For CES, all values of pulse width used for GES and DES were tested. The stimulation frequency tested included 1.1 intrinsic frequency (IF; 10% higher than IF), 1.2 IF, 1.30 IF, 1.38 IF, 1.52 IF for DES (13), and 1.1 IF, 1.38 IF, 2 IF, 3 IF, and 4 IF for GES (14).
Experiment 2: modulatory effects of vagal and sympathetic nerves on electrical stimulation.
Mechanisms involved in the effects of gastrointestinal electrical stimulation were unknown, and we hypothesized that 1) electrical pacing (the entrainment of slow waves by electrical stimulation to a slightly higher frequency but within the normal range) was modulated by the vagal pathway because the entrainment of slow wave is within the normal physiological frequency range and the vagal pathway may be involved in this physiological modulation; and 2) the impairment of slow waves or the induction of slow wave dysrhythmia with electrical stimulation at frequencies higher than the normal frequency of slow waves was modulated by the sympathetic pathway because slow wave dysrhythmia is pathophysiological and the sympathetic pathway may be involved in this pathophysiological modulation. In experiment 1, CES was found incapable of altering slow waves in the colon, and therefore CES was not performed in experiment 2.
This experiment was designed to test the above hypotheses. To test the involvement of the sympathetic pathway, GES and DES were performed in three more sessions in the same normal dogs: in one session, an α-adrenergic receptor antagonist, phentolamine (1 mg/kg bolus + 1 mg·kg−1·h−1 infusion for 60 min) was given; in another session, a β-adrenergic receptor antagonist, propranolol (1 mg/kg + 1 mg·kg−1·h−1 for 60 min) was given; and in the third session, normal saline as control was given for 60 min at the rate of 60 ml/h. GES or DES was given for 20 min each in a randomized order after the initiation of the infusion. GES and DES were performed using stimulation frequencies of 1.1 IF and 1.38 IF. The pulse width was 100 ms to 500 ms for GES, and 10–30 ms for DES. The pulse amplitude was fixed at 4 mA.
To test the modulatory effects of the vagal pathway, GES/DES was performed for 20 min at a stimulation frequency of 1.1 IF and 1.38 IF in six vagotomized dogs, and the entrainment of slow waves was assessed. The pulse width was 100 to 500 ms for GES and 10–30 ms for DES. The pulse amplitude was fixed at 4 mA.
Measurement and analysis of gastrointestinal slow waves.
A multichannel recorder (Acqknowledge EOG 100A; Biopac Systems, Santa Barbara, CA) was used to record slow waves from the stomach, small intestine, and colon throughout the study (27). The signals were displayed on a computer monitor and saved to the computer's hard disk. Low and high cutoff frequencies of the amplifiers were 0.05 Hz and 35 Hz, respectively. Signals were initially sampled at a frequency of 100 Hz and then down-sampled at 2 Hz after low-pass filtering with a cut off frequency of 1 Hz.
Spectral analysis was performed to derive the following parameters related to slow waves.
DOMINANT FREQUENCY AND POWER OF THE SLOW WAVE.
The frequency at which the power spectrum of a specific recording had a peak power was defined as the dominant frequency. The power at the dominant frequency in the power spectrum was defined as the dominant power of the slow wave. These two parameters were calculated by using the smooth power spectral analysis method. Decibel units were used to represent the power of the slow wave.
PERCENTAGE OF NORMAL SLOW WAVES.
The percentage of normal slow waves was defined as the percentage of time during which regular slow waves were present over the entire recording period. The normal gastric slow wave range was defined as 4–6 cpm (27). The normal duodenal slow wave range was defined as 18–22 cpm (13). Both were computed by using the adaptive spectral analysis method (2). In this method, each recording was divided into blocks of 1 min without overlapping. The power spectrum of each 1-min recording was calculated and examined to see whether the peak power was within the range of 4–6 cpm for gastric slow wave or 18–22 cpm for duodenal slow waves. The 1-min recording was categorized as normal if the peak power was within the 4–6 or 18–22 cpm range; otherwise it was categorized as dysrhythmia.
