Abstract
Both, low (<7 g/dl) and high (>14.5 g/dl), maternal hemoglobin (Hb) levels have been related to poor fetal outcome. Most studies have been done at low altitude (LA). Here, we have sought to determine whether this relationship exists at both high and low altitude, and also whether there is an adverse effect of high altitude (HA) on fetal outcome independent of level of maternal hemoglobin. The study is based on a retrospective multicenter analysis of 35,449 pregnancies at LA and six other cities above 3000 meters. In analyses of all women at both LA and HA, those with Hb <9 g/dl had odds ratios (ORs) and 95% confidence intervals (CI) of 4.4 (CI: 2.8–6.7), 2.5 (CI: 1.9–3.2), and 1.4 (CI: 1.1–1.9) for stillbirths, preterm, and small for gestational age (SGA) births, respectively, compared with women with 11–12.9 g/dl of Hb, after adjustment for confounders. These risks by hemoglobin level differed little between women at LA and HA, suggesting that no correction of the definition of anemia is necessary for women at HA. Women living at high altitude with hemoglobin >15.5 g/dl had higher risks for stillbirths (OR: 1.3; CI: 1.05–1.3), preterm (OR: 1.5; CI 1.3–1.8), and SGA births (OR: 2.1, CI 1.8–2.3). There was also a significant adverse effect of living at HA, independent of hemoglobin level for all three outcomes (OR: 3.9, 1.7, and 2.3; CI: 2.8–5.2, 1.5–1.9, and 2.1–2.5) for stillbirths, preterms, and SGA respectively, after adjusting for hemoglobin level. Both, high and low maternal hemoglobin levels were related to poor pregnancy outcome, with similar effect of low hemoglobin in both LA and HA. Our data suggest, that maternal hemoglobin above 11 g/dl but below 13 g/dl is the area of minimal risk of poor adverse outcomes. Living at HA had an adverse effect independent of hemoglobin level.
Keywords: stillbirth, small for gestational age, preterm, altitude
more than 140 million persons live permanently at high altitude (HA) (>2,500 m) in North, Central, and South America; East Africa; and Asia (16). Approximately 9 million people in Peru (30% of the population) live at high altitude (HA) (16). Some previous data have shown that the incidence of low birthweight, stillbirth, and neonatal mortality rates are higher at HA in Peru (16). Most populations living at HA in Peru characteristically have an increase of hemoglobin level (48) due to the effect of hypoxia as a mechanism of compensation. Elevated hemoglobin at HA has also been seen in Tibet (2, 63). The World Health Organization (WHO) has proposed that hemoglobin values should be adjusted for altitude (61, 62). With this adjustment, the cutoff hemoglobin value to define anemia increases as altitude increases (62).
Both low (<7 g/dl) (31) and high (>14.5 g/dl) (25, 36, 53, 55) maternal hemoglobin levels have been related to poor fetal outcome at low altitude. Severe anemia (hemoglobin <7 g/dl) has been associated with late stillbirth, preterm deliveries, and small for gestational age (SGA) (31). It is not known whether these same relationships occur at high altitude (>2,500 m).
Iron supplementation is a common treatment for women with low hemoglobin levels (50). Hemoconcentration is common in women who receive daily iron supplementation (5, 47). A Cochrane review of randomized trial of iron supplementation in nonanemic women during pregnancy, carried out almost exclusively among women living at sea level, was inconclusive about whether supplementation affected either maternal or fetal outcome (29). However, some more recent studies not assessed in the Cochrane review have shown an adverse effect of supplementation. In one study of nonanemic women supplemented daily with iron, some hemoglobin values increased to more than 14.5 g/dl, and these values were associated with low-birth weight and preterm deliveries (5). One recent randomized trial of iron supplementation has shown increased risk of preterm birth for supplemented women (66). Other studies have also found a high risk of perinatal mortality (31), small for gestational age (SGA) births (53), stillbirths (55), and preterm births (25) for women who had high hemoglobin levels in pregnancy. All of these studies were conducted at low or moderate altitude.
The present study has been designed to determine the maternal hemoglobin levels at which adverse perinatal outcomes are observed at both low altitude (LA) and HA. Our a priori hypothesis was that both low and high hemoglobin levels might increase risk at both altitudes. Furthermore, we wished to know whether the known negative effect of HA on reproductive outcome (42) exists independently of maternal hemoglobin levels. To our knowledge, there are no published data on this question.
We have used a large Peruvian database with data to study pregnancy outcomes (stillbirths, preterm, and SGA births) in relationship to maternal hemoglobin in six large cohorts of women living in high altitudes, ranging from 3,070 m to 4,340 m. Data from Lima, a population located at 150 m altitude, are also presented.
MATERIAL AND METHODS
Data sources.
This study is an analysis of data from the Perinatal Information System (PIS) database in seven urban public hospitals belonging to the Ministry of Health in Peru. This database includes data on reproductive outcome, maternal hemoglobin, and maternal socio-economic characteristics. The seven hospitals are located in cities at different altitudes ranging from 150 to 4,340 m (Table 1). Data were obtained at each place of study from 2003 to 2006; data were not available prior to that date. The Institutional Review Board at the Universidad Peruana Cayetano Heredia (Lima, Peru) approved the study (Code 51827). The Peruvian Ministry of Health also approved the study.
Table 1.
Average of maternal hemoglobin level (g/dl) taken at first, second, and third trimester of pregnancy
| Place | 1st Trimester |
2nd Trimester |
3rd Trimester |
|---|---|---|---|
| n = 5,003 | n = 16,658 | n = 13,788 | |
| Lima, 150 m* | 11.57±1.20 | 11.42±1.06 | 10.94±1.23 |
| Huaraz, 3,070 m* | 13.32±1.34 | 12.88±1.37 | 12.67±1.32 |
| Huancayo, 3,280 m* | 13.94±1.41 | 13.62±1.34 | 13.37±1.31 |
| Cuzco, 3,400 m* | 13.73±1.38 | 13.61±1.42 | 13.46±1.45 |
| Huancavelica, 3,640 m* | 13.99±1.44 | 13.75±1.36 | 13.98±1.66 |
| Puno, 3,800 m* | 13.79±1.52 | 13.58±1.44 | 13.45±1.44 |
| Cerro de Pasco, 4,340 m* | 15.18±1.89 | 14.60±1.83 | 14.02±1.69 |
Values are expressed as means ± SD; n = number of subjects.
ANOVA P < 0.001. Scheffé test P < 0.001 between all trimesters.
Data from a total of 37,377 mothers were recorded from the seven hospitals. All of these hospitals serve people of low socioeconomic status.
The database includes all deliveries in each hospital. Most of the mothers live in the city in which hospitals are located. At HA, only about 4% came from outside the city and lived at a different altitude from that of the respective city; in these cases, the altitude of their residence differs by only ± 600 m from the city's altitude.
The study was restricted to single births with gestation ages of 22 wk or above. We removed from the study population 320 births with gestational age below 22 wk because they were defined as miscarriages, 321 multiple gestations in a single pregnancy, and 1,287 pregnancies with missing information, including lack of hemoglobin values and clearly erroneous or discordant data. The final study population included 35,449 births with complete information on all covariates: 8,409 were from Lima (low altitude) and the remainder were from HA cities.
The percentage of women included more than once in the database was 1.6% (multiple births). To determine the impact of women included more than once, we analyzed the data using only first births. Results did not change. Therefore, we dropped this restriction.
Dependent variables.
