Abstract
BACKGROUND
Early prenatal androgenization (PA) accelerates follicle differentiation and impairs embryogenesis in adult female rhesus monkeys (Macaca mulatta) undergoing FSH therapy for IVF. To determine whether androgen excess in utero affects follicle development over time, this study examines whether PA exposure, beginning at gestational days 40–44 (early treated) or 100–115 (late treated), alters the decline in serum anti-Mullerian hormone (AMH) levels with age in adult female rhesus monkeys and perturbs their ovarian response to recombinant human FSH (rhFSH) therapy for IVF.
METHODS
Thirteen normal (control), 11 early-treated and 6 late-treated PA adult female monkeys had serum AMH levels measured at random times of the menstrual cycle or anovulatory period. Using some of the same animals, basal serum AMH, gonadotrophins and steroids were also measured in six normal, five early-treated and three late-treated PA female monkeys undergoing FSH therapy for IVF during late-reproductive life (>17 years); serum AMH also was measured on day of HCG administration and at oocyte retrieval.
RESULTS
Serum AMH levels in early-treated PA females declined with age to levels that were significantly lower than those of normal (P ≤ 0.05) and late-treated PA females (P ≤ 0.025) by late-reproductive life. Serum AMH levels positively predicted numbers of total/mature oocytes retrieved, with early-treated PA females having the lowest serum AMH levels, fewest oocytes retrieved and lowest percentage of females with fertilized oocytes that cleaved.
CONCLUSIONS
Based on these animals, early PA appears to program an exaggerated decline in ovarian reserve with age, suggesting that epigenetically induced hormonal factors during fetal development may influence the cohort size of ovarian follicles after birth.
Keywords: prenatal androgens, anti-Mullerian hormone, aging, ovarian reserve, IVF
Introduction
Emerging data implicate critical times during fetal development when steroids permanently alter, or program, the physiology of the fetus to modify its reproductive function after birth. Most notably, experimentally induced prenatal testosterone excess in early gestation programs LH hypersecretion in the late-gestational fetus and newborn female rhesus monkey (Macaca mulatta), leading to hyperandrogenism after birth (Abbott et al., 2008, 2009). In adulthood, such early prenatal testosterone-treated female monkeys show LH hypersecretion, resulting from reduced hypothalamic sensitivity to steroid negative feedback (Dumesic et al., 2007; Abbott et al., 2008) which, serving as a component of ovarian hyperandrogenism, promotes ovulatory dysfunction and formation of polycystic ovaries (Eisner et al., 2002; Abbott et al., 2005, 2007).
In their mid- to late-reproductive years, adult female monkeys with early prenatal testosterone treatment also show accelerated follicle differentiation and impaired embryo development during FSH therapy for IVF (Dumesic et al., 2002), resembling that of IVF patients with diminished ovarian reserve (Foong et al., 2005). Therefore, a clinically relevant research question is whether androgen excess during a critical time of fetal development alters ovarian follicular differentiation, leading to decreased numbers of ovarian follicles and diminished ovarian reserve after birth. If so, androgen excess in utero also should affect the production of anti-Mullerian hormone (AMH), a transforming growth factor-β-related protein produced by granulosa cells of growing pre-antral/small antral follicles and positively correlated with ovarian follicle cohort size or ovarian reserve (Weenen et al., 2004; Seifer and MacLaughlin, 2007). Because serum AMH levels positively predict ovarian follicular activity (young women: Nelson et al., 2007; young adult female cynomolgus monkeys: Appt et al., 2009) and progressively decrease with age to reach barely detectable levels at menopause (women: Burger et al., 2007; female rhesus monkeys: Downs and Urbanski. 2006), the present study examines whether early prenatal androgenization (PA) alters the decline in serum AMH levels with age in adult female rhesus monkeys and perturbs their ovarian response to FSH therapy for IVF.
Materials and Methods
Experimental animals
The general care and housing of rhesus monkeys (Macaca mulatta) at the National Primate Research Center (NPRC), University of Wisconsin, Madison have been described previously (Goy and Robinson, 1982; Goy and Kemnitz, 1983). The Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, as part of the University of Wisconsin Graduate School. Animal protocols and experiments were approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin, Madison. The animals were maintained according to recommendations of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act with its subsequent amendments. All animals were studied between September and May in order to avoid seasonal effects on menstrual cyclicity (Dailey and Neill, 1981; Nusser et al., 2001).
The study comprised 30 sexually mature female rhesus monkeys between 8.7 and 24.6 years of age. The control group consisted of 13 normal adult females; the PA study group comprised 17 females exposed in utero to testosterone propionate (TP). A detailed description of study design and methodology has been reported previously (Goy and Robinson, 1982). Briefly, females with PA were produced by injecting (s.c.) pregnant rhesus monkeys carrying female fetuses with 10–15 mg TP for 15–35 days, starting on either days 40–44 (early treated, n = 11) or days 100–115 (late treated, n = 6) postconception (total gestation, 165 days). This TP dosing schedule elevated circulating testosterone levels in fetal females to those normally found in fetal males during either the beginning of neuroendocrine development and target tissue differentiation, including the functional acquisition of hypothalamic sensitivity to hormone negative feedback (early treated), or ovarian follicle development (late treated) (Resko and Ellinwood, 1984; Resko et al., 1987; Abbott et al., 2008). Prenatal TP treatment completed by day 84 postconception in rhesus monkeys induced external genital masculinization and obliteration of the external vaginal orifice (Thornton et al., 2009) while female offspring exposed to TP beginning after day 110 postconception showed no genital virilization, except for clitoromegaly.