PERCENTAGE OF SLOW WAVE ENTRAINMENT.
Complete entrainment was defined as follows: the frequency of the gut slow wave was the same as the pacing frequency and was phase-locked with the pacing stimulus. The entrainment was identified if the interval between the appearance of a slow wave peak and a superimposed stimulation artifact in one cycle of the slow wave was exactly the same as that in the next cycles. The percentage of entrainment of gastrointestinal slow waves was defined as the ratio of the difference between the recorded slow wave frequency during pacing (f) and the IF before pacing (fi) and the difference between the pacing frequency (fp) and the fi. It is expressed as %entrainment = (f−fi)/(fp−fi) (13).
Statistical Analysis
All data are expressed as means ± SE. ANOVA was used to compare the data among three or more different periods, and the Student's t-test was used to assess the effect of stimulation compared with the baseline. P < 0.05 was considered statistically significant.
RESULTS
Slow Waves in Different Organs Along the Gut
The dominant frequency (in cpm) and dominant power (dB) of slow waves were 4.88 ± 0.23 cpm and −5.40 ± 0.80 dB in the stomach (Fig. 1) and 19.68 ± 0.31 cpm and −4.60 ± 1.60 dB in the duodenum, respectively (Fig. 2). The colon slow waves showed high and low frequencies, alternatively; the low frequency was in a range of ∼3–6 cpm, and the high frequency was in a range of 16–20 cpm (Fig. 3). Sometimes, the low and high frequency slow waves were present simultaneously. There were no consistent dominant frequencies in the colon slow waves in any of the animals. The slow waves in the stomach and small intestine, however, showed one single and consistent dominant peak in the power spectrum.
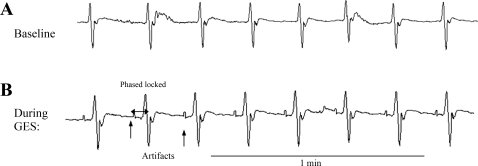
Fig. 1.
Gastric slow waves at baseline and during gastric electrical stimulation (GES). A: baseline gastric slow waves. Regular slow waves are noted. B: completely entrained gastric slow waves, which were identified as the slow waves were phase-locked with the stimuli. The stimulation parameters were: pacing frequency of 1.1 intrinsic frequency (IF), pulse amplitude of 4 mA, and pulse width of 350 ms.
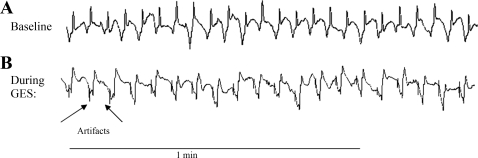
Fig. 2.
Duodenal slow waves at baseline and during duodenal electrical stimulation (DES). A: baseline duodenal slow waves. B: completely entrained duodenal slow waves, where pacing pulse amplitude was 4 mA, pacing pulse width was 15 ms, and pacing frequency was 1.1 IF. Entrainment was identified because each of the slow waves was phase-locked with each stimulus.
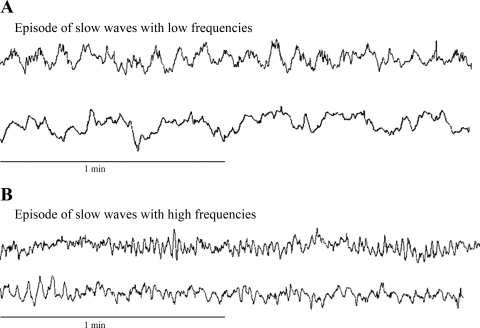
Fig. 3.
Colonic slow waves at baseline. Colonic slow waves included multiple frequencies. A: slow waves at a low frequency band of 3–6 cpm. B: slow waves at a high frequency band of 16–20 cpm. Slow waves were recorded at different time points.