The dependent variables were the following adverse reproductive outcomes: stillbirths, preterm births, and SGA births. Stillbirth was defined as the birth of a fetus at gestational age of 22 wk or later that showed no signs of life after parturition. The time of fetal death was attributed to the week of birth of the stillborn infant (8). Primary cause of fetal death was not available in the database. Most of the cases belong to the category of antepartum fetal deaths; 13% were classified as intrapartum deaths.
Preterm births were defined as birth at a gestational age below 37 wk. Gestational age was estimated from the date of the last menstrual period (LMP) (1, 2). Only infants whose calculated gestational age agreed within 2 wk with that obtained after physical examination were included. Discordant data were observed in 98 cases (0.28%), and these were excluded.
SGA (small for gestational age) was defined as birth weight below the 10th percentile for gestational age using the Latin American Center for Perinatology (CLAP) standard. This is a Latin American chart of reference elaborated by CLAP, Uruguay (6).
Independent variables.
The main independent variable was first maternal hemoglobin value. This value could have occurred at either the first, second, or third trimester. We used the first available hemoglobin measurement in our analyses. Table 1 shows the available hemoglobin data. Women were sampled on the average at 10.73 ± 1.12 wk (mean ± SD), 19.36 ± 3.48, and 34.25 ± 4.27 for first, second, and third trimester, respectively. Generally, hemoglobin values dropped slightly from first to third trimester in all cities. We controlled for time of hemoglobin (Hb) measurement via inclusion of a variable for trimester of Hb measurement in the model. There were no clear trends of change in Hb week to week within trimester, making a variable for “week” unlikely to act as a confounder for Hb. When we included a categorical variable for week within trimester in some models, indeed “week” did not affect the estimated effects (ORs) of hemoglobin or high altitude, which were the parameters of interest. Therefore, we did not control for week of measurement within trimester.
We first considered hemoglobin as a categorical variable in logistic regression analyses, in which the outcome was a yes/no variable. Hemoglobin categories for analyses combining births at low and high altitude were <9, 9–10.9, 11–12.9, and >13 g/dl (the usual cutoff hemoglobin value to define maternal anemia is 11 g/dl) (38). High hemoglobin values in pregnant women were defined as Hb values over 14.5 g/dl (5, 53). These same cutoff values were used at low and high altitude for categorical analyses. The categorization of hemoglobin in these analyses permitted us to evaluate traditional cut-off values for hemoglobin and avoided assuming any particular parametric exposure-response function. Analyses of low- and high-altitude births combined included a variable for high vs. low altitude.
Other independent variables were maternal age, maternal education, marital status, prior stillbirth or preterm birth, prenatal care, parity, maternal body mass index, placental abruption, and gestational hypertension in the current pregnancy. These variables were chosen to include in logistic regression models with the main variable of interest, maternal hemoglobin, because they were of a priori concern and because they proved to be significant predictors (P < 0.05) for at least one of the three outcomes of interest and with the exposure of interest, and hence, they were potential confounders. For simplicity these same set of covariates were included in the logistic regression model for all three outcomes.
Maternal age was defined as completed years at time of delivery, and characterized as <20, 20–34, and >34 years. Maternal education was stratified as no education, some primary education, some secondary education, including high school graduation, and more than secondary education. Marital status was defined as married, consensual union, and single (including widow and divorced). Maternal height and prepregnancy weight were recorded at the woman's first antenatal visit or during delivery in meters and kilograms, respectively. The body mass index (BMI) was calculated as prepregnancy weight in kilograms divided by height (meters)2. Parity was defined as the number of previous births, including stillbirths. Parity was stratified as none, 1–3, and >3 births. According to the Handbook of Maternal Children Care of the Peruvian Ministry of Health, prenatal care was considered as none, little (1–5 visits), and adequate (>5 visits) (17a). Prior infant death or preterm birth was dichotomized as 1 if the woman had a stillbirth or early neonatal death (perinatal mortality) or if the woman had a previous preterm birth, and 0 if not. History of previous SGA or gestational hypertension was not available in the database.
Smoking was not considered since less than 1% of women reported smoking. Placental abruption, a syndrome that occurs in the third trimester of the pregnancy or during labor, is the main cause of late pregnancy bleeding, and it is also responsible for a high-stillbirth rate (44), so it was also included as independent variable (0/1). Only 0.4% of women had placental abruption.
Statistical analysis.
Data were analyzed using the STATA program (Stata ver. 8.0) for personal computer (Stata, College Station, TX).
The principal method of analyses was logistic regression in which the outcomes (stillbirths, SGA, and preterm deliveries) were regressed on hemoglobin (categorized), and the covariates described above, for women at high and low altitude were combined. A dichotomous variable for high vs. low altitude was also included in these models. Logistic regression models result in odds ratios (ORs), which are the ratios of odds of the outcome in an exposed group vs. a nonexposed group (e.g., the odds of SGA at high altitude vs. low altitude).
We also ran some analyses separately for low altitude (Lima) and for the HA cities as a group.
As noted above, we used first hemoglobin measurement as our predictor variable, including an indicator variable for trimester, in which hemoglobin was measured. Grouping the six HA cities together was done after city-specific analyses showed that they were similar regarding the effect of hemoglobin on adverse outcome, and to increase the stability of the estimated effects by avoiding small numbers in some cities.
Because of sparse data for some outcomes for women at LA with hemoglobin below 7 g/dl and above 13 g/dl, in logistic regression analyses, including both LA and the HA cities, we included only four categories of hemoglobin, <9, 9–10.9, 11–12.9, and >13 g/dl. In supplemental analyses focusing more specifically on the high-altitude cities, we included more categories of hemoglobin values [<7 g/dl; 7 to <9 g/dl; 9 to <11 g/dl; 11 to <13 g/dl (reference); 13 to <14.5 g/dl; 14.5 to <15.5 g/dl, and ≥15.5 g/dl]. The three first categories correspond to the definition at sea level of severe, moderate, and mild anemia (11), respectively.
We also ran some logistic regression analyses, in which hemoglobin was categorized by each unit of hemoglobin, i.e., separate categories were created for hemoglobin levels of 7, 8, . … 18, with 11 as the referent group. The same covariates were included as in prior analyses. Odds ratios for each adverse outcome for each of these hemoglobin levels were then plotted against the hemoglobin levels, and results are presented graphically. In addition, we fitted a simple quadratic model to these points, and the resulting quadratic curves are also presented in the graphs.
Significance was considered to be at P < 0.05 for all statistical analyses. Confidence intervals are also presented.
RESULTS
Characteristics of the populations at low and at high altitudes.
Pregnant women at high altitude had higher hemoglobin values (P = 0.001), lower education (P = 0.001), a higher proportion of subjects with normal BMI (P = 0.006), higher parity ≥4 (P = 0.001), more antenatal care (P = 0.001), and higher rate of previous stillbirths or preterm births (P = 0.001) than women residing at LA (150 m) (Table 2).
Table 2.