Serum AMH determinations
Forty-seven blood samples were collected at random times of the menstrual cycle or anovulatory period from these 30 adult female rhesus monkeys, between 8.7 and 24.6 years of age, with some females contributing more than one blood sample as they aged. In addition, blood samples collected from four normal, five early-treated and three late-treated PA perimenopausal females [22–25 years (menopause, 26–28 years)] participating in a dietary restriction study were used to determine nadir levels of AMH during the perimenopause.
Gonadotrophin stimulation for IVF
Owing mostly to age-related mortality typical for this species in captivity (Colman and Anderson, in press), only six normal, five early-treated and three late-treated PA females (total females, n = 14) also underwent ovarian stimulation for IVF during late-reproductive life (age >17 years, Study 2). All five surviving early-treated PA females contributed data, in contrast to only three out of four late-treated PA females. Attempting to balance number, age and BMI of normal females to those of both early-treated and late-treated PA female groups, final normal female group size of six was attained, with three controls also excluded due to IVF cycle cancellation. Each female received twice-daily i.m. injections of 30–45 IU recombinant human (rh) FSH (Follistim: Schering-Plough Pharmaceuticals, Kenilworth, NJ, USA), beginning on days 1–3 of the menstrual cycle [day 1 = the first day of menses (Dumesic et al., 2002, 2003)], or beginning during a period of anovulation. Serial blood samples (5 ml) were drawn from the saphenous vein during rhFSH therapy to quantify changes in circulating estradiol (E2) levels. rhFSH was administered until at least one follicle measuring ≥5 mm in diameter was detected using transabdominal ultrasonography (7.5 MHz convex probe; Aloka SSD-1400 scxanner; Wallingford, CT, USA). Recombinant HCG [rHCG, 1000 IU, i.m. (Ares Serono, NJ, USA)] was administered 1 day later to induce oocyte maturation, and laparoscopic oocyte retrieval was performed 27 h after rHCG. Blood samples taken on the day of rHCG administration confirmed that no animal experienced a spontaneous LH surge. Blood samples (5 ml) drawn before rhFSH treatment were used to quantify basal FSH, LH, AMH, E2, progesterone, 17-hydroxyprogesterone (17-OHP4), androstenedione (A4), testosterone and dihydrotestosterone (DHT) levels; additional blood samples drawn on the day of HCG administration and at oocyte retrieval were used to measure AMH and E2.
Laparoscopic ovarian retrieval
All large follicles (5–7 mm) on each ovary were aspirated individually into separate collection tubes with 200 µl protein-free TL-Hepes medium containing 0.1 mg/ml polyvinyl alcohol, as previously described (Dumesic et al., 2002, 2003). Oocytes from each of these large follicles were cultured separately in individual culture drops of modified CMRL medium containing 20% bovine calf serum so that their meiotic and developmental competence could be directly examined.
IVF and embryo culture
Oocytes were examined for nuclear maturation every 2 h and then inseminated approximately 2–4 h following extrusion of the first polar body (Dumesic et al., 2002, 2003). Metaphase II oocytes possessed one polar body in the perivitelline space and no visible nuclear structure in the cytoplasm. Metaphase I oocytes displayed no polar body in the perivitelline space and no visible nuclear structure in the cytoplasm. Prophase I oocytes displayed no polar body in the perivitelline space and a germinal vesicle (GV) in the cytoplasm. Spermatozoa collected by penile electroejaculation were co-incubated with mature oocytes for 12–16 h at 37°C in a humidified atmosphere of 5% CO2 in air, after which oocytes were examined for fertilization. All diploid zygotes were cultured in G1/G2 medium (Gardner and Lane, 1997) in 5% CO2, 5% O2 and 90% N2 at 37°C for up to 11 days, and were examined daily using Nomarski optics (×200–400 magnification) on a Nikon Eclipse TE300 inverted microscope with a heated (37°C) environmental control chamber (Bavister et al., 1983).
