Abstract
Nitric oxide (NO) participates in the cutaneous vasodilation caused by increased local skin temperature (Tloc) and whole body heat stress in humans. In forearm skin, endothelial NO synthase (eNOS) participates in vasodilation due to elevated Tloc and neuronal NO synthase (nNOS) participates in vasodilation due to heat stress. To explore the relative roles and interactions of these isoforms, we examined the effects of a relatively specific eNOS inhibitor, Nω-amino-l-arginine (LNAA), and a specific nNOS inhibitor, Nω-propyl-l-arginine (NPLA), both separately and in combination, on skin blood flow (SkBF) responses to increased Tloc and heat stress in two protocols. In each protocol, SkBF was monitored by laser-Doppler flowmetry (LDF) and mean arterial pressure (MAP) by Finapres. Cutaneous vascular conductance (CVC) was calculated (CVC = LDF/MAP). Intradermal microdialysis was used to treat one site with 5 mM LNAA, another with 5 mM NPLA, a third with combined 5 mM LNAA and 5 mM NPLA (Mix), and a fourth site with Ringer only. In protocol 1, Tloc was controlled with combined LDF/local heating units. Tloc was increased from 34°C to 41.5°C to cause local vasodilation. In protocol 2, after a period of normothermia, whole body heat stress was induced (water-perfused suits). At the end of each protocol, all sites were perfused with 58 mM nitroprusside to effect maximal vasodilation for data normalization. In protocol 1, at Tloc = 34°C, CVC did not differ between sites (P > 0.05). LNAA and Mix attenuated CVC increases at Tloc = 41.5°C to similar extents (P < 0.05, LNAA or Mix vs. untreated or NPLA). In protocol 2, in normothermia, CVC did not differ between sites (P > 0.05). During heat stress, NPLA and Mix attenuated CVC increases to similar extents, but no significant attenuation occurred with LNAA (P < 0.05, NPLA or Mix vs. untreated or LNAA). In forearm skin, eNOS mediates the vasodilator response to increased Tloc and nNOS mediates the vasodilator response to heat stress. The two isoforms do not appear to interact during either response.
Keywords: skin, nitric oxide, nitric oxide synthase 1, nitric oxide synthase 3, microdialysis
nitric oxide (NO) generation by nitric oxide synthase (NOS) is a well-established neuroendothelial effector mechanism involved in the control of the human cutaneous vasculature. NO generation participates in vasodilatory processes involved in direct application of heat to the skin and in thermoregulatory reflex-mediated vasodilation during whole body heat stress (25, 27, 28, 37, 49). While both direct skin heating and whole body heat stress lead to cutaneous vasodilation, the mechanisms involved in those processes appear to be largely independent. This independence was established when it was demonstrated that botulinum toxin does not alter the vasodilation induced by direct local skin warming yet all but abolishes the reflex vasodilation induced by whole body heat stress (28).
Local vasodilatory mechanisms involved in direct application of heat to skin, i.e., local warming, can increase skin blood flow (SkBF) to maximal levels (54). This vasodilation is biphasic with sensory nerves mediating an initial transient vasodilatory “peak” followed by a prolonged vasodilatory “plateau” that mediated primarily by NOS generation of NO (27, 37).
Thermoregulatory reflex vasodilator responses to heat stress involve increases in SkBF and sweat production to remove heat from the body to the environment and thus maintain thermal homeostasis in response to increases in internal and skin temperature (44, 45). As with local vasodilatory mechanisms in skin, these responses require NOS generation of NO, but also require activation of a branch of the sympathetic nervous system: the cutaneous active vasodilator system. This system involves efferent innervation of cutaneous arterioles and sweat glands by sympathetic cholinergic nerves in glabrous (hairy) skin. It has also been established that NO generation by NOS is involved in effecting approximately 30–40% of the cutaneous vasodilatory reflex response to heat stress (25, 49).
Recent in vivo experiments support the involvement of eNOS in the cutaneous vasodilation induced by increased Tloc in the human forearm (30) and of the nNOS isoform in the human calf (52). For skin vasodilation during whole body heat stress, in vivo studies support the involvement of nNOS in the human forearm (30). These studies used isoform selective antagonists administered separately and thus addressed the roles of eNOS and nNOS in isolation. Interactions between these two isoforms have been reported during vasodilatory responses in skeletal muscle where activity of both isoforms appears to be necessary for full NO-dependent vasodilation (13, 24, 34). No studies have examined interactions between the two NOS isoforms in skin. In the present study, we chose to use both separate and combined NOS isoform-specific antagonists to further examine the roles and potential interactions between eNOS and nNOS in the control of the cutaneous vasculature.
The primary hypotheses examined in the study were whether simultaneous eNOS and nNOS activity was necessary to cause full vasodilation during 1) local skin warming in the human forearm and/or 2) whole body heat stress. Our approach was to examine the effects of two water-soluble NOS antagonists, the relatively selective eNOS antagonist Nω-amino-l-arginine (LNAA) and the selective nNOS antagonist Nω-propyl-l-arginine (NPLA), administered separately and in combination, on SkBF responses to local skin warming and heat stress. If the combination of the antagonists attenuated vasodilatory responses to a greater extent than either antagonist separately, that would indicate that activity of both isoforms is required and that they interact in the response under investigation.