Characteristic of pregnant Peruvian women residing at low (150 m) and at high altitudes (>3,000 m), 2003–2006
| Characteristic | Altitude |
|||
|---|---|---|---|---|
| Low (n = 8,409) |
High (n = 27,040) |
|||
| n | % CI 95% | n | % CI 95% | |
| Hemoglobin level* | ||||
| <9 g/dl | 213 | 2.5 (2.20–2.89) | 183 | 0.6 (0.58–0.78) |
| 9–10.9 g/dl | 2481 | 29.5 (28.5–30.4) | 1083 | 4.0 (3.77–4.24) |
| 11.0–12.9 g/dl | 5081 | 60.4 (59.3–61.4) | 8049 | 29.7 (29.2–30.3) |
| ≥13.0 g/dl | 634 | 7.5 (6.98–8.12) | 17725 | 65.5 (64.9–66.1) |
| Maternal age | ||||
| <20yr | 1304 | 15.5 (14.7–16.2) | 4935 | 18.2 (17.7–18.8) |
| 20–34 yr | 6051 | 71.9 (70.9–72.9) | 18982 | 70.1 (69.6–70.7) |
| >34 yr | 1054 | 12.5 (11.8–13.2) | 3123 | 11.5 (11.2–11.9) |
| Education* | ||||
| None | 65 | 0.7 (0.59–0.98) | 837 | 3.1 (2.89–3.30) |
| Primary | 388 | 4.6 (4.17–5.08) | 5352 | 19.7 (19.3–20.2) |
| Secondary | 5730 | 68.1 (67.1–69.1) | 12656 | 46.8 (46.2–47.4) |
| Superior | 2226 | 26.4 (25.5–27.4) | 8195 | 30.3 (29.7–30.8) |
| BMI, kg/m2 | ||||
| <19.9* | 1008 | 11.9 (11.3–12.7) | 2575 | 9.5 (9.17–9.87) |
| 19.9–25.0* | 5129 | 60.9 (59.9–62.0) | 17412 | 64.3 (63.8–64.9) |
| >25 | 2272 | 27.0 (26.0–27.9) | 7053 | 26.1 (25.5–26.6) |
| Parity | ||||
| None* | 3827 | 45.5 (44.4–46.5) | 11486 | 42.4 (41.8–43.1) |
| 1–3 | 4166 | 49.5 (48.4–50.6) | 12855 | 47.5 (46.9–48.1) |
| ≥4* | 416 | 4.9 (4.49–5.43) | 2699 | 9.9 (9.62–10.3) |
| Prenatal care* | ||||
| None | 3007 | 35.7 (34.7–36.7) | 3727 | 13.7 (13.3–14.1) |
| 1–5 | 1827 | 21.7 (20.8–22.6) | 7076 | 26.1 (25.6–26.6) |
| >5 | 3575 | 42.5 (41.4–43.5) | 16237 | 60.0 (59.4–60.6) |
| Previous perinatal death/preterm* | 228 | 2.7 (2.37–3.08) | 1564 | 5.8 (5.50–6.06) |
| Gestational hypertension | 275 | 3.3 (2.90–3.67) | 954 | 3.5 (3.31–3.75) |
| Abruptio Placentae | 30 | 0.3 (0.24–0.50) | 92 | 0.3 (0.27–0.41) |
| Migration* | 8 | 0.1 (0.04–0.18) | 981 | 3.6 (3.40–3.85) |
Confidence intervals (CIs) appear in parentheses.
χ2-test of differences between high and low altitude. BMI, body mass index.
Rate of gestational hypertension was 3.3% [confidence interval, CI: 2.90–3.67%) at LA and 3.5% (CI: 3.31–3.75%) at HA (P > 0.05)]. Twenty cases (0.05%) of ante partum hemorrhage were observed in the overall population studied. All cases were associated with stillbirths. Abruptio placentae were observed in 122 mothers (0.35%). No differences were observed in these two variables between LA and HA (P > 0.05).
Proportion of low (<11 g/dl) and high maternal hemoglobin levels (>14.5 g/dl), stillbirths, preterm deliveries, and SGA at low and at high altitudes, and for all women combined.
The proportion of low hemoglobin values (<11 g/dl) for all women combined was 11.5% (CI: 11.2–11.8%) but markedly higher in LA (33.3%, CI: 32.3–34.3%) than in the HA cities (3.0–7.2%) (Table 3). The proportion of high hemoglobin values (Hb > 14.5 g/dl) increased markedly in HA compared with sea level, although there was still considerable variation at HA (Table 3). Mean birthweight was lower in all cities at HA than at LA.
Table 3.
Descriptive statistics of pregnancies in women from different altitudes of Peru, 2003–2006
| Place (altitude) | Number of Pregnancies | Hemoglobin, g/dl Means ± SD | Prevalence of Hb, <11 g/dl n % CI 95% | Prevalence of Hb, >14.5 g/dl n % CI 95% | Mean Birth Weight, g, ±SD | Stillbirths n % CI 95% | Preterm n % CI 95% | SGA n % CI 95% |
|---|---|---|---|---|---|---|---|---|
| Lima, 150 m | 8409 | 11.28±1.15 | 2804 | 15 | 3260±553 | 64 | 539 | 721 |
| 33.3 | 0.2 | 0.8 | 6.4 | 8.6 | ||||
| (32.3–34.3) | (0.09–0.29) | (0.66–1.17) | (5.89–6.95) | (7.99–9.19) | ||||
| Huaraz, 3070 m | 10,279 | 12.84±1.36 | 747 | 1063 | 3065±548 | 215 | 857 | 1635 |
| 7.2 | 10.3 | 2.1 | 8.3 | 15.9 | ||||
| (6.77–7.78) | (9.75–10.9) | (1.82–2.38) | (7.80–8.88) | (15.2–16.6) | ||||
| Huancayo, 3280 m | 6613 | 13.55±1.34 | 196 | 1646 | 3040±499 | 125 | 493 | 1417 |
| 3.0 | 24.9 | 1.9 | 7.5 | 21.4 | ||||
| (2.68–3.40) | (23.8–25.9) | (1.57–2.24) | (6.83–8.11) | (20.4–22.4) | ||||
| Cuzco, 3400 m | 2995 | 13.61±1.42 | 97 | 710 | 3090±506 | 64 | 175 | 538 |
| 3.2 | 23.7 | 2.1 | 6.0 | 17.9 | ||||
| (2.63–3.93) | (22.2–25.3) | (1.64–2.72) | (5.02–0.74) | (16.6–19.3) | ||||
| Huancavelica, 3640 m | 2202 | 13.92±1.57 | 75 | 800 | 2930±519 | 91 | 228 | 550 |
| 3.4 | 36.3 | 4.1 | 10.4 | 25.0 | ||||
| (2.68–4.25) | (34.1–38.3) | (3.34–5.04) | (9.11–11.7) | (23.1–26.8) | ||||
| Puno, 3800 m | 2744 | 13.53±1.45 | 89 | 642 | 3100±527 | 56 | 184 | 433 |
| 3.2 | 23.4 | 2.0 | 6.7 | 15.7 | ||||
| (2.61–3.97) | (21.8–25.0) | (1.54–2.64) | (5.79–7.70) | (14.4–17.1) | ||||
| Cerro de Pasco, 4340 m | 2207 | 14.46±1.83 | 72 | 1184 | 2838±468 | 51 | 404 | 717 |
| 3.3 | 53.6 | 2.3 | 18.3 | 32.4 | ||||
| (2.56–4.09) | (51.5–55.7) | (1.72–3.02) | (16.7–19.9) | (30.5–34.4) | ||||
| Overall population | 35449 | 12.89±1.70 | 4080 | 6060 | 3089±541 | 666 | 2880 | 6011 |
| 11.5 | 17.1 | 1.9 | 8.1 | 16.9 | ||||
| (11.2–11.8) | (16.7–17.5) | (1.73–2.02) | (7.84–8.41) | (16.5–17.3) |
CI 95%, 95% confidence interval; SGA, small for gestational age. n = number of subjects with the characteristic.
Proportions of stillbirths, preterm deliveries, and SGA were higher at HA than at LA (Table 3).
Risk of still births, preterm and small for gestational age deliveries, according to maternal hemoglobin value for all women combined.