Optimal Stimulation Parameters for the Stomach, Duodenum, and Colon
For gastric electrical stimulation, the optimal parameters to completely entrain gastric slow waves were as follows: pulse width of 150–450 ms (mean: 320.0 ± 85.4 ms) and pacing frequency of 1.1 IF. With these parameters, a 100 ± 0% entrainment of the gastric slow waves was achieved in all dogs (Fig. 1).
For duodenal electrical stimulation, the optimal parameters to completely entrain intestinal slow waves were as follows: pulse width of 10–20 ms (mean: 15.2 ± 2.8 ms, P < 0.0001, vs. the corresponding value for the stomach) and pacing frequency of 1.1 IF. With these parameters, DES resulted in 100 ± 0% entrainment of the duodenal slow waves in these dogs (Fig. 2). The pulse width required to entrain the duodenal slow waves was only ∼4.28 ± 1.8% of that required for GES to entrain gastric slow waves.
Colon electrical stimulation was unable to entrain or pace colon slow waves (Fig. 4). CES was performed at pulse widths from 100 ms to 500 ms or until the maximum tolerance of the dogs and stimulation frequencies were in a high range of 18–20 cpm and a low range of 4–6 cpm. No colon slow waves were entrained with any of these parameters.
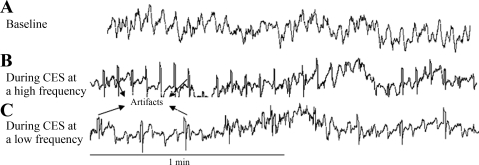
Fig. 4.
Colonic electrical stimulation (CES) was not able to entrain colonic slow waves. A: baseline slow waves in a dog. B: slow waves in the dog during CES at a high frequency. No entrainment was noted as the slow waves were not phase-locked with stimuli. C: slow waves in the dog during CES at a low frequency.
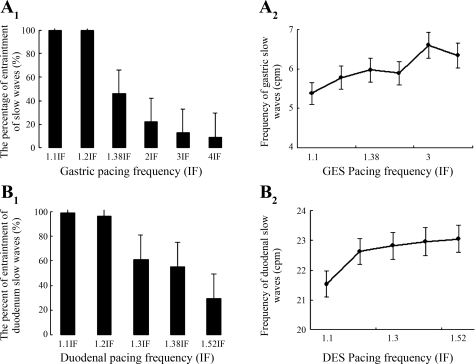
While the gastric and duodenal slow waves could be entrained to a higher frequency, there existed a maximum driven frequency with the slow waves in the stomach and small intestine. The maximal driven frequency was 1.27 ± 0.08 IF in the stomach and 1.21 ± 0.03 IF in the duodenum (P > 0.05, stomach vs. small intestine). The entrainment percentage at the maximum driven frequency was 44.0 ± 3.9% in the stomach and 65.0 ± 1.5% in the duodenum. The effects of the pacing frequency on the gastric and duodenal slow wave frequencies are shown in Fig. 5. The percentage of entrainment decreased as a function of the pacing frequency (Fig. 5), i.e., beyond the pacing frequency of 1.1 IF the percentage of slow wave entrainment was decreased as the pacing or stimulation frequency increased.
Fig. 5.
Effects of GES and DES on slow waves in the stomach and duodenum. A1: %entrainment of gastric slow waves during GES at different frequencies. B1: %entrainment of duodenal slow waves during DES at different frequencies. A2 and B2: frequencies of gastric/duodenal slow waves during GES/DES at different frequencies. %Entrainment was reduced when stimulation was performed at a higher frequency and the frequency of the gastric/duodenal slow waves that could be paced by the electrical stimulation was limited and lower than the pacing frequency.
