Abstract
We used recombinant adeno-associated virus (rAAV)-mediated gene delivery to overexpress a mutant of rat leptin yielding a protein that acts as a neutral leptin receptor antagonist. The long-term consequences of this overexpression on body weight homeostasis and physical activity, as assessed by voluntary wheel running (WR), were determined in F344 × Brown Norway (BN) rats. Leptin antagonist overexpression was confirmed by examination of mRNA levels in the hypothalamus. Food consumption and body weight gain were exacerbated in the antagonist group during both chow and high-fat feeding periods over the 192-day experiment. In a second experiment, a lower dose of antagonist vector was used that resulted in no change in food consumption but still increased body weight. The degree of antagonist overexpression was sufficient to partially block signal transducer and activator of transcription 3 (STAT3) phosphorylation due to administration of an acute submaximal dose of leptin. Rats were provided free access to running wheels for 4 days during both the chow and high-fat feeding periods. With both antagonist doses and during both chow and high-fat feeding, WR was substantially less with antagonist overexpression. In contrast, when leptin was overexpressed in the hypothalamus, WR activity was increased by greater than twofold. At death, adiposity and serum leptin levels were greater in the antagonist group. These data indicate that submaximal central leptin receptor blockade promotes obesity and diminishes WR activity. These findings underscore the critical role of unrestrained leptin receptor activity in long-term energy homeostasis and suggest that even minor disruption of leptin receptor function can promote obesity.
Keywords: leptin resistance, gene therapy, high-fat feeding, voluntary wheel running
at the time of its discovery, the adipocyte-derived hormone leptin sparked great interest as a potential therapy for obesity (1, 10). The interest in this compound has waned because of the reduced efficacy and sensitivity of leptin in obese humans (15) and animals (22). Although of questionable therapeutic potential, leptin is an essential hormone for maintaining energy homeostasis. Nowhere is this more evident than in the overt obesity phenotypes resultant from the absence of leptin or leptin receptors due to genetic mutations (9, 10, 27). Extensive studies in animals with normal genetic background have documented the potent role of leptin in regulating feeding and energy expenditure. These studies invariably involve introduction of exogenous leptin. On the other hand, it is more challenging to evaluate the importance of endogenous leptin and leptin receptor activity in long-term body weight homeostasis. Such knowledge is pivotal for understanding the role of leptin in body weight homeostasis and obesity prevention. Long-term leptin receptor blockade would be one approach to tackle this important issue.
Evolutionary pressure has resulted in redundancy in both anorexic and orexigenic pathways to preserve and regulate energy stores in order to protect against starvation. For the most part, leptin and other anorexic agents cause only transient feeding and weight reduction (11, 33). Emerging evidence has led to theories suggesting that the dominant tone within the central nervous system for energy intake is orexigenic (23). Moreover, despite this dominance, the orexigenic pathway is mostly quiescent, whereas the more feeble anorexic pathways are nearly fully activated (9). Thus one might predict that small deficits in anorexic input such as a suppression of endogenous leptin function would result in consequential weight gain, especially if other anorexic pathways did not compensate for the deficit.
In addition to this balance between anorexic and orexigenic tone, energy expenditure also critically impacts long-term body weight regulation. As such, physical activity is an important component of energy expenditure. Leptin is known to enhance overall energy expenditure by increasing brown fat thermogenesis (16) and white fat lipolysis (32). Despite several lines of evidence indicating leptin's participation in the regulation of physical activity, it was only recently demonstrated that exogenous administration of leptin elevates spontaneous physical activity (4). The importance of normal endogenous levels of leptin in modulating physical activity, such as voluntary wheel running (WR), is unsubstantiated.
In the face of these complex body weight homeostatic mechanisms, we set out to examine the role of central leptin receptor activity in long-term body weight regulation through submaximal leptin receptor blockade. To this end, we used recombinant adeno-associated virus (rAAV)-mediated gene delivery to overexpress a mutant of rat leptin, yielding a protein that acts as a neutral leptin receptor antagonist, and examined the long-term consequences on body weight homeostasis and voluntary WR during periods of chow and high-fat (HF) feeding.
RESEARCH DESIGN AND METHODS
Experimental Animals
Three-month-old male F344 × Brown Norway (F344×BN) rats were obtained from Harlan Sprague Dawley (Indianapolis, IN). On arrival, rats were examined and remained in quarantine for 1 wk. Animals were cared for in accordance with the principles of the Guide for the Care and Use of Laboratory Animals, and protocols were approved by the University of Florida Institutional Animal Care and Use Committee (IACUC). Rats were housed individually with a 12:12-h light-dark cycle (0700 to 1900). During the experimental period, rats were fed either a standard rodent chow (15% kcal from fat, 3.41 kcal/g; Diet 7912, Harlan Teklad, Madison, WI) or a HF diet (60% kcal from fat, 5.24 kcal/g; D12492, Research Diets, New Brunswick, NJ).