METHODS
All subjects were in good health, were nonsmokers, and were taking no medications. All subjects gave their written informed consent to participate in these studies. The studies conformed to the standards set by the Declaration of Helsinki. Local ethics committees approved all procedures.
Nine subjects participated in these experiments (5 men and 4 women). Their average age was 32 ± 3 yr, average weight was 70 ± 3 kg, and average height was 172 ± 2 cm. Subjects were instructed to forego caffeinated products on the day of the study. The menstrual phase of the female subjects was not controlled for because subjects served as their own control within each protocol. Although qualitative changes in thermoregulatory reflexes occur over the course of the menstrual cycle, the basic mechanisms involved do not differ between sexes (5, 6). In the present study, responses of female subjects did not differ perceptively from male subjects; therefore the results from the two sexes were combined.
For these experiments, LNAA (Axxora, San Diego, CA) was chosen as the eNOS antagonist. This water-soluble agent was chosen based on eNOS selectivity demonstrated by in vivo physiological studies done by our lab and others (30, 53). A perfusate concentration of 5 mM LNAA was used based on our prior experiments that showed administration of this concentration for 30 min reliably attenuated the vasodilatory response to exogenous ACh (30).
NPLA (Axxora) was chosen as the nNOS antagonist. Based on in vitro studies, NPLA is a highly selective antagonist of the nNOS isoform based on in vitro and in vivo studies (8, 60). This agent was chosen over the selective nNOS antagonist 7-nitroindazole (7-NI) that we had used in our prior studies of nNOS, because NPLA is water soluble and thus obviates the need to use DMSO as is required to dissolve 7-NI (31). Because DMSO was not needed, we were able to use a higher concentration of NPLA than was possible with 7-NI where the maximal attainable concentration for in vivo studies was limited to 2 mM due to potentially confounding DMSO effects (47). We chose a 5 mM concentration of NPLA based on preliminary studies that showed that perfusion of this concentration for 30 min did not attenuate vasodilator responses to exogenous ACh and hence was unlikely to inhibit eNOS (30, 31, 52).
Two protocols were employed: one with local warming of skin and another with whole body heat stress. In each protocol, four microdialysis probes of our own manufacture were inserted into forearm skin after ice anesthesia as previously described (12, 25). After insertion, the microdialysis probes were perfused with Ringer solution at a rate of 3 μl/min by a microinfusion pump for 140 min or longer to permit insertion trauma to resolve (1).
Protocol 1.
This protocol examined the effects of NOS antagonists on cutaneous vascular responses to local skin warming.
On arrival in the laboratory, each subject had four microdialysis probes placed on the ventral surface of the left forearm as outlined above. For experiments, subjects were placed in a supine position and instrumented to measure LDF from skin at all four microdialysis sites. Each LDF probe was equipped with a special probe holder equipped with heating elements and thermocouples to permit simultaneous LDF measurements and control of Tloc (27). Finally, a Finapres cuff was placed on a finger for continual measurement of pulse rate (PR) and mean arterial pressure (MAP; Finapres BP Monitor, Ohmeda, Madison, WI).
Data collection began with a 5- to 10-min control period with Tloc maintained at 34°C. After this period, the perfusate of one microdialysis site was maintained with Ringer solution, while the perfusate at a second microdialysis site was changed to 5 mM LNAA in Ringer solution. The perfusate at a third microdialysis site was changed to 5 mM NPLA in Ringer solution. The perfusate at fourth microdialysis site was changed to 5 mM LNAA and 5 mM NPLA in Ringer. Perfusion rates at all sites were 3 μl/min.
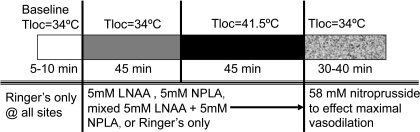
Tloc was maintained at 34°C for 45 min after which Tloc was increased slowly to 41.5°C at all sites to evoke vasodilation. A slow increase in temperature to 41.5°C was chosen to obviate activation of pain fibers, which evokes a vasodilation through mechanisms that are not solely dependent on NO generation by NOS (27). Finally, the perfusates at all sites were changed to 58 mM sodium nitroprusside (SNP) in Ringer solution to effect maximal vasodilation by an endothelium independent mechanism for data normalization (29, 33). The protocol is illustrated in Fig. 1.
Fig. 1.
Illustration of protocol 1. This protocol was designed to examine the effects of separate and combined blockade of endothelial nitric oxide synthase (eNOS) and neuronal NOS (nNOS) on the vasodilation induced by local skin warming. The perfusion rate at all microdialysis sites was 3 μl/min. Tloc, local skin temperature; LNAA, Nω-amino-l-arginine; NPLA, Nω-propyl-l-arginine.
Data are presented as means ± SE. For data analysis, cutaneous vascular conductance (CVC) was indexed as LDF (in mV) divided by MAP (in mmHg) to control for any fluctuations in blood pressure during data collection. Vasomotor responses were analyzed by comparing the mean levels of CVCs during the final 3 min of the two thermal periods. CVC responses were analyzed by repeated-measures ANOVA with the level of statistical significance defined as 0.05.
Protocol 2.