Induction of Slow Wave Dysrhythmia with Gastric/Duodenal Electrical Stimulation
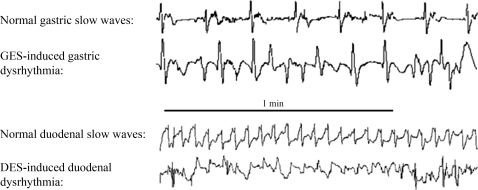
Gastric/duodenal electrical stimulation at a frequency higher than the normal slow wave frequency was able to induce slow wave dysrhythmias in both stomach and small intestine. With the fixed amplitude of 4 mA and pulse width that was about to completely entrain slow waves, GES at a frequency of 1.38–4.00 IF was able to induce dysrhythmia in the stomach. The percentage of normal gastric slow waves was decreased from 98 ± 2% at the baseline to 50 ± 8% with GES at 1.38 IF, 44 ± 5% at 2 IF, 33 ± 6% at 3 IF, and 38 ± 5% at 4 IF (P < 0.05 vs. baseline for all stimulation frequencies) (Fig. 6). No significant difference was noted in the percentage of normal gastric slow waves during GES at these different frequencies, indicating that the induction of slow wave dysrhythmia is not stimulation frequency dependent as long as GES was delivered at a frequency higher than the normal slow wave frequency. The major dysrhythmia was mainly tachygastria and arrhythmia.
Fig. 6.
Effects of electrical stimulation at a higher frequency on slow waves in a dog. Top: regular gastric slow waves at baseline and tachyarrhythmia during GES at a higher frequency. Bottom: regular duodenal slow waves at baseline and bradyarrhythmia during DES at a higher frequency.
Similarly, DES at a frequency of 1.38–1.58 IF was also found to induce dysrhythmia in the duodenum. The percentage of normal duodenum slow waves was decreased from 96 ± 2% at the baseline to 65 ± 5% with DES at 1.2 IF, 67 ± 6% with DES at 1.3 IF, 61 ± 8% with DES at 1.38 IF, and 68 ± 10% with DES at 1.58 IF (P < 0.05 vs. baseline for all stimulation frequencies). Stimulation pulses were fixed at the amplitude of 4 mA and pulse width at the value that induced 100% entrainment (Fig. 6). No significant difference was noted in the percentage of normal duodenal slow waves during DES at these different frequencies.
Modulatory Effects of Sympathetic and Vagal Nerves on Electrical Stimulation
To test possible modulatory effects of the vagal and sympathetic pathways on electrical stimulation, we repeated GES and DES experiments in vagotomized dogs and also in normal dogs after the administration of α- or β-adrenergic receptor antagonists. As shown in Table 1, in the vagotomized dogs, GES and DES, at the frequency of 1.1 IF, were still able to entrain the gastric and intestinal slow waves as the frequency of the gastric and intestinal slow waves were increased proportionally during electrical stimulation, suggesting that the entrainment or pacing does not involve the vagal pathway. In addition, it can also be seen from Table 1 that the application of an α- or β-adrenergic receptor antagonist did not block the entrainment effects of GES or DES on gastric/intestinal slow waves; the increase in slow wave frequency in these sessions was similar to that in the control session.
Table 1.
Vagal and sympathetic mechanisms involved in the effects of gastric/duodenal electrical stimulation on slow waves in dogs
| Dominant Frequency, cpm |
Dominant Power, dB |
%Normal Slow Waves |
||||
|---|---|---|---|---|---|---|
| Stomach | Duodenum | Stomach | Duodenum | Stomach | Duodenum | |
| Normal Dogs, Control Session | ||||||
| Baseline | 4.88±0.23 | 19.68±0.31 | −5.40±0.80 | −4.60±1.60 | 98±2 | 96±2 |
| Saline | 4.95±0.15 | 19.66±0.23 | −5.81±1.20 | −3.55±2.0 | 97±1 | 99±1 |
| ES at 1.1 IF | 5.38±0.11 | 21.56±0.20 | −5.61±0.55 | −4.36±1.23 | 95±3 | 92±6 |