Table 4 reports the results for analyses of low- and high-altitude births combined. Using four categories of hemoglobin, there were increased risks for all three adverse outcomes at low hemoglobin levels. There was also a significant independent adverse effect of living at HA for all three outcomes, after adjusting for hemoglobin level. Odd ratios (CI) were 3.9 (CI: 2.8–5.2), 1.7 (CI: 1.5–1.9), and 2.3 (CI: 2.1–2.5) for stillbirths, preterm deliveries, and SGA, respectively (Table 4). Importantly, interaction terms between hemoglobin and altitude analyzed in the model did not show statistical significance (P value = 0.10).
Table 4.
Model for logistic regression assessing risk of still births, preterms, and small for gestational age, according to maternal hemoglobin value in Peruvian pregnant women, 2003–2006
| Characteristic | Stillbirth (666) |
Preterm Births (2,880) |
SGA Births (6,011) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | (CI 95%) | OR | CI 95% | n | % | (CI 95%) | OR | CI 95% | n | % | (CI 95%) | OR | CI 95% | |
| Hemoglobin, g/dl | |||||||||||||||
| <9 | 29 | 7.2 | (4.8–10.1) | 4.42 | 2.82–6.71 | 75 | 18.6 | (14.9–22.7) | 2.5 | 1.9–3.2 | 68 | 16.9 | (13.3–20.8) | 1.4 | 1.1–1.9 |
| 9–10.9 | 71 | 1.9 | (1.5–2.4) | 1.78 | 1.32–2.38 | 313 | 8.5 | (7.6–9.4) | 1.2 | 1.1–1.4 | 389 | 10.6 | (9.6–11.6) | 0.9 | 0.8–1.1 |
| 11–12.9 | 183 | 1.4 | (1.2–1.6) | 1.0 | referent | 978 | 7.6 | (7.2–8.1) | 1.0 | referent | 1724 | 13.4 | (12.8–14.0) | 1.0 | referent |
| >12.9 | 383 | 2.1 | (1.8–2.3) | 1.07 | 0.89–1.29 | 1514 | 8.2 | (7.7–8.6) | 0.9 | 0.8–1.01 | 3830 | 20.7 | (20.1–21.2) | 1.3 | 1.2–1.4 |
| Altitude | |||||||||||||||
| Low | 64 | 0.8 | (0.5–1.0) | 1.0 | referent | 539 | 6.4 | (5.8–6.9) | 1.0 | referent | 721 | 8.5 | (7.9–9.2) | 1.0 | referent |
| High | 602 | 2.2 | (2.0–2.4) | 3.90 | 2.89–5.28 | 2342 | 8.7 | (8.3–8.9) | 1.83 | 1.6–2.1 | 5287 | 19.6 | (19.1–20.0) | 2.2 | 2.0–2.4 |
| Age (years) | |||||||||||||||
| <20 | 96 | 1.6 | (1.2–1.8) | 0.84 | 0.65–1.08 | 573 | 9.2 | (8.4–9.9) | 1.24 | 1.1–1.4 | 1375 | 22.0 | (21.0–23.1) | 1.0 | 0.9–1.1 |
| 20–34 | 429 | 1.7 | (1.5–1.8) | 1.0 | referent | 1859 | 7.4 | (7.1–7.7) | 1.0 | referent | 4002 | 15.9 | (15.5–16.4) | 1.0 | referent |
| >34 | 141 | 3.5 | (2.8–3.9) | 1.20 | 0.96–1.52 | 449 | 10.7 | (9.8–11.7) | 1.16 | 1.02–1.3 | 631 | 15.1 | (14.0 16.2) | 1.1 | 1.0–1.3 |
| Education | |||||||||||||||
| None | 71 | 7.9 | (6.2–9.8) | 3.19 | 2.37–4.31 | 132 | 14.6 | (12.3–17.4) | 1.56 | 1.3–1.9 | 193 | 21.4 | (18.7–24.2) | 1.3 | 1.1–1.6 |
| Primary | 198 | 3.6 | (3.0–3.9) | 1.71 | 1.40–2.08 | 617 | 10.7 | (9.9–11.5) | 1.26 | 1.1–1.4 | 1178 | 20.5 | (19.4–21.5) | 1.2 | 1.1–1.3 |
| Secondary | 280 | 1.5 | (1.3–1.7) | 1.0 | referent | 1421 | 7.72 | (7.3–8.1) | 1.00 | referent | 3053 | 16.6 | (16.1–17.1) | 1.0 | referent |
| Superior | 117 | 1.1 | (0.9–1.3) | 0.73 | 0.58–0.91 | 711 | 6.83 | (6.3–7.3) | 0.92 | 0.8–1.02 | 1584 | 15.2 | (14.5–15.9) | 0.8 | 0.7–0.9 |
| Parity | |||||||||||||||
| None | 223 | 1.5 | (1.2–1.7) | 1.0 | referent | 1171 | 7.7 | (7.2–8.1) | 1.0 | referent | 3164 | 20.6 | (20.0 21.3) | 1.0 | referent |
| 1–3 | 305 | 1.8 | (1.5–2.0) | 1.03 | 0.84–1.25 | 1332 | 7.8 | (7.4–8.2) | 1.07 | 0.98–1.2 | 2392 | 14.0 | (13.5–14.5) | 2.0 | 1.8–2.3 |
| >3 | 138 | 4.4 | (3.7–5.2) | 1.26 | 0.93–1.70 | 378 | 12.1 | (11–13.3) | 1.28 | 1.1–1.5 | 452 | 14.5 | (13.3–15.8) | 1.3 | 1.1–1.5 |
| Prev Perin death/preterm* | 88 | 4.9 | (4.1–7.3) | 2.05 | 1.46–2.87 | 269 | 15.0 | (12–17.1) | 1.71 | 1.4–2.1 | 342 | 19.0 | (15.4–20.8) | 1.2 | 1.1–1.5 |
| Placental abruption | 17 | 13.9 | (8.9–22.3) | 7.52 | 4.40–12.8 | 44 | 36.0 | (27–45.2) | 5.42 | 3.6–8.0 | 25 | 20.4 | (13.7–15.7) | 1.3 | 0.8–2.0 |
| Gestational hypertension | 30 | 2.4 | (1.2–1.6) | 1.23 | 0.84–1.80 | 280 | 22.7 | (20.3–25) | 3.88 | 3.4–4.5 | 294 | 23.9 | (21.5–26.4) | 1.6 | 1.4–1.8 |
| Prenatal care | |||||||||||||||
| None | 211 | 3.13 | (2.7–3.5) | 3.08 | 2.52–3.76 | 762 | 11.3 | (10.5–12.1) | 2.81 | 2.5–3.1 | 1119 | 16.6 | (15.7–17.5) | 1.2 | 1.1–1.3 |
| 1–5 | 227 | 2.55 | (2.2–2.8) | 2.12 | 1.75–2.56 | 1141 | 12.8 | (12.1–13.5) | 3.15 | 2.9–3.5 | 1570 | 17.6 | (16.8 18.4) | 1.0 | 0.96–1.1 |
| >5 | 228 | 1.15 | (1.0–1.3) | 1.0 | referent | 978 | 4.9 | (4.6–5.2) | 1.0 | referent | 3319 | 16.7 | (16.2–17.3) | 1.0 | referent |
| BMI, kg/m2 | |||||||||||||||
| <19.9 | 67 | 1.87 | (1.4–2.3) | 0.98 | 0.74–1.31 | 335 | 9.4 | (8.4–10.3) | 1.13 | 1.0–1.3 | 765 | 21.3 | (20.0–22.7) | 1.3 | 1.2–1.4 |
| 19.9–25 | 413 | 1.83 | (1.6–2.0) | 1.0 | referent | 1865 | 8.3 | (7.9–8.6) | 1.0 | referent | 4018 | 17.8 | (17.3–18.3) | 1.0 | referent |
| >25 | 186 | 1.99 | (1.7–2.3) | 1.03 | 0.85–1.23 | 681 | 7.3 | (6.7–7.8) | 0.83 | 0.8–0.9 | 1225 | 13.1 | (16.2–17.3) | 0.7 | 0.6–0.8 |
| Measurement of Hb | |||||||||||||||
| 1st trimester | 72 | 1.4 | (1.1–1.8) | 1.0 | referent | 386 | 7.7 | (6.9–8.5) | 1.0 | referent | 741 | 14.8 | (13.8–15.8) | 1.0 | referent |
| 2nd trimester | 279 | 1.6 | (1.4–1.8) | 1.0 | 0.7–1.3 | 1394 | 8.3 | (7.9–8.8) | 1.1 | 0.9–1.2 | 2628 | 15.8 | (15.2–16.3) | 0.9 | 0.8–1.1 |
| 3rd trimester | 315 | 2.2 | (2.0–2.5) | 1.1 | 0.8–1.4 | 1100 | 7.9 | (7.5–8.4) | 0.7 | 0.6–0.8 | 2642 | 19.2 | (18.5–19.8) | 1.23 | 1.1–1.4 |
n = number of cases with fetal adverse outcome.