This protocol examined the effects of NOS antagonists on cutaneous vascular responses to whole body heat stress.
After arriving in the laboratory on the morning of the study, subjects were instrumented with four microdialysis probes as outlined above. Subjects were then placed in a supine position and instrumented to measure LDF from skin at all microdialysis sites (Moorlab Flowmeter, Moor Instruments, Devon, UK). Finally, a Finapres cuff was placed on a finger for PR and MAP measurement.
To induce thermoregulatory reflexes, subjects wore a tube-lined suit that was used to control skin temperature (Tsk) by perfusion with water of different temperatures. By varying the temperature of the water used to perfuse the suit, periods of normothermia, cold stress, and heat stress could be evoked (21, 46). The suit was perfused with cold water to lower Tsk and induce cold stress and warm water to increase Tsk to 39°C during heating periods. Over the suit, subjects wore a water-impermeable plastic garment to insulate them from the room environment and prevent evaporation of sweat. The water-perfused suit and water-impermeable garment covered the entire body with the exception of the arm from which LDF measurements were made. The head, hands, and feet of subjects were also left uncovered.
Internal temperature was monitored with a thermocouple placed in the sublingual sulcus (Tor). Tsk was recorded as the weighted electrical average from six thermocouples taped on the skin surface (21, 46). Pulse rate (PR) and MAP were recorded continuously from a finger (Finapres BP Monitor).
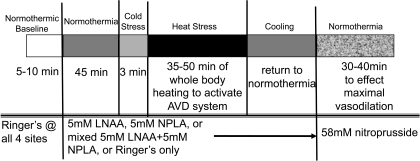
Data collection began with a 5- to 10-min normothermic control period during which Tsk was maintained at a level of 34°C to minimize any extant vasoconstrictor tone (22, 26, 41), and all microdialysis sites were perfused with Ringer solution at 3 μl/min. After this period, the perfusion of one microdialysis site was continued with Ringer solution. The perfusate at a second microdialysis site was changed to 5 mM LNAA in Ringer solution. The perfusate at a third microdialysis site was changed to 5 mM NPLA in Ringer solution. The perfusate at a fourth microdialysis site was changed to a solution of both 5 mM LNAA and 5 mM NPLA in Ringer. Perfusion rates at all sites were maintained at 3 μl/min. After perfusing these solutions for 30–35 min to allow for the antagonists to enter the intradermal space, Tsk was decreased to induce cold stress for 3 min after which Tsk was returned to normothermia. Tsk was then raised to 38–39°C and maintained at that level for 35–50 min to induce heat stress and thus activate the vasodilator system. After heat stress, subjects were cooled and returned to a normothermic Tsk of 34°C. All microdialysis sites were then perfused with 58 mM SNP to effect maximal vasodilation at each site. CVC values were normalized to these maximal levels for data analysis. The protocol is illustrated in Fig. 2.
Fig. 2.
Illustration of protocol 2. This protocol was designed to examine the effects of separate and combined blockade of eNOS and nNOS on the vasodilation induced by whole body heat stress. The perfusion rate at all microdialysis sites was 3 μl/min. AVD, active vasodilation.
Data are presented as means ± SE. For data analysis, CVC values were indexed as LDF/MAP and were normalized to their respective maxima as elicited by SNP at each site. This normalization allowed for comparisons between sites both within and between subjects. The vasomotor and NO responses to heat stress were analyzed by comparing the internal temperature thresholds for the initial increases in CVC at the different microdialysis sites. The internal temperature threshold for the onset of vasodilation for each site was defined as the level of Tor at which a sustained increase in CVC began during whole body heating. Tor thresholds were chosen from separate graphs of CVC vs. time by an investigator blinded as to the conditions, subjects, and antagonist treatment. The thresholds for cutaneous vasodilation were compared by ANOVA for repeated measures. Normothermic baseline CVCs, CVCs during the final minute of cold stress, and CVCs from the final 3 min of heat stress were compared by ANOVA. MAP and PR changes from normothermia to the end of heat stress were compared by paired t-tests. The level of statistical significance was defined as 0.05.
RESULTS
Protocol 1: local warming.
At Tloc = 34°C, CVC averaged 16 ± 3%max at untreated sites, 12 ± 2%max at LNAA-treated sites, 13 ± 2%max at NPLA-treated sites, and 14 ± 4%max at sites that were treated with the combination of LNAA and NPLA. These CVC values did not differ significantly between sites (P > 0.05 between sites).
When Tloc was increased to 41.5°C, CVC at all sites increased significantly to stable plateau levels (P < 0.05 vs. 34°C). These CVC values were 92 ± 6%max at untreated sites, 46 ± 7%max at LNAA-treated sites, 84 ± 8%max at NPLA-treated sites, and 56 ± 7%max at sites that were treated with the combination of LNAA and NPLA. The CVC plateaus at sites treated with LNAA and the combination of LNAA and NPLA (Mix) were attenuated to similar extents compared with CVCs attained at untreated and NPLA-treated sites (P < 0.05, LNAA or Mix vs. untreated or NPLA). CVC increases at untreated and NPLA treated sites did not differ (P > 0.05 between sites). These results are summarized in Fig. 3.
Fig. 3.