Experimental Design
The first experiment consisted of two parts, each involving separate rats administered either control vector or different doses of rAAV-leptin antagonist by intracerebroventricular injection. In the first part of this experiment, rats were administered control vector or the higher dose of rAAV-leptin antagonist vector. Rats (n = 5/group) were allowed access to food and water ad libitum, and food consumption and body weight were recorded daily to weekly. During the 192-day treatment period, whole body adiposity was periodically assessed by time-domain nuclear magnetic resonance (TD-NMR) with a Minispec lean fat analyzer (Bruker Optics, The Woodlands, TX). Validation of TD-NMR methodology has been provided elsewhere (29). From day 83 to day 140, the diet was switched from standard chow to a 60% HF diet (5.24 kcal/g); during periods of both chow and HF feeding rats were allowed free access to running wheels for 4 days, and the extent of WR was recorded daily.
In the second part of this first experiment, rats were administered control vector or the lower dose of rAAV-leptin antagonist (n = 12 rats/group), and only the chow diet was provided. At day 34, running wheels were provided for 4 days and the extent of WR was assessed. At the termination of the experiment (day 87) leptin signaling was determined in the hypothalamus by administration of two separate doses of intracerebroventricular leptin.
In a second experiment, rats (n = 8–10/group) were administered control vector and rAAV-leptin by intracerebroventricular injection. At day 105, after rAAV-leptin or control vector delivery, the rats were allowed free access to running wheels for 4 days and the extent of WR was recorded daily.
Production of rAAV Vectors
Rat leptin antagonist DNA was constructed by PCR-based mutagenesis of the rat leptin gene (SeqWright, Houston, TX) to yield the sequence of a protein previously characterized to block leptin receptor signaling and leptin-induced physiological responses (35). The wild-type sequence, TTGGACCTT, corresponding to amino acids leucine (L39), aspartic acid (D40), and phenylalanine (F41), was mutated to GCAGCCGCC, corresponding to alanine, alanine, alanine. The sequence of the leptin antagonist construct was verified after PCR-based mutagenesis but before vector production.
Rat leptin or leptin antagonist cDNA under the control of a chicken β-actin promoter from pTR-betaObW (20) was subcloned into pUCDM transfer plasmid. Recombinant baculoviruses were constructed with the MultiBac Expression System (2). Serum-free medium-adapted Sf9 cells were used for large-scale rAAV type 1 preparations (31). Vectors were purified and concentrated and physical rAAV particle titers were determined as described previously (36).
rAAV Vector Administration
A single dose (5 × 1012 physical particles/ml) of control vector encoding green fluorescent protein (GFP, 5 μl), rAAV-leptin antagonist (either 2 or 5 μl), or rAAV-leptin (5 μl) was delivered by intracerebroventricular injection into the third cerebral ventricle as previously described (20). The coordinates for injection are 1.3 mm anterior to bregma, 9.4 mm ventral from the skull surface, at an angle of 20° anterior to posterior.
Wheel Running
Rats were housed in cages equipped with Nalgene Activity Wheels (1.081-m circumference, Fisher Scientific, Pittsburgh, PA) that allowed free access to the wheel. Each wheel was equipped with a magnetic switch and counter. The number of revolutions was recorded daily.
Tissue Harvesting
Rats were killed by thoracotomy under 150 mg/kg pentobarbital anesthetic. The circulatory system was perfused with 20 ml of cold saline, and brown adipose tissue (BAT), epididymal, perirenal, and retroperitoneal white adipose tissues (EWAT, PWAT, and RTWAT, respectively), and hypothalami were excised. Protein concentrations were determined with the DC protein assay kit (Bio-Rad, Hercules, CA).
Western Blot Analysis
Protein homogenate (20 μg) was separated on a SDS-PAGE gel and electrotransferred to nitrocellulose membranes (17). Immunoreactivity was assessed with antibodies specific to phospho-tyrosine 705 of signal transducer and activator of transcription 3 (STAT3), either phosphorylated or unphosphorylated STAT3 (Cell Signaling, Danvers, MA), protein tyrosine phosphatase 1B (PTP1B; Calbiochem, San Diego, CA), or BAT uncoupling protein 1 (UCP1, Millipore, Billerica, MA).
RT-PCR
Expression levels of hypothalamic leptin receptor, neuropeptide Y (NPY), and suppressor of cytokine signaling 3 (SOCS3) were identified by relative quantitative RT-PCR with the QuantumRNA 18S Internal Standards kit (Ambion, Austin, TX) as described previously (34). Leptin antagonist transgene expression was determined under identical conditions with primers 5′-TGACACCAAAACCCTCATCA-3′ (sense) and 5′-TGAGCTATCTGCAGCACGTT-3′ (antisense). Brain-specific homeobox transcription factor (Bsx) expression levels were determined with primers 5′-CTTATTTCCTGGCCACCTCA (sense) and 5′-CTGGAACCACGTTTTCACCT (antisense).
Serum Leptin
Radioimmunoassay was used to determine serum leptin (Millipore).
Statistical Analysis
Data were analyzed by two-way ANOVA with repeated measures when appropriate. A post hoc test (Bonferroni) was applied to determine individual differences between means. A P value of <0.05 was considered significant.