Hormone assays
All AMH, gonadotrophin and steroid assays were performed in the NPRC Hormone Assay Services Laboratory, as previously described (Foong et al., 2006; Dumesic et al., 2009). AMH was measured by enzyme immunoassay (Diagnostic Systems Laboratories, Minneapolis, MN, USA) and the intra- and inter-assay coefficients of variation (CVs) for AMH were 6.2 and 5.3%, respectively. The lower level of AMH detection was 0.2 pmol/l. The AMH enzyme immunoassay measures total AMH since both its capture and detection antibodies recognize epitopes in the pro-region of the AMH molecule (Al-Qahtani et al., 2005). Progesterone, testosterone and DHT also were measured by enzyme immunoassay. The intra-assay CVs were: progesterone, 10.0%; testosterone, 4.3% and DHT, 8.8%. The inter-assay CVs were: progesterone, 17.3%; testosterone, 16.9% and DHT, 20.1%. FSH, E2, 17OHP4 and A4 were measured by radioimmunoassay. The intra-assay CVs were: FSH, 4.5%; E2, 4.3%; 17OHP4, 5.6% and A4, 4.7%. The inter-assay CVs were: FSH, 10.8%; E2, 6.6%; 17OHP4, 9.8% and A4, 6.7%. Bioactive LH (bioLH) was measured by the mouse Leydig cell bioassay using the rhLH-RP1 reference preparation. The intra- and inter-assay CVs for LH were 9.1 and 18.1%, respectively.
Statistical analysis
Log-transformation of the hormonal data and arcsine transformation of the oocyte/embryo proportional data were performed to achieve homogeneity of variance and to increase linearity (Sokal and Rohlf, 1995). Regression models with estimation by generalized estimating equations (GEE) used female age and female type (i.e. experimental group) to predict serum AMH levels during adult life, while adjusting for intra-subject correlations due to more than one serum AMH determination per animal. A backward elimination procedure was used to select the final GEE models. Two-way analysis of variance (ANOVA) was used to examine the serum AMH levels, using female type and IVF cycle phase as factors to determine the independent effects of these variables and their possible interaction. One-way ANOVA was used to compare female type differences in IVF cycle characteristics, oocyte fertilization and embryo development, and serum hormone concentrations. Kruskal–Wallis one-way ANOVA was performed when data were not normally distributed. Linear regression was used to compare serum AMH levels basally, after rhFSH therapy and at oocyte retrieval with numbers of total and mature oocytes retrieved. All hormonal and oocyte/embryo proportional data are expressed as mean ± SE, back-transformed log10 mean (95% confidence intervals) or median (25, 75%ile), as appropriate. A P < 0.05 value was considered significant.
Results
Study 1: Effect of female age and type on serum AMH level
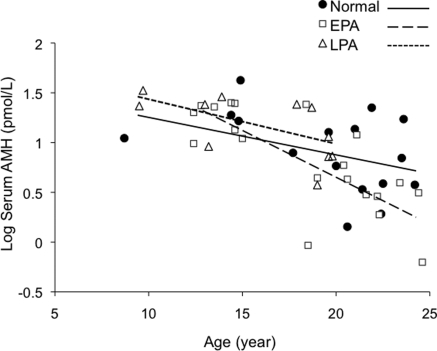
Within the age ranges observed, inverse linear relationships between log(AMH) and age were not dissimilar for normal females [log(AMH) = 2.019 − 0.097(AGE)] and late-treated PA females [log(AMH) = 2.279 − 0.100(AGE)] (Fig. 1). Since there were no significant differences between normal and late-treated PA females in the vertical shift in serum AMH levels (P = 0.8) or the slopes of the inverse linear relationships between log(AMH) and age (P = 0.9), data from these two female types were combined for statistical analysis. In contrast, the inverse linear relationship between log(AMH) and age for early-treated PA females [log(AMH) = 3.926 – 0.216(AGE)] showed a steeper negative slope with age. As a result, the age-related decline in serum AMH level over the 8.7 –24.6 year age range (age effect, P < 0.001, Fig. 1) was greater in early-treated PA compared with the other two female types (female type effect, P < 0.02). The negative slope of the regression line for serum AMH level versus female age in early-treated PA females crossed those of normal and late-treated PA females during mid-reproductive age (age-female type interaction, P = 0.006). By perimenopause (22–25 years), serum AMH levels in four normal, five early-treated PA and three late-treated PA females had reached the same nadir values, without a female type effect (normal, 3.6 ± 0.7; early-treated PA, 2.9 ± 0.7; late-treated PA, 2.9 ± 1.4 pmol/l females; P > 0.6).
Figure 1.
Regression model using female age and treatment type to predict serum AMH values. The inverse linear relationships between age and serum AMH level for normal (closed circles) and late-treated PA (open triangles) females were not dissimilar so that data from these two female types were combined. The age-related decline in serum AMH level over the 8.7–24.6 year age range (age effect, P ≤ 0.001) was greater in early-treated PA (EPA, open squares) versus the other female types (female type effect, P ≤ 0.02). Consequently, the negative slope of the regression line for serum AMH level versus female age in early-treated PA females (large dashed line) crossed those of normal females (solid line) and late-treated PA females (LPA small dashed line) during mid-reproductive age (age-female type interaction, P < 0.006).
Study 2: Effect of female type on IVF during late reproduction
Animal characteristics
Normal and early-treated PA females undergoing rhFSH treatment for IVF were similar in age (P = 0.2), as were normal and late-treated PA females (P = 0.1, Table I). Late-treated PA females were younger than early-treated PA females (P < 0.025). Normal, early-treated and late-treated PA females were comparable in BMI (P = 0.06). Ten animals were ovulatory, based on two serum progesterone levels above 1 ng/ml within 15 days of menses (Goy and Robinson, 1982), while two early-treated and two late-treated PA females were oligo-ovulatory.