Summary of cutaneous vascular conductance (CVC) responses to local skin warming. At a local skin temperature (Tloc) of 34°C, CVC values, normalized to their respective maxima as induced by 56 mM nitroprusside perfusion, did not differ significantly between perfused with Ringer solution alone, 5 mM LNAA or NPLA in Ringer, or a combination of the 2 NOS antagonists in Ringer (P > 0.05 between sites). CVC increased in response to local skin warming at all sites (P < 0.05, 34°C vs. 41.5°C). At a Tloc of 41.5°C, these increased CVC values were significantly different between those sites perfused with either Ringer solution alone or NPLA alone compared with CVC values at sites perfused with either LNAA alone or a combination of the LNAA and NPLA in Ringer (P > 0.05 Ringer vs. NPLA; *P < 0.05 Ringer or NPLA vs. LNAA or combined LNAA and NPLA; P > 0.05 LNAA vs. combined LNAA and NPLA). Thus LNAA treatment attenuated the CVC response to local skin heating, but NPLA treatment added no further attenuation.
Protocol 2: heat stress.
In normothermia, CVC at untreated sites averaged 16 ± 3%max, 12 ± 2%max at LNAA-treated sites, 13 ± 2 at NPLA-treated sites, and 14 ± 4%max at sites treated with the combination of LNAA and NPLA. These levels did not differ among sites (P > 0.05 between sites).
In response to cold stress, CVC decreased at all sites regardless of treatment. By the final minute of cold stress, CVC had decreased to 10 ± 2%max at untreated sites, to 10 ± 2%max at LNAA-treated sites, to 9 ± 2%max at NPLA-treated sites, and to 10 ± 2%max at sites treated with the combination of LNAA and NPLA. These responses did not differ between sites (P > 0.05 between all sites).
MAP averaged 72 ± 3 mmHg and PR averaged 62 ± 3 beats/min under normothermic conditions. During the final 3 min of heat stress, MAP was 72 ± 4 mmHg and PR was 96 ± 3 beats/min. These MAP values did not differ significantly (P > 0.05, normothermia vs. heat stress); however, the increase in PR was significant (P < 0.05, normothermia vs. heat stress).
In response to whole body heating, Tor rose from 36.87 ± 0.13°C in normothermia to 37.57 ± 0.12°C at the peak of heat stress (P < 0.05, normothermia vs. heat stress). During whole body heating, CVC began to increase when Tor reached 37.04 ± 0.14°C at untreated sites, 37.01 ± 0.14°C at LNAA-treated sites, 37.02 ± 0.14°C at NPLA-treated sites, and 37.00 ± 0.22°C at sites treated with the combination of LNAA and NPLA. These Tor threshold temperatures for initiation of active vasodilation did not differ between treatments (P > 0.05 between sites).
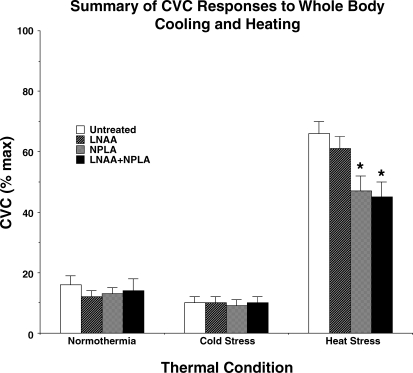
In response to whole body heating, CVC increased at all sites (P < 0.05 normothermia vs. peak heat stress). At the peak of heat stress, CVC had increased to 66 ± 4%max at untreated sites, 61 ± 4%max at LNAA-treated sites, 47 ± 5%max at NPLA-treated sites, and 45 ± 6%max at sites treated with the combination of LNAA and NPLA. CVC increases at sites treated with NPLA and the combination of LNAA and NPLA were attenuated to similar extents compared with CVC increases at untreated and LNAA-treated sites (P < 0.05 NPLA or Mix vs. untreated or LNAA). CVC responses at untreated and LNAA-treated sites did not differ between the two sites (P > 0.05 between sites) nor did responses differ between sites treated with NPLA and the combination of LNAA and NPLA (P > 0.05 between sites). CVC results for whole body cooling and heating results are summarized in Fig. 4.
Fig. 4.
Summary of CVC responses to whole body heat stress. In normothermia, CVC values did not differ between sites (P > 0.05 between sites). In response to whole body cold stress, CVC fell at all sites (P < 0.05). These responses did not differ between sites (P > 0.05 between sites). During whole body heat stress, CVC increased at all sites (P > 0.05). At the peak of heat stress, CVC values were significantly different between those sites perfused with either Ringer solution alone or LNAA alone compared with CVC values at sites perfused with either NPLA alone or a combination of the LNAA and NPLA in Ringer (P > 0.05 Ringer vs. LNAA; *P < 0.05 Ringer or LNAA vs. NPLA or combined LNAA and NPLA; P > 0.05 NPLA vs. combined LNAA and NPLA). Thus NPLA treatment attenuated the CVC response to local skin heating, but LNAA treatment added no further attenuation.