Previous perinatal death and/or preterm births. Marital status was also included in the model but it has no impact on the outcome. Then, it was removed. OR, Odds ratio; CI 95%, confidence interval at 95%; BMI, body mass index.
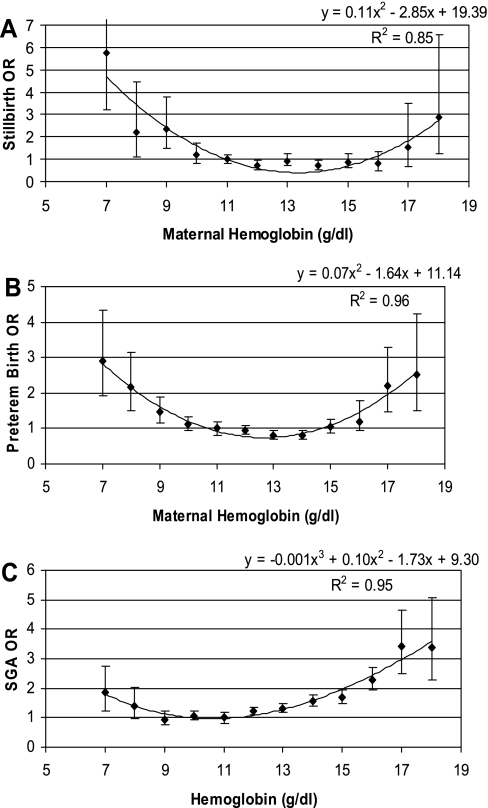
In finer categorical logistic models using each unit of hemoglobin as a category, again combining data from LA and the HA cities and including all covariates, as in Table 4, a U-shaped curve effect for hemoglobin was observed (Fig. 1) for all three outcomes. These figures suggest, as a general rule, that maternal hemoglobin above 11 g/dl, but below 13 g/dl, is the level of minimal risk of poor adverse outcomes for all three outcomes. The R-squares for quadratic curves fit to the points in these figures were quite high for all three outcomes, indicating the good fit of the quadratic model.
Fig. 1.
A: odds ratios (OR) for stillbirths according to maternal hemoglobin levels in populations at sea level and at high altitude combined. B: OR for preterm births according to maternal hemoglobin levels in populations at sea level and at high altitude. C: OR for small for gestational age according to maternal hemoglobin levels in populations at sea level and at high altitude combined.
Risk of still births, preterms, and small for gestational age, according to maternal hemoglobin value in separate analyses of women living at low and high altitude.
The risk of stillbirths at LA increased when Hb was below 11 g/dl, whereas the risks of preterms and SGA increased when Hb was below 9 g/dl (Table 5). No cases with Hb >14.5 g/dl were observed for stillbirths and no cases with Hb >15.5 g/dl were observed for preterms and SGA at LA. Virtually no women at LA had very high hemoglobin levels.
Table 5.
Model for logistic regression assessing risk of still birth, preterm, and small-for-gestational age according to maternal hemoglobin value in pregnant women at Peruvian low altitudes: 2003–2006
| Hemoglobin, g/dl | Stillbirths (64) |
Preterms (539) |
SGA (721) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | OR Adjusted | n | % | OR Adjusted | n | % | OR Adjusted | |
| (CI at 95%) | CI at 95% | (CI at 95%) | CI at 95% | (CI at 95%) | CI at 95% | ||||
| <9 | 2 | 0.88 | 1.41 | 29 | 12.83 | 1.85 | 34 | 15.04 | 1.79 |
| (0.10–0.31) | 0.32–6.13 | (8.76–17.9) | 1.18–2.90 | (10.6–20.3) | 1.21–2.64 | ||||
| 9–10.9 | 28 | 1.09 | 1.73 | 166 | 6.44 | 1.08 | 207 | 8.03 | 0.91 |
| (0.72–1.56) | 1.01–2.96 | (5.52–7.45) | 0.89–1.33 | (7.0–9.14) | 0.76–1.09 | ||||
| 11.0–12.9 | 30 | 0.61 | 1.00 | 297 | 5.99 | 1.00 | 422 | 8.51 | 1.00 |
| (0.40–0.86) | (5.34–6.68) | (7.75–9.32) | |||||||
| 13.0–14.4* | 4 | 0.63 | 1.09 | 46 | 7.26 | 1.22 | 57 | 8.99 | 1.10 |
| (0.17–1.60) | 0.38–3.26 | (5.36–4.55) | 0.88–1.70 | (6.88–11.4) | 0.82–1.47 | ||||
Model adjusted by age, educational status, body mass index, parity, gestational hypertension, health care, and previous perinatal mortality. n = number of cases with fetal adverse outcome. Trimester of gestational age at first hemoglobin measurement was controlled.
Number of pregnancies and adverse outcomes with Hb >14 too small to analyze.
To further explore the effects of high hemoglobin, we then ran a separate model for the HA cities only (Table 6), with more detailed categorization of hemoglobin, especially for high hemoglobin levels, which were very few at low altitude. Again, low Hb values of <7 g/dl, 7–9 g/dl, and 9–10.9 g/dl had significant risks for adverse events for stillbirths and preterm births. Furthermore, hemoglobin levels above 15.5 g/dl showed a significantly increased risk for all three outcomes, and levels ≥ 13.0 g/dl showed significantly increased risk for SGA birth (Table 6).
Table 6.