Table I.
Characteristics and basal hormone levels of normal (control) rhesus monkeys (Macaca mulatta) and monkeys that underwent PA and recombinant human FSH (rhFSH) therapy for IVFa
| Females | Normal (n = 6) | Early-treated PA (n = 5) | Late-treated PA (n = 3) |
|---|---|---|---|
| Age (years)* | 21.6 ± 0.7 | 23.3 ± 0.8 | 19.2 ± 1.1b |
| BMI (kg/m2)* | 43.0 ± 2.1 | 34.7 ± 2.3 | 38.4 ± 2.9 |
| Serum FSH (ng/ml)** | 3.2 (2.3, 4.3) | 2.4 (1.6, 3.5) | 2.4 (1.5, 3.6) |
| Serum bioactive LH (ng/ml)** | 0.3 (0.2, 0.6) | 0.3 (0.2, 0.5) | 0.3 (0.1, 0.6) |
| Serum E2 (nmol/l)** | 0.72 (0.39, 1.35) | 0.14 (0.07, 0.27)c | 0.10 (0.04, 0.26)c |
| Serum progesterone (nmol/l)** | 0.64 (0.32, 0.95) | 0.32 (0.32, 0.64) | 0.32 (0.32, 0.64) |
| Serum 17OHP4 (nmol/l)** | 0.30 (0.30, 0.91) | 0.61 (0.30, 1.21) | 0.61 (0.30, 2.42) |
| Serum A4 (nmol/l)* | 0.69 ± 0.11 | 0.99 ± 0.12 | 1.16 ± 0.15 |
| Serum testosterone (nmol/l)* | 0.68 ± 0.14 | 0.91 ± 0.15 | 0.85 ± 0.19 |
| Serum DHT (nmol/l)* | 0.51 ± 0.10 | 0.45 ± 0.11 | 0.56 ± 0.14 |
17OHP4: 17-hydroxyprogesterone, DHT: dihydrotestosterone, A4: androstenedione, E2: estradiol, early-treated PA: starting PA on days 40–44 postconception (total gestation, 165 days), late treated PA: starting PA on days 100–115 postconception.
aMean ± SE*, back-transformed log10 mean (95% CI)**.
bP < 0.025 versus early-treated PA females.
cP < 0.01 versus normal females.
Serum hormone levels
Basal serum levels of FSH, bioLH, progesterone, 17-OHP4, A4, testosterone and DHT were comparable between normal and both early-treated and late-treated PA females (FSH, P = 0.4; bioLH, P = 0.9; progesterone, P = 0.4; 17-OHP4, P = 0.6; A4, P = 0.07; testosterone, P = 0.5; DHT, P = 0.8, Table I). Basal E2 levels were lower in both PA female groups than in normal females (P < 0.01 for both).
There was a significant female type effect (P ≤ 0.025) on serum AMH levels. Basal serum AMH levels in early-treated PA females of late-reproductive age were significantly lower than those of normal (P ≤ 0.05) and late-treated PA females (P ≤ 0.025, Table II). Serum AMH levels in early-treated PA females on the day of hCG administration and at oocyte retrieval remained significantly below those of normal (P ≤ 0.05, both days) and late-treated PA (P ≤ 0.025, both days) females. Although serum AMH levels tended to increase after rhFSH therapy (IVF cycle phase effect, P = 0.054; female type/IVF cycle phase interaction, P = 0.3), only four of six normal (67%), two of five early-treated PA (40%) and two of three late-treated PA females (67%) showed a rise in serum AMH levels on the day of rHCG administration compared with basal levels (Fig. 2). The rise of serum E2 during rhFSH therapy was highly variable among females so that maximal serum E2 levels on the day of rHCG administration were not significantly different between normal (3.28 ± 0.59), early-treated PA (1.24 ± 0.65) and late-treated PA (2.31 ± 0.84 nmol/l, P = 0.1) females.
Table II.
AMH levels and IVF cycle characteristics in PA and normal rhesus monkeysa
| Females | Normal (n = 6) | Early-treated PA (n = 5) | Late-treated PA (n = 3) |
|---|---|---|---|
| Serum AMH (pmol/l) | |||
| Basal* | 5.7 (3.6, 10.0) | 2.1 (1.4, 3.6)b | 8.6 (4.3, 17.8)c |
| Day of HCG* | 6.4 (3.6, 12.9) | 2.1 (0.7, 4.3)b | 14.3 (5.7, 37.1)c |
| At oocyte retrieval* | 5.7 (2.9, 11.4) | 1.4 (0.7, 3.6)b | 8.6 (3.6, 23.6)c |
| Administered rhFSH (IU)** | 750 (450, 795) | 990 (585, 1080) | 795 (698, 848) |
| Duration of rhFSH (days)** | 9.5 (5.0, 10.0) | 11.0 (6.8, 12.0) | 10.0 (10.0, 10.0) |
| Total oocytes retrieved** | 7.5 (4.0, 14.0) | 2.0 (1.0, 4.5)b | 11.0 (8.5, 12.5)c |
| MII oocytes retrieved** | 4.5 (4.0, 8.0) | 1.0 (0.0, 2.5)d | 7.0 (5.5, 8.0)e |
| Proportion MII oocytes* | 95 (75, 100) | 92 (63, 100)f | 81 (36, 100) |
| Incidence of fertilization* | 81 (53, 97) | 97 (71, 100)g | 55 (4, 90) |
| % females with fertilized oocytes that cleaved | 100 | 25b | 67 |
MII: metaphase II.