DISCUSSION
The present investigation was designed to explore further the roles of both the eNOS and nNOS isoforms in the responses of the forearm cutaneous vasculature to 1) local skin warming and 2) whole body heat stress. In prior experiments, a single NOS antagonist was used to inhibit separately either the eNOS or nNOS isoforms (30, 31, 52). We chose to use separate NOS isoform antagonism and combined NOS isoform antagonism to further characterize how NO is generated in the control of SkBF. Such an approach would allow investigation of whether both activity of both isoforms was required and/or whether the isoforms interact as appears to be the case with NO-dependent vasodilation in skeletal muscle (13, 24, 34). We reasoned that if both NOS isoforms interacted in either of the two responses, the combined blockade of both isoforms would result in a greater attenuation of that response than blockade of either isoform separately. We did not find this to be the case.
We found that the NO-dependent plateau phase of the vasodilator response to local skin warming was attenuated by LNAA antagonism of eNOS, unaltered by NPLA antagonism of nNOS, and that combined LNAA and NPLA antagonism of both isoforms had no greater attenuating effect than LNAA alone. We found that the vasodilation in response to whole body heat stress was unaltered by LNAA antagonism of eNOS, attenuated by NPLA antagonism of nNOS, and that combined LNAA and NPLA antagonism of both isoforms had no greater attenuating effect than NPLA alone. These results support the hypotheses that eNOS mediates NO generation attendant to local warming of the skin with no obvious role for nNOS in the process. Our results also support the hypothesis that nNOS is the sole isoform that mediates NO generation evoked by the cutaneous active vasodilator system during whole body heat stress with no obvious role for eNOS in the process. In no case did combined nNOS and eNOS antagonism have a greater effect than single isoform antagonism; thus simultaneous activation of the two isoforms is not required for either vasodilator response nor do the two isoforms interact during either local skin warming or whole body heating.
The results of our two protocols are consistent with our prior studies of the effects of separate NOS antagonists during local skin heating and whole body heat stress done in human forearm skin (30, 31). They are not consistent with those of Stewart et al. (52) done in human calf skin. They found that nNOS antagonism with Nω-nitro-l-arginine-2,4-l-diaminobutyric amide (Nω), a NOS antagonist with in vitro nNOS selectivity (16), significantly attenuated the NO-dependent plateau of local skin warming to a similar extent as the nonspecific NOS inhibitor, Nω-nitro-l-arginine (LNA). Stewart et al. used exogenous ACh to test their NOS blockade and found that LNA attenuated ACh-mediated vasodilation, but that Nω did not. Differential blockade by the two NOS antagonists on ACh-mediated vasodilation is consistent with selective nNOS antagonism by Nω despite debates as to how exogenous Ach effects vasodilation in skin (14, 29, 52). Their findings show that nNOS mediates the plateau phase of local skin warming in calf skin. In addition Stewart, et al. found that there was no further attenuation of the plateau phase by LNA over Nω alone, suggesting that eNOS did not play a role in the response. At present, data show that NO generated by NOS is the major effector of the plateau phase of the skin response to local warming but also suggest that totally different NOS isoforms mediate the NO-dependent SkBF response to local heating in the human forearm than in the calf. Whether such regional differences extend to other cutaneous vascular responses, such as those attendant to whole body heating, is unknown as most human studies have been done in the forearm. This raises the specter that control mechanisms of the cutaneous vasculature may have heretofore unappreciated anatomically distributed variations (19, 20, 23).
While NO appears to be a direct effector of the vasodilation induced by local skin warming, a number of findings suggest that the role of NO as a direct effector of cutaneous active vasodilation in heat stress may not be so unambiguous. During heat stress, we found that the Tor threshold at which the active vasodilator system begins to increase SkBF during whole body heating was unaltered by nNOS antagonism. This is consistent with our prior findings with the selective nNOS antagonist 7-NI (31) as well as with many (9, 25, 50), but not all (58), studies done with NOS antagonists that are not isoform selective. Immunohistochemical studies show that nNOS is localized in cutaneous nerves around dermal microvessels (17) and that nNOS is found in the same cholinergic neurons as the “classical” neurotransmitter, VIP (55). These findings indicate that cholinergic sympathetic vasodilator nerves possess both a “classical” transmitter and nitrergic system. Failure of NOS inhibition to alter the Tor threshold for initiation of active vasodilation is inconsistent with NO acting as a direct effector of active vasodilation, at least in the early stages of the process. Our observations and immunohistochemical data are consistent with the proposal that NO acts as a neuromodulator that synergistically augments prejunctional classical neurotransmitter release and/or postjunctional effects (57) and that the classical neurotransmitter(s) determines the onset of vasodilation during heat stress.
The foregoing proposal is also consistent with work by Morris and colleagues (40) that examined the physiological function of neurons that contain both classical transmitters and nNOS. They used botulinum toxin A to selectively block the exocytosis of synaptic vesicles that contain classical transmitters. They found that blockade of these vesicles inhibited neurogenic vasodilation by the classical neurotransmitters but had no effect on neurogenic vasodilation mediated by NO generation through nNOS (40). These results show that nNOS activation and NO release can be completely independent from vesicle exocytosis and that nNOS can be activated directly by increased intracellular Ca2+ and calmodulin (CaM) binding (43). We have previously found that botulinum toxin abolishes the cutaneous active vasodilator response to whole body heat stress (28). Given that 1) the release of vesicles that contain classical neurotransmitters from cholinergic nerves is abolished by botulinum toxin (28), 2) cutaneous active vasodilation is all but abolished by botulinum toxin (28), and 3) NO release by nNOS in cholinergic nerves is not abolished by botulinum toxin (40), evidence suggests that the NO-component of cutaneous active vasodilation mediated by nNOS may not act solely as a direct effector of active vasodilation. Current data thus suggest that NO may also act as a neuromodulator, perhaps interacting synergistically with classical neurotransmitters released by vesicles to augment their prejunctional release or postjunctional effects as proposed by Wilkins et al. (57).