RESULTS
Leptin Antagonist Overexpression
Vectors were delivered into a region of the third ventricle that passes through the hypothalamus. Such delivery was previously demonstrated to result in gene expression in cells located along the wall of the ventricle and in the bed nucleus of the anterior commissure, ventrally in the preoptic area, and in the suprachiasmatic nucleus as evidenced by fluorescence of GFP. Caudally, GFP-positive cells were observed in the anterior hypothalamus, paraventricular nucleus, dorsomedial hypothalamus area, and suprachiasmatic nucleus (7). Both the gene construct for leptin and the leptin antagonist include secretory sequences; thus these compounds are secreted into surrounding tissue and into the third ventricle, thus likely reaching target sites throughout the brain. For example, rAAV-leptin gene delivery by this method elevated leptin levels in the cerebral spinal fluid by nearly twofold (21).
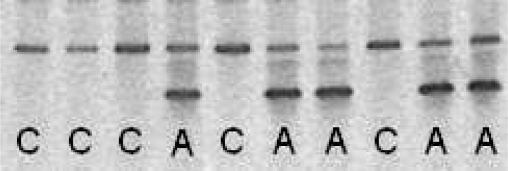
In the present study, leptin antagonist overexpression in hypothalamus was confirmed by RT-PCR. Every rAAV-leptin-antagonist-treated animal displayed expression, whereas no expression was detected in those administered rAAV-control vector (Fig. 1). Semiquantitative RT-PCR analysis of leptin antagonist expression was compared in the rats receiving the higher and lower doses of the antagonist vector. Expression levels in the rats receiving the higher dose were 28% greater than those receiving the lower dose (P = 0.032, data not shown).
Fig. 1.
Leptin antagonist overexpression in hypothalamus. Leptin antagonist transgene expression (lower band), determined by using RT-PCR, was evident in all rats receiving recombinant adeno-associated virus (rAAV)-leptin antagonists (A) and absent in those receiving control vector (C). Upper band represents 18S ribosomal RNA.
Body Weight and Food Consumption with Leptin Antagonist Overexpression
Chow feeding.
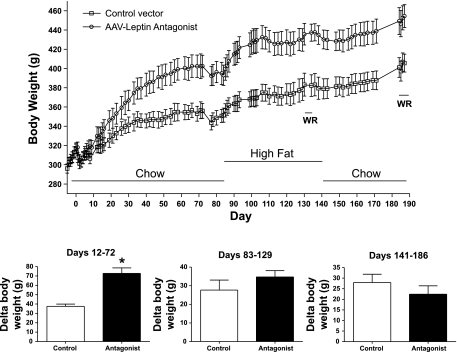
Before vector administration, all rats were steadily gaining weight. This normal growth rate was interrupted by the surgical procedure. As a result, body weight decreased for several days, followed by a resumption of normal weight gain (Fig. 2, top). Beginning at day 10 body weight of the rAAV-leptin antagonist group began to diverge from the rAAV-control rats, and by day 72 they were nearly 50 g heavier (Fig. 2, bottom left).
Fig. 2.
Top: body weight in rats after administration of control vector or high-dose rAAV antagonist. rAAV antagonist or control vectors were administered at day 0 in rats maintained on a chow diet for 82 days. After a series of tests and blood draw, the rats were switched to a 60% high-fat (HF) diet until day 140, after which they were returned to the chow diet for the remainder of the experiment. WR indicates days on which wheel running was evaluated. Values represent means ± SE of 5 rats/group. Bottom: change (Δ) in body weight over the indicated days, representing the initial chow feeding period after recovery from surgery and before blood draw (left), the HF feeding period before evaluation of WR (center), and the final chow feeding period before evaluation of WR (right). *P = 0.0005, difference from control by t-test.
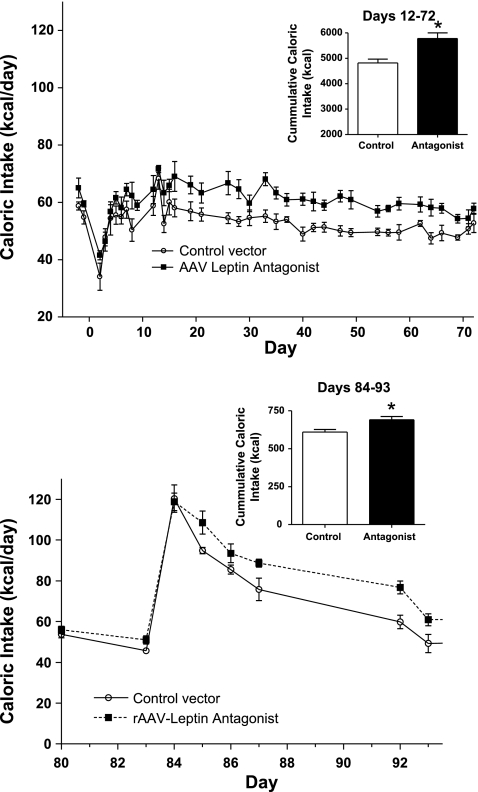
Food consumption also reflected the initial surgical effect, but beginning at day 9 antagonist-treated rats displayed a increase in caloric intake that became significant commencing at day 23 and continuing to day 70 (Fig. 3, top). Cumulative caloric intake over this period demonstrated a modest but significant 16.5% increase compared with control rats (Fig. 3, top, inset).