| ES at high frequency | 50±8 | 61±8 | ||||
| β-Adrenoceptor Antagonist in Normal Dogs | ||||||
| Baseline | 4.89±0.21 | 19.55±0.20 | −5.42±0.23 | −4.90±0.19 | 98±2 | 96±3 |
| Propranolol | 5.04±0.29 | 19.35±0.18 | −5.91±1.20 | −3.65±2.0 | 96±3 | 97±2 |
| ES at 1.1 IF | 5.50±0.12 | 21.60±0.16 | −5.51±0.55 | −4.36±1.23 | 91±6 | 98±1 |
| ES at high frequency | 6.00±0.12 | 20.90±0.15 | −5.61±0.55 | −5.36±1.23 | 89±4 | 90±2 |
| α-Adrenoceptor Antagonist in Normal Dogs | ||||||
| Baseline | 4.89±0.21 | 19.35±0.20 | −5.52±0.23 | −4.90±0.19 | 98±1 | 96±3 |
| Phentolamine | 5.09±0.29 | 19.35±0.18 | −5.81±1.20 | −3.65±2.0 | 96±3 | 97±2 |
| ES at 1.1 IF | 5.50±0.22 | 21.50±0.16 | −5.51±0.55 | −4.36±1.23 | 93±6 | 98±1 |
| ES at high frequency | 5.80±0.20 | 21.60±0.11 | −6.51±0.55 | −5.36±1.23 | 92±5 | 95±3 |
| Vagotomized Dogs | ||||||
| Baseline | 5.01±0.21 | 19.06±0.18 | −5.91±1.20 | −3.75±2.0 | 90±4 | 91±2 |
| ES at 1.1 IF | 5.51±0.18 | 20.98±0.30 | −5.51±0.45 | −3.86±1.23 | 89±6 | 90±1 |
| ES at high frequency | 51±6 | 60±5 | ||||
ES, electrical stimulation of the stomach or small intestine; IF, intrinsic frequency; ES at high frequency, gastric electrical stimulation, or intestinal electrical stimulation at 1.38 IF.
However, the induction of gastric dysrhythmia (or a reduction in the percentage of normal slow waves) with GES or DES at a high frequency was completely blocked by the application of the α- or β-adrenergic receptor antagonist. As shown in Table 1, in the normal dogs with the application of saline, GES, or DES at the frequency of 1.38 IF substantially reduced the percentage of normal slow waves; this effect was, however, completely blocked in the session with the α- or β-adrenergic receptor antagonist. These data suggested that the effect of GES at a high frequency on the induction of slow wave dysrhythmia was modulated by the sympathetic pathway.
Cross-Organ Effects of Electrical Stimulation
Electrical stimulation on the stomach showed no effects on the slow waves in the small intestine or in the colon. The electrical stimulation on the small intestine, however, altered slow waves in the stomach (Fig. 7) but not in the colon; electrical stimulation on the colon had no effects on slow waves in the small intestine or the stomach. The mean frequencies of gastric slow waves were 4.90 ± 0.29 cpm with DES of 1.2 IF (IF of intestinal slow waves), 5.08 ± 0.21 cpm with DES of 1.3 IF, 5.38 ± 0.33 cpm with DES of 1.38 IF, and 6.74 ± 0.68 cpm with DES of 1.52 IF, respectively. The percentages of normal gastric slow waves were reduced from the baseline of 98 ± 2% to 37 ± 2% with DES of 1.2 IF, 47 ± 5% with DES of 1.3 IF, 48 ± 4% with GES of 1.38 IF, and 27 ± 1% with DES of 1.52 IF, respectively (P < 0.05 vs. baseline).
Fig. 7.
DES-induced gastric dysrhythmia in a dog. Bradyarrhythmia followed with tachyarrhythmia.
DISCUSSION
In this study, 1) we found the feasibility of slow wave entrainment with electrical stimulation in the stomach and small intestine but not in the colon and identified optimal stimulation parameters for pacing; 2) we reported that slow wave dysrhythmia could be induced in the stomach and small intestine with electrical stimulation at a higher frequency; and 3) we showed that electrical entrainment of slow waves was a local event that did not involve vagal or sympathetic mechanisms. However, the induction of slow wave dysrhythmia with electrical stimulation was modulated by the sympathetic nerves.