Few studies have addressed the roles of NOS isoforms in humans in vivo due to the technical challenge of proving isoform selectivity of NOS agonists (10). The two constitutively expressed NOS isoforms, eNOS and nNOS, are structurally similar, and the development of isoform-selective antagonists has been challenging. In vitro studies of antagonists on isolated enzymes are used to estimate isoform selectivity as a good initial screen; however, agents that are purported to be highly isoform selective based on isolated enzyme studies often are not so in cell, tissue, or whole organism experiments, or vice versa. For example, inhibitory concentrations (IC50) of 7-NI for eNOS and nNOS are very similar based on in vitro studies with the purified isolated enzyme. While 7-NI cannot be used as an isoform-selective antagonist in vitro, this was not found to be the case from in vivo, cell, and whole animal studies. In vivo, 7-NI has no effects on blood pressure, endothelium-dependent relaxation of blood vessels, or ACh-induced vessel relaxation, all phenomena associated with eNOS activation (2, 18, 51, 59). The selectivity of 7-NI comes from the selective uptake of 7-NI by neurons but not by endothelial cells; thus 7-NI is an in vivo specific inhibitor of nNOS (2, 18, 36, 51, 59).
This complexity extends further to the mechanisms by which different NOS antagonists act. Some NOS blockers, such as LNA and its prodrug Nω-nitro-l-arginine methyl ester (l-NAME), are simple competitive antagonists of l-arginine binding to NOS. Other NOS antagonists are mechanism-based inhibitors (MBIs). MBIs act initially as simple, competitive l-arginine antagonists but are subsequently metabolized to products that irreversibly inactivate NOS. Nω-monomethyl-l-arginine (l-NMMA) is a MBI. Simple, competitive NOS antagonists and MBIs can have different physiological effects. For example, in the rat aorta, both the simple, competitive NOS antagonist LNA and the MBI l-NMMA increase basal tone; however, in this same tissue, LNA antagonizes the effects of exogenous ACh but l-NMMA does not (11). In isolation, this finding might be viewed as evidence that the MBI l-NMMA acts in vivo as a selective nNOS antagonist as it appears to leave ACh-mediated eNOS activation unaltered in the rat aorta; however, l-NMMA does block the vasodilation induced by ACh in the rat mesenteric vasculature (39). Thus the problems of determining NOS isoform specificity derive not only from conflicts between in vitro and in vivo specificity but also from conflicts of within in vivo studies even within a single species. It is not surprising that claims of physiological roles for NOS isoforms based on purportedly isoform-selective agents are met with skepticism.
Both agents used in the present study are MBIs and while it is believed that LNAA is metabolized to an irreversible NOS inactivator, NPLA appears to be metabolized to a slowly reversible NOS antagonist (4, 8). Given conflicted views about the mechanisms by which exogenous ACh causes vasodilation, we chose not to use pharmacological verification of isoform selectivity, but rather to use the dichotomous physiological results from local skin warming and whole body heat stress as evidence of selectivity (7, 14, 29–31). Both local skin warming and cutaneous active vasodilation in heat stress depend on NO generation by NOS for the full response to occur (15, 25, 27, 37, 38, 48, 49). LNAA attenuated the plateau phase of local skin warming but did not attenuate cutaneous active vasodilation. NPLA did not attenuate the plateau phase of local skin warming but did attenuate cutaneous active vasodilation. Neither antagonist added to the attenuating effect of the other. Thus relatively selective eNOS antagonist LNAA and the selective nNOS antagonist NPLA had dichotomous effects on physiological responses known to involve NO generation by NOS. These disparate findings are consistent with selective antagonism of NOS isoforms in vivo in the skin of the human forearm that was preserved when the two agents were combined; however, given the problematic nature of establishing antagonist selectivity, further study in other human tissues and anatomic regions is certainly warranted.
Caveats.
Our study did not specifically evaluate any role for inducible NOS (iNOS) in the normal physiological control mechanisms of SkBF and hence cannot unambiguously exclude a role for this isoform. The rationale for not expanding our work to include the iNOS isoform is based on the following observations.
Both of the constitutively expressed NOS isoforms, eNOS and nNOS, are found in significant levels in human dermis (3); hence either eNOS, nNOS, or both isoforms could generate the NO required for vasodilation in response to local skin warming or whole body heat stress. Although iNOS (type II NOS) has been detected by immunohistochemistry in human skin, it is present in minute amounts when compared with eNOS and nNOS (35). Only inflammatory processes, such as skin burns or wounds, increase iNOS levels to those of eNOS and nNOS (35).