Fig. 3.
Top: daily caloric consumption after administration of control vector or high-dose rAAV antagonist during the period of chow feeding. Inset: cumulative food consumption is significantly elevated between days 12 and 72. *P = 0.016, difference in cumulative food consumption during this period. Bottom: caloric consumption after initiation of HF feeding at day 83 after administration of control vector or rAAV antagonist. Inset: cumulative food consumption is significantly elevated between days 84 and 93. Values represent means ± SE of 5 rats/group. *P = 0.019 for difference in cumulative food consumption.
High-fat feeding.
Previous studies revealed that leptin receptor blockade magnified and prolonged the hyperphagic response to HF feeding (35); thus HF feeding was posed as a challenge to test whether the extent of transgene expression of the leptin antagonist fully or submaximally blocks endogenous central leptin receptor activity. The rats were switched to a HF diet (60% of kcal from fat) at day 83, and daily food consumption was examined. As expected, the control rats initially doubled their caloric intake, followed by a period of normalization to a level isocaloric to chow feeding (Fig. 3, bottom). Earlier studies with pharmacological leptin antagonist treatment at a concentration of the leptin antagonist that fully blocked leptin receptor activity demonstrated increases in both the maximum level of caloric consumption and cumulative caloric intake with a HF challenge. Moreover, antagonist treatment delayed the initiation of the normalization by 4 days (35). In contrast to those findings, in the present study the rAAV-leptin antagonist neither increased the peak elevation in caloric intake nor delayed the initiation of the normalization. However, overall cumulative caloric intake during this period was elevated by 12% (Fig. 3, bottom, inset). These data are consistent with a submaximal blockade of the leptin receptor.
Introduction of HF food accelerated body weight gain in both control and antagonist vector-treated animals (Fig. 2, top), but the weight gains were not different from each other (Fig. 2, bottom center). Moreover, during both the chow and HF feeding periods, feed efficiency, i.e., weight gain per unit food consumption, was also greater in the antagonist vector-treated rats compared with control rats (Table 1).
Table 1.
Feed efficiency (body weight gain per unit of caloric consumption) with chow and HF feeding
| rAAV-Control | rAAV-Leptin Antagonist | |
|---|---|---|
| Chow feeding | 7.13±0.42 | 15.38±1.45* |
| HF feeding | 14.87±1.40 | 21.63±1.24* |
Data, in mg/kcal, represent means ± SE of 5 rats/group. rAAV, recombinant adeno-associated virus; HF, high fat.
P < 0.01 for difference from corresponding rAAV-Control by t-test.
At day 141 the HF food was removed, and all rats were provided chow food for the remainder of the study. During this period, food consumption was not different between the antagonist vector-treated and control groups (data not shown). Body weight, however, remained elevated with a growth rate parallel to that of the control group (Fig. 2, top), and the change in body weight over this period was not different (Fig. 2, bottom right).
Body Weight and Food Consumption After Lower Dose of rAAV-Leptin Antagonist
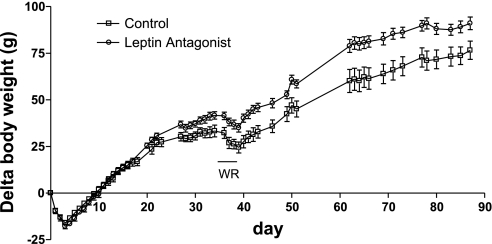
In this experiment, a lower dose of rAAV-leptin antagonist was employed, and as a result food consumption was nearly identical between the control rats and those with antagonist expression throughout the 87-day experiment (control 18.7 ± 0.5 vs. antagonist 18.1 ± 0.6 g/day). This afforded the opportunity to examine the rate of body weight gain and level of WR activity without the complications associated with changes in food intake. In these animals, despite the lack of change in food consumption, body weight gradually increased at a greater rate than in control animals over the 85-day experimental period (Fig. 4).
Fig. 4.
Change in body weight in rats after administration of control vector or the lower dose of rAAV antagonist. rAAV antagonist or control vectors were administered at day 0 in rats maintained on a chow diet for 87 days. Body weight was significantly different between groups commencing at day 27.