Model for logistic regression assessing risk of still birth, preterm, and small-for-gestational age according to maternal hemoglobin value in pregnant women at Peruvian high altitudes: 2003–2006
| Hemoglobin (g/dl) | n | Rate (CI at 95%) | Stillbirth (602) |
n | Rate (CI at 95%) | Pre term (2,341) |
n | Rate (CI at 95%) | SGA (5,290) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR* | CI 95% | OR* | CI 95 % | OR* | CI 95 % | ||||||||||
| <7 | 11 | 26.2 | 13.6 | 6.3 | 29.0 | 10 | 23.8 | 2.7 | 1.3 | 5.6 | 1.0 | 0.4 | 2.3 | ||
| (13.1–40.3) | (11.4–37.8) | 7 | 16.6 | ||||||||||||
| 7–8.9 | 16 | 11.9 | 3.8 | 2.1 | 6.8 | 36 | 26.7 | 2.8 | 1.8 | 4.2 | (8.19–32.7) | 1.2 | 0.8 | 1.8 | |
| (7.20–18.85) | (19.4–34.7) | 27 | 20.0 | ||||||||||||
| 9–10.9 | 43 | 3.9 | 1.8 | 1.2 | 2.6 | 147 | 13.4 | 1.5 | 1.2 | 1.8 | (13.8–27.7) | 0.9 | 0.8 | 1.2 | |
| (2.72–5.09) | (11.2–15.3) | 182 | 16.6 | ||||||||||||
| 11.0–12.9 (Ref) | 153 | 1.9 | 1.0 | 681 | 8.7 | 1.0 | (13.1–17.5) | 1.0 | |||||||
| (1.64–2.26) | (7.98–9.21) | 1302 | 16.5 | ||||||||||||
| 13.0–14.4 | 253 | 2.1 | 1.1 | 0.9 | 1.4 | 864 | 7.3 | 0.8 | 0.7 | 0.9 | (16.1–17.7) | 1.2 | 1.1 | 1.3 | |
| (1.86–2.39) | (6.78–7.74) | 2303 | 19.4 | ||||||||||||
| 14.5–15.5 | 73 | 1.8 | 0.9 | 0.7 | 1.2 | 370 | 9.0 | 1.0 | 0.9 | 1.2 | (18.5–19.9) | 1.4 | 1.3 | 1.6 | |
| (1.47–2.31) | (8.17–9.92) | 912 | 22.3 | ||||||||||||
| >15.5 | 53 | 2.7 | 1.3 | 1.05 | 1.7 | 233 | 11.9 | 1.5 | 1.3 | 1.8 | (20.8–23.3) | 2.1 | 1.8 | 2.3 | |
| (1.99–3.51) | (10.9–13.9) | 557 | 28.5 | ||||||||||||
| (26.8–31.0) | |||||||||||||||
Values are adjusted by age, educational status, body mass index, parity, gestational hypertension, health care, and previous perinatal mortality. n = number of cases with fetal adverse outcome. Rate is expressed as a percentage. Trimester of gestational age at first hemoglobin measurement was controlled.
DISCUSSION
The present study demonstrated a U-shaped curve of increased risk by hemoglobin level for adverse birth outcomes (stillbirths, preterm deliveries, SGA), for births at low and high altitude combined. The pattern of increased risks due to low hemoglobin did not differ significantly between low and high altitudes. The optimal hemoglobin level, with the lowest risk, was between 11 and 13 g/dl.
We also found strong evidence of increased risk for all three reproductive outcomes for women living at HA vs. women living at LA, independent of any effects of hemoglobin. To our knowledge, this is the first such finding in the literature after controlling for the effect of hemoglobin. However, it has been well established by many previous studies that HA was associated with reduced fetal growth, as manifested by reduced mean birth weight and increased risk of SGA, based on a sea-level reference (17, 20, 34, 35, 37, 39, 56, 65). In all of these previous studies, the maternal hemoglobin value was not controlled.
Our results suggest that part of the reduction in birthweight at high altitude was due to aberrant hemoglobin values and partly due to an independent effect of high altitude. The reduction in fetal weight at high altitude is generally thought to be the result of the “brain sparing” effect of fetal hypoxia (30), due, in turn, to uteroplacental ischemia and/or reduced oxygen or other nutrient delivery, and the consequent stunting of fetal postcranial growth. Neonatal mortality is increased (under conditions of similar medical care) at high altitude, and there is abundant literature at low altitude, indicating that lowering birth weight raises mortality risk (32, 57). These two observations suggest that the reduction in fetal growth at high altitude is not adaptive. Our results demonstrate that increases in hemoglobin values are highly associated with low birth weight (higher rate of SGA) at high altitude. High maternal hemoglobin levels seem to be related to low uterine arterial blood flow. A low uterine arterial flow results in reduced oxygen delivery to the fetus (21). Thus, our results demonstrate that high hemoglobin at high altitude is also not adaptive.
Our study had significant strengths, including large sample size at both low and high altitudes, and detailed data on outcomes, on hemoglobin, and on relevant covariates.
One potential problem with this study concerns gestational age assessment, which was based on LMP. Ultrasound has been established as the gold standard. However, in small infants ultrasound may result in assignment of incorrect lower gestational age (18, 40). Furthermore, ultrasound and LMP estimates of gestational age have been shown to be highly correlated. For example, estimated preterm birth rates using LMP-based estimated vs. ultrasound estimates were, respectively, 8.7% and 7.9% in California (12). In our study, we found overall singleton preterm birth rates of 8.3% for the period 2003–2006. It is common to use LMP in large reproductive studies since ultrasounds are often not available for large populations.
Below we discuss the importance of our two main findings, a similar effect of low hemoglobin at low and high altitude, and an increase in risk of adverse outcome at high altitude independent of hemoglobin.
The finding of no important difference in risks by hemoglobin level for women at low and high altitude is important in light of suggestions that anemia should be defined differently at high altitude, and the possible use of iron supplementation during pregnancy (5) to treat anemia. Our findings indicate no such changed definition of anemia is necessary at high altitude. On the basis of our data, significant increased risks for all three outcomes occur for hemoglobin below 9 g/dl, suggesting that this traditional cutoff to define moderate anemia is an appropriate definition of anemia, at both high and low altitude.
Iron supplementation to correct supposed anemia at high altitude involves risks. Daily iron supplementation to nonanemic women has been shown to result in values of hemoglobin over 14.5 g/dl (5). It has been suggested that high hemoglobin restricts intrauterine growth as a consequence of high blood viscosity (55). The increased viscosity may reduce blood flow within the placenta. A number of studies have found high hemoglobin to be associated with adverse reproductive outcome (5, 25, 42, 53, 55, 66), similar to our own findings at high altitude. Experimental studies in pregnant mice with excessive erythrocytosis produced poor arteriogenesis and uterine perfusion, resulting in a lower number of pups. These effects were reversed after reduction of the excessive erythrocytosis (14).
Most populations living at high altitude show an increase in Hb concentration as a compensation mechanism to the effect of hypoxia (48). Considering this situation, the World Health Organization (WHO) has proposed that Hb values should be adjusted for altitude (61, 62). The first equation to correct hemoglobin in defining anemia at high altitude was performed using data from Peruvian men at high altitudes (19). Currently, there are several suggested cutoffs to define anemia at high altitude (3, 10, 13, 19, 58, 64).
Cohen and Haas (7), using hemoglobin correction factors, have estimated the prevalence of iron deficiency anemia in pregnant women residing at high altitudes in Bolivia. However, there are no findings that show that these changes in the cut-off of hemoglobin to define anemia are effectively related with any clinical problem. On the contrary, previous data indicate that populations at HA are not lacking in iron (9, 49). In adult Bolivian women, body iron measurements indicated that only 5.7% had tissue iron deficiency severe enough to produce anemia (9).
Our SIP database had no information about consumption of iron during pregnancy. However, an increase of at least 1 g/dl in hemoglobin after 1 or 2 mo of supplementation is indicative of iron deficiency (62). According to data in Table 1, almost all data from the study showed a reduction in Hb values from the first to third trimester, except Huancavelica, where a 0.23 g/dl increase was observed from second to third trimester. These data suggest that large numbers of women did not take iron supplementation. Daily administration of iron supplementation may result in an increase in Hb values (>14.5 g/dl) in 11% of pregnant women, and this was associated with low birth weight and preterm births (5).
One important unanswered question is why hemoglobin falls during pregnancy. We believe the changes in hemoglobin concentration are due mainly to changes in plasma volume (54). Failure of the plasma volume to expand adequately can lead to restricted fetal growth, resulting in a newborn small for gestational age at birth (54).