The activities of the eNOS and nNOS isoforms are regulated by intracellular Ca2+ levels, indicating that these isoforms can be activated on a relatively short time scale of seconds to minutes. In contrast with eNOS and nNOS, iNOS regulation is Ca2+ independent and is regulated at a transcriptional level, only generating significant amounts of NO hours after transcription is stimulated (32, 42, 56).
All NOS isoforms are cofactor-requiring enzymes that consist of an oxygenase domain that contains heme, tetrahydrobiopterin, and an arginine binding site as well as a reductase domain that contains two flavins and a NADPH binding site. The oxygenase and reductase domains of all isoforms are linked by a CaM binding site that facilitates electron flow between the flavins, as well as from the reductase to the oxygenase domain. CaM thus controls NO generation by all NOS isoforms by regulating electron transfer. In iNOS, the CaM binding site is always occupied, regardless of intracellular Ca2+ levels; thus NO generation by iNOS is not Ca2+ regulated, in contrast to the eNOS or nNOS isoforms. In addition, eNOS and nNOS have an autoregulatory element in the reductase domain that stabilizes the CaM bound confirmation of the enzyme and facilitates NO generation; this element is lacking in iNOS (43). Since only eNOS and nNOS contain the regulatory mechanisms required to participate in rapidly activated physiological mechanisms such as those attendant to local skin warming and cutaneous active vasodilation in heat stress, evidence favors either eNOS and/or nNOS as the source of NO, but not iNOS.
In summary, we found in the human forearm that a relatively selective eNOS antagonist attenuated the plateau phase of the cutaneous vasodilation induced by local warming, but had no effect on the cutaneous active vasodilator response to whole body heat stress. In addition, we found that a selective nNOS antagonist did not attenuate the plateau phase of the cutaneous vasodilation induced by local warming, but attenuated the cutaneous active vasodilator response to whole body heat stress. Combined eNOS and nNOS antagonism added no attenuation to either response over that achieved by separate eNOS or nNOS blockade. We conclude that in human forearm skin 1) eNOS mediates NO generation in response to local skin warming and appears to have no specific role in the response to whole body heat stress; 2) nNOS mediates NO generation in response to whole body heat stress and appears to have no specific role in the response to local skin warming; and 3) the two constitutively expressed isoforms do not interact during either response.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-065599.
Glossary
- CVC
- Cutaneous vascular conductance
- eNOS
- Endothelial nitric oxide synthase (NOS3)
- iNOS
- Inducible nitric oxide synthase (NOS2)
- LDF
- Laser-Doppler flowmetry
- LNA
- Nω-nitro-l-arginine
- LNAA
- Nω-amino-l-arginine
- l-NAME
- Nω-nitro-l-argininemethyl ester
- l-NMMA
- Nω-monomethyl-l-arginine
- nNOS
- neuronal nitric oxide synthase (NOS1)
- Nω
- Nω-nitro-l-arginine-2,4-l-diaminobutyric amide
- NPLA
- Nω-propyl-l-arginine
- PR
- Pulse rate
- SkBF
- Skin blood flow
- Tloc
- Local skin temperature
- Tor
- Oral temperature
- Tsk
- Skin temperature
- 7-NI
- 7-Nitroindazole
REFERENCES
- 1. Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser-Doppler perfusion imaging. J Invest Dermatol 102: 808–811, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Babbage RC, Bland-Ward PA, Hart SL, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substances. Br J Pharmacol 110: 225–228, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol 110: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Byrk R, Wolff DJ. Pharmacological modulation of nitric oxide synthesis by mechanism-based inactivators and related inhibitors. Pharmacol Ther 157–178, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Charkoudian N. Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin Auton Res 11: 295–301, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Charkoudian N, Johnson JM. Reflex control of cutaneous vasoconstrictor system is reset by exogenous female reproductive hormones. J Appl Physiol 87: 381–385, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Christopoulos A, El-Fakahany EE. The generation of nitric oxide by G protein-coupled receptors. Life Sci 64: 1–15, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cooper GR, Mialkowski K, Wolff DJ. Cellular and enzymatic studies of Nω-propyl-l-arginine and S-ethyl-N-[4-trifluoromethyl)phenyl]isothiourea as reversible, slowly dissociating inhibitors selective for the neuronal nitric oxide synthase isoform. Arch Biochem Biophys 375: 183–194, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Dietz NM, Rivera JM, Warner DO, Joyner MJ. Is nitric oxide involved in cutaneous vasodilation during body heating in humans? J Appl Physiol 76: 2047–2053, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Fang J, Silverman RB. A cellular model for screening neuronal nitric oxide synthase inhibitors. Anal Biochem 390: 74–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frew JD, Paisley K, Martin W. Selective inhibition of basal but not agonist-stimulated activity of nitric oxide in rat aorta by NG-monomethyl-l-arginine. Br J Pharmacol 110: 1003–1008, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodges GJ, Chu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol 106: 1112–1118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmqvist B, Olsson CF, Svensson M, Svanborg C, Forsell J, Alm P. Expression of nitric oxide synthase isoforms in the mouse kidney: cellular localization and influence by lipopolysaccharide and Toll-like receptor 4. J Mol Histol 36: 499–516, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilation in young and aged human skin. J Physiol 563: 965–973, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang H, Martesek P, Roman LJ, Masters BS, Silverman