Wheel Running Activity
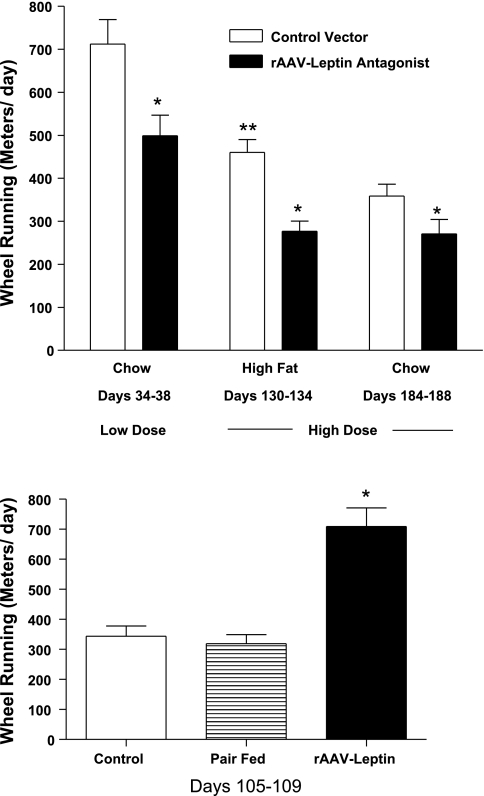
As one measure of physical activity, voluntary WR activity was assessed for a 4-day period during which the animals had free access to running wheels. WR was recorded after delivery of the high dose of rAAV-leptin antagonist during chow and HF feeding periods and after the lower dose of the antagonist vector treatment with chow feeding. With the high dose, the distance run was lower in leptin antagonist-treated rats compared with control rats, with a 25% decrease during chow feeding (days 184–188) and a 40% decrease during HF feeding (days 130–134) (Fig 5, top). In the experiment with the lower dose of rAAV-leptin antagonist, WR was assessed before the administration of the vectors and between days 34 and 38 after treatment. Rats provided the antagonist vector ran 30% less than their control counterparts (Fig 5, top). When individual rats were compared with their own preinjection WR running activity, there was a small, nonsignificant decrease in distance run in rats subsequently given control vector (80 ± 41 m), whereas there was a highly significant decrease (314 ± 68 m, P = 0.0007) in rats subsequently treated with rAAV-leptin antagonist.
Fig. 5.
Top: WR activity during chow and HF feeding periods in rats after administration of control vector or rAAV-leptin antagonist. WR was assessed for a 4-day period during chow feeding after the low dose of the antagonist vector (days 34–38) and during HF feeding (days 130–134) and chow feeding (days 184–188) after the high dose of antagonist vector. Values represent means ± SE of 5 or 6 rats/group. *P = 0.001, difference with antagonist treatment; **P = 0.03, difference between control chow-fed and control HF-fed rats after high dose of antagonist vector. Bottom: WR activity after administration of control vector, rAAV-leptin, or control vector pair fed the amount of food consumed by the rAAV-leptin group. WR was assessed for a 4-day period starting at day 105 after vector delivery. Values represent means ± SE of 8–10 rats/group. *P = 0.001 for difference with leptin treatment from control or pair fed by 1-way ANOVA.
For comparison, in a separate experiment we examined WR activity following long-term leptin treatment as opposed to antagonist treatment. Central leptin levels were elevated by rAAV-leptin-mediated overexpression. The extent of WR activity was assessed at day 105 after rAAV-leptin vector administration. At this point in the treatment, body weight was considerably lower in leptin-treated rats compared with control rats (322 ± 9 vs. 234 ± 6 g) and food consumption was ∼2 g/day less. WR activity was nearly twofold greater in leptin-treated compared with control rats. A portion of the control vector-treated rats were pair fed the amount of food consumed by the leptin-treated rats, and WR activity in this group was found to be unchanged from control activity (Fig. 5, bottom).
Adiposity, Serum Leptin Levels, and UCP1
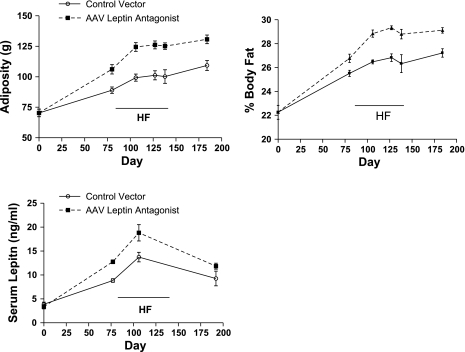
Adiposity levels were determined by TD-NMR in conscious rats periodically during the high-dose antagonist treatment. At all time points examined during both the chow and HF feeding periods, total body adiposity was greater in the antagonist-treated rats compared with the control rats (Fig. 6, top left). When expressed as percent body fat relative to body weight, a similar pattern was observed (Fig. 6, top right).
Fig. 6.
Top: whole body adiposity (left) and % adiposity relative to body weight (right) before vector delivery (day 0), before HF feeding (day 80), during HF feeding (days 106, 127, and 138), and after return to a chow diet (day 183) in control and high-dose rAAV antagonist-treated rats. Values represent means ± SE of 5 rats/group. P < 0.01 for difference between control and rAAV antagonist at each assessment day except day 0. Bottom: serum leptin before vector delivery (day 0), before HF feeding (day 77), during HF feeding (day 106), and after return to a chow diet (day 183) in control and high-dose rAAV antagonist-treated rats. Values represent means ± SE of 5 rats/group. P < 0.001 (day 77) and P < 0.05 (day 106) for difference between control and rAAV antagonist.
Serum leptin levels, another marker of adiposity, somewhat paralleled the NMR assessment of adiposity, with serum leptin levels greater with antagonist treatment compared with the control vector at all time points examined. In particular, serum leptin levels spiked during the period of HF feeding in both treatment groups (Fig. 6, bottom), consistent with dietary elevation of serum leptin (35).