Effects of Electrical Stimulation on Slow Waves and Optimal Stimulation Parameters
Our results showed that slow waves in the stomach and duodenum but not in the colon could be completely entrained by electrical stimulation and that optimal frequency for complete entrainment was 10% higher than the IF of the slow waves. These findings were in agreement with previous studies (13, 14). It was also found in this study that the stimulation pulse width required to entrain slow waves was much shorter for DES (10–20 ms) than GES (150–450 ms). This may be attributed to different characteristics of the histological structures: 1) the stomach is much larger than the small intestine, and the stimulation energy diminishes more quickly in the stomach than in the small intestine; 2) the stomach has extra oblique muscles, although both stomach and duodenum have circular and longitudinal muscles; 3) the stomach smooth muscle layers are much thicker than those in the duodenum.
To the best of our knowledge, this was the first study to show that slow waves in the colon could not be entrained by electrical pacing. The findings of this study (pacing was possible only in the stomach and small intestine but not in the colon) suggest that pacing or complete entrainment of slow waves with electrical stimulation is only possible in an organ that has regular slow waves with one dominant frequency. In other words, electrical stimulation is only able to drive the intrinsic slow wave to a certain higher frequency but not able to induce slow waves of any frequencies. Similar to the findings in this study, numerous previous in vivo and in vitro studies in dogs and humans showed irregular slow waves in the colon. In one in vitro study in human colon tissue, Riezzo et al. (22) reported that slow waves in the colon were present for only 24.5% and 12% of the recording time on the longitudinal and circular layers, respectively, and they appeared as localized activity that was irregular in amplitude and varying in frequency. Electrical coupling between the two muscle layers was rarely seen, and slow waves were not associated with pressure changes. In a human in vivo study with bipolar serosal electrodes, Sarna et al. (23) reported that the ascending colon had a low level signal that showed the simultaneous presence of variable and multiple frequency components in each of two frequency ranges of 2 to 9 cpm and 9 to 13 cpm.
The effects of pacing parameters were systematically and quantitatively investigated in this study. A parameter called the percentage of entrainment was defined. Using this parameter as a standard, we were able to quantitatively compare the effect of different pacing parameters on the entrainment of gut slow waves. In this study, we found that although the slow waves could be entrained to a higher frequency, there existed a maximum driven frequency. Furthermore, it was found that the percentage of entrainment was inversely proportional to the stimulation frequency. From these results, we could conclude that the stomach and duodenum had the same electrical pacing features, as they had similar maximum driving frequencies. The feasibility of entraining gastric/intestinal slow waves suggests that electrical pacing may be used to treat patients with motility disorders attributed to slow wave dysrhythmia in the stomach or small intestine. Previous studies have indicated a higher percentage of slow wave dysrhythmia in patients with gastroparesis, functional dyspepsia, chronic intestinal pseudo-obstruction, etc. (2, 33). In addition, we also showed that electrical stimulation at frequencies higher than the normal frequency of the slow waves could induce dysrhythmia in the stomach and duodenum. While induction of slow wave dysrhythmia with GES was previously reported (34), this was the first study to show that intestinal dysrhythmia could also be induced with electrical stimulation. Since slow wave dysrhythmia is associated with dysmotility, electrical stimulation designed to induce dysrhythmia may be applied to treat obesity (by slowing the digestive process) or treat hypertensive gastrointestinal motility.
Retrogastric inhibitory feedback is a common phenomenon along the gut. A number of previous studies reported that duodenal nutrients or distension reduced proximal gastric tone, delayed gastric emptying, and increased pyloric contractions (17, 32). Colon-rectal distension has been reported to induce delayed gastric emptying (20, 31), abnormalities of gastric slow waves (1, 21), and inhibition of proximal gastric tone (12). In the present study, a similar retrogastric inhibitory effect was also noted with electrical stimulation of the duodenum but not the colon; gastric slow wave dysrhythmia was induced during electrical stimulation of the duodenum at high frequencies. However, it was not clear why electrical stimulation of the colon did not produce such an inhibitory effect on gastric slow waves.