aBack-transformed log10 mean [95% confidence limits (CI)]*, median (25, 75%ile)**.
bP < 0.05 versus normal.
cP < 0.025 versus early-treated PA.
dP < 0.025 versus normal.
eP < 0.05 versus early-treated PA.
fn = 4, one female failed to produce oocytes.
gn = 3, one female failed to produce oocytes; another produced only two GV oocytes.
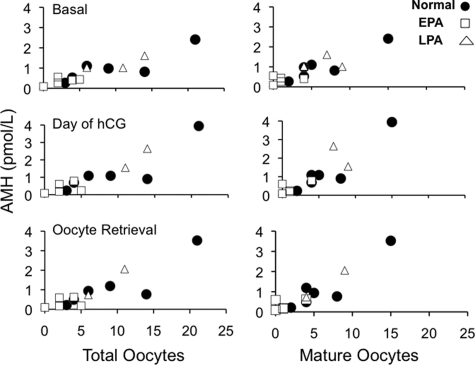
Figure 2.
Correlations between serum AMH levels and numbers of total and mature oocytes retrieved for six normal, five early-treated and three late-treated PA female monkeys that underwent ovarian stimulation for IVF during late-reproductive life (>17 years). Serum AMH levels basally, after recombinant human FSH therapy (day of HCG) and at oocyte retrieval positively correlated with numbers of total oocytes (basally: r2 = 0.81, P ≤ 2 × 10−5; after rhFSH: r2 = 0.80, P ≤ 4 × 10−5; at oocyte retrieval: r2 = 0.77, P ≤ 8 × 10−5) and mature oocytes retrieved (basally: r2 = 0.77, P ≤ 5 × 10−5; after rhFSH: r2 = 0.80, P ≤ 5 × 10−5; at oocyte retrieval: r2 = 0.84, P ≤ 1 × 10−5).
IVF cycle characteristics
The amount of rhFSH administered and the duration of rhFSH treatment were similar in all three groups (P = 0.4, both variables, Table II). Despite comparable amounts of rhFSH administered, however, fewer numbers of total and mature (metaphase II) oocytes were retrieved from early-treated PA females than from normal and late-treated PA females (total oocytes: P ≤ 0.05 versus normal, P ≤ 0.025 versus late-treated PA; mature oocytes: P ≤ 0.025 versus normal, P ≤ 0.05 versus late-treated PA). Serum AMH levels basally, after rhFSH therapy and at oocyte retrieval positively correlated with numbers of total oocytes (basally: r2 = 0.81, P ≤ 2 × 10−5; after rhFSH: r2 = 0.80, P ≤ 4 × 10−5; at oocyte retrieval: r2 = 0.77, P ≤ 8 × 10−5) and mature oocytes retrieved (basally: r2 = 0.77, P ≤ 5 × 10−5; after rhFSH: r2 = 0.80, P ≤ 5 × 10 ×10−5; at oocyte retrieval: r2 = 0.84, P ≤ 1 × 10 ×10−5, Fig. 2).
The proportion of oocytes completing meiotic maturation (P = 0.7) and the incidence of fertilization (P = 0.2) were comparable among the three groups (Table II). One early-treated PA female failed to produce oocytes at laparoscopic oocyte retrieval; another produced only two GV oocytes that did not further mature. Consequently, early-treated PA females received significantly higher amounts of rhFSH per mature oocyte retrieved (343 ± 73, n = 3) than late-treated PA (67 ± 73, n = 3) or normal (79 ± 51 IU/mature oocyte, n = 6) females (P < 0.05 versus both female groups). Moreover, the percentage of early-treated PA females having fertilized oocytes that cleaved was significantly lower than that of normal females (P < 0.05).
Discussion
The present study demonstrates that an exaggerated decline in AMH production with age occurs in early-treated PA adult female rhesus monkeys and accompanies diminished ovarian reserve following rhFSH therapy for IVF during late-reproductive life. These data suggest that epigenetically induced hormonal factors during fetal development influence the cohort size of ovarian follicles after birth. Our findings also confirm the value of AMH as an endocrine marker of ovarian follicular activity (Lee et al., 1996; Knight and Glister, 2003; Weenen et al., 2004; Burger et al., 2007; Seifer and MacLaughlin, 2007), given its negative interaction with female age in predicting ovarian responsiveness in IVF patients (Nelson et al., 2007) and its strong positive value in predicting ovarian follicle number in female cynomolgus monkeys (Appt et al., 2009). Importantly, rhFSH was administered alone, and in specified amounts, because FSH therapy lowers AMH production in some normal patients (Eldar-Geva et al., 2005) and patients with polycystic ovary syndrome (PCOS) (Eldar-Geva et al., 2005; Catteau-Jonard et al., 2007), but not in all individuals with PCOS (Laven et al., 2004), while LH/HCG may stimulate AMH secretion in some patients with PCOS as well (Laven et al., 2004). In addition, here we matched normal and PA female monkeys for BMI because AMH production in normal women and patients with PCOS is both negatively (Chen et al., 2008; Piouka et al., 2008) and positively correlated with insulin resistance (Piltonen et al., 2005; Crisosto et al., 2007).