At death (day 192), relative adiposity levels coincided with serum leptin and NMR assessment. The individual and sum of three adiposity tissues, PWAT, RTWAT, and EWAT, were elevated by nearly 50% in the leptin antagonist-treated rats compared with control rats (Table 2). In contrast, after long-term leptin antagonist overexpression, neither BAT levels (533 ± 32 vs. 569 ± 17 mg) nor UCP1 protein levels in BAT (Table 2) were elevated.
Table 2.
Rat weight, adiposity, and UCP1 protein levels at death
| rAAV-Control | rAAV-Leptin Antagonist | |
|---|---|---|
| Rat weight, g | 404±10 | 449±13* |
| PWAT, g | 1.46±0.11 | 1.94±0.07† |
| RTWAT, g | 5.57±0.69 | 9.04±0.51† |
| EWAT, g | 7.51±0.81 | 10.49±0.19† |
| Adiposity, g | 14.54±1.57 | 21.47±0.58† |
| UCP1 per BAT | 100±20.4 | 102±19.6 |
Data were derived from rats given the higher dose of leptin antagonist and represent means ± SE of 5 rats/group. Adiposity represents the sum of perirenal (PWAT), epididymal (EWAT), and retroperitoneal (RTWAT) white adipose tissue. Uncoupling protein 1 (UCP1) levels in rAAV-Control rats were set to 100, with SE adjusted proportionally; BAT, brown adipose tissue.
P < 0.05,
P < 0.01 for difference from corresponding rAAV-Control by t-test.
Leptin Signaling in Hypothalamus
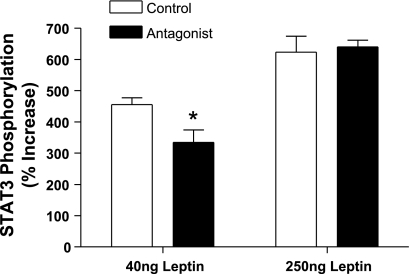
Leptin signaling following acute injection of two doses of leptin was examined at day 87 in the rats administered the lower dose of antagonist vector and corresponding control rats. The two doses of leptin corresponded to submaximal (40 ng) and supramaximal (250 ng) doses of leptin based on a previously determined full dose-response curve (16). In the present study, there was a dose-dependent increase in hypothalamic STAT3 phosphorylation, with stimulation of 4.5-fold with the submaximal dose and >6-fold with the supramaximal dose (Fig. 7). In the antagonist vector-treated rats, the submaximal dose of leptin was partially blocked whereas the supramaximal dose resulted in full leptin stimulation even in the presence of the leptin antagonist (Fig. 7).
Fig. 7.
Signal transducer and activator of transcription 3 (STAT3) phosphorylation following a single intracerebroventricular injection of either a submaximal (40 ng) or a supramaximal (250 ng) of leptin in control vector- or low-dose rAAV-leptin antagonist-treated rats. STAT3 phosphorylation was assessed at day 87 before termination of the experiment. Values represent % increase over vehicle-injected rats and are means ± SE of 6 rats/group. *P = 0.034, difference from control vector by t-test.
In rats receiving the higher dose of antagonist vector acute leptin signaling was not assessed, but several hypothalamic signaling factors associated with leptin signaling were examined at death. Basal STAT3 phosphorylation and PTP1B protein levels as well as leptin receptor, NPY, and SOCS3 expression levels were unchanged with antagonist treatment (Table 3). In addition, we examined expression of Bsx, which has a role in locomotor activity (14), but found no difference with treatment (Table 3).
Table 3.
Hypothalamic signaling factors
| rAAV-Control | rAAV-Leptin Antagonist | |
|---|---|---|
| p-STAT3, arbitrary units | 100±10.5 | 94.8±7.8 |
| PTP1B, arbitrary units | 100±12.2 | 85.4±3.9 |
| Leptin receptor (mRNA/18S rRNA) | 0.24±0.03 | 0.24±0.02 |
| NPY (mRNA/18S rRNA) | 0.60±0.01 | 0.64±0.03 |
| SOCS3 (mRNA/18S rRNA) | 0.31±0.01 | 0.37±0.02 |
| Bsx (mRNA/18S rRNA) | 0.53±0.01 | 0.54±0.02 |
Data were derived from rats given the higher dose of leptin antagonist and represent means ± SE of 5 rats/group. Phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and protein tyrosine phosphatase 1B (PTP1B) levels in rAAV-Control rats were set to 100, with SE adjusted proportionally. All other data represent the ratio of individual mRNA to 18S rRNA by RT-PCR. NPY, neuropeptide Y; SOCS3, suppressor of cytokine signaling 3; Bsx, brain-specific homeobox transcription factor.