Vagal and Sympathetic Modulatory Effects on Gastrointestinal Electrical Stimulation
The neural involvement of electrical stimulation-induced entrainment has never been reported before. In this study we found that GES- or DES-induced entrainment of gastric or duodenal slow waves was not affected by propranolol, phentolamine, or vagotomy, suggesting that the sympathetic and vagal pathway is not involved in the entrainment induced by electrical stimulation. This was also in agreement with a previous study showing that the sympathetic pathway did not play a role in the regulation of gastric or intestinal slow waves under normal conditions (15). These findings indicate that electrical stimulation at a physiological frequency is a local event directly acting on smooth muscle cells. On the contrary, dysrhythmia induced by GES or DES was blocked by propranolol or phentolamine, suggesting that the sympathetic pathway played a role in electrical stimulation-induced dysrhythmia. The sympathetic and vagal activities are known major regulative factors on gastrointestinal motility (29, 30). It was also reported that the sympathetic activation might cause tachygastria and decrease action potentials on slow waves in the canine antrum (6, 19). On the basis of the findings of the present study we may conclude that the systematic autonomic nerve system is not involved in the entrainment of slow waves under the physiological condition but plays an important role in the electrical stimulation-induced dysrhythymia under the pathophysiological condition.
Perspectives and Significance
From this study we know that slow waves in the stomach and small intestine can be electrically altered; however, appropriate stimulation parameters are required to entrain slow waves or induce dysrhythmia. The findings of this study are of great clinical significance. By properly pacing the stomach, we may be able to entrain slow waves and therefore normalize gastric dysrhythmia. This suggests that electrical pacing may be used to treat patients with various motility disorders involving slow wave dysrhythmia, such as gastroparesis and intestinal pseudo-obstruction. However, wide pulses are required for the electrical stimulation to be effective. On the other hand, by electrically impairing slow waves, we may be able to impair gastric motility and slow down the digestive process and therefore apply the method of electrical stimulation to treat obesity. Similarly, appropriate selection of stimulation parameters is also important for this kind of electrical stimulation. Although the optimal stimulation parameters presented in this study were derived from dogs, a recent study has demonstrated the similarity in the selection of stimulation parameters among different species (28). Accordingly, the optimal stimulation parameters reported in this study may also be applicable in humans. It should be acknowledged that the current study did not address physiological effects of electrical stimulation such as gastric emptying or intestinal transit.
In conclusion, electrical pacing or entrainment is feasible for the stomach and small intestine but not the colon. The energy required to pace the slow waves in the duodenum is substantially lower than that for the stomach. There is a retrogastric inhibitory effect on slow waves with duodenal stimulation but not colon stimulation. The entrainment of slow waves with gastrointestinal electrical stimulation is not modulated by the vagal or sympathetic pathway.
GRANT
This work is partially supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-055437.
REFERENCES
- 1.Abo M, Kono T, Wang Z, Chen JD. Impairment of gastric and jejunal myoelectrical activity during rectal distension in dogs. Dig Dis Sci 45: 1731–1736, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Chen J, McCallum RW. Gastric slow wave abnormalities in patients with gastroparesis. Am J Gastroenterol 87: 477–482, 1992 [PubMed] [Google Scholar]
- 3.Christensen J. Myoelectric control of the colon. Gastroenterology 68: 601–609, 1975 [PubMed] [Google Scholar]
- 4.Cigaina V. Long-term follow-up of gastric stimulation for obesity: the Mestre 8-year experience. Obes Surg 14, Suppl 1: S14–S22, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res 11: 1456–1462, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Daniel EE. Electrical and contractile responses of the pyloric region to adrenergic and cholinergic drugs. Can J Physiol Pharmacol 44: 951–979, 1966 [DOI] [PubMed] [Google Scholar]
- 7.Diamant NE, Bortoff A. Effects of transsection on the intestinal slow-wave frequency gradient. Am J Physiol 216: 734–743, 1969 [DOI] [PubMed] [Google Scholar]