Given that circulating AMH levels decline with age in adult female rhesus monkeys (Downs and Urbanski, 2006), our observation of an exaggerated age-related decline of AMH production in early-treated PA females has clinical implications. Specifically, serum AMH levels before and throughout rhFSH therapy were significantly reduced in early- versus late-treated PA females and versus controls during late-reproductive age when the early-treated PA females also showed decreased ovarian responsiveness to FSH. At all stages of IVF, serum AMH levels positively predicted the numbers of total and mature oocytes retrieved, with early-treated PA females having both the lowest serum AMH levels and the fewest oocytes retrieved. In early-treated PA females, decreased serum AMH levels and diminished ovarian reserve accompanied normal basal serum FSH levels, agreeing with clinical IVF studies showing serum AMH levels superior to those of FSH in predicting oocyte numbers (Ebner et al., 2006; Nelson et al., 2007).
Early PA programs LH hypersecretion owing to reduced hypothalamic sensitivity to steroid negative feedback (Dumesic et al., 2007; Abbott et al., 2008), with epigenetically induced neuroendocrine dysfunction more pronounced than intrinsic theca cell hyperandrogenism (Norman et al., 2007; Abbott et al., 2009). In terms of the endocrinology of ovarian aging, therefore, early PA closely resembles congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency and virilizing tumors, in which androgen excess in utero entrains LH hypersecretion, causing secondary ovarian hyperandrogenism (Barnes et al., 1994; Merke and Cutler, 2001; Stikkelbroeck et al., 2003). Consequently, early PA may enhance follicle recruitment in utero, uninhibited by AMH, which first appears in the primate ovary at the end of fetal life (Rajpert-De Meyts et al., 1999). After birth, such programmed development of ovarian function may then predispose to polyfollicular ovaries (Abbott et al., 1998, 2002), followed by an exaggerated decline of AMH production with age and diminished ovarian reserve. Such a mechanism differs from AMH overproduction in PCOS (Eldar-Geva et al., 2005; Piltonen et al., 2005; Pigny et al., 2006; Pellatt et al., 2007), perhaps because PA adult female rhesus monkeys do not exhibit the same degree of robust theca cell hyperandrogenism found in PCOS (Abbott et al., 2009) resulting from obvious augmented expression of several steroidogenic enzymes (Nelson et al., 1999, 2001).
An important study limitation is the small number of adult female monkeys in each group. Therefore, we combined data in Study 1 from normal and late-treated PA female groups because their inverse linear relationships between serum AMH levels and age were not dissimilar and an automated backwards statistical model of serum AMH levels and age eliminated both age (late-treated PA versus normal females, P = 0.9) and age/female type interaction (late-treated PA versus normal females, P = 0.9) as predictors. Sensitivity analyses comparing data between early-treated PA females and normal females alone (excluding late-treated PA females), and restricting analysis to females aged less than 20 years, did not alter data interpretation, with linear associations between serum AMH values and age maintained in each female group within this age range.
In addition, only three of the presently reported females (two early-treated PA, one normal) had stored sera available from their previous IVF cycle performed 4 years earlier (Dumesic et al., 2002), with serum AMH levels in the two early-treated PA females only 39% (4.3, 5.7 pmol/l) of normal (12.9 pmol/l) at this younger age. Therefore, diminished ovarian reserve already existed in these females during their earlier IVF cycle, and was unlikely to have been exaggerated by the greater numbers of previous IVF cycles performed in early-treated PA [3.0 (2.0, 3.5)] than in normal [1.0 (1.0, 1.0), P < 0.01] or late-treated PA [1.0 (0.5, 1.5), median (25, 75%ile), P < 0.05] females (Elder et al., 2008). Nor did our study examine fecundity of PA females, or granulosa cell-derived paracrine factors, although reduced inhibin production from ovarian aging (Elting et al., 2003) may have advanced follicle selection (Klein and Soules, 1998) in normal monkeys, thus increasing basal E2 levels.
An important question is how early PA exaggerates the age-related loss of ovarian response to FSH. In adult female rhesus monkeys, androgens promote follicle recruitment and granulosa cell proliferation via up-regulation of genes for FSH receptor, insulin-like growth factor I (IGF-I) receptor and IGF-I in granulosa cells and for IGF-I receptor and IGF-I in primordial follicle oocytes (Vendola et al., 1998, 1999a, b; Weil et al., 1998, 1999). Through this mechanism, early PA in monkeys could hasten depletion of the primordial follicle pool. As a more complex effect of PA on early follicle growth, PA in sheep increases follicle recruitment, while decreasing total follicle numbers in the fetal ovary (Steckler et al., 2005). In this regard, enhanced follicle recruitment in AMH null mice followed by pre-antral oocyte degeneration and early follicle atresia accompanies an exaggerated age-related loss of ovarian response to FSH (Visser et al., 2007). Therefore, early PA in female rhesus monkeys may alter the balance of follicle growth and atresia to reduce AMH in late-reproductive life and thereby exaggerate an age-related loss of ovarian response to FSH and oocyte quality (Ebner et al., 2006). These findings may be relevant to women with congenital adrenal 21-hydroxylase deficiency who often postpone conception and experience age-related menstrual dysfunction (Hagenfeldt et al., 2008).