DISCUSSION
Rodents (3, 12) and humans (13) lacking leptin exhibit overt obesity that is corrected by administration of leptin. In contrast, leptin therapy in obese humans with elevated leptin has proven to be futile, presumably because of pronounced leptin resistance, and this approach has been mostly abandoned (15). Leptin resistance thus is generally considered as a hindrance to pharmacological treatment for obesity, and little attention has been paid to its potential role in abnormal body weight homeostasis. Accumulating evidence in rodents suggests that leptin resistance increases susceptibility to dietary obesity. For instance, rats rendered leptin resistant by chronic elevation of leptin (18), a combination of HF feeding and elevated leptin (24), or chronic dietary consumption of fructose (25) subsequently exhibit exacerbated weight and adiposity gain on a HF diet compared with their respective HF-fed leptin-responsiveness controls, suggesting that leptin resistance, in and of itself, is one causative factor in obesity.
Leptin resistance is characterized by diminished central leptin receptor-mediated signaling (8), a characteristic that is pharmacologically similar to a submaximal leptin receptor blockade. Virus-mediated gene delivery of a leptin antagonist is a unique approach to achieve partial blockade of endogenous leptin receptor activity. This enabled us to explore the impact of deficient leptin receptor activity, a model for leptin resistance, on body weight homeostasis. ob/ob mice or obese fa/fa Zucker rats represent severe cases of diminished leptin receptor activity that lead to extreme obesity, profound metabolic disturbances, and other adverse manifestations (5, 9, 12). These genetic models reflect mixed outcomes of leptin function on maintenance of energy balance during both development and adulthood. Our model of partial leptin receptor blockade avoids the leptin-associated developmental abnormalities and addresses directly the importance of endogenous leptin receptor activity in energy homeostasis against a normal genetic background. Our model also enjoys the advantage of directly targeting central leptin action while minimally disturbing peripheral responses to leptin.
Use of this model system yielded two salient findings. First, a partial blockade of leptin receptor activity in the brain is sufficient to elevate obesity in the presence or absence of augmented food intake. Second, submaximal leptin receptor blockade diminishes the propensity to engage in WR activity, whereas overexpression of leptin increases WR activity. Moreover, these decreases and increases in WR occur in the absence of changes in food consumption, highlighting the role of leptin in modulating physical activity levels.
Submaximal leptin receptor blockade was achieved by central overexpression of a leptin mutant. This protein was previously characterized as a neutral leptin antagonist capable of full blockade of central leptin receptor activity in vivo (35). Simultaneous central administration of exogenous leptin and increasing doses of the leptin antagonist revealed a dose-dependent inhibition of leptin-induced hypothalamic STAT3 phosphorylation (35). When delivered with exogenous leptin in a 7-day central infusion study, the leptin antagonist blocked the leptin-mediated anorexic effects as well as the increase in BAT UCP1 protein and STAT3 phosphorylation (35).
Overexpression of this antagonist resulted in a moderate increase in caloric intake accompanied by increases in both body weight and adiposity. A second, lower dose of the antagonist vector did not alter food consumption but augmented body weight, suggesting that energy expenditure was diminished. Although no direct measure of energy expenditure was performed, food efficiency was enhanced and the propensity for voluntary WR was diminished. Although it is well established that leptin enhances energy expenditure, it has only recently been demonstrated that leptin increases spontaneous physical activity in Sprague-Dawley rats (4). The present study supports this finding with the observation that leptin overexpression increases voluntary WR. On the other hand, in the absence of leptin or leptin receptor function in either the ob/ob mouse (6, 30) or the obese Zucker rat (26, 28), physical activity levels are reduced. Moreover, the diminished WR activity in ob/ob mice is partially reversed by leptin administration (30). In the present study, overexpression of the leptin antagonist against a normal genetic background in rats decreased WR, thus highlighting the importance of leptin in maintaining daily volitional activity. Moreover, the decrease in WR due to antagonism of the leptin receptor was independent of food intake and occurred both early after initiation of the antagonist treatment, when body weights were similar, and some 185 days after treatment, when body weights were considerably different between control and antagonist groups, suggesting that the decreased WR was a direct action of the antagonist and not secondary to body weight differences.
Evidence that overexpression of the leptin antagonist resulted in a partial blockade of leptin receptor signaling stems from both signaling and physiological assessments. First, leptin receptor activation triggers several downstream signals including STAT3 phosphorylation. Previous studies detailing the dose-response leptin stimulation of hypothalamic STAT3 phosphorylation indicated that the Kact for leptin is 41 ng, with a maximum stimulation achieved at 100 ng after intracerebroventricular injection (17). Thus in the present study the efficacy of the leptin antagonist was assessed by an acute injection of leptin at a dose equal to the Kact and a dose that was 2.5-fold greater than the dose necessary for maximum stimulation. In our previous study (19, 35), infusion of a pharmacological dose of the leptin antagonist protein was able to fully block stimulation of STAT3 phosphorylation by exogenous leptin administration. The present study demonstrated that acute administration of leptin at a dose equivalent to the Kact was partially blocked in rats with antagonist overexpression, whereas the supramaximal dose was not. These results suggest that the expression level of the leptin antagonist was capable of inhibition of leptin doses within the physiological range but easily overcome by a supramaximal dose of leptin. Overt changes in other components of the leptin signaling cascade were lacking. PTP1B levels, NPY mRNA, and leptin receptor expression were unchanged. Similarly, basal phosphorylated STAT3 levels were unaltered by antagonist overexpression.