- 8.Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol 61: 19–43, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Huizinga JD, Stern HS, Chow E, Diamant NE, El-Sharkawy TY. Electrophysiologic control of motility in the human colon. Gastroenterology 88: 500–511, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373: 347–349, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol 220: 112–118, 1971 [DOI] [PubMed] [Google Scholar]
- 12.Lei Y, Zhu H, Xing J, Chen JD. Rectal distension modulates canine gastric tone and accommodation. Dig Dis Sci 50: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lin X, Peters LJ, Hayes J, Chen JD. Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Dig Dis Sci 45: 652–656, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol Gastrointest Liver Physiol 274: G186–G191, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Xu J, Chen J. Roles of putative neurotransmitters in the regulation of gastric and intestinal slow waves in conscious dogs. J Gastroenterol Hepatol 22: 1044–1050, 2007 [DOI] [PubMed] [Google Scholar]
- 16.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology 114: 456–461, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen NQ, Fraser RJ, Bryant LK, Holloway RH. Functional association between proximal and distal gastric motility during fasting and duodenal nutrient stimulation in humans. Neurogastroenterol Motil 19: 638–645, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Ordog T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol 518: 257–269, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang H, Chen JD. Long-pulse gastric electrical stimulation at tachygastrial frequency reduces food intake by inhibiting proximal gastric tone. Scand J Gastroenterol 42: 702–707, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Penning C, Vu MK, Delemarre JB, Masclee AA. Proximal gastric motor and sensory function in slow transit constipation. Scand J Gastroenterol 36: 1267–1273, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Qian L, Orr WC, Chen JD. Inhibitory reflexive effect of rectal distension on postprandial gastric myoelectrical activity. Dig Dis Sci 47: 2473–2479, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Riezzo G, Maselli MA, Pezzolla F, Thouvenot J, Giorgio I. In vitro electro-mechanical activity of the human colon. Simultaneous recording of the electrical patterns of the two muscle layers. Arch Int Physiol Biochim Biophys 100: 93–100, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Sarna SK, Bardakjian BL, Waterfall WE, Lind JF. Human colonic electrical control activity (ECA). Gastroenterology 78: 1526–1536, 1980 [PubMed] [Google Scholar]
- 24.Sarna SK, Bowes KL, Daniel EE. Gastric pacemakers. Gastroenterology 70: 226–231, 1976 [PubMed] [Google Scholar]
- 25.Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol Cell Physiol 252: C290–C299, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Snape WJ., Jr Myoelectric and motor activity of the colon in normal and abnormal states. Scand J Gastroenterol Suppl 96: 55–60, 1984 [PubMed] [Google Scholar]
- 27.Song G, Hou X, Yang B, Liu J, Qian W, Chen JD. Two-channel gastric electrical stimulation accelerates delayed gastric emptying induced by vasopressin. Dig Dis Sci 50: 662–668, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Song GQ, Lei Y, Xu X, Chen JD. Gastric electrical stimulation in humans and animals: can data obtained in animals be replicated in humans? Neuromodulation. doi: 10.1111/j.1525-1403.2009.00241.x. In press. [DOI] [PubMed] [Google Scholar]
- 29.Tada H, Fujita M, Harris M, Tatewaki M, Nakagawa K, Yamamura T, Pappas TN, Takahashi T. Neural mechanism of acupuncture-induced gastric relaxations in rats. Dig Dis Sci 48: 59–68, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T, Kendrick ML, Zyromski NJ, Meile T, Sarr MG. Vagal innervation modulates motor pattern but not initiation of canine gastric migrating motor complex. Am J Physiol Gastrointest Liver Physiol 281: G283–G292, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Tjeerdsma HC, Smout AJ, Akkermans LM. Voluntary suppression of defecation delays gastric emptying. Dig Dis Sci 38: 832–836, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Chen JD. Intestinal electrical stimulation improves delayed gastric emptying and vomiting induced by duodenal distension in dogs. Neurogastroenterol Motil 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Chen JD. Pacing the gut in motility disorders. Curr Treat Options Gastroenterol 9: 351–360, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Xu X, Chen JD. Chronic tachygastrial electrical stimulation reduces food intake in dogs. Obesity (Silver Spring) 15: 330–339, 2007 [DOI] [PubMed] [Google Scholar]