Funding
NIH Grants U01 HD044650 and P51 RR000167; Schering-Plough Pharmaceuticals.
Acknowledgements
The authors thank the following personnel at the NPRC: D. Wade, S. Maves, S.L. Knowles, M. Shotsko and M. Brown for assistance with animal procedures; S.G. Eisele and the animal care staff of the NPRC for maintenance of the animals and computerized records; K. Brunner DVM and C. Cruzen DVM for veterinary care; Ann Marie Paprocki and R. Dee Schramm for technical assistance; and F.W. Wegner, D.J. Wittwer, S.T. Baum and S. Jacoris for assistance with hormone assays. The authors also thank Rebekah R. Herrmann and Bob Becker for preparation of the manuscript.
References
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–67. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Colman RJ, Kemnitz JW, Eisner JR, Dumesic DA. Prenatal androgen excess programs for polycystic ovarian syndrome in female rhesus monkeys. In: Chang J, Heindel JJ, Dunaif A, editors. Polycystic Ovary Syndrome. New York: Marcel Dekker Inc; 2002. pp. 119–133. [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Bruns CM, Barnett DK, Dumesic DA. Fetal programming of polycystic ovary syndrome. In: Kovacs G, Norman R, editors. Polycystic Ovary Syndrome. 2nd edn. Cambridge: Cambridge University Press; 2007. pp. 262–287. [Google Scholar]
- Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79:154–163. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:1–9. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtani A, Muttukrishna S, Appasamy M, Johns J, Cranfield M, Visser JA, Themmen AP, Groome NP. Development of a sensitive enzyme immunoassay for anti-Müllerian hormone and the evaluation of potential clinical applications in males and females. Clin Endocrinol (Oxf) 2005;63:267–273. doi: 10.1111/j.1365-2265.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- Appt SE, Clarkson TB, Chen H, Adams MR, Christian PJ, Hoyer PB, Wilson ME, Kaplan JR. Serum antimüllerian hormone predicts ovarian reserve in a monkey model. Menopause. 2009;16:597–601. doi: 10.1097/gme.0b013e3181906fb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Boatman DE, Leibfried LM, Loose M, Vernon MW. Fertilization and cleavage of rhesus monkey oocytes in vitro. Biol Reprod. 1983;28:983–999. doi: 10.1095/biolreprod28.4.983. [DOI] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Robertson DM, Dennerste L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update. 2007;13:559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- Catteau-Jonard S, Pigny P, Reyss AC, Decanter C, Poncelet E, Dewailly D. Changes in serum anti-mullerian hormone level during low-dose recombinant follicular-stimulating hormone therapy for anovulation in polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4138–4143. doi: 10.1210/jc.2007-0868. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yang WS, Chen CL, Wu MY, Yang YS, Ho HN. The relationship between anti-Mullerian hormone, androgen and insulin resistance on the number of antral follicles in women with polycystic ovary syndrome. Hum Reprod. 2008;23:952–957. doi: 10.1093/humrep/den015. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson R. Nonhuman primate calorie restriction. Antioxid Redox Signal. doi: 10.1089/ars.2010.3224. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisosto N, Codner E, Maliqueo M, Echiburú B, Sánchez F, Cassorla F, Sir-Petermann T. Anti-Müllerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2739–2743. doi: 10.1210/jc.2007-0267. [DOI] [PubMed] [Google Scholar]
- Dailey RA, Neill JD. Seasonal variation in reproductive hormones of rhesus monkeys: anovulatory and short luteal phase menstrual cycles. Biol Reprod. 1981;25:560–567. doi: 10.1095/biolreprod25.3.560. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female Rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Bird IM, Peterson E, Paprocki AM, Zhou R, Abbott DH. Reduced intrafollicular androstenedione and estradiol levels in early-treated prenatally androgenized female rhesus monkeys receiving FSH therapy for in vitro fertilization. Biol Reprod. 2003;69:1213–1219. doi: 10.1095/biolreprod.102.015164. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. PCOS and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimullerian hormone (AMH) levels predict follicle responsiveness to FSH in normoandrogenic ovulatory women undergoing GnRH analog/recombinant human FSH therapy for IVF-ET. Fertil Steril. 2009;92:217–221. doi: 10.1016/j.fertnstert.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77:167–172. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Margalioth EJ, Gal M, Ben-Chetrit A, Algur N, Zylber-Haran E, Brooks B, Huerta M, Spitz IM. Serum anti-mullerian hormone levels during controlled ovarian hyperstimulation in women with polycystic ovaries with and without hyperandrogenism. Hum Reprod. 2005;20:1814–1819. doi: 10.1093/humrep/deh873. [DOI] [PubMed] [Google Scholar]
- Elder K, Mathews T, Kutner E, Kim E, Espenberg D, Faddy M, Gosden R. Impact of gonadotrophin stimulation for assisted reproductive technology on ovarian ageing and menopause. Reprod Biomed Online. 2008;16:611–616. doi: 10.1016/s1472-6483(10)60472-5. [DOI] [PubMed] [Google Scholar]