Second, our contention that the central antagonist overexpression achieved a partial leptin receptor blockade is also supported by the HF-related feeding response in the antagonist group. Normally, when rats are provided a HF diet they consume a greater amount of calories for a limited period, and gradually intake is normalized until it is isocaloric with chow-fed animals. This normalization of caloric intake following HF feeding is leptin dependent (35). Indeed, when we employed a concentration of the leptin antagonist capable of full blockade of leptin receptor activity (35), the peak of the HF-evoked hyperphagia was greater and the initiation of the normalization process was delayed compared with control rats. In contrast, in the present study, in response to HF feeding, antagonist overexpression neither increased the peak of caloric intake nor delayed the initiation of the normalization process. Overall, cumulative calorie consumption during the 24-day HF feeding period was elevated relative to that of control rats, suggesting some inhibition of exogenous leptin action, yet not to the degree realized with antagonist infusion in our previous study (35). Collectively, these signaling and physiological data suggest that the level of central antagonist overexpression in the present study achieves only submaximal leptin receptor blockade.
Perspectives and Significance
These findings indicate that even a partial blockade of leptin receptor activity in the brain is sufficient to diminish WR activity as well as elevate obesity. The latter occurs both in conjunction with augmented food consumption and in the absence of increased food intake. These data indicate the critical role of unfettered leptin receptor activity in long-term energy homeostasis, including physical activity, and suggest that even minor disruption of leptin receptor function, similar to that which occurs with partial leptin resistance, can promote obesity.
GRANTS
Supported by National Institute on Aging Grant AG-26159, the University of Florida Institute on Aging, Claude D. Pepper Older Americans Independence Center NIH P30-AG-028740, and the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol 22: 1583–1587, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Choi YH, Li C, Hartzell DL, Little DE, Della-Fera MA, Baile CA. ICV leptin effects on spontaneous physical activity and feeding behavior in rats. Behav Brain Res 188: 100–108, 2008 [DOI] [PubMed] [Google Scholar]
- 5.da Silva BA, Bjorbaek C, Uotani S, Flier JS. Functional properties of leptin receptor isoforms containing the gln→pro extracellular domain mutation of the fatty rat. Endocrinology 139: 3681–3690, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q J Exp Physiol 72: 549–559, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Dhillon H, Kalra SP, Prima V, Zolotukhin S, Scarpace PJ, Moldawer LL, Muzyczka N, Kalra PS. Central leptin gene therapy suppresses body weight gain, adiposity and serum insulin without affecting food consumption in normal rats: a long-term study. Regul Pept 99: 69–77, 2001 [DOI] [PubMed] [Google Scholar]
- 8.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96: 1658–1663, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101: 4531–4536, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueiras R, Lopez M, Lage R, Perez-Tilve D, Pfluger P, Mendieta-Zeron H, Sakkou M, Wiedmer P, Benoit S, Datta R, Dong JZ, Culler M, Sleeman M, Vidal-Puig A, Horvath T, Treier M, Dieguez C, Tschop MH. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology 149: 3009–3015, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proietto J, Thorburn AW. The therapeutic potential of leptin. Expert Opin Investig Drugs 12: 373–378, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol Endocrinol Metab 273: E226–E230, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 104: 1111–1117, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia 48: 1075–1083, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Scarpace PJ, Matheny M, Zhang Y, Cheng KY, Tumer N. Leptin antagonist reveals an uncoupling between leptin receptor signal transducer and activator of transcription 3 signaling and metabolic responses with central leptin resistance. J Pharmacol Exp Ther 320: 706–712, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tumer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology 143: 3026–3035, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Scarpace PJ, Matheny M, Zhang Y, Tumer N, Frase CD, Shek EW, Hong B, Prima V, Zolotukhin S. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology 42: 548–561, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Scarpace PJ, Zhang Y. Elevated leptin: consequence or cause of obesity? Front Biosci 12: 3531–3544, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Shapiro A, Matheny M, Zhang Y, Tumer N, Cheng KY, Rogrigues E, Zolotukhin S, Scarpace PJ. Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats. Diabetes 57: 614–622, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 295: R1370–R1375, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern JS, Johnson PR. Spontaneous activity and adipose cellularity in the genetically obese Zucker rat (fafa). Metabolism 26: 371–380, 1977 [DOI] [PubMed] [Google Scholar]
- 27.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Thorburn AW, Proietto J. Biological determinants of spontaneous physical activity. Obes Rev 1: 87–94, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12: 150–160, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Tou JC, Wade CE. Determinants affecting physical activity levels in animal models. Exp Biol Med (Maywood) 227: 587–600, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Urabe M, Nakakura T, Xin KQ, Obara Y, Mizukami H, Kume A, Kotin RM, Ozawa K. Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J Virol 80: 1874–1885, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem 274: 17541–17544, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes 46: 1782–1785, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 285: R1011–R1020, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Matheny MK, Tumer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after high-fat feeding are leptin dependent. Am J Physiol Regul Integr Comp Physiol 292: R868–R874, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28: 158–167, 2002 [DOI] [PubMed] [Google Scholar]