RB. Nω-nitroarginine-containing dipeptide amides. Potent and highly selective inhibitors of neuronal nitric oxide synthase. J Med Chem 46: 3147–3153, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Ibba-Manneschi L, Niissalo S, Milia AF, Allanore Y, Del Rosso A, Pacini A, Manetti M, Toscano A, Cipriani P, Liakouli V, Giacomelli R, Kahan A, Konttinen YT, Mattucci-Cerinic M. Variations on neuronal nitric oxide synthase in systemic sclerosis. Skin Arthritis Rheumat 54: 202–213, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Jiang MH, Kaku T, Hada J, Hayashi Y. 7-Nitroindazole reduces nitric oxide concentration in rat hippocampus after forebrain ischemia. Eur J Pharmacol 380: 117–121, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Johnson JM. How does skin blood flow get so high? J Physiol 577: 768, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson JM. The cutaneous circulation. In: Laser Doppler Blood Flowmetry, edited by Shepherd A, Öberg P. Boston, MA: Kluwer Academic, 1990, p. 121–140 [Google Scholar]
- 21. Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 50: 814–818, 1981 [DOI] [PubMed] [Google Scholar]
- 22. Johnson JM, Park MK. Reflex control of skin blood flow by skin temperature: role of core temperature. J Appl Physiol 47: 1188–1193, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Joyner MJ. Cutaneous blood flow: uncomfortable in our own skin? Am J Physiol Heart Circ Physiol 296: H29–H30, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol 97: 293–301, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Kellogg DL, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kellogg DL, Jr, Pérgola PE, Kosiba WA, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 295: H123–H129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kellogg DL, Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kleinhart H, Pautz A, Linker K, Scharz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol 500: 255–226, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Kreidstein ML, Pang CY, Carlsen LN, Xu N. Evidence for endothelium-dependent and endothelium-independent vasodilation in human skin flaps. Can J Physiol Pharmacol 70: 1208–1216, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Levin NW, Morris AT, Lavarias VA, Wang Y, Glabman MB, Leung JP, Yusurf SA, LeVoci AL, Polaschegg HD, Kaufman AM. Effects of body core temperature reduction on haemodynamic stability and haemodialysis efficacy at constant ultrafiltration. Nephrol Dial Transplant 11: 31–34, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Meng W, Ayata C, Waeber C, Huang PL, Moskowitz MA. Neuronal NOS-cGMP-dependent Ach-induced relaxation in pial arterioles of endothelial NOS knockout mice. Am J Physiol Heart Circ Physiol 274: H411–H415, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Moore PK, al-Swayeh OA, Chong NW, Evans RA, Gibson A. l-NG-nitro arginine (l-NOARG), a novel, l-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol 99: 408–412, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morris JL, Jobling P, Gibbins IL. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am J Physiol Heart Circ Physiol 281: H2124–H2132, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Pérgola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA. Reflex control of active cutaneous vasodilation by skin temperature in humans. Am J Physiol Heart Circ Physiol 266: H1979–H1984, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Preiser JC, Zhang H, Vray B, Hrabak A, Vincent JL. Time course of inducible nitric oxide synthase activity following endotoxin administration in dogs. Nitric Oxide 5: 208–211, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Roman LJ, Masters BS. Electron transfer by neuronal nitric-oxide synthase is regulated by concerted interaction of calmodulin and two intrinsic regulatory elements. J Biol Chem 281: 23111–23118, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974 [DOI] [PubMed] [Google Scholar]
- 45. Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol 69: 154–166, 1977 [DOI] [PubMed] [Google Scholar]
- 46. Rowell LB, Murray JA, Brengelmann GL, Kraning KK., II Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Circ Res 24: 711–724, 1969 [DOI] [PubMed] [Google Scholar]
- 47. Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol 65: 1035–1041, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Shastry S, Minson CT, Wilson SA, Dietz NK, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 88: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Shastry S, Reed AS, Halliwill JR, Dietz NM, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol 93: 1947–1951, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol 51: 383–394, 1996 [DOI] [PubMed] [Google Scholar]
- 52. Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol 290: F1315–F1319, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984 [DOI] [PubMed] [Google Scholar]
- 55. Ventura S, Bavetta S, Milner P, Ralevic V, Burnstock G. Nitric oxide synthase is co-localized with vasoactive intestinal polypeptide in postganglionic parasympathetic nerves innervating the rat vas deferens. Neuroscience 83: 607–616, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Wang R, Ghahary A, Shen YJ, Scott PG, Tredget EE. Human dermal fibroblasts produce both nitric oxide and express both constitutive and inducible nitric oxide synthase isoforms. J Invest Dermatol 106: 419–427, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilation in humans. J Physiol 548: 963–969, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshida T, Limmroth V, Irikura K, Moskowitz MA. The NOS inhibitor, 7-nitroindazole, decreases focal infarct size but not the response to topical acetylcholine in pial vessels. J Cereb Blood Flow Metab 14: 924–929, 1994 [DOI] [PubMed] [Google Scholar]
- 60. Zhang HQ, Fast W, Marletta MA, Martesek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by Nω-propyl-l-arginine. J Med Chem 40: 3869–3870, 1997 [DOI] [PubMed] [Google Scholar]