- Elting MW, Kwee J, Korsen TJM, Rekers-Mombarg LTM, Schoemaker J. Aging women with polycystic ovary syndrome who achieve regular menstrual cycles have a smaller follicle cohort than those who continue to have irregular cycles. Fertil Steril. 2003;79:1154–1160. doi: 10.1016/s0015-0282(03)00152-3. [DOI] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Lesnick TG, Session DR, Walker DL, Dumesic DA. Diminished intrafollicular estradiol (E2) levels in women with reduced ovarian responsiveness to recombinant human follicle stimulating hormone (FSH) therapy for in vitro fertilization (IVF) Fertil Steril. 2005;83:1377–1383. doi: 10.1016/j.fertnstert.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome (PCOS) patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91:2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- Goy RW, Robinson JA. Prenatal exposure of rhesus monkeys to patent androgens: morphological, behavioral, and physiological consequences. Banbury Rep. 1982;11:355–378. [Google Scholar]
- Goy RW, Kemnitz JW. Early, persistent and delayed effects of virilizing substances delivered transplacentally to female rhesus monkeys. In: Zbinden G, Cuomo V, Racagni G, Weiss B, editors. Applications of Behavioral Pharmacology in Toxicology. New York: Raven Press; 1983. pp. 303–314. [Google Scholar]
- Hagenfeldt K, Janson PO, Holmdahl G, Falhammar H, Filipsson H, Frisen L, Thoren M, Nordenskjold A. Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod. 2008;23:1607–1613. doi: 10.1093/humrep/den118. [DOI] [PubMed] [Google Scholar]
- Klein NA, Soules MR. Endocrine changes of the perimenopause. Clin Obstet Gynecol. 1998;41:912–920. doi: 10.1097/00003081-199812000-00017. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. Local roles of TGF-β superfamily members in the control of ovarian follicle development. Anim Reprod Sci. 2003;78:165–183. doi: 10.1016/s0378-4320(03)00089-7. [DOI] [PubMed] [Google Scholar]
- Laven JSE, Mulders AGMGJ, Visser JA, Themmen AP, de Jong FH, Fauser BCJM. Anti-mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–323. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, Hasegawa Y, Noto RA, Schoenfeld D, MacLaughlin DT. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- Merke DP, Cutler GB., Jr New ideas for medical treatment of congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30:121–135. doi: 10.1016/s0889-8529(08)70022-7. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycysitc ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin K, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles-implications for individualization of therapy. Hum Reprod. 2007;22:2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- Nusser KD, Mitalipov S, Widmann A, Gerami-Naini B, Yeoman RR, Wolf DP. Developmental competence of oocytes after ICSI in the rhesus monkey. Hum Reprod. 2001;16:130–137. doi: 10.1093/humrep/16.1.130. [DOI] [PubMed] [Google Scholar]
- Pellatt L, Hanna L, Brincat M, Galae R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-mullerian hormone is increased in polycystic ovaries. J Clin Endocinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–945. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Müllerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–1826. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D. Antimullerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab. 2009;296:E238–E243. doi: 10.1152/ajpendo.90684.2008. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Jørgensen N, Graem N, Müller J, Cate RL, Skakkebaek NE. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- Resko JA, Ellinwood WE. Sexual differentiation of the brain of primates. In: Serio M, Motta M, Zanisi M, Martini L, editors. Sexual Differentiation: Basic and Clinical Aspects. New York: Raven Press; 1984. pp. 169–181. [Google Scholar]
- Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol Reprod. 1987;37:1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- Seifer DB, MacLaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88:539–546. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry, The Principles and Practice of Statistics in Biological Research. 3rd edn. New York: WH Freeman and Co; 1995. pp. 413–422. [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ. Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv. 2003;58:275–284. doi: 10.1097/01.OGX.0000062966.93819.5B. [DOI] [PubMed] [Google Scholar]
- Thornton J, Zehr JL, Loose MD. Effects of prenatal androgens on rhesus monkeys: a model system to explore the organizational hypothesis in primates. Horm Behav. 2009;55:633–645. doi: 10.1016/j.yhbeh.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicle growth in the primate ovary. J Clin Invest. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod. 1999a;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999b;61:353–357. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- Visser JA, Durlinger ALL, Peters IJJ, van den Heuvel ER, Rose UM, Kramer P, de Jong FH, Themmen APN. Increased oocyte degeneration and follicular atresia during the estrus cycle in anti-mullerian hormone null mice. Endocrinology. 2007;148:2301–2308. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BCJM, Themmen APN. Anti-mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83:2479–2485. doi: 10.1210/jcem.83.7.4917. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–2956. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]