At the first trimester of pregnancy, blood and plasma viscosity are increased, and they subsequently fall with advancing gestation (22), as a mechanism to avoid reduction in arterial uterine flow. This also occurs at HA (60). A drop in viscosity would thus promote efficient blood flow within the placenta and vice versa (54). Pregnancy at high altitude compared with sea level is characterized by increased blood viscosity as a result of increased hemoglobin and plasma viscosity (23). This will decrease uterine arterial flow resulting in low birthweight (21).
Our second important finding was an adverse effect of high vs. low altitude, independent of hemoglobin level. We believe that differences in the oxygen-carrying capacity may partly explain the effect of altitude on perinatal outcome.
In Peru, others have observed that elevation in arterial O2 saturation offsets the pregnancy-induced fall in hemoglobin concentration, so that arterial O2 is preserved at nonpregnant levels. The Peruvian women with the greatest rise in ventilation and ventilatory sensitivity to hypoxia produced the heaviest birthweight infants, suggesting that maternal arterial oxygenation was an important determinant of fetal growth (35). An increase in hypoxic ventilatory response may be an important contributor to increased maternal ventilation with pregnancy and infant birth weight at high altitude (38). Babies with heavier birth weights were born to Andean women with higher ventilation during pregnancy (33, 60).
Another mechanism through which high hemoglobin values might affect growth is the development of preeclampsia (41, 54). Some authors found higher preeclampsia rates at high altitude (24, 45, 59). Surprisingly, we do not find differences in gestational hypertension between mothers from LA and HA.
Populations residing at HA may have health, social, and communication infrastructures less developed than those residing at LA, particularly in Lima, the Peruvian capital. The Lima metropolitan area has lower percentages of poor and extremely poor population: 37.1% and 4.2%, respectively, compared with the respective figures at HA of 7.1% and 34.1% (46). Maternal education was the only variable we had to control for socio-economic status. Therefore, we cannot exclude the possibility that residual confounding by socio-economic status (SES) could explain the increased risk of adverse reproductive outcome at HA. However, it should be noted that low maternal education, while statistically significant, generally resulted in odds ratios less than 2.0 (particularly for preterm and SGA), and this may indicate the SES is not an overwhelming risk factor, so that residual confounding by SES is not likely to be able to explain the high risk for HA vs. LA (ORs of 3.9, 1.7, and 2.3 for stillbirth, preterm birth, and SGA, respectively). There are studies indicating that low SES is not the cause of the altitude-associated reduction in birth weight (20, 15).
All hospitals studied are located in urban cities and in the capitals of their respective departments. However, using the low altitude population in Lima as a referent has the advantage that Lima is the capital of Peru and may offer better medical care. It is possible that this difference may explain differences in the birth outcomes between LA and HA populations after maternal hemoglobin values have been controlled. More intrapartum stillbirths were observed at HA (13.6%) than at sea level (8.05%). In developed countries, intrapartum stillbirths comprise less than 10% of all stillbirths (28). When intrapartum stillbirths occur, they likely represent inadequate access to, or poor quality of, essential obstetric care (51).
Perspectives and Significance
It is important to determine risk factors associated with adverse perinatal outcome, like stillbirths, preterm birth, and small for gestational age. Our data show a U-shaped curve of increased risk of adverse outcomes at very high and very low hemoglobin levels; the increased risk at low hemoglobin levels did not differ between low- and high-altitude births. Results from the present study demonstrate that high maternal hemoglobin values in high-altitude populations are associated with adverse perinatal outcomes, suggesting that an increase in hemoglobin during pregnancy at high altitude is not adaptive. We also found an adverse effect of high vs. low altitude, independent of maternal hemoglobin levels.
GRANTS
This work was supported by a National Institutes of Health Research Grant 5-D43TW005746-04 funded by the Fogarty International Center, National Institutes on Environmental Health Services, National Institute for Occupational Safety and Health. The Agency for Toxic Substances and Disease Registry also supported this project.
ACKNOWLEDGMENTS
We acknowledge the support of the Ministry of Health in Peru and professional and technical staff at the hospitals from Huaraz, Huancayo, Huancavelica, Cuzco, Puno, and Cerro de Pasco for the facilities to perform the study.
REFERENCES
- 1.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcome. Paediatr Perinat Epidemiol 21Suppl 2: 22–30, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Beall CM, Reichsman AB. Hemoglobin levels in a Himalayan high altitude population. Am J Phys Anthropol 63: 301–306, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Berger J, Aguayo VM, San Miguel JL, Lujan C, Tellez W, Traissac P. Definition and prevalence of anemia in Bolivian women of childbearing age living at high altitudes: the effect of iron-folate supplementation. Nutr Rev 55: 247–256, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr 93: 120–122, 1978 [DOI] [PubMed] [Google Scholar]
- 5.Casanueva E, Viteri FE, Mares-Galindo M, Meza-Camacho C, Loría A, Schnaas L, Valdés-Ramos R. Weekly iron as a safe alternative to daily supplementation for non-anemic pregnant women. Arch Med Res 37: 674–682, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Centro Latinoamericano de Perinatología/World Health Organization/Pan American Health Organization Growth and perinatal development [Spanish]. Bol Salud Perinatal (Uruguay) 3: 1–28, 1991 [Google Scholar]
- 7.Cohen JH, Haas JD. Hemoglobin correction factors for estimating the prevalence of iron deficiency anemia in pregnant women residing at high altitudes in Bolivia. Rev Panam Salud Publica 6: 392–399, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Conde-Agudelo A, Belizan JM, Diaz-Rosello JL. Epidemiology of fetal death in Latin America. Acta Obstet Gynecol Scand 79: 371–378, 2000 [PubMed] [Google Scholar]
- 9.Cook JD, Boy E, Flowers C, del Carmen Daroca M. The influence of high-altitude living on body iron. Blood 106: 1441–1446, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Dallman PR, Siimes MA, Steckel A. Iron deficiency in infancy and childhood. Am J Clin Nutr 33: 86–118, 1980 [DOI] [PubMed] [Google Scholar]
- 11.De Maeyer EM. Preventing and Controlling Iron Deficiency Anemia Through Primary Health Care Geneva: World Health Organization, 1989 [Google Scholar]
- 12.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound based estimates of gestational age using linked California live births and prenatal screening records. Paediat Perinat Epidemiol 21Suppl: 62–71, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dirren H, Logman MHGM, Barclay DV, Freire WB. Altitude correction for hemoglobin. Eur J Clin Nutr 48: 625–632, 1994 [PubMed] [Google Scholar]
- 14.Gassmann M, Manini A, Stallmach T, Saam B, Kuhn G, Grenacher B, Bogdanova AY, Vogel J. Abortion in mice with excessive erythrocytosis is due to impaired arteriogenesis of the uterine arcade. Biol Reprod 78: 1049–1057, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49: 490–494, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gonzales GF. Peruvian contributions to the study on human reproduction at high altitude: From the chronicles of the Spanish conquest to the present. Respir Physiol Neurobiol 158: 172–179, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hass JD, Frongillo EA, Jr, Stepick CD, Beard JL, Hurtado L. Altitude, ethnic and sex differences in birth weight and length in Bolivia. Hum Biol 52: 459–477, 1980 [PubMed] [Google Scholar]
- 17a.Health Ministry of Peru National Guide of Sexual Reproductive Health Integral Attention: Lima, Peru: Health Ministry, 2004 [Google Scholar]
- 18.Henriksen TB, Wilcox AJ, Hedegaar M, Secher NJ. Bias in studies of pre-term and post-term delivery due to ultrasound assessment of gestational age. Epidemiology 6: 533–537, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Hurtado A, Merino C, Delgado E. Influence of anoxemia on the hemopoietic activity. Arch Intern Med 75: 284–323, 1945 [Google Scholar]
- 20.Jensen GM, Moore L. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health 87: 1003–1007, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 296: R1564–R1575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kametas NA, Krampl E, McAuliffe F, Rampling MW, Nicolaides KH. Haemorheological adaptation during pregnancy in a Latin American population. Eur J Haematol 66: 305–311, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kametas NA, Krampl E, McAuliffe F, Rampling MW, Nicolaides KH. Pregnancy at high altitude: a hyperviscosity state. Acta Obstet Gynecol Scand 83: 627–633, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DY, Moore LG. Intrauterine growth restriction, preeclampsia and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Knottnerus JA, Delgado LR, Knipschild PG, Essed GG, Smits F. Haematologic parameters and pregnancy outcome. A prospective cohort study in the third trimester. J Clin Epidemiol 43: 461–466, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Krampl E. Pregnancy at high altitude. Ultrasound Obstet Gynecol 19: 535–539, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lam CM, Wong SF, Chow KM, Ho LC. Women with placenta praevia and antepartum haemorraghe have a worse outcome than those who do not bleed before delivery. J Obstet Gynecol 20: 27–31, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirth and intrapartum-related neonatal deaths. Bull World Health Organ 83: 409–417, 2005 [PMC free article] [PubMed] [Google Scholar]
- 29.Mahomed K. Withdrawn: Iron supplementation in pregnancy. Cochrane Database Syst Rev 3: CD000117, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malamitsi-Puchner A, Nikolaou KE, Puchner KP. Intrauterine growth restriction, brain-sparing effect, and neurotrophins. Ann NY Acad Sci 1092: 293–296, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mamun AA, Padmadas SS, Khatun M. Maternal health during pregnancy and perinatal mortality in Bangladesh: evidence from a large-scale community-based clinical trial. Paediatr Perinat Epidemiol 20: 482–490, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Mari G, Hanif F. Intrauterine growth restriction: how to manage and when to deliver. Clin Obstet Gynecol 50: 497–509, 2007 [DOI] [PubMed] [Google Scholar]
- 33.McAuliffe F, Kametas N, Krampl E, Ernsting J, Nicolaides K. Blood gases in pregnancy at sea level and at high altitude. BJOG 108: 980–985, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Monge CC, Bonavia D, León-Velarde F, Arregui A. Adaptation to hypoxia in high altitude populations that reside in Nepal and the Andes. In: Hypoxia The Adaptations, edited by Sutton JR, Coates G, Remmers JE. Toronto, Canada: B. C. Decker, 1990, p. 53–58 [Google Scholar]
- 35.Moore LG. Maternal oxygen transport and fetal growth in Colorado, Peru; and Tibet high-altitude residents. Am J Hum Biol 2: 627–637, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol 2: 257–279, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol 4: 141–156, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Moore LG, Brouder P, Chumbe O, D'Brot J, Hofmeister S, Monge C. Maternal hypoxic ventilatory response, ventilation and infant birth weight at 4,300 m. J Appl Physiol 60: 1401–1406, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol Suppl 27: 25–64, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Morin I, Morin L, Zhang X, Platt RW, Blondel B, Bréart G, Usher R, Kramer MS. Determinants and consequences of discrepancies in menstrual and ultrasonographic gestational age estimates. BJOG 112: 145–152, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Murphy JF, O'Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet 1: 992–995, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Nahum GG, Stanislaw H. Hemoglobin, altitude and birth weight: does maternal anemia during pregnancy influence fetal growth? J Reprod Med 49: 297–305, 2004 [PubMed] [Google Scholar]
- 44.Nayama M, Tamakloè-Azamesu D, Garba M, Idi N, Djibril B, Kamayé M, Marafa A, Touré A, Diallo FZ, Houfflin-Debarge V. Abruptio placentae. Management in a reference Nigerian maternity. Prospective study about 118 cases during one year. Gynecol Obstet Fertil 35: 975–981, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Palmer SK, Moore LG, Young DZ, Cregger B, Berman JC, Zamudio S. Increased preeclampsia and altered blood pressure course during normal pregnancy at high (3100 m) altitude in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Pan American Health Organization Peru. In: Health in the Americas Washington, D. C.: Pan American Health Organization, 1988, vol. II, p. 413–427 [Google Scholar]
- 47.Pena-Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy (Abstract). Cochrane Databse Syst Rev 3: CD004736, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Reeves JT, Leon-Velarde F. Chronic mountain sickness: recent studies of the relationship between hemoglobin concentration and oxygen transport. High Alt Med Biol 5: 147–155, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Reynafarje Hurtado C. Iron metabolism during pregnancy at high altitudes. Arch Biol Med Exp 20: 31–37, 1987 [PubMed] [Google Scholar]
- 50.Reveiz L, Gyte G, Cuervo L. Treatments for iron-deficiency anaemia in pregnancy (Abstract). Cochrane Database Syst Rev 2: CD003094, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Ronsmans C, De Brouwere V, Dubourg D, Dieltiens G. Measuring the need for life-saving obstetric surgery in developing countries. BJOG 111: 1027–1030, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Rupert JL, Hochachka PW. Genetic approaches to understanding human adaptation to altitude in the Andes. J Exp Biol 204: 3151–3160, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol 96: 741–748, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Steer PJ. Maternal hemoglobin concentration and birth weight. Am J Clin Nutr 71 Suppl: 1285S–1287S, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA 284: 2611–2617, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Tripathy V, Gupta R. Birth weight among Tibetans at different altitudes in India: Are Tibetans better protected from IUGR? Am J Hum Biol 17: 442–450, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA 259: 3427–3432, 1988 [PubMed] [Google Scholar]
- 58.US Centers for Disease Control and Prevention Recommendations to prevent and control iron deficiency in the United States. MMWR 47 (RR-3): 1–36, 1988 [PubMed] [Google Scholar]
- 59.van Patot MC, Valdez M, Becky V, Cindrova-Davies T, Johns J, Zwerdling L, Jauniaux E, Burton GJ. Impact of pregnancy at high altitude on placental morphology in non-native women with and without preeclampsia. Placenta 30: 523–528, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Vargas M, Vargas E, Julian CG, Armaza JF, Rodriguez A, Tellez W, Niermeyer S, Wilson M, Parra E, Shriver M, Moore LG. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude. Am J Physiol Regul Integr Comp Physiol 293: R1303–R1312, 2007 [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization Prevalence of Anaemia in Women in Reproductive Health Indicators Guidelines for their Generation, Interpretation and Analysis for Global Monitoring Geneva, Switzerland: WHO, 2006, p. 41–43 [Google Scholar]
- 62.WHO/NHD Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Programme Managers Geneva, Switzerland: WHO, 2001, 140 pp. [Google Scholar]
- 63.Wu T, Wang X, Wei C, Cheng H, Wang X, Li Y, Ge-Dong Zhao H, Young P, Li G, Wang Z. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol 98: 598–604, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Yepez R, Estevez E, Galan P, Chauliac M, Davila M, Calle A, Estrella R, Masse-Raimbault AM, Hercberg S. [High altitude anemia: validity of definition criteria] Sante 4: 9–13, 1994. [Article in French]. [PubMed] [Google Scholar]
- 65.Yip R. Altitude and birth weight. J Pediatr 111: 869–876, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomized placebo-controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with hemoglobin ≥13.2 g/dl. BJOG 114: 684–688, 2007 [DOI] [PubMed] [Google Scholar]